Abstract
The occurrence of metastasis, an important breast cancer prognostic factor, depends on cell migration/invasion mechanisms, which can be controlled by regulatory and effector molecules such as Rho-associated kinase protein (ROCK-1). Increased expression of this protein promotes tumor growth and metastasis, which can be restricted by ROCK-1 inhibitors. Melatonin has shown oncostatic, antimetastatic, and anti-angiogenic effects and can modulate ROCK-1 expression. Metastatic and nonmetastatic breast cancer cell lines were treated with melatonin as well as with specific ROCK-1 inhibitor (Y27632). Cell viability, cell migration/invasion, and ROCK-1 gene expression and protein expression were determined in vitro. In vivo lung metastasis study was performed using female athymic nude mice treated with either melatonin or Y27832 for 2 and 5 wk. The metastases were evaluated by X-ray computed tomography and single photon emission computed tomography (SPECT) and by immunohistochemistry for ROCK-1 and cytokeratin proteins. Melatonin and Y27632 treatments reduced cell viability and invasion/migration of both cell lines and decreased ROCK-1 gene expression in metastatic cells and protein expression in nonmetastatic cell line. The numbers of ‘hot’ spots (lung metastasis) identified by SPECT images were significantly lower in treated groups. ROCK-1 protein expression also was decreased in metastatic foci of treated groups. Melatonin has shown to be effective in controlling metastatic breast cancer in vitro and in vivo, not only via inhibition of the proliferation of tumor cells but also through direct antagonism of metastatic mechanism of cells rendered by ROCK-1 inhibition. When Y27632 was used, the effects were similar to those found with melatonin treatment.
Keywords: 99mTc-tetrofosmin, breast cancer, lung metastasis, MCF-7 cells, MDA-MB-231 cells, melatonin, Rho-associated kinase protein-1 inhibitor, single photon emission computed tomography
Background
Breast cancer is the most common nonskin neoplasm and responsible for higher cancer-related death in women worldwide [1]. The mortality rate is intrinsically related to the occurrence of metastasis, as observed in over 90% of the fatal cases [2, 3]. Although the molecular mechanisms responsible for the metastasis in breast cancer have not been fully elucidated, it is known that the loss of cellular adhesion and subsequent anchoring-independent survival are crucial steps in the metastatic process [4]. Cell migration and substratum binding are regulated by external signaling molecules which alter cell structure by activating surface receptors [5].
Rho-associated kinase and its isoforms (ROCK-1 and ROCK-2) play a central role in the invasion and migration process by regulating the actin rearrangements in the cytoskeleton [6]. The ROCK-mediated signal plays a fundamental role in cellular morphology, adhesion, and mobility [7]. Liu et al. [7] verified an increased expression of ROCK gene, mainly ROCK-1, in metastatic breast cancers than in nonmetastatic tumors. It has, furthermore, shown that a specific ROCK-1 inhibitor, Y27632, was able to affect in vitro cell mobility and in vivo metastasis.
Melatonin (N-acetyl-5-methoxytryptamine), the main hormone of the pineal gland, was derived from serotonin. Melatonin is also produced by immune cells and a number of peripheral tissues [8], and it holds an oncostatic and cytoskeleton modulatory properties, capable of inhibiting cancer cells’ invasiveness, and tumor growth both in vivo and in vitro [9]. Among other functions, melatonin participates in the regulation of the circadian rhythms and has been reported to be oncostatic in estrogen receptor (ERα)-positive cells at physiological concentrations, but not in ERα-negative cells [10]. Its antitumoral effects have been noticed in breast neoplasms both in vivo using rat models with chemically induced cancer and in vitro in cell lines [11].
The use of melatonin in breast cancer therapy is based on its selective estrogen receptor modulator (SERM) and selective estrogen enzyme modulator (SEEM) properties [12]. The SERM actions include estrogen-regulated cell proliferation, cell invasiveness, protein expression, growth factors, and oncogene expression. These actions are perceived in cells expressing ERα and are mediated by MT1 melatonin receptor. The SEEM behavior is due to the inhibition of expression and activity of P450 aromatase, estrogen sulfatase, and 17β-hydroxysteroid dehydrogenase and to the stimulus of estrogen sulfotransferase. This dual-action mechanism (SERM and SEEM) and ERα specificity provide melatonin potential advantages to breast cancer treatment [13]. However, there has not been any mechanism or pathway described for the treatment of triple-negative breast cancer (TNBC) using melatonin.
We have recently shown that melatonin treatment in vivo decrease TNBC tumor growth and downregulating pro-angiogenic factors [14]. Similarly, Luchetti et al. [9] have already demonstrated the inhibitory effect of melatonin on tumor growth in vitro and in vivo. There are a few studies evaluating the effect of melatonin in tumor metastasis, but nothing has been reported about the inhibitory effect of melatonin on ROCK-mediated metastasis of breast cancer [6, 7, 10]. The purpose of this study was to determine the effect of melatonin on ROCK-mediated (mainly ROCK-1) human TNBC metastasis in a mouse model, possibly representing a new therapeutic alternative to suppress breast cancer metastasis.
Materials and methods
This study was approved by the ethics committee on animal use (CEUA) of the Faculty of Medicine of Sao Jose do Rio Preto (FAMERP), # 3210/2011 and the Institutional Animal Care and Use Committees (IACUC) of Henry Ford Health System in the United States, # 1228, which are developed following national and international standards of ethics in animal experimentation.
Cell culture
This study was performed using human breast cancer cell lines MDA-MB-231 (metastatic, ERα-negative) (ATCC, Manassas, VA, USA) and MCF-7 (nonmetastatic, ERα-positive) (ATCC). Both cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (4.5 g/L) (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (GIBCO), 2 mm l-glutamine (GIBCO), and 1% antimycotic and antibiotic (GIBCO). All cell lines were cultured in a humidified chamber with 5% CO2 and at 37°C.
Cell viability assessment by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cell viability was measured using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Vibrant MTT Cell Proliferation Assay Kit; Molecular Probes, Eugene, OR, USA). Briefly, MDA-MB-231 and MCF-7 cells were plated at a density of 5 × 104 cells/well in 96-well plates in 100 µL DMEM with 2% FBS. Then, the cells in different groups were treated with melatonin (Sigma-Aldrich, St. Louis, MO, USA) (0.0001, 0.001, 0.01, 0.1, 1 mm) and Y27632 (Sigma-Aldrich) (2.5, 10, 50, 100 µm) for 24 and 48 hr. Melatonin was diluted in ethanol (0.01%) and Y27632 was diluted in phosphate- buffered saline (PBS). Control groups contained ethanol corresponding to the concentration present in the highest melatonin dosage of 1 mm to exclude possible effects of the solvent. Thereafter, 10 µL of MTT solution was added to each well and the plates were incubated at 37°C for an additional 4 hr. To solubilize the MTT formazan crystals, the cells were incubated with dimethyl sulfoxide (DMSO) (Sigma-Aldrich) for 10 min. The absorbance of each well was measured using a 570-nm Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA). Medium with 2% FBS was used as background and subtracted from the samples. Cell viability (%) was calculated for all groups compared to control sample and performed in triplicate.
Based on the results of MTT assay, four treatment groups were established: group I (control) containing only cells in culture medium with vehicle (0.01% ethanol), group II containing 1 mm of melatonin, group III containing 10 µm of Y27632, and group IV containing a combination of 1 mm of melatonin and 10 µm of Y27632.
Migration and invasion assay
The invasiveness of breast cancer cells was tested in 24-well plates with 8-µm inserts Matrigel membranes (BD Biosciences, Billerica, MA, USA). In the upper compartment of the chamber, approximately 2.5 × 104 cells/insert were added into culture medium without serum, while 750 µL of culture medium (with 10% FBS) was added to the lower compartment with different treatments (melatonin or Y27632 alone or in combination). For negative and positive controls, 0.5% and 10% FBS, respectively, were used.
After 24 hr, those membranes were washed, paraformaldehyde-fixed, and permeabilized, and they were stained with hematoxylin to detect the migrated cells. The counting was made with an inverted optic microscope (Nikon Eclipse E200; Nikon, Melville, NY, USA) and photographed by putting the insert over a plate containing glycerol at 50%. The invasion rate was calculated by dividing the average number of treated cells that migrated and invaded the Matrigel membrane by the average number cells that migrated in positive control samples.
