Abstract
Objective
Giant cell tumor of bone (GCTB) is a locally aggressive primary bone tumor. Its malignant counterpart is quite rare. Rarely, a conventional GCTB shows marked nuclear atypia, referred to as symplastic/pseudoanaplastic change, which can mimic sarcomatous transformation. Recently, somatic driver mutations of histone H3.3 exclusively in H3F3A have been described in GCTB. We report a series of 9 cases of GCTB with symplastic/pseudoanaplastic change, along with analysis of H3F3A variants.
Materials and methods
Nine cases of GCTB with symplastic change were identified. Clinico-radiological features, morphological features, and immunohistochemical stain for Ki-67 stain were reviewed. H3F3A variants were also analyzed using Sanger sequencing.
Results
Histologically, conventional giant cell tumor areas with scattered foci of markedly atypical cells were seen in all of the cases and all showed rare if any Ki-67 labeling. One patient had received denosumab treatment and another radiation therapy. Radiological features were characteristic of conventional GCTB. Mutation in H3F3A (p.Gly34Trp [G34W]) was found in 6 of the 7 cases. Clinical follow-up ranged from 6 to 208 months. Local recurrences were seen in 4 cases (44 %).
Conclusions
GCTB with symplastic/pseudoanaplastic change is an uncommon variant of conventional GCTB, which can mimic primary sarcoma or sarcomatous transformation. These tumors possess the same missense mutation in histone H3.3 as conventional GCTB.
Keywords: Symplastic/pseudoanaplastic, Giant cell tumor of bone
Introduction
Giant cell tumor of bone (GCTB), a locally aggressive primary bone tumor, represents about 5 % of all primary bone tumors. It most frequently occurs in skeletally mature young adults between 20 and 40 years of age, and is more common in women [1]. They usually affect epimetaphyseal regions of long bones, mostly around the knee joint. The stromal cells constitute the main proliferating cell population in GCTB and express receptor activator for NF-kB ligand (RANKL), a factor critical in the development and activation of receptor activator for NF-kB (RANK), expressing osteoclasts such as giant cells and their precursors [2]. Malignant transformation that primarily involves a neoplastic stromal cell component, is a rare phenomenon and constitutes about 1.8 % of GCTB [3]. It can be in the form of primary malignant GCTB, with synchronous high-grade sarcomatous growth adjacent to areas of benign giant cell tumor, or as a secondary phenomenon in a recurrence of a previously conventional GCTB, treated with irradiation or surgery.
Traditionally, GCTB is primarily treated with curettage or en bloc resection. Radiotherapy has also been used, especially in tumors in surgically difficult locations. Classically, the recurrence rates for primary treated lesions range from 0 to 65 % depending on the type of treatment [4–6]. In recent years, they have also been treated with osteoclast inhibitor or anti-RANKL (denosumab), which has been shown to reduce or arrest tumor growth. Recently, somatic driver mutations of histone H3.3, exclusively in the H3F3A gene, leading to p.Gly34Trp (G34W) or p.Gly34Leu (G34L) alterations, have been described in 92 % of GCTB [7]. These mutations were restricted to the stromal cell population and were not detected in osteoclasts or their precursors. This high prevalence of his-tone H3.3 alterations in GCTB and their high specificity allows detection of this mutation as a good diagnostic marker, especially in characterizing morphologically ambiguous cases.
Rarely, conventional GCTB shows marked nuclear atypia referred to as symplastic/pseudoanaplastic change, which can mimic sarcomatous transformation. Only 5 cases with such changes have been described in the literature [8, 9]. Herein, we report a series of 9 cases of GCTB with symplastic/pseudoanaplastic change, along with molecular findings and a comprehensive literature review that summarizes the characteristics of this entity.
Materials and methods
This is a retrospective study. After Institutional Review Board approval, the pathology database from 1993 to 2011 was searched and a total of 181 cases of GCTB were identified. All cases of GCTB were reviewed by a musculoskeletal pathologist. There were 102 men and 79 women with a mean age of 36.6 years (median: 35 years). Twenty-nine cases were located in the axial skeleton, whereas 152 were located in the appendicular bones. Of these, 9 cases were diagnosed as GCTB with symplastic changes. GCTB with symplastic change was defined as a tumor with areas of conventional GCTB, along with interspersed atypical cells with marked nuclear pleomorphism, enlarged hyperchromatic nuclei, irregular nuclear contours, smudged nuclear chromatin, and nuclear pseudo-inclusions. Electronic medical records were reviewed for clinical follow-up. The radiology images available were re-reviewed by a musculoskeletal radiologist (S.H.). Pathology slides and blocks of these cases were retrieved. All tissues had been fixed in 10 % neutral-buffered formalin and embedded in paraffin as part of a routine surgical pathology procedure. Five-micron-thick sections stained with hematoxylin and eosin (H&E) were reviewed.
