Abstract
Objective
To determine the degree to which a high-frequency, low-magnitude vibration (HLV) signal emitted by a floor-based platform transmits to the distal tibia and distal femur of children with spastic cerebral palsy (CP) during standing.
Design
Cross-sectional study
Setting
University research laboratory
Participants
4 to 12 year-old children with spastic CP who could stand independently (n=18) and typically developing children (n=10) participated in the study.
Intervention
Not applicable
Main outcome measures
The vibration signal at the HLV platform (~33 Hz and 0.3 g), distal tibia and distal femur was measured using accelerometers. Degree of plantar flexor spasticity was assessed using the Modified Ashworth Scale.
Results
The HLV signal was greater (p<0.001) at the distal tibia than at the platform in children with CP (0.36±0.06 vs. 0.29±0.05 g) and controls (0.40 ± 0.09 vs. 0.24 ± 0.07 g). Although the HLV signal was also higher at the distal femur (0.35±0.09 g, p<0.001) than at the platform in controls, it was lower in children with CP (0.20±0.07 g, p<0.001). The degree of spasticity was negatively related to the HLV signal transmitted to the distal tibia (rs=−0.547) and distal femur (rs=−0.566) in children with CP (both p<0.05).
Conclusions
An HLV signal from a floor-based platform was amplified at the distal tibia, attenuated at the distal femur and inversely related to the degree of muscle spasticity in children with spastic CP. Whether this transmission pattern affects the adaptation of their bones to HLV requires further investigation.
Keywords: cerebral palsy, muscle spasticity, vibration, bone
Introduction
Children with physical disabilities such as cerebral palsy (CP) have reduced muscle1 and bone2, 3 mass and quality, especially in the lower extremities. This musculoskeletal deficiency in children with CP is associated with less force generating capacity of the muscles4 and a higher incidence of low-energy fractures in the lower extremities.5 Because children with CP have difficulty participating in physical activities6 leading to reduced mechanical loading on their skeletal system, identifying alternate nonpharmacologic treatments is of interest.7
Studies have shown that a floor based, high-frequency, low-magnitude vibration (HLV) signal has an anabolic effect on bone in various populations8, 9 including children with CP.10, 11 There are also studies showing no effect of HLV on bone12, 13 or an inconsistent effect across sites.14, 15 It is plausible that the effectiveness of HLV is dictated by the degree to which the HLV signal is transmitted to a particular bone site, similar to the site-specific effects of exercise.16 The tibia and femur are of interest in children with CP because they are the most commonly fractured bones and the distal femur is the most commonly fractured site.5 Unfortunately, the transmission of HLV to key bone sites in children with CP has not been studied.
The primary aim of this study was to determine the degree to which an HLV signal emitted by a floor-based platform transmits to the distal tibia and distal femur of children with spastic CP during standing. An amplification of vibration at the ankle and an attenuation at the knee has been observed in typically developing children.17 Whether a similar profile is exhibited in children with spastic CP is unknown. Toe-standing, which is common in children with spastic CP, has been shown to attenuate vibration at the ankle and the knee.18 Therefore, we hypothesized that the HLV transmission would be lower in children with spastic CP than typically developing children. We also hypothesized that there would be an inverse relationship between the degree of spasticity and HLV transmission in children with CP.
Methods
Participants
Children with spastic CP, 4–12 years of age, at the level of I to III using the Gross Motor Function Classification System (GMFCS) were recruited from the AI duPont Hospital for Children, Wilmington, DE. Children were excluded if they were unable to stand independently, if they had any metal rods in their thigh or leg, or if they had a surgery involving the musculoskeletal system or any anti-spastic medication, such as botulinum toxin, within the past year. Eighteen children with CP were tested. Eight of the children had one of more previous surgeries which include: adductor lengthening (n=1), adductor tenotomy (n=1), adductor release (n=1), hamstring lengthening (n=6), tendoachilles lengthening (n=1), gastrocnemius recession/lengthening (n=4) and botulinum toxin (n=8). Thirteen of the children had equinus deformity and walked on their toes. Five of the children were taking antiepileptic medication. Typically developing children (n=10) in the same age range as the children with CP, between the 5th and 95th percentiles for height and body mass and without a history of chronic medication use were recruited from the Newark, DE community to serve as controls. This study was approved by the AI duPont Hospital for Children and the University of Delaware Institutional Review Boards. Participants and their parents gave written assent and consent, respectively, before testing.
