Abstract
Neurons are highly polarized cells with axonal and dendritic projections that extend over long distances. Target-derived neurotrophins provide local axonal cues that function in developing neurons, while physical or chemical injuries to long axons initiate local environmental cues in mature neurons. In both instances initial responses at the location of stimulation or injury must be coordinated with changes in the transcriptional program and subsequent changes in axonal protein content. To achieve this coordination, intracellular signals move ‘there and back again’ between axons and the nucleus. Here, we review new findings on neuronal responses to growth factors and injury and highlight the coordination of transcription, translation and transport required to mediate communication between axons and cell bodies.
Introduction
Intracellular communication is a major challenge for neurons due to the great distances traversed by long axons. Signals generated at axons in response to external stimuli must be retrogradely transported to the nucleus to elicit transcriptional changes and enable adaptations in response to the stimuli. Subsequently, new transcripts or proteins are transported back to axons from the soma to generate a multifaceted adaptive response. Thus, bidirectional transport between axon terminals and cell nuclei is an essential aspect of executing these responses. Kinesins and cytoplasmic dynein, respectively, are the molecular motors that move components along microtubules in the anterograde and retrograde direction. Disruption of axonal transport in either direction impedes responses to neurotrophins and injury, resulting in axonal degeneration and functional deterioration. For detailed information on axonal transport mechanisms, please refer to recent reviews: [1,2]. In this review, we focus on recent findings on retrograde and anterograde mechanisms coordinating transcription and local translation in response to neurotrophins and axonal injury.
There: retrograde signaling from axons to cell bodies
During development, neurons undergo complex changes in cellular morphology as they assemble into functional circuits. Target-derived neurotrophins (NGF, BDNF, NT3/4) initiate signaling cascades at axonal terminals by binding to their receptors (TrkA, TrkB, TrkC, p75) to regulate axonal growth and innervation, circuit refinement and survival [3]. The signaling endosome model proposes that neurotrophin binding causes activation and internalization of its receptor. The resultant vesicles are transported retrogradely via dynein motors [4,5] and elicit transcriptional changes at the soma [6–11]. Inhibition of Trk internalization and dynein-based retrograde transport reduces neuron viability [12]. Molecular characterization of signaling endosomes and how they attach to dynein remains an area of active investigation [5,13].
Axonal injuries may disrupt basal neurotrophin signaling. In addition, injury itself initiates signals conveyed from sites of injury to the nucleus to alter the transcriptional state of the neuron. Propagation of calcium waves from injury sites to the soma is a very rapid mechanism that induces transcriptional changes [14]. Additional signals initiated at the injury site are more slowly retrogradely transported to the soma by dynein to initiate additional transcriptional changes necessary for axonal regeneration after injury. While dynein is a fast motor, it moves with velocities between 1 and 3 μm/s [15]. Below, we discuss recently described mechanisms of retrograde signaling events in development and in injury responses.
Retrograde signaling during development
Initial studies identified several mRNAs that are transported to axons and are essential for axonal outgrowth, including β-actin. Surprisingly however, axonal mRNAs also include transcripts encoding proteins necessary for nuclear functions. Studies from Jaffrey and colleagues indicated that NGF stimulation of axon terminals results in axonal translation of the transcription factor CREB, and that newly synthesized CREB associates with NGF-TrkA signaling endosomes. Retrograde transport of phosphorylated CREB with signaling endosomes elicits transcriptional responses essential for neuronal survival [16]. As the location of NGF stimulation specifies the nature of transcriptional responses, it will be important to determine whether CREB or other transcription factors are critical for spatially distinctive responses. Notably, Creb1 was not detected in axons of sympathetic, hippocampal or retinal neurons [17–19], suggesting that some signaling mechanisms may vary among distinct neuronal populations.
Recent studies have identified Calcineurin as a component responsible for forming signaling endosomes. Calcineurin interacts with, and dephosphorylates Dynamin1 in an NGF-dependent manner [20]. A recent study from the Kuruvilla lab indicated that formation of signaling endosomes is altered in Down syndrome due to increased expression of RCAN1. RCAN1, a gene triplicated in Down syndrome, encodes a Calcineurin inhibitor. Excess RCAN1 inhibited dynamin dephosphorylation and TrkA endocytosis, thereby attenuating retrograde signaling in vitro and in vivo and leading to decreased innervation of target tissues [21] (Figure 1).