Ki-67 immunohistochemistry (IHC) analysis was performed in all 9 cases. IHC was performed on 5-μm thick sections by Ventana, Benchmark XT immunohistochemical stainer. The sections were deparaffinized and subjected to heat-induced antigen retrieval using CC1 at a high pH for 40 min before primary incubation with Ki67 rabbit monoclonal antibody, clone 30–9 from Ventana. Slides were then counterstained with hematoxylin, dehydrated, and cover-slipped. The IHC stains were scored subjectively (by M.H. and J.S.).
Genomic DNA was extracted from 5-μm thick sections of undecalcified blocks of formalin-fixed, paraffin-embedded tumor tissues using a DNA FFPE Tissue Kit according to the manufacturer’s instructions. PCR amplification, followed by Sanger sequencing of exons covering hot spot mutations between codons 29 and 63 of the H3F3A gene was performed in all 9 cases. The primers used for PCR amplification are as follows: forward, 5′-GGTCTCTGTACCATGGCTCG-3′; and reverse, 5′-GGTCTCCTTAGACCTCCAGGTAA-3′.
Results
The 9 cases of GCTB with symplastic change included 7 women and 2 men, with a mean age of 43.3 years (range: 28–64 years). Except for one, all patients had pain as the initial presenting symptom. One patient was discovered incidentally. Anatomical locations included distal femur (2), vertebra (3), proximal tibia (1), fibula (1), and ischium (2). Thus, the tumors were almost equally distributed between the axial skeleton and the lower limbs.
Radiological imaging features were characteristic of GCTB. The lesions were eccentrically located, with an expansile lytic appearance, and had cortical involvement. Preoperative radiograph, which was available in 4 cases, showed a lytic lesion in all 4, with sclerotic rim (<50 % of lesion border) in 3 of these (3 out of 4); 1 case also showed multiple septations (Figs. 1, 2). Preoperative CT was available in 3 cases and all 3 had an expansile lytic lesion with marked cortical thinning and partial destruction with a sclerotic rim (<50 % of lesion border; Fig. 2b). In 2 out of 3 CT examinations, intravenous contrast medium was administered and two thirds of the tumor showed enhancement in both cases. No periosteal reaction or mineralized matrix was identified in any of the 3 cases. Preoperative bone scans were available in 4 cases and all showed predominantly peripheral radiotracer uptake with heterogeneous internal photopenia (Fig. 1c). Preoperative magnetic resonance imaging (MRI) was available in 5 cases. According to MRI, the size ranged from 4.2 cm to 6.8 cm (mean: 4.9 cm). T1-weighted images were available in 4 out of 5 cases, and the T1 signal of the tumor was diffusely iso- or hypointense compared with that of muscle in all cases (Fig. 2). Fluid-sensitive images were available in 5 out of 5 cases, and the signal was heterogeneously high compared with that of muscle in more than one third of the tumor in all cases. Although there was a periosteal reaction on CT or radiography, periosteal edema in bone or soft tissue was present in all 5 cases on MRI. An extraosseous mass was also observed in 4 out of 5 cases.
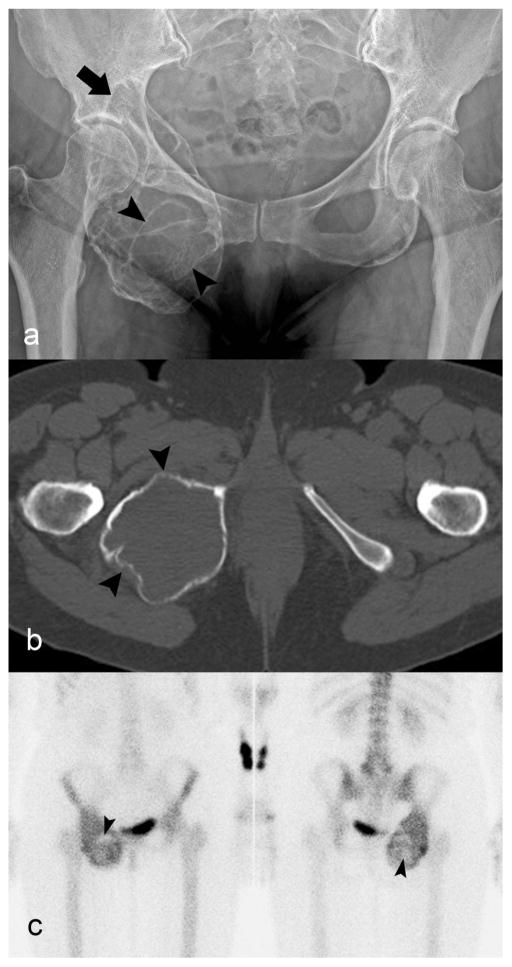
Fig. 1.