Quantitative real-time RT-polymerase chain reaction
The expression of ROCK-1 gene was determined by real-time polymerase chain reaction (PCR). Total RNA was isolated from MDA-MB-231 and MCF-7 cells using the TRIzol reagent (GIBCO) according to the manufacturer’s instructions. Complementary DNA (cDNA) corresponding to 2 µg of total RNA was used per reaction (20 µL) in a real-time quantitative PCR performed on StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) and using TaqMan Universal Master Mix kit (Applied Biosystems). TaqMan inventoried probes were used for ROCK-1 (Hs01127699_m1) detection and, actin beta (ACTB) (Hs99999903_m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1) as endogenous control for reaction standardizers.
The relative quantification (RQ) value of the expression of interest genes was determined with DataAssist 3.0 software (Applied Biosystems), using the average of standardizer genes (ΔΔCt) [15, 16]. The samples were tested in triplicate and all experiments included negative controls.
Protein extraction and Western blotting
Cells treated with or without melatonin or Y27632 alone or in combination, were washed in ice-cold PBS and lysed in NP40 cell lysis buffer (Invitrogen, Camarillo, CA, USA) supplemented with 1 mm phenylmethanesulfonyl (PMSF) (Sigma-Aldrich) and protease inhibitor cocktail (Sigma-Aldrich). After incubation for 30 min with intermittent vortexing, the cell lysate was centrifuged and the proteins were collected from supernatant. Proteins extracts were quantified by the bicinchoninic acid (BCA) with Pierce™ CA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA), separated on 8% SDS-PAGE and transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated with the desired primary antibody for ROCK-1 at 1:1000 (Sigma-Aldrich) and tubulin at 1:5000 (Sigma-Aldrich) overnight at 4°C, followed by incubation with the appropriate secondary antibody for 2 hr at room temperature. The detection of tubulin was used as a loading control. ROCK-1 and tubulin were detected using the Clarity Western ECL kit (Bio-Rad), and quantification was performed using Image J software (NIH, Bethesda, MD, USA) as image analyzer. The values were obtained in arbitrary units (au) and showed the mean optical density (MOD) to each sample.
Animal model and treatment schedules
Athymic nude female mice (n = 35) 6–8 wk old and weighing 20–25 g were randomly separated into different groups of treatment, kept under pathogen-free conditions at room temperature (21–25°C) on exposure to light for 12 hr and 12 hr in the dark. Food and water were offered ad libitum. The mice were purchased from Charles River Laboratory (Frederick, MD, USA) and housed at Henry Ford Hospital (Detroit, MI, USA).
For lung metastasis induction, as described by Chen et al. [17], 2.5 × 105 viable MDA-MB-231 cells (washed with PBS and resuspended in 0.1 mL of serum free RPMI 1640 medium; GIBCO) were injected intravenous (i.v.) into the tail vein after prior restraint of animals.
After 1 wk of tumor induction, the animals received treatment for 2 and 5 wk with melatonin (100 mg/kg per day) or Y27632 (10 mg/kg per day) or vehicle as control containing PBS with 10% of DMSO (Sigma, St. Louise, MO, USA) and 10% cremophor (Sigma), intraperitoneally (i.p.) for 5 days during the week (Monday through Friday). Melatonin was administered at 17:00 hr just before turning off the light of the animal facility. To better evaluate the effect of melatonin treatment, animals underwent single photon emission computed tomography (SPECT) on weeks 3 or 6 following induction of lung metastasis (i.v. injection of cells). However, for Y27632, animals underwent SPECT on week 3 (2 wk after treatment) following induction of lung metastasis. As an indicator of overall animal health, the body weight was measured twice weekly.
Single photon emission computed tomography study and image analysis
To identify the metastases formation in treated and untreated animals with melatonin and Y27632, animals underwent SPECT imaging according to the protocol established by Dr. Arbab modified from Cowey et al. [18] and Gambini et al. [19]. The animals were restrained using proper retainer for mice and were injected in the tail vein with 1 mCi of 99mTc-tetrofosmin. 99mTc-tetrofosmin accumulates in larger amounts in tumor cells due to increased mitochondrial activity [20–22].
After 2 hr, the animals were anesthetized with ketamine/xylazine (100/15 mg/kg) and images were acquired with a gamma camera (PRISM 3000 system, Bioscan, Washington, DC, USA) converted to a micro SPECT system for small animal scanning. The images were acquired with mouse specific pinhole collimators (Bioscan) in 360 degree rotation, using 3 collimators obtaining 10 projections for each collimator (total 30 projections), was acquired a minimum 90 s per projection (minimum 40,000 counts/projection). The total acquisition time for each animal was approximately 15 min.
Images were reconstructed with HiSPECT software (Bioscan), and the generated images in DICOM format were analyzed by Image J software (NIH). The intensity of radioactivity was detected in lung and submitted to three times of subtracting the standard deviation for all groups (IntDen − 3 × S.D.) to eliminate excessive background. The positive control was considered as the basis for the analysis between groups. The presence of metastasis was confirmed by histology with hematoxylin and eosin (H&E) stain and cytokeratin immunohistochemistry.
The X-ray computed tomography (CT) was performed to detect the metastatic foci in the lungs using the Albira SPECT-CT imaging station (Bruker Biospin Corp., Woodbridge, CT, USA) as described by Wathen et al. [23]. Imaging was acquired in high resolution with 80-mm axial field of view (FOV). The CT images were reconstructed at a resolution of 25 µm.
Histopathology and immunohistochemistry
Following 2 or 5 wk (3 or 6 wk following tumor induction) of treatment, the animals were euthanized and perfused with PBS and 4% paraformaldehyde (Acros Organics, Morris Plains, NJ, USA). The lung was collected and fixed in 4% paraformaldehyde containing 3% sucrose (Fisher Chemical, Waltham, MA, USA). Collected tissues were prepared for paraffin blocks and sectioning. Standard H&E staining was performed to evaluate the presence of metastases, and immunohistochemical staining procedures were performed as recommended by the suppliers of primary antibodies. The following antibodies were used to delineate the expression of corresponding antigens: ROCK-1 (Santa Cruz Biotechnology, Dallas, TX, USA) and cytokeratin (clone AE1/AE3; Dako, Carpinteria, CA, USA), a marker of epithelial cells.
Briefly, the slides containing tissues were deparaffinized and rehydrated and then incubated with citrate buffer at 96°C for 45 min. Once it washed with PBS, the sections were incubated with PBS + filtered 1% albumin for 20 min. Following this process, sections were incubated with the primary antibody (anti-ROCK-1 or anticytokeratin) diluted in PBS + albumin 0.5% at 4°C overnight. Then, the sections were stabilized in room temperature, washed with PBS, and incubated with Starr Trek Universal HRP Detection System kit (Biocare Medical, Concord, CA, USA) containing the secondary antibody (biotinylated anti-mouse, anti-rabbit, anti-goat immunoglobulins). The sections were once again rinsed with PBS and incubated with the peroxidase–streptavidin conjugates, washed, and incubated with diaminobenzidine tetrachloride (DAB) chromogenic substrate. At last, all sections were counterstained with hematoxylin, dehydrated, and coverslipped.
Data analysis and statistics
The immunohistochemistry analyses were performed following the method described by Jardim-Perassi et al. [14]. All results were submitted to descriptive analysis to determine statistical normality. An analysis of variance (ANOVA) was performed, followed by Bonferroni’s test. Values of P ≤ 0.05 were considered statistically significant. The GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA) was used.