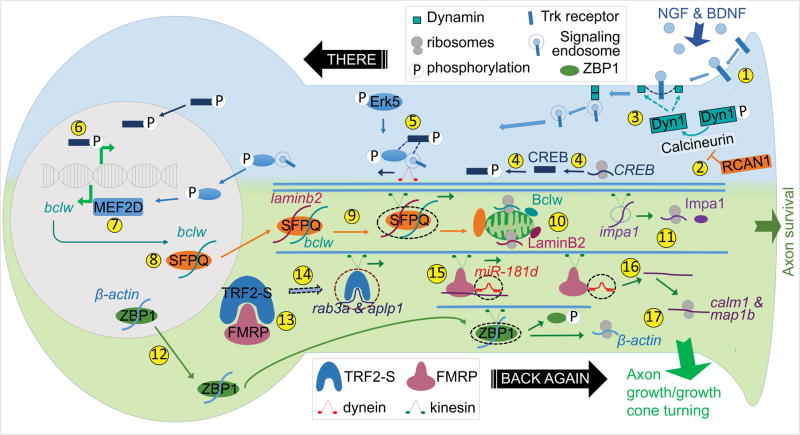
Figure 1. Anterograde and retrograde mechanisms in response to target-derived neurotrophins.
(1) NGF or BDNF binds and activates Trk receptors. (2) Formation of signaling endosome is enabled by Calcineurin, which is inhibited by RCAN1. (3) Calcineurin dephosphorylates Dynamin thereby promotes endocytosis of ligand-Trk complex. (4) Neurotrophin stimulation induces local synthesis and phosphorylation of CREB, (5) which associates with retrogradely transported signaling endosome along with phosphorylated Erk5 (pErk5) and (6) activates transcriptional responses in the soma. (7) MEF2D, a downstream transcription factor of pErk5, induces bclw transcription. (8) SFPQ binds bclw for nuclear export. (9) In the soma, laminb2 unites with SFPQ-bclw to be trafficked to axon via kinesin. (10) Axonal translation of bclw and laminb2 at the mitochondria and (11) translation of impa1 promote axon survival. (12) ZBP1-β-actin is exported from nucleus and transported via kinesin to the axon. (13) In the soma, FMRP may sequester TRF2-S, (14) which otherwise binds to rab3a and aplp1 mRNAs to be transported to the axon. (15) Both calm1 and map1b are anterogradely transported by FMRP-miR-181d complex via kinesin and (16) then released from this complex in the axon. Axonal TRF2-S and (17) local translation of calm1, map1b and β-actin promote axon growth and growth cone turning.
Retrograde signaling in injury response
Axonal translation also contributes to retrograde responses to injury. The demonstration that importin β1 is translated within injured axons was initially surprising, as Importins bind nuclear localization signals (NLSs) of molecules destined for the nucleus and promote nuclear import. Following injury, locally translated Importin β1 binds Importin α, forming a functional NLS binding complex that associates with dynein. This allows nuclear factors with an NLS sequence to bind this complex and to be retrogradely transported from axons after injury. Once this cargo arrives at the soma, Importins escort the cargo through the nuclear pore, so the cargo components can elicit transcriptional changes. Blocking NLS sites on Importins leads to decreased injury response, demonstrating the importance of this communication for biological functions [22]. Fainzilber and colleagues demonstrated that only importin β1 isoforms with a long 3′UTR are targeted to and translated within axons. Disruption of the 3′UTR axonal targeting motif greatly attenuates the majority of cell body transcriptional responses to injury and delays recovery [23].
Importin-mediated nuclear transport is regulated by a small G-protein Ran. Within the nucleus, RanGTP binds Importin β1, causing release of the cargo and nuclear export of Importin-Ran complex. Within the axons, RanBP1, along with RanGAP, catalyzes dissociation of RanGTP from the Importin complex and its hydrolysis, thereby providing access for new cargoes to bind the Importin complex. Like importin β1, RanBP1 is translated in axons after injury. Together, locally translated Importin β1 and RanBP1 orchestrate the movement of critical transcription factors from axons to nuclei upon injury [24]. Although the precise axonal localization motif of RanBP1 is not yet known, the long 3′UTR variant of RanBP1 is required to target this mRNA to axons.