Giant cell tumor of bone of the ischium. a) Radiograph of pelvis shows an expansile lytic lesion causing marked cortical thinning. The rim is partially sclerotic (arrow) and there are septations (arrowheads) in the lesion. b) Axial unenhanced CT image demonstrates marked cortical thinning (arrowhead) without periosteal reaction or mineralized matrix. c) Bone scan shows the radiotracer uptake predominantly at the periphery and central photopenia (arrowheads)
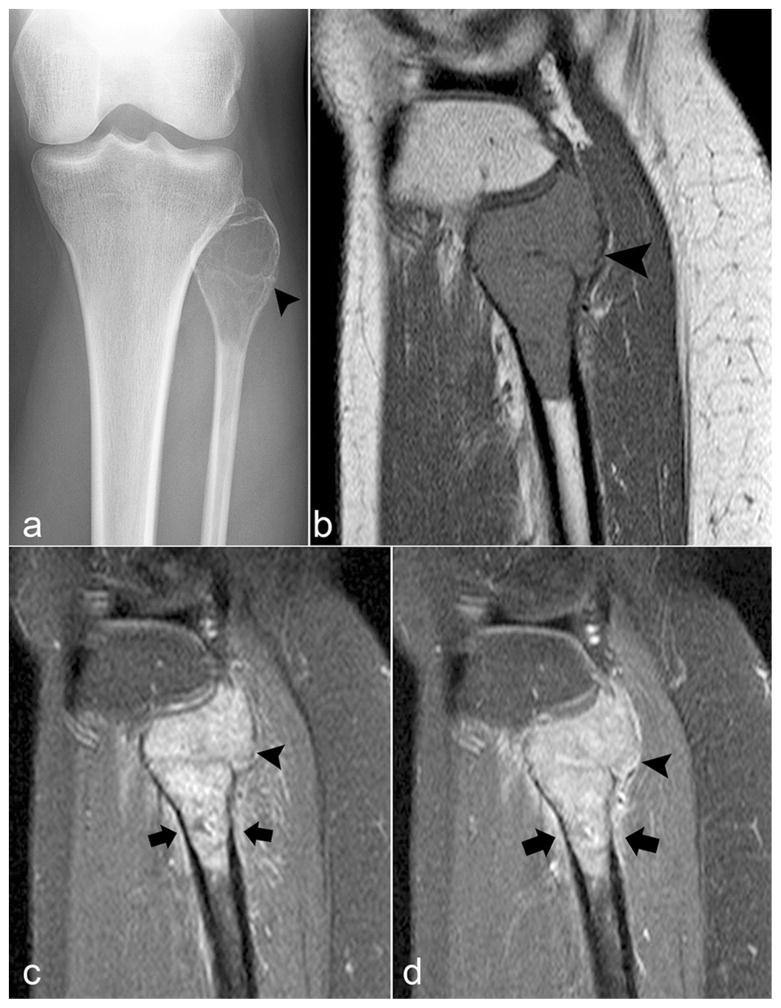
Fig. 2.
Giant cell tumor of bone of the fibula. a) Anteroposterior view radiograph shows an expansile lytic lesion in the proximal fibula causing marked cortical thinning (arrowhead). b) Sagittal T1-weighted image demonstrates the diffusely low signal. c) Fat-suppressed sagittal T2-weighted image and d) fat-suppressed contrast-enhanced sagittal T1-weighted image demonstrate mild extraosseous soft tissue (arrowhead) and periosteal edema and enhancement (arrows) in the adjacent bone and soft tissue
Histological examination showed the majority of the tumors with uniformly distributed giant cells interspersed with mononuclear cells, showing round, oval, or (occasionally) spindle-shaped nuclei with fine nuclear chromatin, small prominent nucleoli, and a scant to moderate amount of eosin-ophilic cytoplasm (Fig. 3). Rare mitotic figures were seen in these stromal cells. In addition, all 9 cases demonstrated scattered foci of cells with marked nuclear pleomorphism, with enlarged hyperchromatic nuclei, irregular nuclear contours, smudged nuclear chromatin, and nuclear pseudo-inclusions (Fig. 4). Mitotic figures were not seen in these atypical cells. Although the overall Ki-67 proliferative index ranged from 0 to 10 %, most of these atypical and pleomorphic cells did not label with Ki-67 or showed only rare Ki-67 positivity (Fig. 5). The clinical findings and Ki-67 index are summarized in Table 1.
Fig. 3.