Results
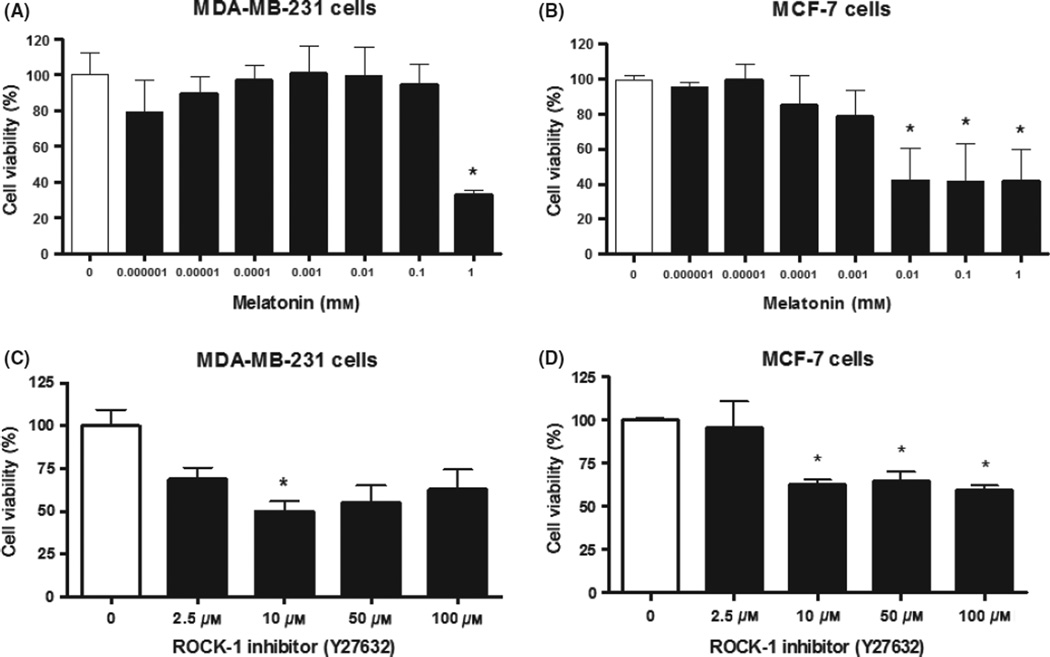
Both cell lines were subjected to MTT cell viability testing, after being treated with melatonin and Y27632. We previously [14] showed that the MDA-MB-231 cells were sensitive to 1 mm of melatonin after 24 hr of incubation, showing a statistically significant reduction in cell viability compared to control (P < 0.05). In 48 hr of treatment with a concentration of 1 mm melatonin, cell viability remained significantly different when compared to control cells (32.89 ± 2.56%; P < 0.05; Fig. 1A). Based on the results of MTT assay, we have selected 1 mm concentration of melatonin as the standard dose for subsequent studies.
Fig. 1.
Evaluation of cell viability by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (A) MDA-MB-231 and (B) MCF-7 breast cancer cell lines after 48 hr of melatonin treatment; (C) MDA-MB-231 and (D) MCF-7 breast cancer cell lines after 24 hr of Y27632 treatment. Significant value in ANOVA followed by Bonferroni’s test (±S.E.M. *P < 0.05).
Cell viability was also affected by the Y27632 with most concentrations after 24 hr of treatment; however, only the 10 µm concentration was able to produce a statistically significant decrease in cell viability compared to control (50.1 ± 5.7%; P < 0.05; Fig. 1C). After 48 hr of Y27632 treatment, the different concentrations tested did not show significant difference compared to control cells, thus demonstrating the loss of drug action within this range (data not shown).
The similar MTT assay was used for the nonmetastatic cell line, MCF-7. For melatonin, previously we also showed [24] that the concentrations of 0.001–1 mm were able to inhibit cell viability significantly compared to control at 24 hr (P < 0.05). Following 48 hr of melatonin treatment, only the concentrations between 0.01 and 1 mm showed statistically significant differences when compared to control cells (42.48 ± 18.03%, 41.43 ± 21.76%, 41.50 ± 18.21%, respectively; P < 0.05; Fig. 1B). MCF-7 cells demonstrated to be more sensitive to melatonin treatment than MDA-MB-231 cells. For Y27632 treatment, almost all concentrations were effective (P ≤ 0.0002), especially 10 µm that caused a 59.7% (±2.6%; P < 0.0001) in reducing MCF-7 cell viability compared to control at 24 hr (Fig. 1D). Similar to that of MDA-MB-231 in 48 hr, Y27632 treatment had no response in MCF-7 cells (data not shown).
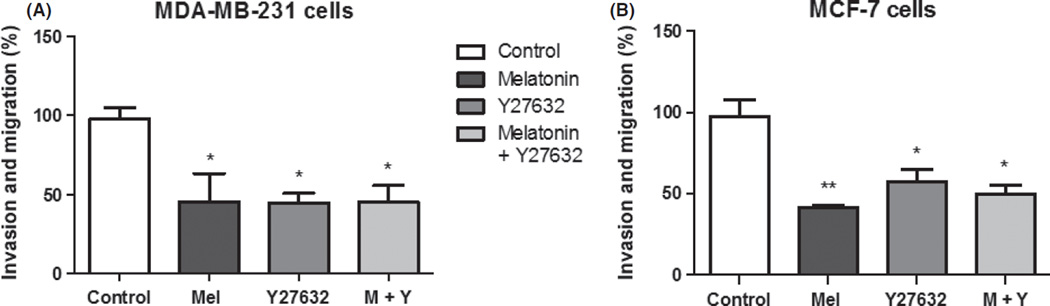
To verify whether melatonin or Y27632 alone or in combination would decrease the migration and invasive potential of breast cancer cell lines, both cell lines were subjected to migration and invasion assay (Fig. 2A,B). After 24 hr of melatonin treatment, there was a significant decrease (55 ± 18.0%; P < 0.05) in invasion and migration of MDA-MB-231 cells and there was also significant decrease in migration and invasion of MCF-7 cells (58 ± 1.6%; P < 0.05). Y27632 treatment decreased 55.3 ± 6.0% (P < 0.05) for MDA-MB-231 and 42.5 ± 7.7% (P < 0.05) for MCF-7 cells. For the combined treatments, there was a 54.7 ± 10.2% (P < 0.05) reduction for MDA-MB-231 cells and 49.7 ± 5.5% (P < 0.05) for MCF-7 cells. Melatonin showed the same competence as Y27632 to inhibit the migration and invasion of both cell lines. For this assay, the positive control was used to compare with treatment results, and negative control assay showed a 50 ± 10.2% reduction in the migration of the cells compared to that of positive control (P < 0.05), indicating the validity of the results (data not shown).
Fig 2.
Analysis of migration and invasion rate after melatonin and Y27632 treatments. (A) MDA-MB-231 and (B) MCF-7 breast cancer cell lines. Significant value in ANOVA followed by Bonferroni’s test (±S.E.M. *P < 0.05, **P < 0.001).
