Abstract
Background:
Among advanced therapeutic interventions for wounds, hyperbaric oxygen therapy (HBOT) has the unique ability to ameliorate tissue hypoxia, reduce pathologic inflammation, and mitigate ischemia reperfusion injury. Most of the conditions for which it is utilized have few successful alternative treatments, and the morbidity and mortality associated with treatment failure are significant. Data on the efficacy and effectiveness of HBOT were reviewed, comparative effectiveness research of HBOT was explained, and a new paradigm for the appropriate use of HBOT was described.
Methods:
Systematic reviews and randomized controlled trials that have evaluated HBOT were reviewed.
Results:
Although numerous small randomized controlled trials provide compelling support for HBOT, the physics of the hyperbaric environment create significant barriers to trial design. The electronic health record infrastructure created to satisfy mandatory quality and registry reporting requirements as part of healthcare reform can be harnessed to facilitate the acquisition of real world data for HBOT comparative effectiveness studies and clinical decision support.
Conclusions:
Predictive models can identify patients unlikely to heal spontaneously and most likely to benefit from HBOT. Although electronic health records can automate the calculation of predictive models making them available at the point of care, using them in clinical decision making is complicated. It is not clear whether stakeholders will support the allocation of healthcare resources using mathematical models, but the current patient selection process mandates a 30-day delay for all patients who might benefit and allows treatment for at least some patients who cannot benefit.
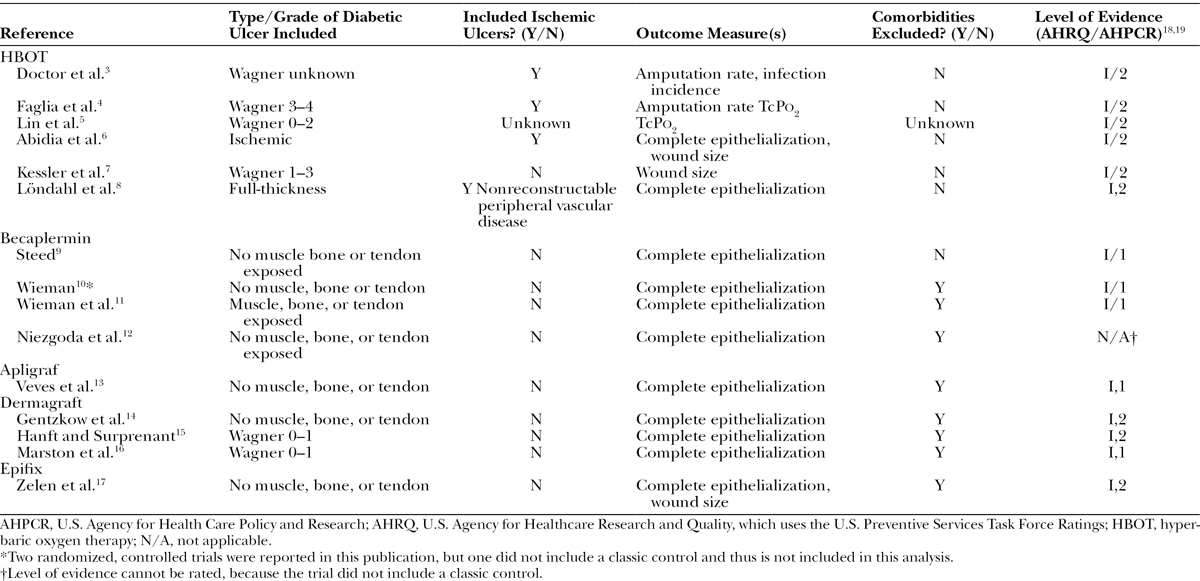
Tissue hypoxia, usually due to ischemia, is the common denominator among most nonhealing wounds.1 Tissue oxygen levels control angiogenesis and the neutrophil function that mitigates infection. Nevertheless, in a review of 1172 randomized controlled trials (RCTs) in wound care, none were directed at improving angiogenesis in ischemic ulcers except those involving hyperbaric oxygen therapy (HBOT) and vascular endothelial growth factor.2 Since 1996, a myriad of advanced therapeutics have become available, but their proven efficacy is limited to superficial ulcers that do not involve tendon or joint capsule and that occur in patients without peripheral arterial disease. Table 1 includes a comparative sample of criteria used for HBOT RCTs on diabetic foot ulcers (DFUs)3–17 versus criteria used for RCTs evaluating Becaplermin,9–12 Apligraf,13 Dermagraft,14–16 and Epifix.17 The HBOT trials were generally comparable in terms of their level of evidence as ranked by the U.S. Agency for Healthcare Research and Quality18 and the U.S. Agency for Health Care Policy and Research,19 but only the HBOT trials included patients with significant comorbidities, Wagner Grade 3 or greater ulcers, and ischemic vascular disease. The superficial ulcers enrolled in cellular product and growth factor trials used epithelialization as the primary outcome, but the more serious ulcers enrolled in HBOT trials measured outcomes such as amputation rate, infection incidence, and/or improvement in transcutaneous oximetry values (TcPo2). Thus, although the HBOT trials are smaller, their results are more generalizable to real-world patients who may need limb-saving intervention. The success of nearly all the advanced therapeutics currently available for treating chronic wounds presupposes the availability of adequate tissue oxygen tensions to support cellular replication. The unique ability of HBOT to induce neovascularization accounts for its persistence in the clinical armamentarium of chronic wound treatments, despite ongoing controversy regarding its cost effectiveness.1,20,21
Table 1.