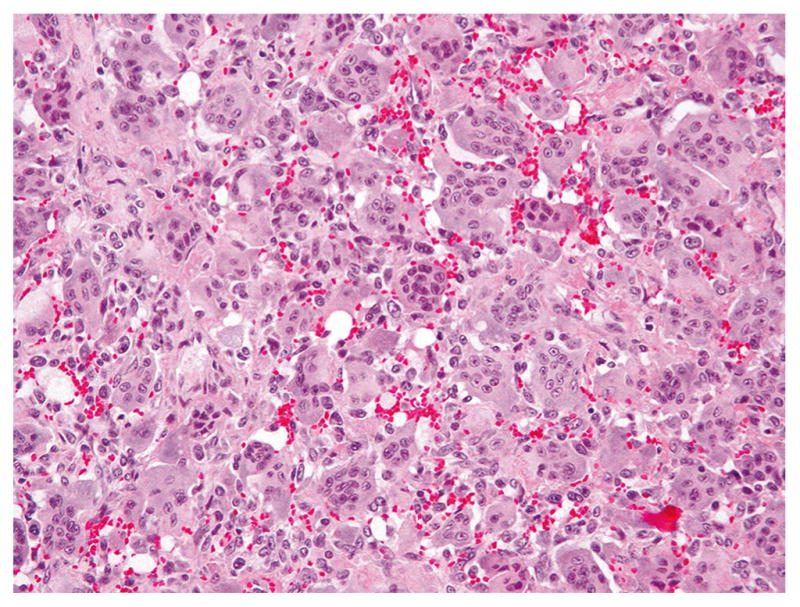
Giant cell tumor without atypia with uniformly dispersed giant cells and interspersed mononuclear cells, whose nuclei have a similar morphological appearance to the nuclei of giant cells
Fig. 4.
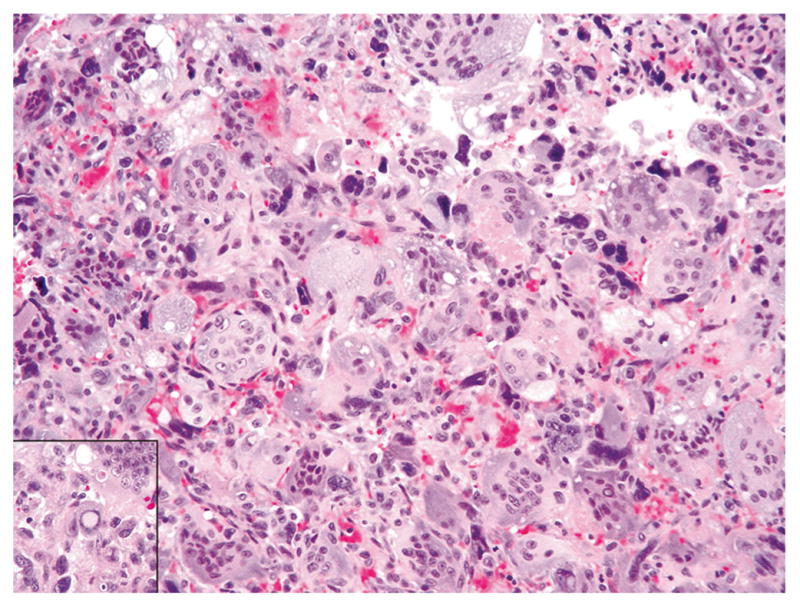
Giant cell tumor with stromal cells showing atypia, with scattered foci of marked nuclear pleomorphism, enlarged hyperchromatic nuclei, smudged nuclear chromatin, and nuclear pseudo-inclusion (inset)
Fig. 5.
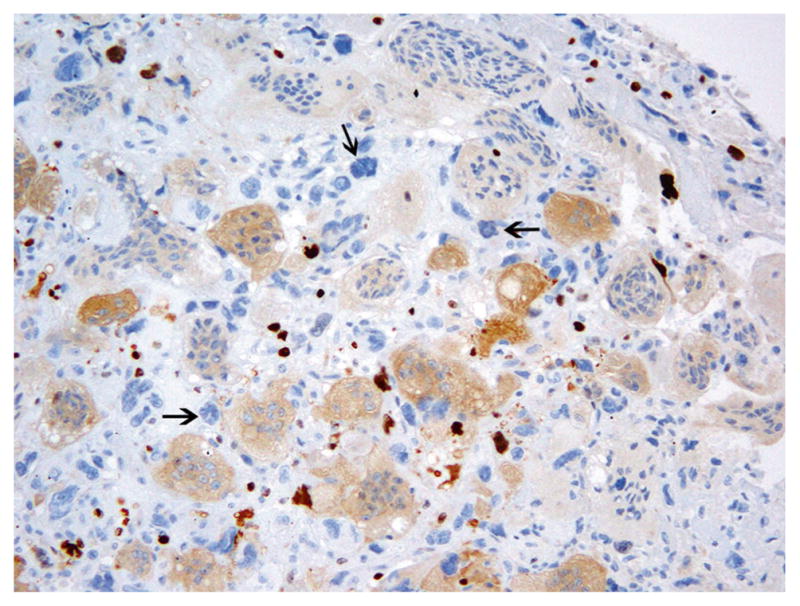
Atypical cells show an absence of Ki67 staining (arrows). Giant cells show cytoplasmic staining
Table 1.