Critical cargoes transported by the Importin complex from axons to soma following injury include classical Importin cargoes such as STAT3, and non-classical Importin cargoes such as Vimentin. STAT3 is a transcription factor that is synthesized in axons following injury. Phosphorylated STAT3 (pSTAT3) is then retrogradely transported to nuclei. Together, local translation of importin β1 and STAT3 enables a specific signal to travel from a site of injury and initiate a proregenerative program [25,26]. Verge and colleagues identified Luman/CREB3 as another classical importin cargo that is locally synthesized in response to injury. Luman is a transmembrane transcription factor required for axonal outgrowth following injury. Local translation produces endoplasmic reticulum-associated Luman protein. Cleavage of the N-terminus of Luman enables both association with Importins and retrograde transport of the transcriptionally active protein [27]. Among the transcriptional targets regulated by Luman are several components of the Unfolded Protein Response (UPR) [28].
The non-classical importin cargo Vimentin has also been implicated in injury responses. A soluble form of Vimentin is locally synthesized and cleaved following injury, binds pErk1/2 (pErk) and thereby links pErk to importin/dynein complexes. Upon arrival at the soma, pErk dissociates from the complex and phosphorylates and activates transcription factors such as Elk1 [29] (Figure 2).
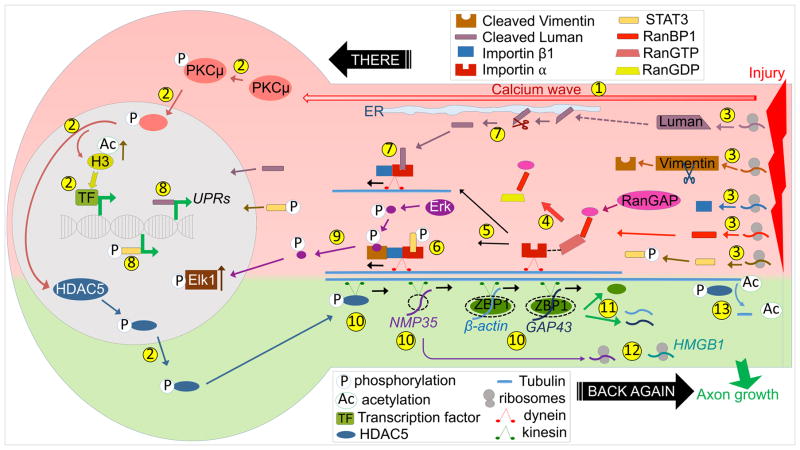
Figure 2. Anterograde and retrograde mechanisms for injury responses.
(1) Injury induces back-propagating calcium wave towards the soma. (2) Ca+2 increase activates PKCμ and causes its nuclear translocation, which triggers HDAC5 nuclear export, increased acetylated Histone H3, and subsequent changes in transcription. (3) Injury induces local translation of RanBP1, Importin β1, Vimentin, STAT3 and Luman. (4) RanBP1 and RanGAP trigger dissociation and hydrolysis of RanGTP. (5) This leads to formation of the importin complex by binding of Importin β1 to Importin α. (6) pSTAT3 and (7) cleaved Luman are retrogradely transported by the Importin complex via dynein, (8) and then alter transcription. (9) Cleaved Vimentin is a non-classical cargo that enables transport of pErk, which dissociates from the Importin complex and activates nuclear Elk1. (10) HDAC5, NMP35, and ZBP1 carrying β-actin or GAP43 are delivered to axons via kinesins. (11) Release of cargo mRNAs from ZBP1, (12) local translation of NMP35 and HMGB1 and (13) tubulin deacetylation by HDAC5 promotes axon regrowth.
Back again: anterograde signaling from cell body to axons
Neurotrophins or injury signals induce transcriptional changes, and the results of the new transcriptional program must be communicated back to axons to execute changes in outgrowth or axon maintenance. Transport of newly transcribed mRNAs to axons provides an efficient mechanism for this phase of communication, as multiple copies of the protein(s) can be locally synthesized in a spatially restricted fashion. Targeting of mRNAs to axons requires localization sequences, usually present in the 3′UTR, which bind specific RNA-binding proteins (RBPs). Together, mRNAs and RBPs assemble into RNA granules that are transported by kinesins to distal axons. Below, we provide examples of anterograde signaling events in development and in injury responses.