A Comparison of Criteria Used in RCTs That Evaluated HBOT versus Other Advanced Therapeutics on DFUs
HBOT is administered by placing the entire patient into a pressure vessel (chamber), whereupon 100% oxygen is respired at atmospheric pressures ranging from 2 times sea level (202.65 kPa) to approximately 2.4 times that experienced at sea level (243.18 kPa). HBOT has a very low but predictable occurrence of side effects,22 the most common of which is mild to moderate otic barotrauma from pressure-related changes in the gas volume of the middle ear, occurring in up to 10% of patients.23,24 A very rare side effect is central nervous system oxygen toxicity manifested as a grand mal seizure, the incidence of which is variously reported between 1:10,000 and 1:50,000. Chronic obstructive pulmonary disease is a rare, relative contraindication to HBOT, the air trapping from which can predispose to pulmonary overpressurization, pneumothorax, arterial gas embolism, and even death.23,24 Myopia, usually reversible, has been reported in patients undergoing a prolonged course of daily therapy.25 Reduction in blood glucose after HBOT has been reported among patients with diabetes. However, it is not known if this is due to the timing of treatment in relation to medication administration, or some direct physiological effect of HBOT, the mechanism of which has not been elucidated, although increased peripheral insulin sensitivity has been observed.26 We will review data on the efficacy of HBOT, describe how the specialty registry reporting requirements mandated as part of healthcare payment reform can be harnessed to study the comparative effectiveness of HBOT, and propose a new paradigm for the appropriate use of HBOT.
INDICATIONS AND MECHANISM OF ACTION
The U.S. Food and Drug Administration defers to the Undersea and Hyperbaric Medical Society (UHMS) to establish the list of indications for which HBOT has sufficient evidence to support its use.23,24,27 HBOT chambers are Class II medical devices. The U.S. Food and Drug Administration clears them for marketing and use in 13 indications (Table 2).23,24
Table 2.
The 13 Indications for Hyperbaric Oxygen Therapy Approved by the U.S. Food and Drug Administration for Marketing and for Use23,24

Recently, the Department of Defense has funded trials to evaluate the use of HBOT in chronic traumatic brain injury, which thus far is not supported by the evidence.28–32 Although acute ischemic or traumatic injuries seem logical targets for HBOT research, the significant challenges involved in providing HBOT immediately to very sick patients have limited their application to the chronic phase of these conditions, a time frame during which any beneficial effects of HBOT (assuming they exist) are least likely to be identified.33
The use of HBOT in acute air embolism and decompression illness (DCI) takes advantage of the physical compression of gas bubbles, which occurs as atmospheric pressure increases. Thereafter, the diverse list of conditions listed above may benefit from HBOT via a similar underlying mechanism. In 2011, Thom34 authored a detailed, seminal review of the mechanism of action of HBOT and the clinical rationale for its use. For decades, the HBOT mechanism of action was simplistically focused on the amelioration of tissue hypoxia by the physical dissolution of oxygen in the plasma. This is negligible breathing air at sea level, but increases approximately 2 volumes percent with each additional atmosphere of pressure when respiring 100% oxygen. In fact, a landmark study by Boerema et al.35 published in 1960 demonstrated that during HBOT, unanesthetized pigs without circulating hemoglobin (hemoglobin 0.5%) could function comfortably as a result of the significant amount of plasma-dissolved oxygen achieved under hyperbaric conditions. This is the same mechanism by which HBOT has proved life sustaining to patients with exceptional blood loss anemia who refuse transfusion.
In the past 2 decades, Thom,34 Piantadosi and colleagues,36–38 Zamboni and colleagues,39,40 and others have elucidated the biochemical basis for HBOT, the principal mechanism of which is the controlled generation of reactive oxygen and nitrogen species.1,34 These increases result in higher levels of various growth factors and the activation of growth factor receptors, the mobilization of bone-marrow-derived stem/progenitor cells, an alteration in the function of integrin (resulting in reduced neutrophil adhesion), and changes in monocyte chemokine synthesis, as well as hemoxygenase-1, heat shock proteins, and hypoxia-inducible factor-1 that reduce inflammation. Thus, HBOT helps to resolve the pathologic inflammation and ischemia, which are characteristics of chronic wounds.1,34
CHALLENGES TO RESEARCH
A major criticism of HBOT is the lack of a sufficient number of large, related RCTs. However, the optimal way to design an RCT of HBOT remains in dispute.23,24 Evidence suggests that patients perceive simply going into the chamber to have a salutary effect.28,41,42 To control for this, sham treatments have been successfully devised creating the illusion of the HBOT experience by compressing the chamber only enough to produce the sensation of pressure on the tympanic membranes.24 Another approach has been to compress the chamber to the actual treatment pressure but allowing the control subjects to breathe air (21% oxygen, 21.33 kPa) rather than 100% oxygen. However, since the partial pressure of oxygen (Po2) increases as atmospheric pressure increases, at a total pressure of 202.65 kPa, the Po2 is 42.66 kPa, equivalent to respiring 42% oxygen at sea level. Data suggest a possible therapeutic effect of oxygen even at this low dose, possibly confounding study results.24 More problematic, control subjects are now breathing compressed nitrogen. Depending on the atmospheric pressure and duration of the exposure, they will dissolve this inert gas into their tissues and be exposed to the risk of DCI after exiting the chamber. The study of Löndahl et al.8 provides compelling evidence for the efficacy of HBOT in DFUs. However, in this RCT, control subjects respired air at 243.18 kPa for a total of 95 minutes. This exposure was in excess of the “no decompression limit” for most sport diving tables. There was no discussion in the paper regarding the possible risk of DCI to the controls, and it is not clear whether DCI could have been the reason that a control subject was hospitalized for 24 hours after “temporarily losing consciousness after a treatment session.”8,24
Although the laws of physics have been a major challenge to HBOT research, the greatest obstacle has been the lack of adequate funding.23,24 Most approved conditions garner little funding for clinical research and often have few alternative treatments with even as much evidence of benefit as HBOT. Furthermore, should HBOT fail, the next step may be, for example, limb amputation (in the case of an ischemic DFU) or cystectomy (in the case of refractory radiation-induced hemorrhagic cystitis). Consequently, a new paradigm for comparative effectiveness research and appropriate utilization is required.