Clinico-pathological features
| Cases | Age | Sex | Site | Ki67 (%) | Treatment | Follow up (months) | Recurrence | H3F3A mutation |
|---|---|---|---|---|---|---|---|---|
| GCT1 | 36 | Female | Right distal femur | 3–4 | Curettage, cryosurgery | 30 | No | G34W |
| GCT2 | 28 | Female | Lumbar vertebra—L3 | 1–2 | Resection | 23 | Yes | G34W |
| GCT3 | 60 | Female | Right ischium acetabulum | 1–2 | Denosumab, radical resection | 45 | No | G34W |
| GCT4 | 29 | Female | Left fibula | 5–8 | Curettage, cryosurgery | 14 | No | G34W |
| GCT5 | 41 | Female | Left distal femur | <1 | Curettage excision, cryosurgery | 18 | No | Wild type |
| GCT6 | 42 | Female | C6 spine | ~5 | Multiple excisions, radiation (6,000 cGy) | 148 | Yes | G34W |
| GCT7 | 51 | Male | Right proximal tibia | 3–4 | Curettage, cryosurgery | 208 | Yes | Unsuccessful |
| GCT8 | 64 | Male | Left sacro-iliac | 5–10 | Curettage, cryosurgery | 155 | Yes | Unsuccessful |
| GCT9 | 39 | Female | Right ischium | ~10 | Curettage, grafting | 6 | No | G34W |
H3F3A mutation, p.Gly34Trp (G34W) alteration was found in 6 of the 7 cases (86 %) by Sanger sequencing (Fig. 6). One case (GCT5) was negative for H3F3A mutation. Sanger sequencing was unsuccessful in 2 of the 9 tumors (GCT 7 and GCT8).
Fig. 6.
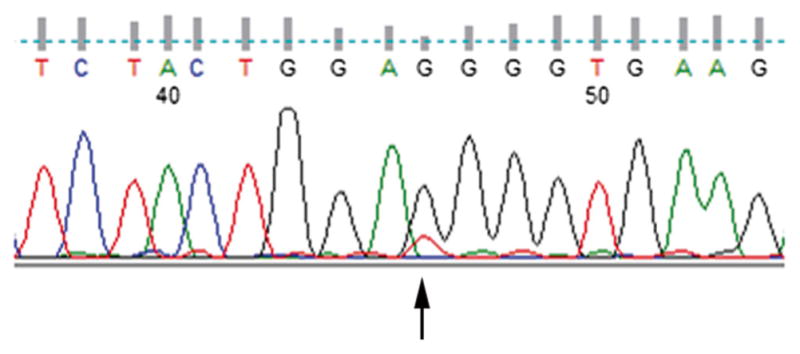
Representative sequencing of the H3F3A electropherograms, with G > T alteration
All patients were treated using resection and/or curettage and cryosurgery. None of the patients were embolized before surgery. One patient (C6 spine, GCT6) with a recurrent giant cell tumor had received radiation therapy with 6,000 cGy of radiation. One case (GCT3) had previous denosumab treatment for tumor of the ischium to reduce the need to resect the acetabulum. Subsequent resection showed denosumab treatment-associated exuberant new bone formation in association with symplastic atypia (Fig. 7). The sections also showed a marked decrease in the number of osteoclast-type giant cells, with large areas showing decreased cellularity, mainly represented by pleomorphic/dysplastic tumor cells, and a marked increase in fibroblastic stroma with myxochondroid and bone matrix formation. On clinical follow-up ranging from 6 to 208 months (mean: 72 months), local recurrence was seen in 4 cases (44 %), 3 of which were in the axial skeleton (lumbar vertebra, cervical spine, and sacro-iliac) and 1 in the proximal tibia. Thus, recurrence occurred in 3 out of 5 axial tumors (60 %) and only 1 out of 4 appendicular tumors (25 %). No pulmonary or other metastases developed in any of the cases.
Fig. 7.
