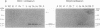Abstract
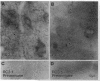
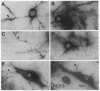
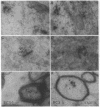
The rodent protein RC3 is expressed mainly by forebrain neurons during postnatal development and maturity. RC3 and its bovine homolog neurogranin/B-50 immunoreactive C-kinase substrate (BICKS) contain overlapping sites for protein kinase C phosphorylation and calmodulin binding that resemble those of the presynaptic 43-kDa growth-associated protein (GAP-43). However, morphological evidence suggests that RC3 has a postsynaptic localization. To test this hypothesis, we used two polyclonal antisera against synthetic peptides corresponding to nonoverlapping sequences within RC3 and compared cellular distributions of their binding in neostriatum of adult rats by immunohistochemistry, Golgi impregnation/gold toning, and correlative light/electron microscopy. Somatic and punctate patterns of RC3 immunoreactivity were observed. Somatic RC3 was found in cyto- and nucleoplasmic compartments of all neuronal phenotypes (medium spiny, medium aspiny, and large aspiny cells). Punctate RC3 was found mostly in dendritic spines. In contrast to the 43-kDa growth-associated protein, RC3 was seen infrequently in axons. We conclude that RC3 accumulates postsynaptically in dendritic spines of neostriatal neurons. We propose that RC3 acts as a "third messenger" substrate of protein kinase C-mediated molecular cascades during synaptic development and remodeling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Alexander K. A., Wakim B. T., Doyle G. S., Walsh K. A., Storm D. R. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. J Biol Chem. 1988 Jun 5;263(16):7544–7549. [PubMed] [Google Scholar]
- Apel E. D., Byford M. F., Au D., Walsh K. A., Storm D. R. Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry. 1990 Mar 6;29(9):2330–2335. doi: 10.1021/bi00461a017. [DOI] [PubMed] [Google Scholar]
- Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J Biol Chem. 1989 Jan 25;264(3):1824–1828. [PubMed] [Google Scholar]
- Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991 Jan 5;266(1):229–237. [PubMed] [Google Scholar]
- Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N., Finklestein S. P., Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988 Jan;8(1):339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990 Jun;10(6):1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins P. J., Zwiers H. Binding of the neuronal protein B-50, but not the metabolite B-60, to calmodulin. J Neurochem. 1990 Jan;54(1):274–277. doi: 10.1111/j.1471-4159.1990.tb13311.x. [DOI] [PubMed] [Google Scholar]
- Coggins P. J., Zwiers H. Evidence for a single protein kinase C-mediated phosphorylation site in rat brain protein B-50. J Neurochem. 1989 Dec;53(6):1895–1901. doi: 10.1111/j.1471-4159.1989.tb09259.x. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990 Jul;13(7):286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Roberts R. C., Benowitz L. I. Immunoreactive GAP-43 in the neuropil of adult rat neostriatum: localization in unmyelinated fibers, axon terminals, and dendritic spines. J Comp Neurol. 1990 Dec 22;302(4):992–1001. doi: 10.1002/cne.903020421. [DOI] [PubMed] [Google Scholar]
- Difiglia M., Pasik T., Pasik P. Ultrastructure of Golgi-impregnated and gold-toned spiny and aspiny neurons in the monkey neostriatum. J Neurocytol. 1980 Aug;9(4):471–492. doi: 10.1007/BF01204837. [DOI] [PubMed] [Google Scholar]
- Dray A. The physiology and pharmacology of mammalian basal ganglia. Prog Neurobiol. 1980;14(4):221–335. doi: 10.1016/0301-0082(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Fairén A., Peters A., Saldanha J. A new procedure for examining Golgi impregnated neurons by light and electron microscopy. J Neurocytol. 1977 Jun;6(3):311–337. doi: 10.1007/BF01175194. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Buchwald N. A., Hull C. D., Levine M. S. GABAergic basal forebrain neurons project to the neocortex: the localization of glutamic acid decarboxylase and choline acetyltransferase in feline corticopetal neurons. J Comp Neurol. 1988 Jun 22;272(4):489–502. doi: 10.1002/cne.902720404. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Somogyi J. The 'single' section Golgi-impregnation procedure: methodological description. J Neurosci Methods. 1984 Sep;11(4):221–230. doi: 10.1016/0165-0270(84)90084-0. [DOI] [PubMed] [Google Scholar]
- Houbre D., Duportail G., Deloulme J. C., Baudier J. The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP 43), with membrane phospholipids. J Biol Chem. 1991 Apr 15;266(11):7121–7131. [PubMed] [Google Scholar]
- Jensen K. F., Ohmstede C. A., Fisher R. S., Sahyoun N. Nuclear and axonal localization of Ca2+/calmodulin-dependent protein kinase type Gr in rat cerebellar cortex. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2850–2853. doi: 10.1073/pnas.88.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M., Colley P. A., Akers R. F., Nelson R. B., Routtenberg A. Direct relation of long-term synaptic potentiation to phosphorylation of membrane protein F1, a substrate for membrane protein kinase C. Brain Res. 1986 Dec 10;399(2):205–211. doi: 10.1016/0006-8993(86)91510-6. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Rodriguez-Peña A., Perez-Castillo A., Ferreiro B., Sutcliffe J. G., Bernal J. Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol. 1991 Feb;5(2):273–280. doi: 10.1210/mend-5-2-273. [DOI] [PubMed] [Google Scholar]
- POWERS M. M., CLARK G. An evaluation of cresyl echt violet acetate as a Nissl stain. Stain Technol. 1955 Mar;30(2):83–88. doi: 10.3109/10520295509113749. [DOI] [PubMed] [Google Scholar]
- Represa A., Deloulme J. C., Sensenbrenner M., Ben-Ari Y., Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990 Dec;10(12):3782–3792. doi: 10.1523/JNEUROSCI.10-12-03782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Shinnick T. M., Bloom F. E. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983 Jul;33(3):671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., Sutcliffe J. G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J Neurosci Res. 1990 Aug;26(4):397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- Wojtkowiak Z., Briggs R. C., Hnilica L. S. A sensitive method for staining proteins transferred to nitrocellulose sheets. Anal Biochem. 1983 Mar;129(2):486–489. doi: 10.1016/0003-2697(83)90581-x. [DOI] [PubMed] [Google Scholar]