EVIDENCE
The UHMS regularly publishes a summary of the current evidence,43 and several excellent systematic reviews have been published.44,45 For example, the Wounds Group of the Cochrane Collaboration performed a systematic review of HBOT for chronic wounds.44 Ten of the 12 RCTs reviewed were DFU studies. Pooled data from 5 trials with 205 participants showed an increase in the rate of ulcer healing with HBOT at 6 weeks, although the benefit was not evident at 1 year (risk ratio: 2.35; 95% confidence interval [CI]: 1.19–4.62; P = 0.01). Huang et al.45 performed the most exhaustive HBOT systematic review to develop the UHMS clinical practice guidelines for the diabetic foot, analyzing 9 RCTs and more than 20 observational studies using the GRADE criteria. There is moderate level evidence that HBOT is beneficial in preventing amputation and promoting complete healing in patients with Wagner Grade 3 or greater DFUs, who have undergone a surgical debridement or have shown no significant improvement after 30 or more days of conservative care. There is inadequate evidence to justify HBOT in Wagner Grade 2 or lower DFUs.45 These conclusions align with the Centers for Medicare and Medicaid Services (CMS) National Coverage Determination for DFUs, which specifies that HBOT coverage is limited to Wagner Grade 3 ulcers that have failed to respond to 30 days of standard wound care.46
Although several well-conducted, albeit small, prospective trials have demonstrated the efficacy of HBOT in DFUs, the effectiveness of HBOT could not be demonstrated in a recent large retrospective analysis.47 Margolis et al.48 studied 6259 individuals with DFUs using data obtained from a wound center management company database. Using propensity score–adjusted models, patients undergoing HBOT were less likely to heal a DFU (hazard ratio: 0.68; 95% CI: 0.63–0.73) and more likely to have an amputation (2.37 [1.84–3.04]). While the authors stated that the patient cohorts in this study were defined by the CMS eligibility criteria for HBOT, the preponderance of patients in the analysis had Wagner Grade 2 ulcers, lesions for which the data do not suggest that HBOT is useful and which are also excluded from Medicare coverage.46,47 Given that the providers failed to ensure that patients met the most basic requirement for HBOT of a Wagner Grade 3 DFU, it is doubtful that all the requirements of conservative care before HBOT were provided. This dataset also did not distinguish “major” amputations (e.g., below or above the knee) from “minor” amputations (e.g., toe or partial foot). Without this vital information, patients whose ambulation was preserved with a partial foot or toe amputation were still considered hyperbaric failures. If all were major amputations that occurred despite HBOT, then this expensive therapy was wasted because it was futile. Additionally, the HBOT provided to a majority of patients at 83 wound centers in 31 states was actually unnecessary (and thus inappropriate) since these Wagner Grade 2 ulcers could have been managed via less costly methods.47
THE APPROPRIATE USE OF HBOT
HBOT is ineffective when it is provided to patients who could heal without it (inappropriate), when it is provided to patients who cannot be helped (wasted), or when more treatments are provided than are needed to achieve the desired benefit (excessive). Medicare has laudably sought to prevent or reduce unnecessary treatments by requiring that HBOT be reserved for patients who exhibit “no measurable signs of healing for 30 days.”46 Wound healing trajectory based on surface area measurement over 4 weeks can be used to identify ulcers unlikely to heal.49 Since some decrease in SA may occur in wounds still destined to fail, limiting HBOT to wounds exhibiting absolutely no evidence of healing is overly restrictive and likely prevents some appropriate use. A more accurate method to predict the likelihood of healing is via mathematical modeling, which can be performed on the first visit. The Wound Healing Index (WHI) is a suite of mathematical models for 7 different wound types that combine patient and wound variables.50 A predictive model specific to DFUs has been validated, which, using the data available on the first visit (C-statistic >0.65, Fig. 1), can predict the likelihood that the DFU will heal with conservative care alone.51 It is not known whether payers will support the use of models to select the patients most in need of advanced therapeutic interventions. However, doing so would allow interventions like HBOT to be employed earlier, at a time when they are more likely to be of benefit, and without the added expense of 4 weeks of care that could have been predicted to fail.