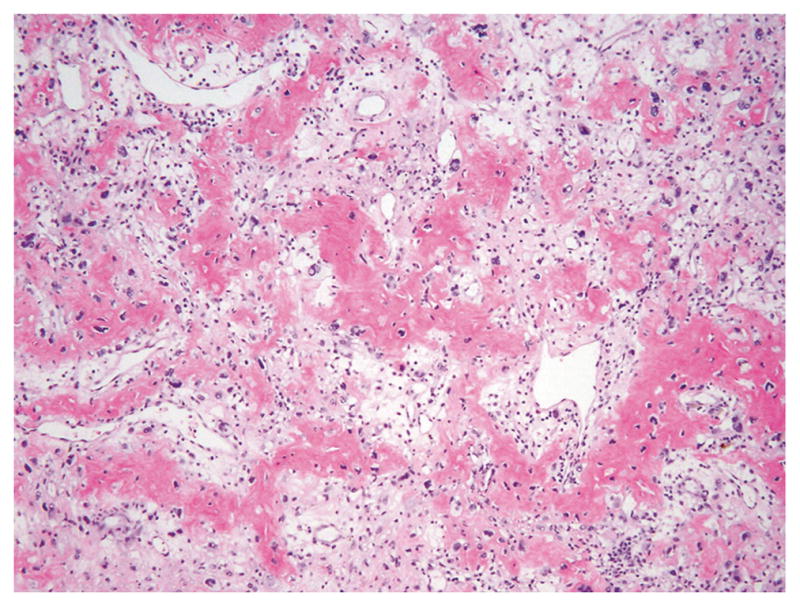
Denosumab-treated giant cell tumor of the bone with exuberant new bone formation
Discussion
Pseudoanaplastic change is a well-known phenomenon described in various mesenchymal neoplasms, most notably in peripheral nerve sheath tumors (ancient schwannoma, neurofibroma) and leiomyoma [10–12]. Although benign, these changes can be alarming and can potentially mimic changes seen in malignant tumors. Pseudoanaplasia/symplastic changes are quite rare in bone tumors and are described only as case reports in GCTB, chondromyxoid fibroma, osteoblastoma, aneurysmal bone cyst (ABC), fibrous dysplasia, and non-ossifying fibroma (NOF) [8, 13–17].
Layfield et al. described pseudoanaplastic change for the first time in a sacro-coccygeal giant cell tumor in 1999, in a 21-year-old woman [9]. In this case report, the authors described islands of atypical (pseudoanaplastic) cells near large areas of hemorrhage or necrosis, suggesting a degenerative or ischemic etiology for this change, associated with preoperative embolization therapy. There was no recurrence or metastases after a 4-year follow-up. Subsequently, 4 more cases were reported by Bahk and Mirra, who noted the stromal cells or stromal giant cells with large and bizarre, hyperchromatic to smudged nuclei, without mitotic figures [8]. None of their cases had developed recurrence or metastasis by 4 years’ follow-up.
We are reporting the largest series of cases of symplastic/pseudoanaplastic changes in GCTB. All of the cases showed focal areas of stromal cells with enlarged hyperchromatic nuclei, smudged chromatin, nuclear pseudo-inclusions, and irregular nuclear borders. In some cases, the changes were florid, thus mimicking sarcomatous transformation. Classically, malignant GCTBs show two components, composed of classic GCTB areas with uniformly distributed giant cells in a vascularized stroma interspersed with oval or spindle-shaped mononuclear cells and a second component of a high-grade pleomorphic sarcoma composed of atypical and pleomorphic stromal cells, which can be in the form of fibrosarcoma, malignant fibrous histiocytoma or osteosarcoma. Mitotic activity is generally high and is seen in both mononuclear cells and giant cells, and atypical mitoses are often present [18]. Mitotic figures, especially atypical ones, have often been used as a feature of malignancy in GCTB. However, mitotic figures and anaplastic changes may be irregularly distributed. Therefore, generous sampling of tumor may be necessary to identify malignant features such as an atypical mitotic figure. In all of our cases, the atypical stromal cells did not show typical or atypical mitotic figures.
Few authors have addressed the application of Ki-67 as a marker to distinguish pseudoanaplastic change from malignant transformation in tumors such as neurofibroma and schwannomas [11, 19]. These studies showed that the proliferation index, as shown by Ki-67, is low in these lesions, with no indication that positive staining of Ki-67 was present in the pseudoanaplastic cells. Proliferative index using Ki-67 immunostain has been studied in conventional GCTB with a reported range between 1 and 20 % [20, 21]. In the study by Ismail et al., no correlation was found between Ki-67 proliferative index and biological behavior (recurrence and metastasis) [20]. All of our 9 cases had low Ki-67 labeling (0–10 %) and Ki-67 staining was essentially negative or only seen in rare atypical stromal cells. Malignant GCTBs show overt pleomorphism accompanied by abnormal mitotic figures. Although the utility of Ki-67 in malignant giant cell tumors is unknown, our observation that the majority of the pseudoanaplastic cells fail to label for Ki-67 suggest that they are in a nonproliferating state. Thus, Ki-67 could be potentially useful in the diagnosis. However, a larger series of cases need to be studied to substantiate this finding.