Study Design and Procedures
A within-subject and between-group comparison design was used. Anthropometrics, pubertal development, degree of spasticity, gross motor function, and vibration transmission were assessed during a single visit at the University of Delaware.
Anthropometrics
Height and body mass were measured while the children were wearing minimal clothing and were without shoes or braces. Height was measured to the nearest 0.1 cm using a stadiometer (Seca 217).a Body mass was measured to the nearest 0.1 kg using a weighing scale (Detecto D1130).b
Tanner Staging
Tanner staging was conducted by a physician assistant to assess sexual maturity of each participant. The Tanner stage rating scale ranges from I to V, with I indicating no sign of sexual maturity and V indicating full sexual maturity.19, 20
Modified Ashworth Scale (MAS)
Ankle plantar flexor tightness was assessed in children with CP while the participant was lying on a table in a supine position using the MAS. The grading system ranges from 0 to 4, with 0 indicating presence of normal tone and 4 indicating muscle rigidity in flexion/extension.21 The grade for each limb was based on an average grade of three trials. The reliability of spasticity assessment of the plantar flexors using the MAS was determined by evaluating 12 children with CP (4–11 years) on two different days one month apart. A Cohen’s Kappa=0.71 (p<0.001) indicates good reliability.
GMFCS
Gross motor function was assessed using the GMFCS, which ranges from I to V. Only those children who were GMFCS level I (walks without restrictions), II (walks without assistive device; limitations walking outdoors and in the community) or III (walks with assistive mobility device; limitations walking outdoors and in the community)22 participated in our study. The reliability of GMFCS was determined by evaluating 12 children with CP (4–11 years) on two different days one month apart. A Cohen’s Kappa=0.74 (p<0.001) indicates good reliability.
Vibration Transmission
Participants stood on an HLV platform (Juvent 1000 Motion Therapy System)c for three consecutive conditions (pre-HLV, HLV and post-HLV) of 30 seconds per condition. The HLV platform delivered a sinusoidal vertical vibration signal of approximately 0.3 g at a frequency of 30–37 Hz. No HLV was transmitted during the pre-HLV and post-HLV conditions, which were immediately before and immediately after the HLV condition, respectively. The platform was divided into right and left halves and the center of each foot was placed in the center of the respective half. The participants stood on the platform without shoes, socks or braces. They were instructed to stand on the platform as still as possible in a relaxed positon and were encouraged to stand without support. Although all children were able to stand without assistance, poor balance is an issue associated with CP which can be exacerbated by antiepileptic medications.23 Therefore, as a precaution, a standard wooden chair was placed in front of the HLV platform to allow intermittent support, if needed, and a spotter stood on each side of the participant to prevent falls. Only data collected during independent standing was used for the analysis. The same HLV platform was used for all participants.