Anterograde signaling during development
ZBP1 (Zipcode binding protein1) is an RBP that interacts with β-actin and GAP-43 mRNAs through zipcode and AU-rich element in their 3′UTRs, respectively, and is required for axonal localization of these transcripts [30,31]. ZBP1 has clear roles in anterograde signaling in developmental responses to target-derived neurotrophins. ZBP1 enables both nuclear export of β-actin mRNA, and its subsequent transport to distal axons and growth cones by kinesin [32]. ZBP1 also regulates translation of β-actin; following neurotrophin stimulation of distal axons, phosphorylation of ZBP1 leads to release and local translation of β-actin mRNA. Regulated synthesis of β-actin within distal axons allows outgrowth and turning of growth cones [30,33–35]. Together these studies identify multi-step regulation of β-actin mRNA by coordinated nuclear export, transport to axons, and local release and translation, which enables spatiotemporal control of axonal β-actin production and axonal navigation.
While β-actin is the most well characterized axonal mRNA, multiple additional transcripts are localized to developing axons and regulated by target-derived neurotrophins [36]. Neurotrophin-regulated axonal genes include impa1, which encodes a key enzyme in inositol cycle that produces phosphatidylinositol (PI). The long isoform mRNA, impa1-L, is enriched in axons and has a novel NGF-responsive sequence within the 3′UTR. Data from Riccio and colleagues suggested that local NGF activates downstream cascades to enable axonal localization and translation of impa1, and therefore generate PI products critical for axon maintenance [17].
Several mRNAs that are transcribed in the nucleus and translated in axons encode mitochondria-associated proteins. These include COXIV [37], laminb2 and bclw. Axonal translation of laminb2 can be regulated by Engrailed, NGF or BDNF. Locally synthesized LaminB2 localizes to mitochondria, and knockdown of laminb2 leads to reduced mitochondria membrane potential and longer mitochondria. These LaminB2-dependent changes in mitochondria likely explain the critical role of LaminB2 in axon viability [38]. Work from our group demonstrated that target-derived neurotrophins also stimulate local translation of bclw to promote axonal viability [39].
SFPQ (splicing factor, polyglutamine rich) is an RBP that coordinates neurotrophin-stimulated anterograde axonal transport of both bclw and laminb2. Intriguingly, bclw and laminb2 colocalize to the same RNA transport granules, and this colocalization requires SFPQ. Like laminb2 and bclw knockdown [38,39], SFPQ knockdown results in axon degeneration [40]. Thus, SFPQ binds and coordinates expression of functionally related axonal mRNAs in space and time to enable a program of axonal survival. Future studies will determine whether co-assembly of functionally related mRNAs into transport granules represents a general phenomenon of axonal RNA regulons.
Fragile X mental retardation protein (FMRP) binds multiple distinct mRNAs, and therefore has the potential of orchestrating additional RNA regulons [41]. Two recent studies uncovered distinct roles of FMRP in axonal targeting of mRNAs. Wang et al showed that FMRP associates with both miRNAs and mRNAs. In DRG neurons, miR181-d binds and inhibits translation of its target mRNAs map1b and calm1. FMRP promotes axonal delivery of miR181-d as well as its target mRNAs, thus delivering the mRNAs in a repressed form. Local NGF stimulation induces dissociation of target mRNAs from FMRP-miR181-d, thereby terminating translational inhibition. This novel regulatory activity of FMRP is necessary for axon outgrowth [42]. Zhang et al demonstrated that FMRP binds TRF2-S, a novel RBP, and that these two RBPs have opposing effects on target mRNAs, including rab3a and aplp1. While TRF2-S promotes delivery of target mRNAs to axons and enables axon outgrowth and synaptic changes, FMRP blocks these processes [43]. In both studies, FMRP has a regulatory role in axonal growth, although this is achieved via distinct mechanisms (Figure 1).
Anterograde signaling in injury response
ZBP1 and its mRNA cargoes are also critical for axon regrowth after injury in adults. Axonal localization of β-actin and GAP-43 are impaired in ZBP1+/- mice, and so these animals exhibit decreased regeneration after nerve injury [44]. Additional mRNAs that are localized and translated in axons upon injury include the chromatin interacting HMGB1 and NMP35 [45,46]. Both mRNAs require their 3′UTRs for axonal localization and contribute to axonal outgrowth after injury [45,46].
Neurons also employ epigenetic mechanisms to change gene expression for adaptation upon injury. Recently, Cavalli and colleagues demonstrated that the histone deacetylase HDAC5 has dual roles in injury responses; it functions as an epigenetic modifier regulating transcription and also functions in axons. Axonal injury induces a back-propagating calcium wave that travels to the soma. Increased intracellular Ca2+ activates PKCμ, leading to phosphorylation and nuclear export of HDAC5 and anterograde transport of this enzyme to the injury site. HDAC5 deacetylates axonal tubulin, reducing microtubule stability and thereby enhancing regeneration. Simultaneously, nuclear export of HDAC5 enhances acetylation of Histone H3, and thereby alters transcription [14,47] (Figure 2).