In 2 of the cases reported by Bahk and Mirra, the atypical cells were associated with previous Gelfoam embolization, leading to the hypothesis of degenerative changes due to ischemia [8]. None of the cases in our series had previous embolization. One patient (cervical spine, C6–T1), with a previous history of a GCTB and multiple recurrences, had previously undergone radiation therapy with 6,000 cGy in 30 fractions before the biopsy; 1 case had received denosumab treatment and subsequently showed denosumab-associated exuberant new bone formation and persistence of the symplastic cells in the resection specimen, thus complicating the histology and mimicking a bone-forming tumor (Fig. 5). Denosumab is a human monoclonal antibody that specifically inhibits RANKL, preventing RANKL–RANK binding, leading to the inhibition of osteoclast-mediated bone destruction. In most cases, denosumab treatment results in a marked reduction of giant cells, replacement of the spindle-shaped stromal cells with a less cellular stroma, and new osteoid formation [22]. Some of these features can mimic an osteosarcoma or malignant transformation of GCTB. Wojcik et al. showed that denosumab-treated GCTBs show less atypia, reduced mitotic activity, and a lack of an infiltrative growth pattern, with Ki-67 proliferation index in the range of 3 % to 8 % [21].
Most conventional GCTBs are found in the epi-metaphyseal region of long bones, and less so in the axial skeleton [6]. Even though this is a small series, 5 of our 9 patients (55 %) had GCTB located in the axial skeleton. Three of the 5 previously reported symplastic GCTB cases were located in the axial skeleton (lumbar spine and sacrum) [8, 9]. Historically, local recurrence rates of 0 % to 65 % have been reported in giant cell tumors of long bones [1, 4–6, 23, 24]. More recent series have reported improved local recurrence rates following adjuvant treatment (10–20 %) [6, 25, 26]. Although 4 of our cases (44 %) showed local recurrence, 3 of these cases were located in the axial skeleton, where complete excision is difficult to achieve with surgery. The follow-up period of our series is relatively small: 5 cases have less than 3 years’ follow-up. However, none of our cases were associated with distant metastasis.
Behjati et al. identified recurrent somatic mutations of H3F3A gene, encoding replication-independent histone H3.3 in GCTB [7]. They found that in 92 % of GCTBs, histone H3.3 alterations are seen exclusively in H3F3A, leading to p.Gly34Trp or p.Gly34Leu alterations. The mutations were restricted to the stromal cell population and were not detected in osteoclasts or their precursors. Subsequently, mutations of glycine 34 in H3F3Awere found in 96 % and 69 % of GCTBs [27, 28]. In our study, we found that symplastic/pseudoanaplastic GCTBs have the same H3F3A mutation (seen in 6 out of 7 cases). The different mutation rates reported in various studies are likely due to the sensitivities of different techniques used for mutation testing. As these H3F3A mutations have not been found in other giant cell-rich lesions, such as the central giant cell lesions/giant cell reparative granulomas of the jaws, brown tumors of hyperparathy-roidism, cherubism, and other giant cell-rich lesions, including ABC, NOF, giant cell granuloma, and osteoclast-rich malignant bone tumors, mutation analysis for H3F3A can be of diagnostic use in small biopsy specimens or needle aspirates [27–29].
It is not known whether primary malignant giant cell tumors harbor this mutation. To our knowledge, only 2 reported cases of H3F3A p.G34W alteration in malignant tumors are found in literature, 1 in an unusual high-grade osteoclast-rich tumor with adamantinoma-like features and a second one, in a case of osteosarcoma [27, 30]. Additional studies are needed to determine the prevalence of this mutation in malignant bone tumors, including malignant GCTBs.
In conclusion, GCTB with symplastic/pseudoanaplastic change is an uncommon variant of a conventional GCTB, which can histologically mimic primary sarcoma or sarcomatous transformation, even though radiological features are similar to those seen in conventional GCTBs. The relative absence of Ki-67 staining in these atypical cells can be useful in distinguishing it from malignant GCTB. Although mutation testing of H3F3A is of diagnostic use for confirming GCTB, especially in small biopsy specimens, at the present time this cannot be relied upon for excluding malignancy.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflicts of interest.
Funding disclosure Chao Lu is the Kandarian Family Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2195-14).
IRB approval The approval number is WA0151-13(1).