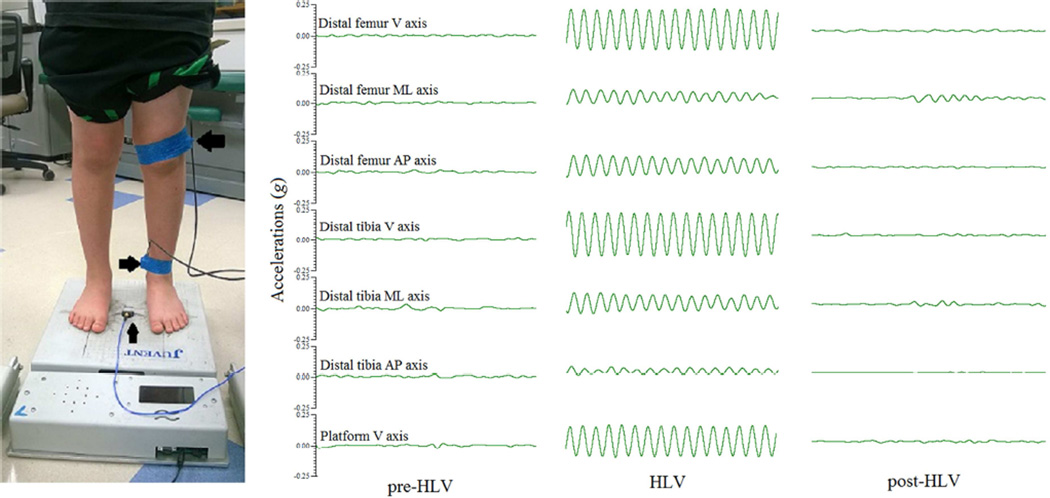
To quantify the amount of vibration emitted by the HLV platform, a uniaxial accelerometer (model 3711B1110G)d was secured to the center of the platform using double-sided tape, as per recommendations by Rauch et al.,24 and further secured with single-sided tape over the top of the accelerometer. To quantify the transmission of HLV signal to the distal tibia and the distal femur, triaxial accelerometers (model 3713B1113G),d were secured to the skin immediately above the medial malleolus of the distal tibia and at the lateral condyle of the distal femur to the limit of the participant’s comfort using a self-adhesive elastic bandage. The placement of all accelerometers was done by a single research assistant for all participants. All accelerometers were calibrated prior to each data collection session. The same accelerometers were used at the same sites for all participants. The data collection setup is displayed in Figure 1.
Figure 1.
On the left (A) is a participant with triaxial accelerometers secured to the distal femur (large arrow) and distal tibia (medium arrow) and a uniaxial accelerometer secured to a platform that emits a high-frequency, low-magnitude vibration (HLV) signal when turned on (small arrow). On the right (B) are sinusoidal waveforms showing signals at the HLV platform, distal tibia, and distal femur in the vertical (V), mediolateral (ML) and anteroposterior (AP) axis while standing before the HLV platform is turned on (pre-HLV), while it is on (HLV) and after it is turned off (post-HLV).
A 12-bit AD converter data acquisition device (model USB-1208FS)e was used to collect the vibration data at a sampling frequency of 2 kHz using DASYLab® (version 11.0).e Spike 2 (version 7.10)f was used to analyze the data after it was filtered using custom MATLABg coding. Peak-to-peak voltage values (mV) were converted to g force per the sensitivity for each respective axis. The resultant g was calculated for the triaxial accelerometers at the distal tibia and the distal femur using the following equation: R = √ (Vg2 + MLg2 + APg2) with Vg, MLg, APg representing g forces in the vertical, mediolateral, and anteroposterior directions. A 5 second period within each 30 second condition (pre-HLV, HLV and post-HLV) was chosen to calculate amplitude and frequency of HLV signals. HLV transmission was defined as the difference in HLV signals at the platform vs. the distal tibia and the distal femur.
The reliability of acceleration measurement at the platform, distal tibia and distal femur was determined by assessing 6 children with CP and 9 typically developing children (4–11 years) on two different days one month apart. Intraclass correlations = 0.89, 0.91 and 0.95, respectively, in the combined sample (all p<0.05) indicate excellent reliability.
Statistical analysis
Data were analyzed using SPSS (version 22.0).i Data were assessed for normality using skewness, kurtosis, and the Shapiro-Wilk test. Group differences in physical characteristics were assessed using independent t-tests. The Chi-square test of independence was used to determine if there group differences in Tanner stage. Paired t-tests were used to determine if pre-HLV and post-HLV signals were different. If they were not different, the pre-HLV condition was used for comparison to the HLV condition. A two-way ANOVA (group × site) with repeated measures on site was used to determine if there were group differences and an interaction effect in HLV transmission at the platform, distal tibia, and distal femur. If a group-by-site interaction was detected, simple contrasts were used to identify specific differences. A Bonferroni adjustment was made for multiple comparisons. Spearman correlation (rs) analysis was used to determine if there were relationships between MAS and the HLV signal transmission. Values are reported as mean±SD. The magnitude of effects of group differences was assessed by using Cohen’s d (d), with 0.2, 0.5, and 0.8 demonstrating small, medium, and large effects.25
Results
Physical characteristics of the participants are reported in Table 1. All data were normally distributed except pubic hair and testicular-penile/breast Tanner stage. There were no group differences in age, Tanner stages, body mass, BMI or BMI percentile (all p>0.20). Children with CP had lower height and height percentile than controls (p<0.05). In addition, height in children with CP was lower than the 50th age-and sex-based percentile (p<0.001). Body mass percentile (p=0.064) was lower in children with CP than controls and body mass in children with CP was also lower than the 50th age-and sex-based percentile (p=0.059), although the differences were marginally insignificant. Height, body mass and BMI in controls were not different from the 50th age- and sex-based percentiles (p>0.380).
