Abstract
We describe an extraordinary case of an immunocompetent patient who developed sacral-distribution zoster, followed 3 months later by neurological disease that progressed for 6 years and was attributed to varicella zoster virus (VZV) infection of the brain. Despite the prolonged infection, neurologic symptoms and signs resolved rapidly and completely after treatment with intravenous acyclovir.
Keywords: VZV, progressive neurological disease
1. Introduction
We describe an extraordinary case of a patient who developed sacral-distribution zoster followed 6 months later by progressive neurological disease that lasted for 6 years and was shown to be caused by varicella zoster virus (VZV) infection of the brain. Despite the prolonged infection, neurologic symptoms and signs resolved rapidly and completely after treatment with intravenous acyclovir.
2. Case report
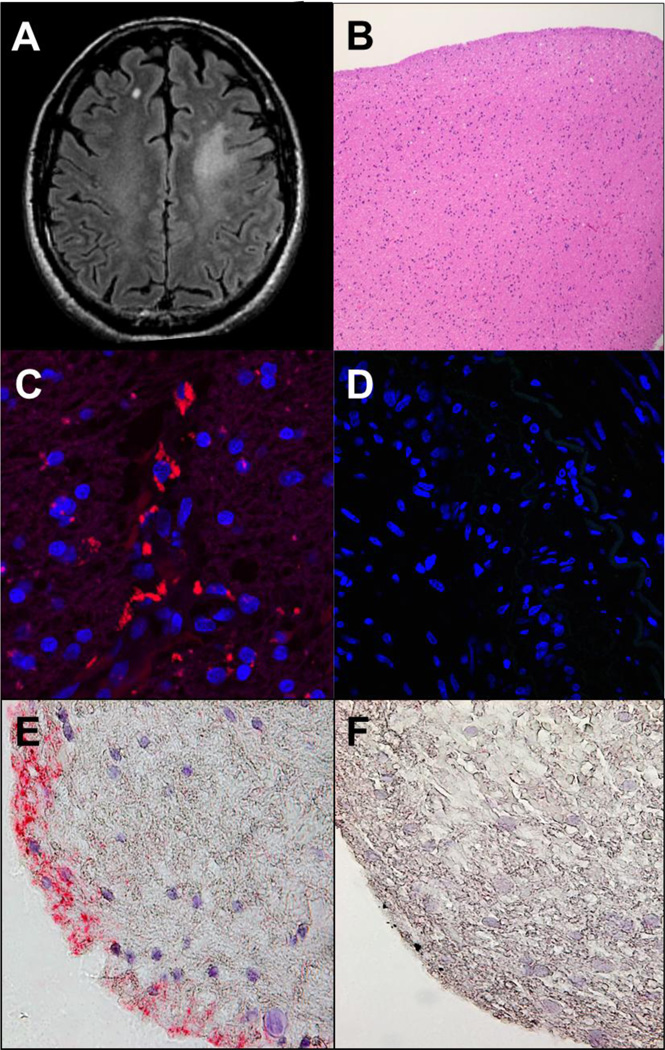
In 2010, a 63-year-old immunocompetent man developed right S1-2 distribution zoster. Three months later, cognitive problems ensued, followed by an episode of transient global amnesia 3 months later. CSF examination a few days later (6 months after zoster and 3 months after the onset of cognitive difficulty) was acellular with normal protein and glucose; CSF PCR for VZV, HSV-1, HSV-2, EBV and CMV DNA were all negative. There was no prior history of diabetes, HIV or other immunodeficiencies. Brain MRI at an outside hospital revealed a focal non-enhancing left centrum semiovale T2 hyperintense lesion with ill-defined margins (Fig. 1A). Brain biopsy revealed hypercellular white matter (Fig. 1B) without neoplasm, inflammation, vasculitis or necrosis. The patient was followed without intervention. In 2011, he noted mild progressive impairment of cognition and executive function. During lectures to students, he had difficulty focusing and was often unable to finish without pausing.
Fig. 1.