Conclusions
Recent studies reveal a multi-step coordination of transcription, local translation and transport that enable multifaceted outcomes in response to neurotrophins and injury. Local translation in axons is a key component that functions both to initiate retrograde signaling and also to implement axonal events in response to these environmental cues. Locally synthesized transcription factors are retrogradely transported to initiate rapid and distinct transcriptional responses at the soma. To implement subsequent changes in axonal composition, several RBPs, including FMRP, ZBP1 and SFPQ, bind elements within the 3′UTR of target mRNAs and coordinate multiple steps of mRNA transport and regulation.
To date, much of the research relies on studies of cultured neurons. New techniques such as Translating Ribosome Affinity purification (TRAP) and RiboTag will enable in vivo analysis of the axonal translatome in response to injury or neurotrophic stimulation. In vivo studies will also provide insights into the functional significance of local translation. While local translation enables fast response at the site of stimuli and spatiotemporal control of protein synthesis, it is unknown why mRNAs of transcription factors are located to axons where they are locally translated and transported back to the cell body. Future studies will be needed to determine whether axonally synthesized transcription factors possess different modifications and properties, thus directing distinct transcriptional responses. In this way, axonally synthesized transcription factors may provide a mechanism to alert the cell body about axonal stimuli. Evidence that RNA regulons enable coherent coordination of transport and translation of functionally related mRNAs in neurons suggests that future studies will be needed to reveal more RNA regulons that orchestrate multiple mRNAs in morphologically complex neurons.
Highlights.
Cues in the axonal microenvironment induce local and global changes in neurons.
Bidirectional transport enables coordination of local and global changes.
Some transcription factors are translated in the axon and transported to the soma.
mRNAs are transported to and locally translated at axons for survival and growth.
Individual RBPs coordinate multiple steps of mRNA transport and regulation.
Acknowledgments
We would like to apologize to authors whose studies were not included in this review due to space constraints. We would like to thank Katharina Cosker, Sarah Pease, Xuesong Zhao, Maria Pazyra-Murphy and Emily Chadwick for helpful comments and editing. Our research is supported by the National Institutes of Health grant RO1 NS050674 (RAS) and T32 AG000222 (OETY).
Footnotes
Conflict of interest statement:
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur ELF. Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlson E, Maday S, Fu M-M, Moughamian AJ, Holzbaur ELF. Retrograde axonal transport: pathways to cell death? Trends in Neurosciences. 2010;33:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- 4.Chowdary PD, Che DL, Cui B. Neurotrophin Signaling via Long-Distance Axonal Transport. Annu Rev Phys Chem. 2012;63:571–594. doi: 10.1146/annurev-physchem-032511-143704. [DOI] [PubMed] [Google Scholar]
- 5.Cosker KE, Segal RA. Neuronal Signaling through Endocytosis. Cold Spring Harbor Perspectives in Biology. 2014;6:a020669–a020669. doi: 10.1101/cshperspect.a020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Current Opinion in Neurobiology. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends in Cell Biology. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 9.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 10.Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. Journal of Neuroscience. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazyra-Murphy MF, Hans A, Courchesne SL, Karch C, Cosker KE, Heerssen HM, Watson FL, Kim T, Greenberg ME, Segal RA. A Retrograde Neuronal Survival Response: Target-Derived Neurotrophins Regulate MEF2D and bcl-w. Journal of Neuroscience. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature Neuroscience. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 13.Schmieg N, Menendez G, Schiavo G, Terenzio M. Signalling endosomes in axonal transport: Travel updates on the molecular highway. Seminars in Cell and Developmental Biology. 2014;27:32–43. doi: 10.1016/j.semcdb.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14**.Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. This study shows that axotomy induces a back-propagating calcium wave that travels to the soma, leading to nuclear export of HDAC5. HDAC5 is transported to the injury site and deacetylates axonal tubulin, reducing microtubule stability and thereby enhancing regeneration. Concurrently, nuclear export of HDAC5 enhances acetylation of Histone H3, and thereby alters transcription. Injury-induced HDAC5 nuclear export fails to occur in central nervous system injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallee RB, McKenney RJ, Ori-McKenney KM. Multiple modes of cytoplasmic dynein regulation. Nature Cell Biology. 2012 doi: 10.1038/ncb2420. [DOI] [PubMed] [Google Scholar]