Table 1.
Physical characteristics of children with cerebral palsy (CP) and typically developing children (Control)
| CP (n = 18) |
Control (n = 10) |
p | d | |
|---|---|---|---|---|
| Age (y) | 8.0 ± 2.5 | 8.7 ± 1.7 | 0.413 | 0.33 |
| Tanner stage (1/2/3) | ||||
| Pubic hair | 13/4/1 | 9/1/0 | 0.261 | 0.47 |
| Testicular-penile/breast | 16/2/0 | 8/1/1 | 0.323 | 0.42 |
| Height (m) | 1.2 ± 0.12a | 1.32 ± 0.07 | 0.011 | 1.11 |
| Height (percentile) | 21 ± 24a,b | 56 ± 32 | 0.003 | 1.32 |
| Body mass (kg) | 25.7 ± 9.3 | 29.8 ± 6.7 | 0.231 | 0.49 |
| Body mass (percentile) | 35 ± 32 | 58 ± 27 | 0.064 | 0.77 |
| BMI (kg/m2) | 17.3 ± 3.8 | 16.9 ± 2.9 | 0.786 | 0.11 |
| BMI (percentile) | 52 ± 37 | 48 ± 33 | 0.791 | 0.11 |
| GMFCS (I/II/III) | 10/7/1 | - | ||
| MAS (1/1.5/2/3) | 6/7/1/4 | - |
Values are means ± SD
Group difference, p < 0.05
Different from the 50th age-based percentile, p < 0.05
Gross motor function classification system (GMFCS)
Modified Ashworth Scale (MAS)
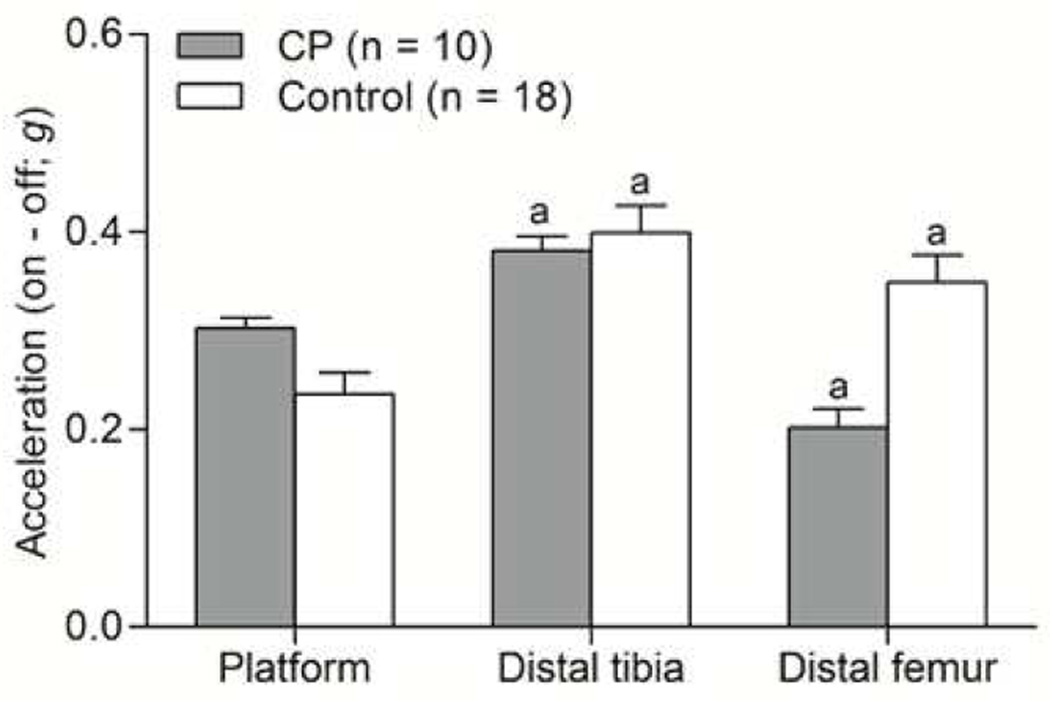
HLV data are reported in Figure 2. No differences in pre-HLV and post-HLV measures were observed at the platform (d=0.18, p=0.850), distal tibia (d=0.19, p=0.805), or distal femur (d=0.01, p=0.450) so the pre-HLV was used for comparison against the HLV condition. There was a significant group-by-site effect (d=1.49, p<0.001). Specifically, in children with CP, the HLV signal was significantly higher at the distal tibia (d=1.28, p<0.001) and lower at the distal femur (d=1.58, p<0.001) than at the platform. In controls, the HLV signal was significantly higher at the distal tibia (d=2.10, p<0.001) and higher at the distal femur (d=1.47, p<0.001) than at the platform. The same patterns were observed when only those children with CP who had equinus deformity (n=13) were compared to controls (p<0.05).