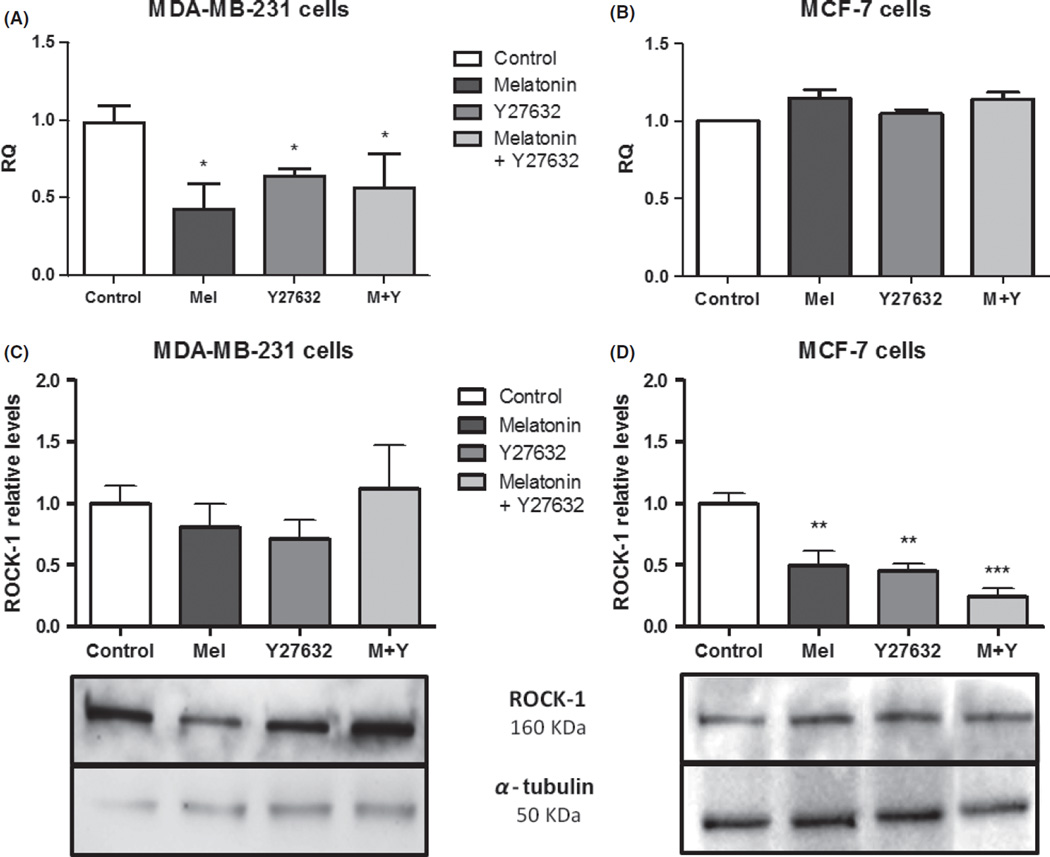
Expression of ROCK-1 gene after treatment with melatonin and specific inhibitor Y27632 in MDA-MB-231 cells is shown in Fig. 3A. The value of RQ for the control group was established as 1.0 arbitrary unit (au). The difference in ROCK-1 expression between control and treated cells with melatonin was 0.50 au (±0.14 au; P < 0.05) and with Y27632 was 0.65 au (±0.03 au; P= 0.05) and with combined melatonin and Y27632 was 0.46 au (±0.18 au; P < 0.05; Fig. 3A). On the other hand, neither melatonin nor specific inhibitor Y27632 decreased the expression of ROCK-1 in MCF-7 cells (Fig. 3B).
Fig. 3.
Analysis of expression of Rho-associated kinase protein (ROCK)-1 gene and protein after 24 hr of treatment with melatonin and Y27632. (A) and (C) MDA-MB-231, and (B) and (D) MCF-7 breast cancer cell lines. The gene expression values were relative quantification (RQ) represented and protein expression values were represented in relative levels. ROCK-1 protein levels were normalized by α-tubulin protein, presented in the boxes. Significant value in ANOVA followed by Bonferroni’s test (±S.E.M. *P < 0.05, **P < 0.001, and ***P < 0.0001).
Melatonin treatment also showed a tendency to reduce ROCK-1 protein expression (0.80 ± 0.19 au) compared to control group (1 ± 0.14 au), similar to that of Y27632 treatment alone (0.70 ± 0.15 au), but not for combined drugs (1.11 ± 0.35 au), showing a comparable decrease to that of gene expression found in the gene expression analysis for 24 hr (Fig. 3C). After 48 hr of treatment, the combined treatment group showed a tendency to decrease the ROCK-1 protein expression; however, the others groups did not showed any difference compared to control (data not shown).
Nonmetastatic cell line MCF-7 did not show statistically significant difference in ROCK-1 gene expression compared to control after 24 hr of treatment with melatonin and specific ROCK-1 inhibitor Y27632 (Fig. 3B). Nevertheless, protein analysis showed an efficient ROCK-1 protein reduction after melatonin (0.49 ± 0.12 au; P ≤ 0.001) and Y27632 (0.44 ± 0.06 au; P ≤ 0.001) treatments, especially when the drugs were combined (0.24 ± 0.06 au; P ≤ 0.0001; Fig. 3D) compared to control (1 ± 0.08 au). After 48 hr, this reduction was maintained for the combined group (0.14 ± 0.01 au; P ≤ 0.0001), but not for the other groups studied (data not shown).
To determine the role of melatonin in metastasis, we investigated its effects using a mouse model of breast cancer metastasis to lung. All mice remained healthy for the duration of experiment, and no clinical or pathologic signs of toxicity from melatonin or Y27632 were found. The identification of metastatic foci was performed by SPECT technique. At the end of treatment period, animals were subjected to 99mTc-tetrofosmin scanning to identify lung metastases. 99mTc-tetrofosmin is accumulated in the lung metastatic foci and give rise of high radioactivity in the lung metastatic foci.
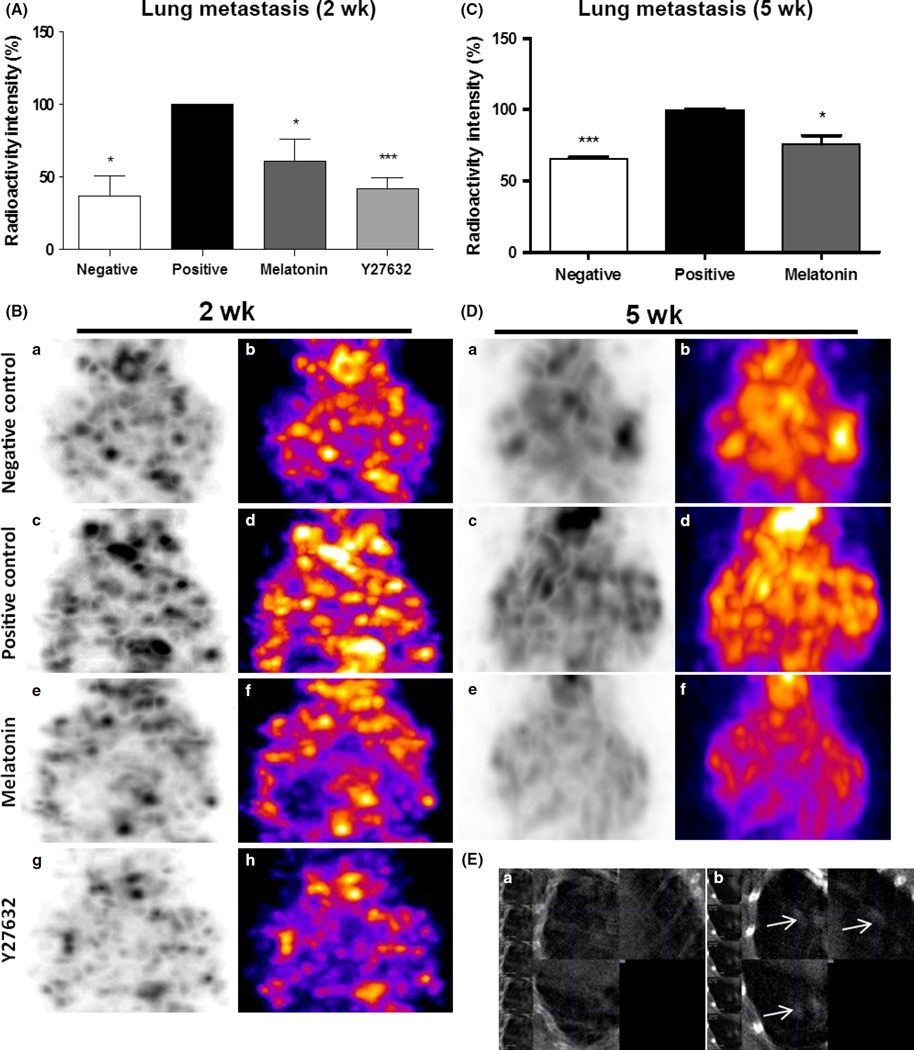
The results demonstrated that animals treated with melatonin (61.0 ± 15.1%; P < 0.05) or Y27632 (42.2 ± 7.2%; P < 0.001) for 2 wk showed a lower intensity of radioactivity in the lungs when compared to that of untreated positive control animals (100 ± 0.2%; Fig. 4A, B). When compared between melatonin and Y27632 treatments, there was no significant difference (P > 0.05).
Fig. 4.