Fig. 1.
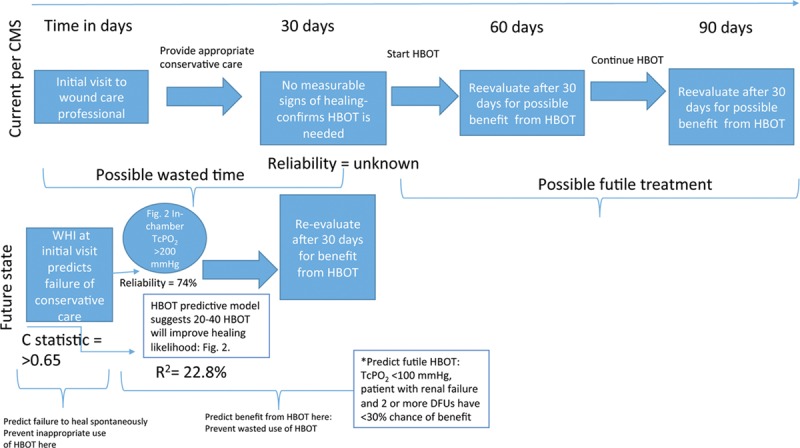
Choosing Wisely for HBOT in DFUs: a mathematical paradigm.50,51,53,54 TcPo2 = transcutaneous oximetry measurement; R2 is the coefficient of determination, a statistical measure of how close data are to the fitted regression line. R2 is always between 0% and 100%, whereby 0% indicates that the model explains none of the variability of the data around its mean, and 100% indicates that the model explains all such variability. A very low R2 is not desirable in models that might be used to influence clinical decisions regarding the use of a medical intervention. However, even in this model with a relatively low R2 but statistically significant predictors, it is still possible to understand how changes in the predictor values are associated with the changes in the likelihood of benefit from HBOT; C-statistic is the probability that predicting the outcome is better than chance. C-statistic is used to compare the goodness of fit of logistic regression models. Values for this measure range from 0.5 to 1.0, whereby a value of 0.5 indicates that the model is no better than chance at making a prediction, and a value of 1.0 indicates that the model is perfect at identifying the desired outcome. Models are typically considered reasonable when the C-statistic is higher than 0.7 and strong when C exceeds 0.8. Therefore, a C-statistic >0.65 for this model predicting the benefit of HBOT is better than chance, but is not a strong model.
How can we reduce wasted HBOT? The single best predictor of benefit from HBOT in DFUs is the TcPo2 obtained while the patient is undergoing an HBOT treatment.52 In-chamber TcPo2 alone is 74% accurate at predicting benefit from HBOT in DFUs.53 In a large retrospective study, when the in-chamber TcPo2 was >200 mm Hg, 84% of DFUs benefited; when it was <100 mm Hg, only 14% benefitted.54 Figure 2 shows the failure rates at different TcPo2 levels.54 Ninety-percent of DFUs failed when the TcPo2 was <100 mm Hg, whereas 35.7% of wounds failed at 101 to 200 mm Hg and 301 to 400 mm Hg, and 18.2% failed at >1000 mm Hg (Fig. 2). Predicting the response to HBOT can be improved with mathematical modeling. A relatively simple model available since 2007 uses baseline TcPo2, pack-year smoking history, Wagner Grade, patient age, and the number of years of diabetes to predict the approximate number of HBOT treatments needed to achieve benefit (R2 = 22.8%).54 The model, which does not require an in-chamber TcPo2, has the advantage that the patient does not have to undergo a hyperbaric treatment to determine their likely benefit from HBOT. The same study suggested that for DFUs, there is no incremental benefit after 40 hyperbaric treatments (Fig. 3). Thus, if the model predicts that more than 40 treatments will be necessary to achieve a positive outcome, HBOT may not be a realistic treatment option. However, if the in-chamber TcPo2 is >200 mm Hg and the predictive model suggests that between 15 and 40 treatments will be required, there is a reasonable likelihood of benefit from HBOT for a DFU.
Fig. 2.
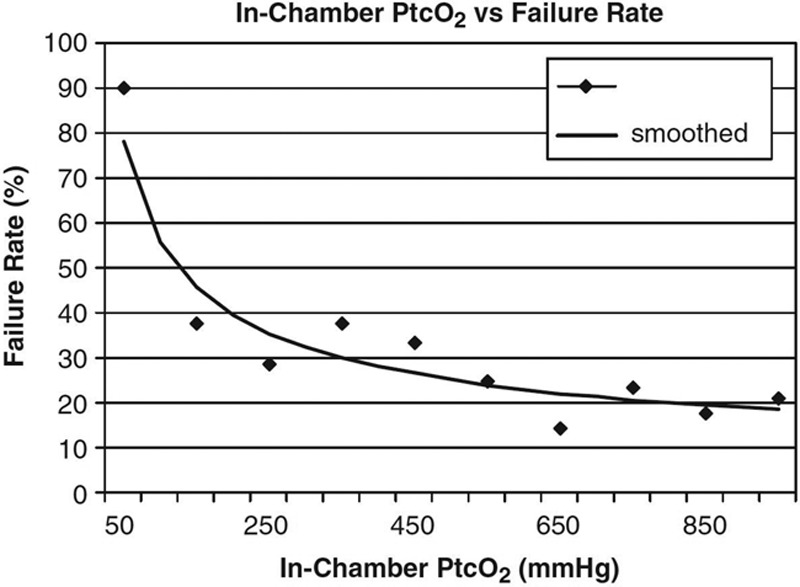
In-chamber failure rates vs Ptco2 achievement. Data from 221 patients with DFUs.53 This figure demonstrates that for Ptco2 levels ≤100, the failure rate was 90.0%; for Ptco2 levels of 101–200 and for 301–400, the failure rate was 35.7%; for Ptco2 levels >1000, the failure rate was 18.2%.