Figure 2.
Bar graphs showing transmission of the high-frequency, low-magnitude vibration (HLV) signal from the HLV platform to the distal tibia and the distal femur. The HLV signals are presented as values in the on condition (HLV) minus the off conditions (pre-HLV).aDifferent from the HLV signal values at the platform, p < 0.05.
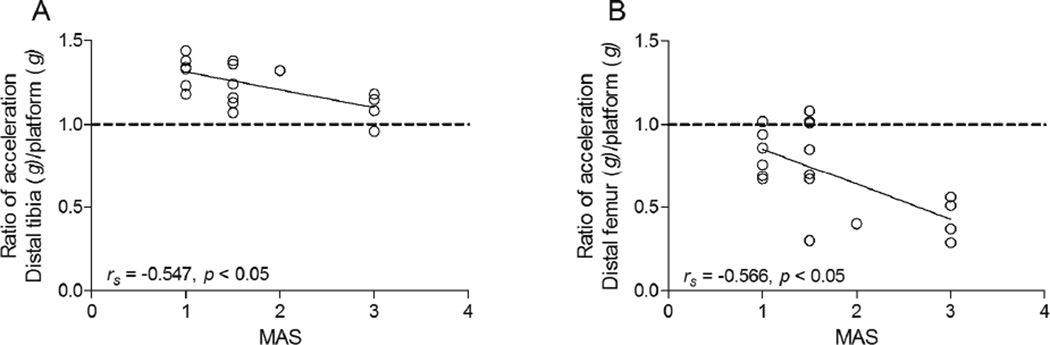
Scatter plots of MAS vs. HLV transmission, as indicated by the ratio of the vibration signal measured at the site (i.e., distal tibia or distal femur) to the vibration signal measured at the platform, in children with CP are depicted in Figure 3. MAS was moderately and negatively related to the transmission of HLV from the platform to the distal tibia (rs=−0.547, p=0.019) and from the platform to the distal femur (rs=−0.566, p=0.014).
Figure 3.
Scatter plots show the relationship between spasticity estimated using the Modified Ashworth Scale (MAS) and the transmission of high-frequency, low-magnitude vibration (HLV) signals emitted from the HLV platform to (A) the distal tibia and (B) the distal femur in children with CP (n = 18). The transmission of HLV signal was expressed as the ratio of the acceleration measured at the bone site divided the acceleration measured at the HLV platform. A value of 1.0 (dotted line) indicates 100 % transmission. Values above 1.0 indicate an amplification of HLV signal and values below 1.0 indicate a loss of HLV signal.