- 16.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nature Cell Biology. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreassi C, Zimmermann C, Mitter R, Fusco S, De Vita S, Devita S, Saiardi A, Riccio A. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nature Neuroscience. 2010;13:291–301. doi: 10.1038/nn.2486. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in Uninjured and Regenerating Cortical Mammalian Axons. Journal of Neuroscience. 2009;29:4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zivraj KH, Tung YCL, Piper M, Gumy L, Fawcett JW, Yeo GSH, Holt CE. Subcellular Profiling Reveals Distinct and Developmentally Regulated Repertoire of Growth Cone mRNAs. Journal of Neuroscience. 2010;30:15464–15478. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodmer D, Ascano M, Kuruvilla R. Isoform-Specific Dephosphorylation of Dynamin1 by Calcineurin Couples Neurotrophin Receptor Endocytosis to Axonal Growth. Neuron. 2011;70:1085–1099. doi: 10.1016/j.neuron.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Patel A, Yamashita N, Ascano M, Bodmer D, Boehm E, Bodkin-Clarke C, Ryu YK, Kuruvilla R. RCAN1 links impaired neurotrophin trafficking to aberrant development of the sympathetic nervous system in Down syndrome. Nature Communications. 2015;6:10119. doi: 10.1038/ncomms10119. This study identified RCAN1, a Calcineurin inhibitor, as a link between regulation of signaling endosome formation and developmental deficits in sympathetic neurons in Down syndrome. In Down syndrome, formation of signaling endosomes is altered due to increased expression of RCAN1, a gene within the Down syndrome critical region. Excess RCAN1 inhibits dynamin dephosphorylation and TrkA endocytosis, thereby attenuating retrograde signaling in vitro and in vivo and leading to decreased innervation of target tissues, eventually leading to neuron loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanz S, Perlson E, Willis D, Zheng J-Q, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van Minnen J, Twiss JL, et al. Axoplasmic Importins Enable Retrograde Injury Signaling in Lesioned Nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 23.Perry RB-T, Doron-Mandel E, Iavnilovitch E, Rishal I, Dagan SY, Tsoory M, Coppola G, McDonald MK, Gomes C, Geschwind DH, et al. Subcellular knockout of importin β1 perturbs axonal retrograde signaling. Neuron. 2012;75:294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, et al. Localized Regulation of Axonal RanGTPase Controls Retrograde Injury Signaling in Peripheral Nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bareyre FM, Garzorz N, Lang C, Misgeld T, Büning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proceedings of the National Academy of Sciences. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Ying Z, Misra V, Verge VMK. Sensing nerve injury at the axonal ER: Activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. Proc Natl Acad Sci USA. 2014;111:16142–16147. doi: 10.1073/pnas.1407462111. This study identified Luman/CREB3, an endoplasmic reticulum-associated transcription factor, as a classical importin cargo that is locally synthesized and required for axonal outgrowth upon injury. Cleavage of the N-terminus of Luman upon injury enables association with Importins and retrograde transport of the transcriptionally active protein. Subsequently, [28*] showed that transcriptional targets regulated by Luman are several components of the Unfolded Protein Response (UPR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Ying Z, Zhai R, McLean NA, Johnston JM, Misra V, Verge VMK. The Unfolded Protein Response and Cholesterol Biosynthesis Link Luman/CREB3 to Regenerative Axon Growth in Sensory Neurons. Journal of Neuroscience. 2015;35:14557–14570. doi: 10.1523/JNEUROSCI.0012-15.2015. See annotation to [27*] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-Dependent Spatial Translocation of an Activated MAP Kinase in Injured Nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Welshhans K, Bassell GJ. Netrin-1-induced local β-actin synthesis and growth cone guidance requires zipcode binding protein 1. Journal of Neuroscience. 