Brain imaging and histology, and immunostaining of brain biopsy for varicella zoster virus antigen. Axial FLAIR brain MRI demonstrates a focal area of T2 hyperintensity in the left anterior centrum semiovale with ill-defined margins, without mass effect or volume loss (A). No post-contrast enhancement was seen (images not shown). Hematoxylin and eosin stained paraffin-embedded section of the brain biopsy shows well-fixed minimally hypercellular white matter without evidence of neoplasm, inflammation, vasculitis or necrosis (B). These findings were confirmed with special immunostains for GFAP, CD68 and IDH1 mutation (data not shown). Indirect immunofluorescence with mouse monoclonal anti-VZV antibodies directed against both the VZV immediate early (IE) 62 and the late VZV gE proteins applied to the brain biopsy, as described by Halling et al. [1], revealed VZV antigen (red) in the cytoplasm of brain cells (C), but not when primary antibody was omitted (D); the same anti-VZV antibody did not stain normal brain tissue [1]. VZV antigen was also detected by immunohistochemistry with rabbit monospecific polyclonal anti-VZV IE 63 antibody (E, pink color), as described by Gilden et al. [2], in a different section of the same brain biopsy, but not in another section of the same brain biopsy when normal rabbit serum was substituted for primary rabbit anti-VZV IE 63 antibody on (F). Magnification: 100X (B); 630X (C and D); 600X (E and F).
In 2014, the patient, a viral immunologist, listened to a case presentation of a person with focal VZV encephalitis without rash who was treated successfully with oral valacyclovir [1]. Wondering if he might also have VZV brain infection, particularly since he had zoster 6 months before the onset of his neurological disease, he arranged for the 2010 brain biopsy to be studied for VZV infection. Immunofluorescence staining of the brain biopsy revealed VZV proteins in the cytoplasm of numerous cells (Fig. 1C). Because the scientific laboratory was not an authorized pathology laboratory, his physicians did not accept the virological findings and did not treat the viral infection. In 2015, a second independent laboratory detected VZV antigen by immunohistochemistry in the same brain biopsy (Fig. 1E). Unfortunately, not enough tissue remained to identify the cell type(s) in brain that were infected by virus or to extract DNA for PCR analysis. During 2014–2015, he experienced episodes of dizziness, difficulty concentrating, continued trouble lecturing and impaired reading comprehension.
In December 2015, he was re-evaluated at the University of Colorado Hospital. There was no evidence of clinical depression or history of mental illness. The neurological examination revealed that he could remember 3/3 cities, 0/3 numbers and 4/5 objects after a 5-minute period. He named months of year backwards, but did so slowly. He had difficulty with similarities and comparisons and trouble interpreting proverbs. The remainder of the mental status examination was normal, as were cranial nerves, motor, sensory, coordination and DTRs, and there were no pathological reflexes. EEG was normal and serial follow-up MRIs were unchanged from 2010. He was treated with intravenous acyclovir, 10 mg/kg 3 times daily, and after only 2 days, he appeared more alert and animated. Dizziness abated. After 2 weeks of treatment, the mental status exam was normal and MRI was unchanged. He has remained neurologically asymptomatic for the past 6 months. While a placebo effect cannot be ruled out with certainty, the patient’s dramatic improvement in mental status correlated with antiviral treatment.