Discussion
This is the first study to investigate the transmission of a floor-based HLV platform (i.e., transmits <1.0 g) across the lower extremity of ambulatory children with spastic CP. Relative to the signal generated at the platform, the signal at the distal tibia was amplified in children with CP and in typically developing children. The signal was also amplified at the distal femur in typically developing children but it was dampened in children with CP to ~70% of the signal emitted at the platform.
One factor that may have contributed to the different pattern of HLV signal transmission in children with CP vs. typically developing children is the spasticity in children with CP. There was an inverse relationship between MAS and the degree of HLV transmission to the distal femur. The reason for this relationship is unclear but it may be related to the effect of spasticity on posture. Some of the children with CP had equinus deformity (n=13 of 18). Therefore, they had difficulty limiting knee and ankle plantar flexion and had difficulty or were unable to place their feet completely flat on the HLV platform, which are common postures in children with spastic CP26. Previous studies have shown that increased knee flexion and toe standing leads to attenuation of a vibration signal at the knee.18, 27 However, the pattern of the results in the present study was the same when only those with equinus deformity were included in the statistical analysis which suggests that other factors may have contributed to the dampened transmission of HLV to the distal femur. Another factor that may have contributed to the dampened transmission of HLV to the distal femur include unequal weight distribution in the lower extremities creating variable degrees of muscle tension or muscular force production. Increased muscular force production has been shown to dampen vibration.28 The loss of HLV signal at the distal femur may also be related to mechanical filtering through altered soft tissue.28 Individuals with CP have a high concentration of fat within29 and surrounding30 their musculature, which might lead to attenuated HLV signals at the distal femur. However, the effect of leg adiposity on HLV transmission has not been investigated. Foot position on the HLV platform is another element that could affect vibration transmission and local site-specific resonance frequency.24 For example, standing in the step position (i.e., standing with one foot behind the other) has been shown to give rise to local resonance at 12.5–25 Hz leading to amplified vibration transmission at the lateral epicondyle of the femur. The potential effect of foot position was minimized in the present study by having all participants placed their feet in the same place at the center of the platform.
The amplified HLV signal at the distal tibia in children with CP and in typically developing children is consistent with previous findings in adults.17, 18, 31 It is also consistent with a study by Bressel et al.17 who reported an amplification of vibration signal to the distal tibia in typically developing children during standing while using a higher magnitude vibration signal (~2.15–5.15 g) than used in the present study (~0.3 g) and not considered an HLV (i.e., not <1 g). The amplification of HLV signal can be attributed to many factors, chief being the resonant frequency. Resonance is the propensity of a structure to oscillate at a greater amplitude at some specific frequencies or over a range of frequencies. Previous studies have determined that the resonance frequency of the ankle lies between 10–63 Hz in healthy adults18, 31 and between 28–33 Hz in children.17 In the current study, a mean frequency of 33 Hz was used. Another factor that may contribute to the amplified HLV signal at the distal tibia is the lack of natural shock absorbers to attenuate the vibration signal at the foot.32 The effect of an amplified signal on bone is unknown and requires further investigation.
The HLV signal at the distal femur of typically developing children was higher than the signal measured at the HLV platform (Figure 2). This is inconsistent with previous studies that have examined vibration transmission to the knee and showed a dampening of the signal.17, 18, 31 However, previous studies have assessed vibration signals18, 31 that were higher in magnitude or were at different sites, such as the lateral tibial tuberosity.17, 31 In the present study, we assessed the transmission of a very low magnitude vibration signal (~0.3 g) at the lateral femoral condyle. There is evidence that the resonance frequency is different along the length of a bone,33 which may partially account for our different results.