Fig. 3.
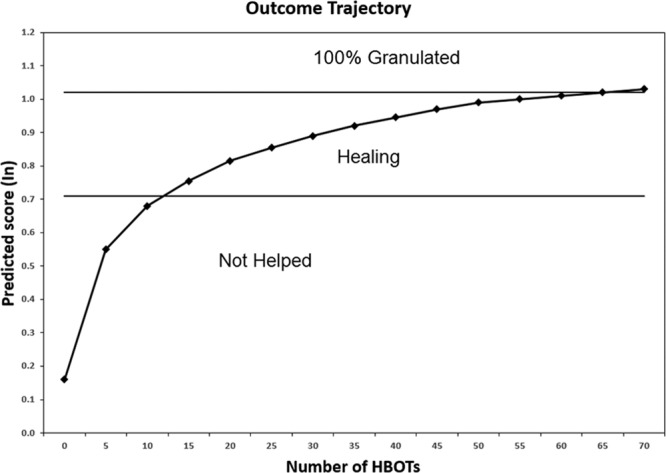
This figure depicts the expected outcome trajectory of a hypothetical patient with the following patient characteristics: age = 60 yr; diabetes = 15 yr; Wagner Grade = 3; Ptco2 (air) = 15 mm Hg; nonsmoker.53 Some improvement in the wound should be visible after 12 hyperbaric treatments, but approximately 35–40 treatments will likely be required to achieve healing. The curve demonstrates that there is little incremental benefit from additional treatments after 40. The clinical use of predictive models like this would require significant provider education to ensure that they are not interpreted as literal recommendations. For example, if the model predicted that 200 treatments would be necessary to achieve improvement, the clinician should understand that benefit from HBOT is not likely to be achieved within a clinically reasonable course of treatment. Conversely, if the model were to predict, for example, that a patient will require fewer than 10 treatments to heal, it is likely that the patient does not need any hyperbaric treatments at all.
Figure 1 incorporates these mathematical predictive models into a new paradigm to guide the care provider’s decision making processes on the use of HBOT for a DFU compared with current Medicare coverage policy. Currently, clinicians implement conservative care and wait 30 days, after which only patients who have no evidence of healing are HBOT candidates. Some patients who need HBOT may then be excluded because they had small reductions in surface area. This approach also delays HBOT for all who need it. After initiation of HBOT, reassessment is performed in 30 days to evaluate benefit, allowing some patients to undergo a potentially futile course of HBOT. An alternative is to perform the DFU WHI on the first visit to identify ulcers unlikely to heal with conservative care, after which either an in-chamber TcPo2 can be performed or the HBOT predictive model can be employed to determine whether benefit can be expected within a reasonable number of treatments. Biochemical markers might identify when the benefit of HBOT has been achieved even before visible signs of healing, facilitating shorter treatment courses.55,56 This stepwise approach would enable the clinician to decrease the inappropriate use of HBOT by accurately predicting which DFUs will fail to heal spontaneously and then predicting whether HBOT is likely to be of benefit in those, thus reducing wasted resources.50,54 The WHI model was created using more than 106,272 wounds, while the HBOT model was based on 971 patients. The WHI underwent validation with a holdout sample at the time it was developed. The HBOT model has had no validation because, for most of the decade since its development, there was no impetus for the creation of such models and there were many practical barriers limiting their use. Since the advent of point of care, electronic health records (EHRs) capable of automating probabilistic model calculations, the patient’s likelihood of healing and estimated benefit from HBOT can be made immediately available to the clinician, removing barriers to practical utilization. However, clinicians need guidance if they are to translate predictions into actionable clinical decisions (Fig. 3).57
Real-world data can be used to elucidate the impact of missed treatments and perhaps the ideal treatment pressure for various conditions. For example, a large retrospective analysis of DFUs treated with HBOT suggested that there was no difference in outcome among DFUs treated at 202.65 kPa versus 243.18 kPa.53 Although patients treated at the higher treatment pressure were more likely to achieve very high in-chamber TcPo2, it appears that no incremental benefit is achieved after an in-chamber TcPo2 of 200 mm Hg is reached. As we make the transition from volume-based reimbursement to a value-based healthcare system, providers will be motivated to determine the least number of treatments that can improve outcome. Real-world data can be used to evaluate the incremental benefit of treatments as well as the impact of various disease states or health practices (e.g., smoking) on likelihood of benefit from HBOT.58
The urgent need for national data to evaluate the cost effectiveness of HBOT may be achievable by leveraging the Meaningful Use requirement for clinicians to submit data to a specialty registry. The Hyperbaric Oxygen Therapy Registry (HBOTR) is cosponsored by the UHMS, under the aegis of the US Wound Registry (USWR).59 Any certified EHR can satisfy the Meaningful Use registry reporting requirement by automatically transmitting patient Continuity of Care Documents (CCDs) to the HBOTR. CCDs provide detailed, structured data on patients, without the need for any laborious secondary data entry, transmitting all ICD-10 diagnosis codes, procedures, medications, laboratory results, and demographics as part of current interoperability requirements. Unfortunately, CCDs do not provide any information on wound outcome, which is necessary if data are to be used for effectiveness research. Therefore, we have linked the collection of outcome data to the reporting of clinical quality measures (QMs). The USWR is a qualified clinical data registry, which has developed hyperbaric oxygen and wound care QMs in collaboration with the UHMS that eligible providers can report to satisfy participation in the Physician Quality Reporting System60 and for Maintenance of Certification in hyperbaric medicine. Participating in QM reporting can improve HBOT effectiveness and adherence to Medicare coverage policy by ensuring compliance with clinical practice guidelines such as DFU offloading, vascular screening, and hemoglobin A1c control. Several individual QMs have been combined to create a larger measure focused on the “Appropriate Use of Hyperbaric Oxygen Therapy for Patients with Diabetic Foot Ulcers,”60 which, like all the USWR measures, is available as an electronic clinical QM that can be downloaded free of charge and installed into any certified EHR, enabling data transmission to the registry via a quality reporting data file. In this way, clinicians who are participating in Physician Quality Reporting System will also be reporting standardized datasets to the HBOTR, which include the outcome of the wounds treated with HBOT.