2011;31:9800–9813. doi: 10.1523/JNEUROSCI.0166-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo S, Kim HH, Kim P, Donnelly CJ, Kalinski AL, Vuppalanchi D, Park M, Lee SJ, Merianda TT, Perrone-Bizzozero NI, et al. A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3′ untranslated region AU-rich regulatory element. Journal of Neurochemistry. 2013;126:792–804. doi: 10.1111/jnc.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki Y, Welshhans K, Wen Z, Yao J, Xu M, Goshima Y, Zheng JQ, Bassell GJ. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. Journal of Neuroscience. 2010;30:9349–9358. doi: 10.1523/JNEUROSCI.0499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hüttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH. Spatial regulation of β-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 35.Ceci M, Welshhans K, Ciotti MT, Brandi R, Parisi C, Paoletti F, Pistillo L, Bassell GJ, Cattaneo A. RACK1 Is a Ribosome Scaffold Protein for β-actin mRNA/ZBP1 Complex. PLoS ONE. 2012;7:e35034–11. doi: 10.1371/journal.pone.0035034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aschrafi A, Natera-Naranjo O, Gioio AE, Kaplan BB. Regulation of axonal trafficking of cytochrome c oxidase IV mRNA. Mol Cell Neurosci. 2010;43:422–430. doi: 10.1016/j.mcn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local Translation of Extranuclear Lamin B Promotes Axon Maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, Segal RA. Target-Derived Neurotrophins Coordinate Transcription and Transport of Bclw to Prevent Axonal Degeneration. Journal of Neuroscience. 2013;33:5195–5207. doi: 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Cosker KE, Fenstermacher SJ, Pazyra-Murphy MF, Elliott HL, Segal RA. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nature Neuroscience. 2016 doi: 10.1038/nn.4280. advance online publication SP - EP.This study identified SFPQ (splicing factor, polyglutamine rich) as an RNA-binding protein that binds and coordinates expression of functionally related axonal mRNAs. Especially, SFPQ coordinates neurotrophin-stimulated anterograde transport of both bclw and laminb2 mRNAs in dorsal root ganglion neurons. SFPQ is required for co-assembly of bclw and laminb2 to the same RNA transport granules. SFPQ-dependent RNA regulon orchestrates localization and expression of axonal mRNAs to enable neurotrophin-dependent axon viability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Wang B, Pan L, Wei M, Wang Q, Liu W-W, Wang N, Jiang X-Y, Zhang X, Bao L. FMRP-Mediated Axonal Delivery of miR-181d Regulates Axon Elongation by Locally Targeting Map1b and Calm1. Cell Reports. 2015;13:2794–2807. doi: 10.1016/j.celrep.2015.11.057. This study shows that FMRP along with axon-enriched miR181-d regulates axon outgrowth by targeting mRNAs map1b and calm1 to axons of DRG neurons. miR181-d binds and inhibits translation of map1b and calm1. FMRP promotes axonal delivery of miR181-d and mRNAs map1b and calm1 in a repressed form. Local NGF triggers release of target mRNAs from FMRP-miR181-d complex, thereby terminating translational inhibition. [DOI] [PubMed] [Google Scholar]
- 43**.Zhang P, Abdelmohsen K, Liu Y, Tominaga-Yamanaka K, Yoon J-H, Ioannis G, Martindale JL, Zhang Y, Becker KG, Yang IH, et al. Novel RNA- and FMRP-binding protein TRF2-S regulates axonal mRNA transport and presynaptic plasticity. Nature Communications. 2015;6:8888. doi: 10.1038/ncomms9888. This study identifies TRF2-S as a novel RBP that is necessary for axonal delivery of its target mRNAs including rab3a and aplp1. FMRP inhibits TRF2-S-mRNA complex formation by occupying the mRNA binding site on TRF2-S. TRF2-S and FMRP have opposite effects on axonal translocation of target mRNAs and on axonal growth; TRF2-S promotes whereas FMRP blocks translocation of target mRNAs and axon outgrowth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly CJ, Willis DE, Xu M, Tep C, Jiang C, Yoo S, Schanen NC, Kirn-Safran CB, van Minnen J, English A, et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011;30:4665–4677. doi: 10.1038/emboj.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merianda TT, Coleman J, Kim HH, Kumar Sahoo P, Gomes C, Brito-Vargas P, Rauvala H, Blesch A, Yoo S, Twiss JL. Axonal Amphoterin mRNA Is Regulated by Translational Control and Enhances Axon Outgrowth. Journal of Neuroscience. 2015;35:5693–5706. doi: 10.1523/JNEUROSCI.3397-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merianda TT, Vuppalanchi D, Yoo S, Blesch A, Twiss JL. Axonal transport of neural membrane protein 35 mRNA increases axon growth. Journal of Cell Science. 2013;126:90–102. doi: 10.1242/jcs.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]