One strength of our study was the homogeneous nature of the participants. All the children with CP were able to stand independently and all had spasticity in their leg muscles. The pattern of findings remained the same when typically developing children were matched to a subgroup of 10 children with CP for age, sex, and race. Moreover, typically developing children were not different from the 50th age-based percentile for height, body mass and BMI. Another strength of this study is that the transmission of HLV was evaluated at key bone sites. More than 80 % of all fractures in children with CP occur in the lower extremities, with almost half of all fractures occurring at the distal femur.5
Study limitations
One of the limitations of this study is the lack of kinematic data. We did not collect any data related to posture of our participants. Skin-mounted accelerometers can overestimate the acceleration signal by ~10%.34 However, even when a 10% correction was made (not reported), the pattern of the findings remained the same.
Conclusions
The results from this study suggest that the HLV signal from a floor-based platform is amplified at the distal tibia but dampened at the distal femur in children with CP. The dampening of HLV is related to the degree of spasticity, with greater spasticity associated with less signal transmission. Future studies are needed to uncover the specific mechanisms underlying the relationship between spasticity and HLV signal transmission are needed. Studies are also needed to determine if the potential HLV-induced benefits to bone mass and architecture in children with CP are influenced by the degree of HLV transmission.
Acknowledgments
The study was supported by the National Institutes of Health (HD071397). We express our deepest gratitude to all research participants and their families.
Abbreviations
- CP
Cerebral palsy
- GMFCS
Gross motor function classification system
- HLV
High-frequency, low-magnitude vibration
- MAS
Modified Ashworth Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of the data was presented at the American Academy of Cerebral Palsy and Medicine 68th Annual Conference, San Diego, CA, September 2014. The clinicaltrials.gov number is NCT01803464.
Conflict of Interest: No commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a benefit on the authors or any organization with which the authors are associated.
Suppliers
Seca Medical Measuring Systems and Scales, Hammer Steindamm 9 – 25, Hamburg, Germany 22089
Detecto, 203 East Daugherty Ave, Webb City, MO 64870
Juvent Inc, 2000 Avenue P, Ste 14, Riviera Beach, FL 33404
PCB Piezotronics, 3425 Walden Avenue, Depew, NY 14043
Measurement Computing Corp., 10 Commerce Way, Norton, MA 02766
Cambridge Electronic Design, 4 Cambridge Science Park, Milton Rd, Cambridge, Cambridgeshire, UK CB4 0FE
The MathWorks, Inc., 3 Apple Hill Drive, Natick, MA 01760
International Business Machines Corp., New Orchard Rd., Armonk, NY 10504.
References
- 1.Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45(8):542–550. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 2.Modlesky CM, Kanoff SA, Johnson DL, Subramanian P, Miller F. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporos Int. 2009;20(4):609–615. doi: 10.1007/s00198-008-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlesky CM, Whitney DG, Singh H, Barbe MF, Kirby JT, Miller F. Underdevelopment of trabecular bone microarchitecture in the distal femur of nonambulatory children with cerebral palsy becomes more pronounced with distance from the growth plate. Osteoporos Int. 2015;26(2):505–512. doi: 10.1007/s00198-014-2873-4. [DOI] [PubMed] [Google Scholar]
- 4.Riad J, Haglund-Akerlind Y, Miller F. Power generation in children with spastic hemiplegic cerebral palsy. Gait Posture. 2008;27(4):641–647. doi: 10.1016/j.gaitpost.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Presedo A, Dabney KW, Miller F. Fractures in patients with cerebral palsy. J Pediatr Orthoped. 2007;27(2):147–153. doi: 10.1097/BPO.0b013e3180317403. [DOI] [PubMed] [Google Scholar]
- 6.Carlon SL, Taylor NF, Dodd KJ, Shields N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35(8):647–655. doi: 10.3109/09638288.2012.715721. [DOI] [PubMed] [Google Scholar]