The soon to be implemented Merit-Based Incentive Payment System61 is intended to incentivize specialty registry data submission and QM reporting, particularly appropriate use and outcome measures. The Appropriate use of HBOT in the Diabetic Foot QM mirrors most of the requirements of the HBOT “prior authorization” program for DFUs, illustrating the way in which EHRs can also be leveraged to automate many auditing functions in a far more efficient and less costly way than human reviewers can achieve. The outcome measure for DFUs treated with HBOT is risk stratified by the WHI, making it possible to create matched cohorts of patients who did and did not undergo HBOT, thus facilitating comparative effectiveness studies from real-world data. Currently, over 2000 clinicians report data to the HBOTR, which contains approximately 25,539 hyperbaric patients. It is not clear whether the impetus provided by Merit-Based Incentive Payment System will be sufficient motivation to expand participation in HBOTR in the absence of any additional CMS requirements or incentives. However, it is hoped that HBOTR will facilitate much needed comparative effectiveness research and more reliable predictive models to establish the optimal role of HBOT in an era of limited resources.
CONCLUSIONS
Among advanced therapeutic interventions for wounds, HBOT has the unique ability to ameliorate tissue hypoxia, reduce pathologic inflammation, and mitigate ischemia reperfusion injury. Most conditions for which it is utilized have few successful alternative treatments, and the morbidity and mortality associated with treatment failure are significant. Although numerous small RCTs provide compelling support for HBOT, the physics of the hyperbaric environment create significant barriers to trial design. The infrastructure created to satisfy mandatory quality and registry reporting requirements as part of healthcare reform can be harnessed to facilitate the acquisition of real-world data for HBOT comparative effectiveness studies, with matched cohorts made possible by the WHI. Predictive models already exist that may be useful in selecting the patients most likely to need HBOT and most likely to benefit from it. Although it is not clear whether patients, payers, or clinicians will support the allocation of healthcare resources by mathematical models, a better paradigm for the appropriate use of HBOT is needed.
ACKNOWLEDGMENTS
Wound Healing Index was funded in part by a grant from KCI, an ACELITY company. Wound Healing Index is currently the property of the University of Utah, although the USWR is currently negotiating a licensing agreement for its use. The hyperbaric oxygen therapy predictive model was developed without funding and is the property of the University of Texas Health Science Center, Houston, and Dr. Gordon Otto.
Footnotes
Disclosure: Dr. Fife is the Executive Director of the Chronic Disease Registry (CDR), a 501(c)(3) nonprofit organization. The registry operates the U.S. Wound Registry, which is recognized by the Centers for Medicare and Medicaid Services as a qualified clinical data registry. Dr. Fife received some grant support from KCI, an ACELITY company. Ms. Eckert and Carter are paid consultants of Dr. Fife for this study and assisted in the preparation of the article.
REFERENCES
- 1.Fife CE, Hopf H. Discussion. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):142S–143S. doi: 10.1097/PRS.0b013e3181fb5443. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 3.Doctor N, Pandya S, Supe A. Hyperbaric oxygen therapy in diabetic foot. J Postgrad Med. 1992;38:112–4, 111. [PubMed] [Google Scholar]
- 4.Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19:1338–1343. doi: 10.2337/diacare.19.12.1338. [DOI] [PubMed] [Google Scholar]
- 5.Lin T, Chen S, Niu K. The vascular effects of hyperbaric oxygen therapy in treatment of early diabetic foot. Undersea Hyperb Med. 2001;28(Suppl):67. [Google Scholar]
- 6.Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513–518. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 7.Kessler L, Bilbault P, Ortéga F, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26:2378–2382. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- 8.Löndahl M, Katzman P, Nilsson A, et al. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg. 1995;21:71–78. doi: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 10.Wieman TJ. Clinical efficacy of becaplermin (rhPDGF-BB) gel. Becaplermin Gel Studies Group. Am J Surg. 1998;176(Suppl 2A):74S–79S. doi: 10.1016/s0002-9610(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 11.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 12.Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS wound matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5 Pt 1):258–266. doi: 10.1097/00129334-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Veves A, Falanga V, Armstrong DG, et al. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 14.Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–354. doi: 10.2337/diacare.19.4.350. [DOI] [PubMed] [Google Scholar]
- 15.Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast-derived dermis. J Foot Ankle Surg. 2002;41:291–299. doi: 10.1016/s1067-2516(02)80047-3. [DOI] [PubMed] [Google Scholar]
- 16.Marston WA, Hanft J, Norwood P, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–1705. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 17.Zelen CM, Serena TE, Denoziere G, et al. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10:502–507. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Grade Definitions. 2014. Oct, Available at: http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions. Accessed March 10, 2016.