Lung metastasis after 2 and 5 wk of melatonin and Y27632 treatments. Semiquantitative analysis of radioactivity intensity by single photon emission computed tomography (SPECT) images following (A) 2 wk of treatment and following (C) 5 wk of treatment. SPECT images obtained after injection of the 99mTc-tetrofosmin showing lung metastasis following (B) 2 wk of treatment and following (D) 5 wk of treatment. (a) and (b) negative control, (c) and (d) positive control, (e) and (f) melatonin treatment, (g) and (h) Rho-associated kinase protein (ROCK)-1 inhibitor (Y27632) treatment. (E) High-resolution computed tomography images at 25-µm thickness. (a) Control animal without metastasis; (b) positive control animal without treatment showing high-density area (white arrow) indicating lung metastasis in animal after 6 wk following induction of lung metastasis. Significant value in ANOVA followed by Bonferroni’s test (±S.E.M. *P < 0.05; ***P < 0.0001).
Similar reduction of metastasis foci and radioactivity were observed in animals that were treated with melatonin for 5 wk (75.9 ± 5.9%) when compared to positive control animals (100 ± 0.5%; P < 0.05), indicating the effect of melatonin for preventing lung metastases for the extended period (Fig. 4C,D). High-resolution images of 25 µm thick acquired by CT identified the presence of metastatic lesions in the lungs of a positive control animal after 6 wk of tumor induction (Fig. 4E).
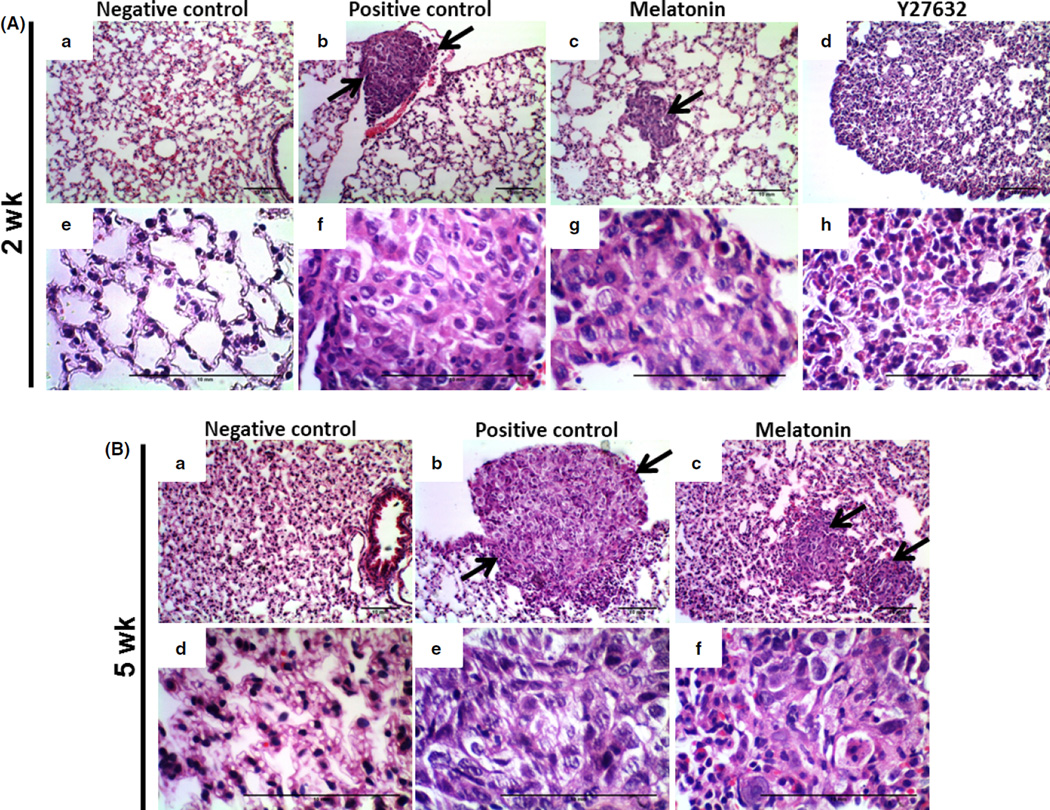
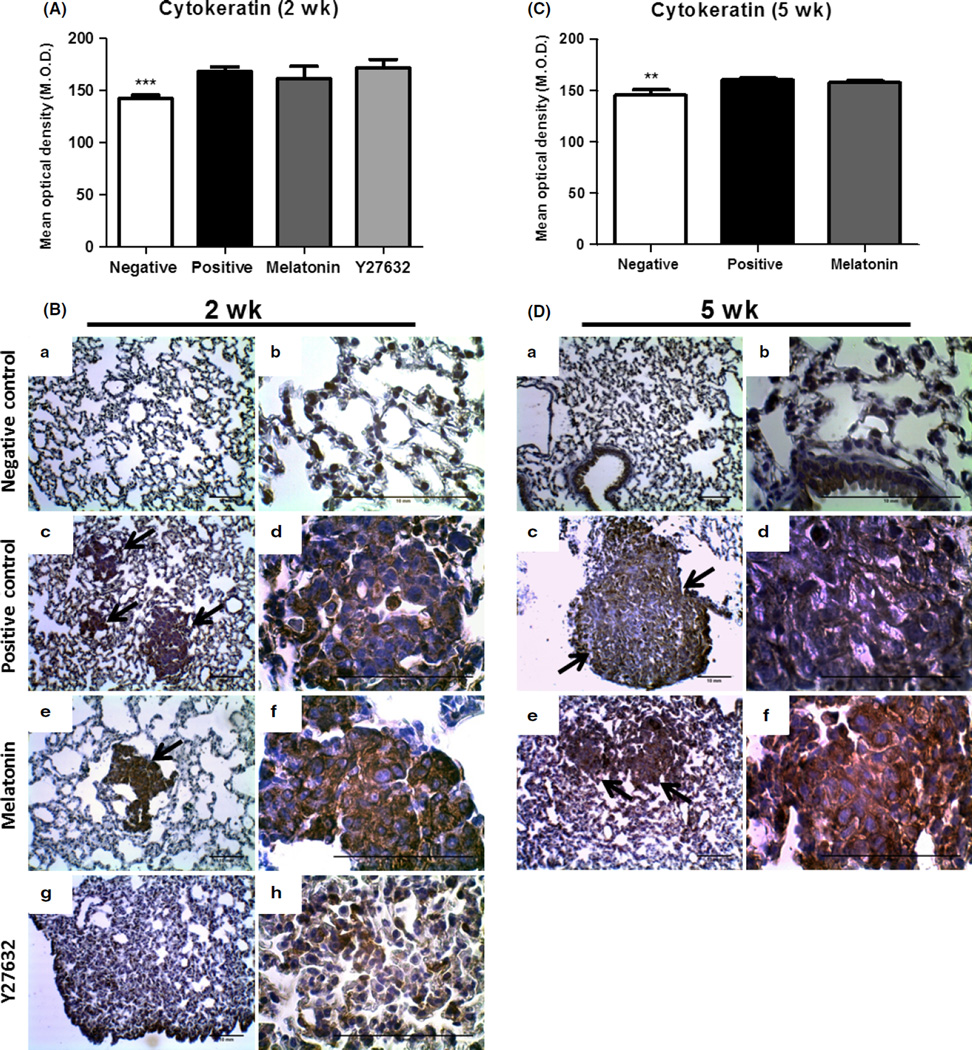
After paraffin section, the lung tissues from all groups were investigated using H&E staining (Fig. 5). Histological examination of the untreated tissue and melatonin group showed compact tumor cells with intact structure for 2 and 5 wk of treatment (Fig. 5A,B), but in Y27632 treatment group, the tumor cells were sparse and separated from each other (Figs 5A and 6B). Breast tumors in the model of lung metastasis were also confirmed by anticytokeratin immunohistochemistry (Fig. 6). The cytokeratin stain was observed in the cytoplasm in all regions of the lung metastases identifying breast cancer cells and epithelial regions of the bronchioles. Nodular pulmonary metastases were found in greater numbers and size in the positive control when compared to the treated groups (Figs 6 and 7).
Fig. 5.
Hematoxylin and eosin staining showing lung tissues in all groups. (A) Representative images from 2 wk of treatment and (B) from 5 wk of treatment. (a) and (e) negative control, (b) and (f) positive control, (c) and (g) melatonin treatment, (d) and (h) Y27632 treatment. Images were taken with 10× and 40× magnification. Arrows show the sites of metastasis. Note the sparsity of tumor cells in Y27632-treated group.