3. Discussion
Except for subacute sclerosing panencephalitis and chronic rubella encephalitis caused by measles and rubella virus, respectively, virtually all neurotropic viruses produce acute neurologic disease. VZV is unique in that it produces both acute and chronic waxing and waning neurologic disease, including VZV vasculopathy, myelopathy and encephalitis. Herein, we describe a patient with the longest known VZV infection of the brain without inflammation of 6 years duration, who responded rapidly and completely to treatment with intravenous acyclovir. The focal white matter lesion in our immunocompetent patient contrasts with 2 earlier cases of fatal multifocal VZV leukoencephalitis with demyelination and necrosis that developed in immunocompromised patients with cancer [3]. Because the brain lesion was focal and the CSF was examined for VZV DNA 3 months after the onset of cognitive problems, it is not surprising that PCR for VZV DNA in CSF was negative for viral DNA. Similarly, in VZV vasculopathy, detection of VZV DNA in CSF is only 30% sensitive due to the chronic nature of disease [4]. While we recognize that VZV was demonstrated in brain only at the onset of neurological disease, the combination of progressive cognitive decline for 6 years and the gratifying response to antiviral treatment provided further support for the notion that his chronic illness was produced by VZV infection
Other protracted cases of VZV infection in the CNS have been reported. The first virologically-verified case of VZV vasculopathy lasted 314 days before the patient died [5]. A second patient with protracted VZV vasculopathy of 6 months duration was completely cured after antiviral treatment [6]. Disease of a third patient with progressive multifocal VZV vasculopathy manifest initially by ischemic optic neuropathy followed by 4 strokes over the next 26 months was arrested upon antiviral treatment [7]. The onset of CNS infection in our patient and the development of VZV vasculopathy in the second case above, occurred 6 months after zoster, a time when a history of zoster might not have been appreciated, resulting in the lack of search for VZV in brain. Importantly, 2 of the above 3 patients with VZV vasculopathy, as well as 3 other cases of VZV encephalitis, all involving the temporal lobe, also had no rash [1, 8, 9].
4. Conclusion
Overall, VZV can cause chronic progressive focal or generalized neurologic disease, with or without a recent history of zoster. Despite the lengthy duration of infection, proper antiviral treatment can result in a favorable outcome.
HIGHLIGHTS.
VZV causes progressive neurological disease
Antivirals can result in favorable outcome even after 6 years of VZV brain infection
VZV causes both acute and chronic neurological disease
Acknowledgments
This work was supported in part by Public Health Service grant AG032958 (D.G., R.J.C., M.A.N.). The authors thank Dr. Clayton Wiley for providing brain biopsy tissue, Marina Hoffman for editorial review and Cathy Allen for word processing and formatting.
Abbreviations
- VZV
varicella zoster virus
- HSV
herpes simplex virus
- EBV
Epstein-Barr virus
- CMV
cytomegalovirus
- DTR
deep tendon reflex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors report no conflicts of interest.
REFERENCES
- 1.Halling G, Giannini C, Britton JW, Lee RW, Watson RE, Jr, Terrell CL, et al. Focal encephalitis following varicella-zoster virus reactivation without rash in a healthy immunized young adult. J. Infect. Dis. 2014;210:713–716. doi: 10.1093/infdis/jiu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84:1948–1955. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horten B, Price RW, Jimenez D. Multifocal varicella-zoster virus leukoencephalitis temporally remote from herpes zoster. Ann. Neurol. 1981;9:251–266. doi: 10.1002/ana.410090308. [DOI] [PubMed] [Google Scholar]
- 4.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani F, Schiller A, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging and virological features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilden HD, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 6.Gilden DH, Lipton HL, Wolf JS, Akenbrandt W, Smith JE, Mahalingam R, Forghani B. Two patients with unusual forms of varicella-zoster virus vasculopathy. N. Eng. J. Med. 2002;347:1500–1503. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 7.Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. Varicella zoster virus vasculopathy: A treatable form of rapidly progressive multi-infarct dementia after 2 years’ duration. J. Neurol. Sci. 2012;323:245–247. doi: 10.1016/j.jns.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tattevin P, Schortgen F, de Broucker T, Dautheville S, Wolff M. Varicella-zoster virus limbic encephalitis in an immunocompromised patient. Scand. J. Infect. Dis. 2001;33:786–788. doi: 10.1080/003655401317074680. [DOI] [PubMed] [Google Scholar]
- 9.Yajima R, Utsumi K, Ishihara T, Kanazawa M, Okamoto K, Kawachi I, Nishizawa M. Varicella-zoster virus encephalitis localized to the bilateral medial temporal lobes. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:3108. doi: 10.1212/NXI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]