- 19.Hadorn DC, Baker D, Hodges JS, et al. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol. 1996;49:749–754. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 20.Boykin JV. Hyperbaric oxygen therapy: a physiological approach to selected problem wound healing. Wounds. 1996;8:183–198. [Google Scholar]
- 21.Löndahl M. Hyperbaric oxygen therapy as adjunctive treatment for diabetic foot ulcers. Int J Low Extrem Wounds. 2013;12:152–157. doi: 10.1177/1534734613486154. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Li L, Yang M, et al. Systematic review of the effectiveness of hyperbaric oxygenation therapy in the management of chronic diabetic foot ulcers. Mayo Clin Proc. 2013;88:166–175. doi: 10.1016/j.mayocp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Fife WP, Fife CE. Hyperbaric oxygen therapy in chronic Lyme disease. In: Jain KK, editor. Textbook of Hyperbaric Medicine. 5th ed. Göttingen, Germany: Hogrefe & Huber; 2009. pp. 149–155. [Google Scholar]
- 24.Fife CE, Eckert KA, Workman WT. Ethical issues, standards and quality control in practice of hyperbaric medicine. In: Jain KK, editor. Textbook of Hyperbaric Medicine. 6th ed. New York, NY: Springer; 2016. [Google Scholar]
- 25.Lyne AJ. Ocular effects of hyperbaric oxygen. Trans Ophthalmol Soc U K. 1978;98:66–68. [PubMed] [Google Scholar]
- 26.Wilkinson D, Chapman IM, Heilbronn LK. Hyperbaric oxygen therapy improves peripheral insulin sensitivity in humans. Diabet Med. 2012;29:986–989. doi: 10.1111/j.1464-5491.2012.03587.x. [DOI] [PubMed] [Google Scholar]
- 27.Undersea & Hyperbaric Medical Society (UHMS) Indications for hyperbaric oxygen therapy. UHMS. 2015 Available at: https://www.uhms.org/resources/hbo-indications.html. Accessed March 7, 2016. [Google Scholar]
- 28.Wolf G, Cifu D, Baugh L, et al. The effect of hyperbaric oxygen on symptoms after mild traumatic brain injury. J Neurotrauma. 2012;29:2606–2612. doi: 10.1089/neu.2012.2549. [DOI] [PubMed] [Google Scholar]
- 29.Walker WC, Franke LM, Cifu DX, et al. Randomized, sham-controlled, feasibility trial of hyperbaric oxygen for service members with postconcussion syndrome: cognitive and psychomotor outcomes 1 week postintervention. Neurorehabil Neural Repair. 2014;28:420–432. doi: 10.1177/1545968313516869. [DOI] [PubMed] [Google Scholar]
- 30.Cifu DX, Hart BB, West SL, et al. The effect of hyperbaric oxygen on persistent postconcussion symptoms. J Head Trauma Rehabil. 2014;29:11–20. doi: 10.1097/HTR.0b013e3182a6aaf0. [DOI] [PubMed] [Google Scholar]
- 31.Cifu DX, Walker WC, West SL, et al. Hyperbaric oxygen for blast-related postconcussion syndrome: three-month outcomes. Ann Neurol. 2014;75:277–286. doi: 10.1002/ana.24067. [DOI] [PubMed] [Google Scholar]
- 32.Miller RS, Weaver LK, Bahraini N, et al. Effects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trial. JAMA Intern Med. 2015;175:43–52. doi: 10.1001/jamainternmed.2014.5479. [DOI] [PubMed] [Google Scholar]
- 33.Bennett MH, Lehm JP, Jepson N. Hyperbaric oxygen therapy for acute coronary syndrome. Cochrane Database Syst Rev. 2015;7:CD004818. doi: 10.1002/14651858.CD004818.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boerema I, Meyne NG, Brummelkamp WK, et al. Life without blood. [Article in Dutch] J Cardiovasc Surg. 1960;1:133–146. [Google Scholar]
- 36.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15:452–460. doi: 10.1016/j.molmed.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demchenko IT, Zhilyaev SY, Moskvin AN, et al. Baroreflex-mediated cardiovascular responses to hyperbaric oxygen. J Appl Physiol (1985) 2013;115(6):819–828. doi: 10.1152/japplphysiol.00625.2013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Sam AD, Klitzman B, et al. Inhibition of nitric oxide synthase on brain oxygenation in anesthetized rats exposed to hyperbaric oxygen. Undersea Hyperb Med. 1995;22:377–382. [PubMed] [Google Scholar]
- 39.Baynosa RC, Naig AL, Murphy PS, et al. The effect of hyperbaric oxygen on nitric oxide synthase activity and expression in ischemia-reperfusion injury. J Surg Res. 2013;183:355–361. doi: 10.1016/j.jss.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Jones SR, Carpin KM, Woodward SM, et al. Hyperbaric oxygen inhibits ischemia-reperfusion-induced neutrophil CD18 polarization by a nitric oxide mechanism. Plast Reconstr Surg. 2010;126:403–411. doi: 10.1097/PRS.0b013e3181df64a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett MH. Hyperbaric medicine and the placebo effect. Diving Hyperb Med. 2014;44:235–240. [PubMed] [Google Scholar]