Fig. 6.
Cytokeratin protein expression analysis in lung metastasis. (A) and (C) Semiquantitative analysis from animals with 2 and 5 wk of treatment, respectively. (B) and (D) Representative images from (a) and (b) negative control, (c) and (d) positive control, (e) and (f) melatonin treatment, (g) and (h) Y27632 treatment. Images were taken with 10× and 40× magnification. Arrows show the sites of metastasis. The values of mean optical density and standard error for each group are shown in arbitrary units (au). (**) represents P ≤ 0.001 and (***) P ≤ 0.0001 by ANOVA followed by Bonferroni’s test.
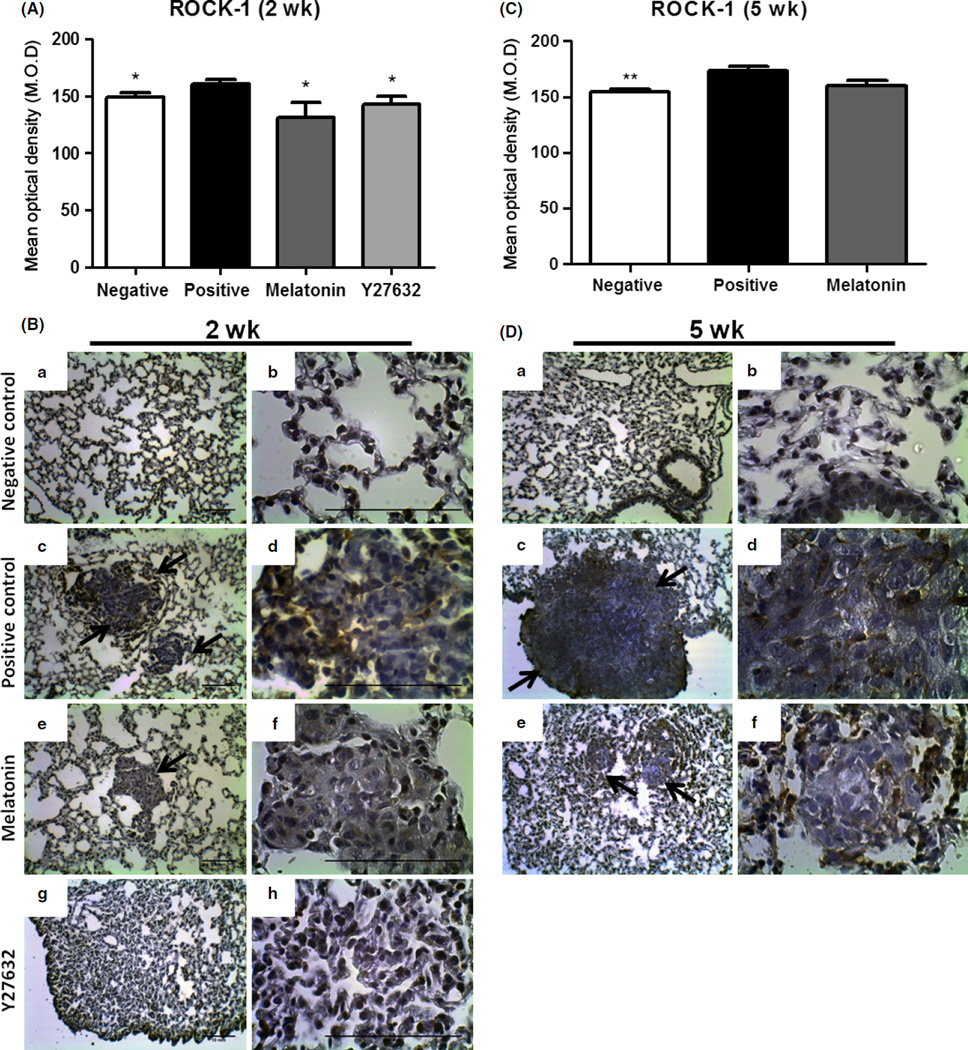
Fig. 7.
Rho-associated kinase protein (ROCK)-1 protein expression analysis in lung metastasis. (A) and (C) Semiquantitative analysis from animals with 2 and 5 wk of treatment, respectively. (B) and (D) Representative images from (a) and (b) negative control, (c) and (d) positive control, (e) and (f) melatonin treatment, (g) and (h) Y27632 treatment. Images were taken with 10× and 40× magnification. Arrows show the sites of metastasis. The values of mean optical density and standard error for each group are shown in arbitrary units (au). (*) represents P ≤ 0.05 and (**) P ≤ 0.001 by ANOVA followed by Bonferroni’s test.
For ROCK-1 protein detection in lung metastasis, we performed the immunohistochemical procedure. The presence of ROCK-1 was observed in all groups, however, with less intensity in the treated groups (Fig. 7). The immunostaining quantification was assessed by optical densitometry, and the results showed a statistically significant decrease in ROCK-1 protein expression in animals treated with melatonin (131.3 ± 13.1 au; P < 0.05) and Y27632 (143.3 ± 6.5 au; P < 0.05) for 2 wk compared to the positive control (160.5 ± 3.7 au; Fig. 7A,B). However, a tendency of reduction in ROCK-1 protein expression was observed in animals treated with melatonin (160.2 ± 4.4 au; P > 0.05) for 5 wk when compared to the positive control (173.2 ± 4.4 au), which indicates a preserved and additive action of melatonin treatment over time (Fig. 7C,D).
Discussion
The metastatic process is divided into several steps, among them the basement membrane invasion and migration through the extracellular matrix, which allows reaching the systemic circulation and anchoring in distant sites [25]. Melatonin has been considered an important natural oncostatic agent, in studies with breast cancer cell lines and with rats with chemically induced breast cancer [11, 26–30]. Our results demonstrated that melatonin and Y27632 were able to reduce cell viability in both MDA-MB-231 and MCF-7 breast cancer cell lines, assuming an oncostatic role. In additional, MCF-7 cells showed higher responsiveness than MDA-MB-321 cells to melatonin treatment.
To achieve therapeutic effects, endogenous substances are commonly administered in much higher concentrations than those corresponding to the physiological concentration [31]. Concentrations above 1 µm are described as pharmacological, while physiological concentration includes those below 1 nm [32, 33]. In this study, only the highest concentration (1 mm) was effective in metastatic and ERα-negative cells. High concentrations of melatonin (>0.01 mm) showed effectiveness in reducing the cell proliferation of melanoma cells (B16 cells line), being 1 mm the most effective. Moreover, the authors demonstrated that the efficacy of melatonin was dependent on the number of cells in culture, and the higher the availability of melatonin per cell, the greater their antiproliferative action [31].
Regarding the antitumor effect of melatonin in ERα-positive and ERα-negative breast cancer cells, Hill et al. [34] have shown that, at physiological concentrations (serum levels of 1–0.01 nm in humans corresponding to the peak at night- and at daytime, respectively), melatonin can inhibit the growth of ERα-positive breast tumor cell lines, such as MCF-7, T47D, and ZR 75-1, and ERα-negative as MDA-MB-468. Furthermore, melatonin at high concentrations (10 µm–1 mm) inhibits the proliferation of ERα-positive lines (MCF-7, T47D, ZR-75-1) more efficiently than ERα-negative lines such as BT-20, MDA-MB-231, MDA-MB-364, and Hs587t T47Dco, suggesting that part of its antiproliferative effect is mediated by estrogen signaling pathway [35, 36].
Melatonin binds to membrane receptors coupled to the G protein, known as MT1 and MT2. Mammary tumor cells which expressing MT1 receptor, acts in the regulation of the antiproliferative effects [37]. Both ERα-positive and ERα-negative cell lines express the MT1 receptor [37]; however, relevant studies have shown that ERα-positive tumors have an increased expression of MT1 compared to triple-receptor-negative tumors such as MDA-MB-231 line [38, 39].
In our in vivo study, melatonin treatment was performed at a dose of 100 mg/kg in mice with lung metastases. Liu et al. [40] and Rao et al. [41] analyzed different melatonin doses in animal tumors models, testing low (25 mg/kg), moderate (50 mg/kg), and high (100–200 mg/kg) doses. In agreement with our findings, these authors showed that melatonin has dose-dependent oncostatic action, with high efficiency around 100 mg/kg and cytotoxicity at a dose of 200 mg/kg.