References
- 1.Beebe-Dimmer JL, Cetin K, Fryzek JP, Schuetze SM, Schwartz K. The epidemiology of malignant giant cell tumors of bone: an analysis of data from the surveillance, epidemiology and end results program (1975–2004) Rare Tumors. 2009;1(2):e52. doi: 10.4081/rt.2009.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117(2):210–6. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520–9. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 4.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134(9):969–78. doi: 10.1007/s00432-008-0370-x. [DOI] [PubMed] [Google Scholar]
- 5.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591–9. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, et al. Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop Relat Res. 2002;397:248–58. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45(12):1479–82. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahk WJ, Mirra JM. Pseudoanaplastic tumors of bone. Skeletal Radiol. 2004;33(11):641–8. doi: 10.1007/s00256-004-0826-2. [DOI] [PubMed] [Google Scholar]
- 9.Layfield LJ, Bentley RC, Mirra JM. Pseudoanaplastic giant cell tumor of bone. Arch Pathol Lab Med. 1999;123(2):163–6. doi: 10.5858/1999-123-0163-PGCTOB. [DOI] [PubMed] [Google Scholar]
- 10.Dahl I, Hagmar B, Idvall I. Benign solitary neurilemmoma (Schwannoma). A correlative cytological and histological study of 28 cases. Acta Pathol Microbiol Scand A Pathol. 1984;92(2):91–101. [PubMed] [Google Scholar]
- 11.Lin BT, Weiss LM, Medeiros LJ. Neurofibroma and cellular neurofibroma with atypia: a report of 14 tumors. Am J Surg Pathol. 1997;21(12):1443–9. doi: 10.1097/00000478-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Siti-Aishah MA, Noriah O, Malini MN, Zainul-Rashid MR, Das S. Atypical (symplastic) leiomyoma of the uterus—a case report. Clin Ter. 2011;162(5):447–50. [PubMed] [Google Scholar]
- 13.Bertoni F, Fernando Arias L, Alberghini M, Bacchini P. Fibrous dysplasia with degenerative atypia: a benign lesion potentially mistaken for sarcoma. Arch Pathol Lab Med. 2004;128(7):794–6. doi: 10.5858/2004-128-794-FDWDAA. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni F, Unni KK, McLeod RA, Dahlin DC. Osteosarcoma resembling osteoblastoma. Cancer. 1985;55(2):416–26. doi: 10.1002/1097-0142(19850115)55:2<416::aid-cncr2820550221>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Cheung FM, Wu WC, Lam CK, Fu YK. Diagnostic criteria for pseudomalignant osteoblastoma. Histopathology. 1997;31(2):196–200. doi: 10.1046/j.1365-2559.1997.5870820.x. [DOI] [PubMed] [Google Scholar]
- 16.Craver RD, Heinrich S, Mirra J. Fibrous cortical defect with bizarre nuclear features. Ann Diagn Pathol. 1997;1(1):26–30. doi: 10.1016/s1092-9134(97)80006-8. [DOI] [PubMed] [Google Scholar]
- 17.Desai SS, Jambhekar NA, Samanthray S, Merchant NH, Puri A, Agarwal M. Chondromyxoid fibromas: a study of 10 cases. J Surg Oncol. 2005;89(1):28–31. doi: 10.1002/jso.20113. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento AG, Huvos AG, Marcove RC. Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer. 1979;44(4):1393–402. doi: 10.1002/1097-0142(197910)44:4<1393::aid-cncr2820440433>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki K, Mera Y, Uchimiya H, Katahira Y, Kanzaki T. Plexiform schwannoma. Clin Exp Dermatol. 2003;28(4):372–4. doi: 10.1046/j.1365-2230.2003.01286.x. [DOI] [PubMed] [Google Scholar]
- 20.Ismail FW, Shamsudin AM, Wan Z, Daud SM, Samarendra MS. Ki-67 immunohistochemistry index in stage III giant cell tumor of the bone. J Exp Clin Cancer Res. 2010;29:25. doi: 10.1186/1756-9966-29-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wojcik J, Rosenberg AE, Bredella MA, Choy E, Hornicek FJ, Nielsen GP, et al. Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone. Am J Surg Pathol. 2016;40(1):72–80. doi: 10.1097/PAS.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 22.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–80. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 23.Dahlin DC, Cupps RE, Johnson EW., Jr Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061–70. doi: 10.1002/1097-0142(197005)25:5<1061::aid-cncr2820250509>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64(5):755–61. [PubMed] [Google Scholar]
- 25.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81(6):811–20. doi: 10.2106/00004623-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Saiz P, Virkus W, Piasecki P, Templeton A, Shott S, Gitelis S. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop Relat Res. 2004;424:221–6. doi: 10.1097/01.blo.0000128280.59965.e3. [DOI] [PubMed] [Google Scholar]
- 27.Presneau N, Baumhoer D, Behjati S, Pillay N, Tarpey P, Campbell PJ, et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J Pathol Clin Res. 2015;1(2):113–23. doi: 10.1002/cjp2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleven AH, Hocker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovee JV. Mutation analysis of H3F3A and H3F3B as a diagnostic tool for giant cell tumor of bone and chondroblastoma. Am J Surg Pathol. 2015;39(11):1576–83. doi: 10.1097/PAS.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 29.Gomes CC, Diniz MG, Amaral FR, Antonini Guimaraes BV, Gomez RS. The highly prevalent H3F3A mutation in giant cell tumours of bone is not shared by sporadic central giant cell lesion of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(5):583–5. doi: 10.1016/j.oooo.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Joseph CG, Hwang H, Jiao Y, Wood LD, Kinde I, Wu J, et al. Exomic analysis of myxoid liposarcomas, synovial sarcomas, and osteosarcomas. Genes Chromosomes Cancer. 2014;53(1):15–24. doi: 10.1002/gcc.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]