- 42.Boussi-Gross R, Golan H, Fishlev G, et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury - randomized prospective trial. PLoS One. 2013;8:e79995. doi: 10.1371/journal.pone.0079995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver L, editor. Hyperbaric Oxygen Therapy Indications. 13th ed. North Palm Beach, FL: Undersea and Hyperbaric Medical Society; 2014. [PubMed] [Google Scholar]
- 44.Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;6:CD004123. doi: 10.1002/14651858.CD004123.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang ET, Mansouri J, Murad MH, et al. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers. Undersea Hyperb Med. 2015;42(3):205–247. [PubMed] [Google Scholar]
- 46.Centers for Medicare & Medicaid Services. National coverage determination (NCD) for hyperbaric oxygen therapy (20.29). Available at: https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=12&ncdver=3&NCAId=37&ver=7&NcaName=Hyperbaric+Oxygen+Therapy+for+Hypoxic+Wounds+and+Diabetic+Wounds+of+the+Lower+Extremities&fromdb=true&IsPopup=y&bc=AAAAAAAAEAgA. Accessed March 4, 2016. [PubMed]
- 47.Le PN. Interpretation of the study “Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation”. Undersea Hyperb Med. 2013;40:307–310. [PubMed] [Google Scholar]
- 48.Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36:1961–1966. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheehan P, Jones P, Giurini JM, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006;117(Suppl 7):239S–244S. doi: 10.1097/01.prs.0000222891.74489.33. [DOI] [PubMed] [Google Scholar]
- 50.Horn SD, Fife CE, Smout RJ, et al. Development of a wound healing index for patients with chronic wounds. Wound Repair Regen. 2013;21:823–832. doi: 10.1111/wrr.12107. [DOI] [PubMed] [Google Scholar]
- 51.Fife CE, Horn SD, Smout RJ, et al. A predictive model for diabetic foot ulcer outcome: the Wound Healing Index. Adv Wound Care (New Rochelle) 2016;5:279–287. doi: 10.1089/wound.2015.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wattel FE, Mathieu DM, Fossati P, et al. Hyperbaric oxygen in the treatment of diabetic foot lesions: search for healing predictive factors. J Hyperbaric Med. 1991;6:263–267. [Google Scholar]
- 53.Fife CE, Buyukcakir C, Otto GH, et al. The predictive value of transcutaneous oxygen tension measurement in diabetic lower extremity ulcers treated with hyperbaric oxygen therapy: a retrospective analysis of 1,144 patients. Wound Repair Regen. 2002;10:198–207. doi: 10.1046/j.1524-475x.2002.10402.x. [DOI] [PubMed] [Google Scholar]
- 54.Fife CE, Buyukcakir C, Otto G, et al. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15:322–331. doi: 10.1111/j.1524-475X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 55.Gürdöl F, Cimşit M, Oner-Iyidoğan Y, et al. Early and late effects of hyperbaric oxygen treatment on oxidative stress parameters in diabetic patients. Physiol Res. 2008;57:41–47. doi: 10.33549/physiolres.931139. [DOI] [PubMed] [Google Scholar]
- 56.Heyboer M, 3rd, Milovanova TN, Wojcik S, et al. CD34+/CD45-dim stem cell mobilization by hyperbaric oxygen - changes with oxygen dosage. Stem Cell Res. 2014;12:638–645. doi: 10.1016/j.scr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pencina MJ, Peterson ED. Moving from clinical trials to precision medicine: the role for predictive modeling. JAMA. 2016;315:1713–1714. doi: 10.1001/jama.2016.4839. [DOI] [PubMed] [Google Scholar]
- 58.Otto GH, Buyukcakir C, Fife CE. Effects of smoking on cost and duration of hyperbaric oxygen therapy for diabetic patients with non-healing wounds. Undersea Hyperb Med. 2000;27:83–89. [PubMed] [Google Scholar]
- 59.ClinicalTrials.gov. Hyperbaric Oxygen Therapy Registry (HBOTR) Bethesda, MD: National Library of Medicine; 2015. NCT02483650. Available at: https://clinicaltrials.gov/ct2/show/NCT02483650. Updated June 24, 2015. Accessed March 7, 2016. [Google Scholar]
- 60.U.S. Wound Registry (USWR) Quality Measures in Wound Care. USWR: The Woodlands, TX; 2015. Available at: https://www.uswoundregistry.com/Specifications.aspx. Accessed March 9, 2016. [Google Scholar]
- 61.Centers for Medicare & Medicaid Services. The merit-based incentive payment system (MIPS) & alternative payment models (APMs). Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMS/MACRA-MIPS-and-APMS.hml. Accessed May 27, 2016.