We also demonstrated that melatonin and Y27632 were able to reduce around 50% of migration and invasion in both cell lines tested. Interestingly, melatonin showed similar ability to inhibit these processes compared to Y27632 treatment. Corroborating with our findings, Liu et al. [7] demonstrated a cell migration reduction around 30–40% in MDA-MB-231 and SUM1315 (human mammary epithelial) cells after Y27632 treatment. In contrast, Yang and Kim [42] observed that ROCK inhibition was effective to increase proliferation, migration, and invasion of nonmetastatic MCF-7 cells, but not the metastatic MDA-MB-231 cells.
Mao et al. [29] and Cos et al. [10] demonstrated the role of melatonin in breast cancer cell invasion and metastasis using invasive clones from MCF-7 breast cancer cells and noted that nanomolar concentrations of melatonin are capable of inhibiting cell proliferation and increase the expression of adhesion proteins (E-cadherin and β1-integrin), leading to reduced invasiveness of breast tumor cells. Melatonin can act as a modulator of cellular cytoskeleton to promote an increase in tubulin polymerization and microtubule rearrangement through Ca2+/calmodulin antagonism [43, 44].
Metastatic cells, characterized by poorly structured microfilaments and scarce anchorage on its substrate forming impaired stress fibers, leading to decrease focal adhesion causing no cell adhesion, increase migration and cell growth [45]. The protrusion migration of these cells occurs by means of microfilaments and microtubules arranged in membrane ruffles, lamellipodia and filopodia, whereas contraction of these structures at the cellular cytoskeleton occurs at the opposite side. [6]. Moreover, nonmigratory cells remain attached to the substrate via anchor cell produced by a highly specialized microfilament organization in adhesion plaques [46]. Focal adhesion formation involves microfilaments arrangement in stress fibers, which interact with vinculin, among other proteins [47]. Thus, minimally invasive cells, such as MCF-7 cells, attach to the substrate through actin stress fibers forming focal adhesion in combination with adhesion proteins such as integrins and cadherins, among other proteins [10].
In the present study, treatment with melatonin and Y27632 was able to inhibit the ROCK-1 gene expression in MDA-MB-231 metastatic cell line and protein expression in MCF-7 nonmetastatic cell line. Liu et al. [7] demonstrated that ROCK expression is much higher in metastatic human mammary tumors compared with nonmetastatic tumors as well in metastatic breast cancer cells as compared to nonmetastatic cells in vitro, thus suggesting that initial higher expression of ROCK-1 gene in metastatic cells could be the reason for resistance to decrease in ROCK-1 protein expression levels in the initial period of melatonin treatment. Decrease in ROCK-1 protein expression could be observed in our in vivo study, where animals were treated for a longer period of time (2–5 wk).
Besides that, when MCF-7 nonmetastatic cells were subjected a ROCK-1 gene overexpression, it was possible to verify an increased activity of this protein, giving a metastatic phenotype of these cells. For Joshi et al. [48], ROCK signaling is regulated by translocation of its mRNA-promoting cellular protrusion and invasive response due to hypoxia, suggesting that ROCK participates in many important roles in cell migration and invasion. Raviraj et al. [49] demonstrated that ROCK-1 activity is increased in tumor cells when grown in high-density matrix, indicating that the higher the barrier and resistance to tumor cell migration, the higher the production of this protein is. However, they also showed that the requirement of ROCK-1 is conditional upon the availability of other mechanisms such as proteolysis-assisted migration.
Ramírez-Rodríguez et al. [50] showed that melatonin can reorganize actin microfilaments in dense stress fibers and increase focal adhesion contacts formation in epithelial canine kidney MDCK cells by stimulating ROCK downstream protein kinase C (PKC) pathway. Melatonin selectively activates PKC-alpha causing the translocation of this enzyme into the cytoskeleton, concomitant leading to increase vimentin phosphorylation and reorganize vimentin intermediate filaments [44]. The same authors [47] also noted the relevance between melatonin and ROCK, wherein melatonin could decrease ROCK activity similar to that of specific inhibitor Y27632, when compared to the untreated cells.
To validate the results from our in vitro study, we developed a lung metastasis model via tail vein injection in nude athymic mice, wherein we observed lower 99mTc-tetrofosmin radioactivity intensity in the animal’s lungs treated with melatonin and Y27632, independent of treatment period. When it was compared the early and delayed melatonin treatments, we succeeded in identifying a preserved melatonin action for a long time. In addition, melatonin showed a similar response to modulate the lung metastasis as Y27632 treatment when it was compared on 2 wk of treatment. Corroborating with our findings, Leon-Blanco et al. [51] in their studies with melatonin and mammary tumors using MCF-7 cell line did not find metastatic focus in treated animals. Conversely, 62.5% of the animals of nontreated group developed secondary tumors. Similarly, Anisimov et al. [52] observed lower metastasis incidence in spontaneous mammary tumors models in HER2/neu transgenic mice during 9 months of melatonin treatment. Cos et al. [10] also noticed less tumor size compared to animal control in athymic nude mice treated with melatonin for 10 wk. Further, in the same study it was demonstrated that melatonin treatment led to reduced development of distant metastasis and increased animal survival. However, only Liu et al. [7] showed the role of ROCK in breast cancer metastasis using specifically a bone metastasis model.
In this study, we found less metastasis in treated animals and consequently lower intensity staining for cytokeratin. Expression of ROCK-1 protein was reduced in animals treated with Y27632 and melatonin after 2 and 5 wk when compared to positive control animals.
Taken together, the results have shown that melatonin is able to delay cancer progression not only via inhibition of the proliferation of tumor cells, but also through direct antagonism of metastatic cell functions rendered by the inhibition of ROCK-1. MDA-MB-321 cells were more sensitive to high and prolonged doses of melatonin both in vitro and in vivo. When Y27632 was used, the effects were similar to those found with melatonin treatments.
Melatonin has proved to be effective in reducing metastasis in vitro and in vivo. Melatonin tends to preserve pulmonary parenchyma in lung metastasis cases. Therefore, research with melatonin should be prioritized as a possible new agent in the fight against breast cancer and its metastasis.
Acknowledgments
We thank Jucimara Colombo for revising the manuscript critically and Igor Benedick Coimbra who provided medical writing services and performed language editing.
We also thank Fundacao de Amparo a Pesquisa do Estado de Sao Paulo – FAPESP (grants n°2011/13154-0, n°2011/20850-3, n°2012/12114-8, n°2011/18986-4 and n°2011/18987-0) and Fundacao de Apoio a Pesquisa e Extensao de Sao Jose do Rio Preto – FAPERP (grant n°176/2014) which funded this research, Faculdade de Medicina de São Jose do Rio Preto – FAMERP and the Laboratory of Molecular Research in Cancer – LIMC/FAMERP for providing material and structure to carry out this project.