- 7.Houlihan CM, Stevenson RD. Bone Density in Cerebral Palsy. Phys Medi Rehabil Clin N Am. 2009;20(3):493-+. doi: 10.1016/j.pmr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 9.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21(9):1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 10.Wren TAL, Lee DC, Hara R, Rethlefsen SA, Kay RM, Dorey FJ, et al. Effect of High-frequency, Low-magnitude Vibration on Bone and Muscle in Children With Cerebral Palsy. J Pediatr Orthoped. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19(3):360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 12.Slatkovska L, Alibhai SMH, Beyene J, Hu HX, Demaras A, Cheung AM. Effect of 12 Months of Whole-Body Vibration Therapy on Bone Density and Structure in Postmenopausal Women A Randomized Trial. Ann Intern Med. 2011;155(10):668–U45. doi: 10.7326/0003-4819-155-10-201111150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Leung KS, Li CY, Tse YK, Choy TK, Leung PC, Hung VWY, et al. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly-a cluster-randomized controlled trial. Osteoporos Int. 2014;25(6):1785–1795. doi: 10.1007/s00198-014-2693-6. [DOI] [PubMed] [Google Scholar]
- 14.Lam TP, Ng BKW, Cheung LWH, Lee KM, Qin L, Cheng JCY. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–1636. doi: 10.1007/s00198-012-2144-1. [DOI] [PubMed] [Google Scholar]
- 15.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Haapasalo H, Kannus P, Sievanen H, Heinonen A, Oja P, Vuori I. Long-term unilateral loading and bone-mineral density and content in female squash players. CalcifTissue Int. 1994;54(4):249–255. doi: 10.1007/BF00295946. [DOI] [PubMed] [Google Scholar]
- 17.Bressel E, Smith G, Branscomb J. Transmission of whole body vibration in children while standing. Clin Biomech. 2010;25(2):181–186. doi: 10.1016/j.clinbiomech.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Harazin B, Grzesik J. The transmission of vertical whole-body vibration to the body segments of standing subjects. J Sound Vib. 1998;215(4):775–787. [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in Pattern of Pubertal Changes in Girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in Pattern of Pubertal Changes in Boys. Archives of Disease in Childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashworth B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. Practitioner. 1964;192(115):540–542. [PubMed] [Google Scholar]
- 22.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 23.van Gaalen J, Kerstens FG, Maas RP, Harmark L, van de Warrenburg BP. Drug-induced cerebellar ataxia: a systematic review. CNS Drugs. 2014;28(12):1139–1153. doi: 10.1007/s40263-014-0200-4. [DOI] [PubMed] [Google Scholar]
- 24.Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, et al. Reporting whole-body vibration intervention studies: Recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for Behavioral Sciences. 2nd. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
- 26.Lidbeck CM, Gutierrez-Farewik EM, Brostrom E, Bartonek A. Postural Orientation During Standing in Children With Bilateral Cerebral Palsy. Pediatr PhysTher. 2014;26(2):223–229. doi: 10.1097/PEP.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Griffin MJ. Dynamic response of the standing human body exposed to vertical vibration: Influence of posture and vibration magnitude. J Sound Vib. 1998;212(1):85–107. [Google Scholar]
- 28.Wakeling JM, Nigg BM. Modification of soft tissue vibrations in the leg by muscular activity. J Appl Physiol. 2001;90(2):412–420. doi: 10.1152/jappl.2001.90.2.412. [DOI] [PubMed] [Google Scholar]
- 29.Noble JJ, Charles-Edwards GD, Keevil SF, Lewis AP, Gough M, Shortland AP. Intramuscular fat in ambulant young adults with bilateral spastic cerebral palsy. BMC Musculoskelet Disord. 2014;15(1):236. doi: 10.1186/1471-2474-15-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154(5):715–720. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiiski J, Heinonen A, Jaervinen TL, Kannus P, Sievanen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23(8):1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 32.Voloshin A, Wosk J. An in vivo study of low back pain and shock absorption in the human locomotor system. Journal of Biomechanics. 1982;15(1):21–27. doi: 10.1016/0021-9290(82)90031-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhao LM, Dodge T, Nemani A, Yokota H. Resonance in the mouse tibia as a predictor of frequencies and locations of loading-induced bone formation. Biomech Model Mechan. 2014;13(1):141–151. doi: 10.1007/s10237-013-0491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim W, Voloshin AS, Johnson SH, Simkin A. Measurement of the impulsive bone motion by skin-mounted accelerometers. J Biomech Eng. 1993;115(1):47–52. doi: 10.1115/1.2895470. [DOI] [PubMed] [Google Scholar]