Footnotes
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Moreau JE, Anderson K, Mauney JR, et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 2007;67:10304–10308. doi: 10.1158/0008-5472.CAN-07-2483. [DOI] [PubMed] [Google Scholar]
- 3.Song NR, Hwang MK, Heo YS, et al. Piceatannol suppresses the metastatic potential of MCF10A human breast epithelial cells harboring mutated H-ras by inhibiting MMP-2 expression. Int J Mol Med. 2013;32:775–784. doi: 10.3892/ijmm.2013.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schackmann RC, van Amersfoort M, Haarhuis JH, et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J Clin Invest. 2011;121:3176–3188. doi: 10.1172/JCI41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 6.Ortíz-López L, Morales-Mulia S, Ramírez-Rodríguez G, et al. ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. J Pineal Res. 2009;46:15–21. doi: 10.1111/j.1600-079X.2008.00600.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Goldstein RH, Scepansky EM, et al. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 8.Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 10.Cos S, Fernández R, Güézmes A, et al. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–4390. [PubMed] [Google Scholar]
- 11.Proietti S, Cucina A, Reiter RJ, et al. Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci. 2013;70:2139–2157. doi: 10.1007/s00018-012-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cos S, Recio J, Sánchez-Barceló EJ. Modulation of the length of the cell cycle time of MCF-7 human breast cancer cells by melatonin. Life Sci. 1996;58:811–816. doi: 10.1016/0024-3205(95)02359-3. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, et al. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin Investig Drugs. 2012;21:819–831. doi: 10.1517/13543784.2012.681045. [DOI] [PubMed] [Google Scholar]
- 14.Jardim-Perassi BV, Arbab AS, Ferreira LC, et al. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE. 2014;9:e85311. doi: 10.1371/journal.pone.0085311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Manning CD, Millar H, et al. CNTO 95, a fully human anti alphav integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- 18.Cowey S, Szafran AA, Kappes J, et al. Breast cancer metastasis to bone: evaluation of bioluminescent imaging and microSPECT/CT for detecting bone metastasis in immunodeficient mice. Clin Exp Metastasis. 2007;24:389–401. doi: 10.1007/s10585-007-9076-8. [DOI] [PubMed] [Google Scholar]
- 19.Gambini JP, Cabral P, Alonso O, et al. Evaluation of 99 mTc-glucarate as a breast cancer imaging agent in a xenograft animal model. Nucl Med Biol. 2011;38:255–260. doi: 10.1016/j.nucmedbio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Arbab AS, Koizumi K, Arai T, et al. Application of Tc-99m-tetrofosmin as a tumor imaging agent: comparison with Tl-201. Ann Nucl Med. 1996;10:271–274. doi: 10.1007/BF03165405. [DOI] [PubMed] [Google Scholar]
- 21.Buccheri G, Ferrigno D. Lung tumour markers in oncology practice: a study of TPA and CA125. Br J Cancer. 2002;87:1112–1118. doi: 10.1038/sj.bjc.6600577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schillaci O, Spanu A, Scopinaro F, et al. Mediastinal lymph node involvement in non-small cell lung cancer: evaluation with 99mTc-tetrofosmin SPECT and comparison with CT. J Nucl Med. 2003;44:1219–1224. [PubMed] [Google Scholar]
- 23.Wathen CA, Foje N, van Avermaete T, et al. In vivo X-ray computed tomographic imaging of soft tissue with native, intravenous, or oral contrast. Sensors (Basel) 2013;13:6957–6980. doi: 10.3390/s130606957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jardim-Perassi BV, Lourenco MR, Doho GM, et al. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer Agents Med Chem. 2015;15 doi: 10.2174/1871520615666150511094201. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Liu D, Duan H, et al. Metastasis-related miRNAs, active players in breast cancer invasion, and metastasis. Cancer Metastasis Rev. 2010;29:785–799. doi: 10.1007/s10555-010-9265-9. [DOI] [PubMed] [Google Scholar]
- 26.Eck KM, Yuan L, Duffy L, et al. A sequential treatment regimen with melatonin and all-trans retinoic acid induces apoptosis in MCF-7 tumour cells. Br J Cancer. 1998;77:2129–2137. doi: 10.1038/bjc.1998.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cos S, Mediavilla MD, Fernández R, et al. Does melatonin induce apoptosis in MCF-7 human breast cancer cells in vitro? J Pineal Res. 2002;32:90–96. doi: 10.1034/j.1600-079x.2002.1821.x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan L, Collins AR, Dai J, et al. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192:147–156. doi: 10.1016/s0303-7207(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Mao L, Yuan L, Slakey LM, et al. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12:R107. doi: 10.1186/bcr2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirunalini S, Karthishwaran K, Dhamodharan G, et al. Studies on the chemopreventive potential of melatonin on 7, 12- dimethylbenz(a)anthracene induced mammary carcinogenesis in rats. J Appl Sci Res. 2010;6:245–253. [Google Scholar]
- 31.Yerneni LK, Jayaraman S. Pharmacological action of high doses of melatonin on B16 murine melanoma cells depends on cell number at time of exposure. Melanoma Res. 2003;13:113–117. doi: 10.1097/00008390-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Dubocovich ML, Delagrange P, Krause DN, et al. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juszczak M, Roszczyk M, Kowalczyk E, et al. The influence od melatonin receptors antagonists, luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on melatonin-dependent vasopressin and adrenocorticotropic hormone (ACTH) release from the rat hypothalamo-hypophysial system. In vitro and in vivo studies. J Physiol Pharmacol. 2014;65:777–784. [PubMed] [Google Scholar]
- 34.Hill SM, Frasch T, Xiang S, et al. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8:337–346. doi: 10.1177/1534735409353332. [DOI] [PubMed] [Google Scholar]
- 35.Hill SM, Spriggs LL, Simon MA, et al. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992;64:249–256. doi: 10.1016/0304-3835(92)90050-6. [DOI] [PubMed] [Google Scholar]
- 36.Cos S, Sánchez-Barceló EJ. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histol Histopathol. 2000;15:637–647. doi: 10.14670/HH-15.637. [DOI] [PubMed] [Google Scholar]
- 37.Mao L, Yuan L, Xiang S, et al. Molecular deficiency (ies) in MT1 melatonin signaling pathway underlies the melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer cells. J Pineal Res. 2014;56:246–253. doi: 10.1111/jpi.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill SM, Cheng C, Yuan L, et al. Declining melatonin levels and MT1 receptor expression in aging rats is associated with enhanced mammary tumor growth and decreased sensitivity to melatonin. Breast Cancer Res Treat. 2011;127:91–98. doi: 10.1007/s10549-010-0958-0. [DOI] [PubMed] [Google Scholar]
- 39.Jablonska K, Pula B, Zemla A, et al. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res. 2013;54:334–345. doi: 10.1111/jpi.12032. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Xu L, Wei JE, et al. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat Rec (Hoboken) 2011;294:781–788. doi: 10.1002/ar.21361. [DOI] [PubMed] [Google Scholar]
- 41.Rao GN, Ney E, Herbert RA. Effect of melatonin and linolenic acid on mammary cancer in transgenic mice with c-neu breast cancer oncogene. Breast Cancer Res Treat. 2000;64:287–296. doi: 10.1023/a:1026552405042. [DOI] [PubMed] [Google Scholar]
- 42.Yang S, Kim HM. ROCK inhibition activates MCF-7 cells. PLoS ONE. 2014;9:e88489. doi: 10.1371/journal.pone.0088489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui DH, Machado-Santelli GM. Alterations in F-actin distribution in cells treated with melatonin. J Pineal Res. 1997;23:169–175. doi: 10.1111/j.1600-079x.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 44.Benítez-King G. PKC activation by melatonin modulates vimentin intermediate filament organization in N1E-115 cells. J Pineal Res. 2000;29:8–14. doi: 10.1034/j.1600-079x.2000.290102.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang DS, Dou KF, Li KZ, et al. Enhancement of migration and invasion of hepatoma cells via a Rho GTPase signaling pathway. World J Gastroenterol. 2004;10:299–302. doi: 10.3748/wjg.v10.i2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkan D, Kleinman H, Simmons JL, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramírez-Rodríguez G, Ortiz-López L, Benítez-King G. Melatonin increases stress fibers and focal adhesions in MDCK cells: participation of Rho-associated kinase and protein kinase C. J Pineal Res. 2007;42:180–190. doi: 10.1111/j.1600-079X.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 48.Joshi B, Strugnell SS, Goetz JG, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 49.Raviraj V, Fok S, Zhao J, et al. Regulation of ROCK1 via Notch1 during breast cancer cell migration into dense matrices. BMC Cell Biol. 2012;13:12. doi: 10.1186/1471-2121-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramírez-Rodríguez G, Meza I, Hernández ME, et al. Melatonin induced cyclic modulation of vectorial water transport in kidney-derived MDCK cells. Kidney Int. 2003;63:1356–1364. doi: 10.1046/j.1523-1755.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 51.Leon-Blanco MM, Guerrero JM, Reiter RJ, et al. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–211. doi: 10.1034/j.1600-079x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 52.Anisimov VN, Alimova IN, Baturin DA, et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer. 2003;103:300–305. doi: 10.1002/ijc.10827. [DOI] [PubMed] [Google Scholar]