This study demonstrates that preischemic administration of uncultured stromal vascular fraction could increase cell retention and then improve renal function and structure at both early and long-term stage after renal ischemia/reperfusion (IR), which may provide a novel therapeutic approach for IR injury.
Keywords: Adipose stem cells, Autologous stem cell transplantation, Cellular therapy, Renal, Stromal vascular fraction, Ischemia/reperfusion
Abstract
Ischemia/reperfusion (IR)-induced acute kidney injury (AKI) is a common clinical syndrome. Stem/progenitor cell therapy is a promising option to foster the intrinsic capacity for kidney regeneration. However, there are still several challenges to be resolved, including the potential risks during cell culture, low retention rate after transplantation, and unclear effect on the progression of chronic kidney disease (CKD). Recently, nonexpanded adipose stromal vascular fraction (SVF) has been regarded as an attractive cell source for cell-based therapy. Preconditioning with ischemia has been suggested as a useful method to promote the retention and survival of transplanted cells in vivo. In this study, freshly isolated autologous SVF was transplanted to the kidney of rats before ischemia, and then an IR-induced AKI model was established. Postischemic administration of SVF to the kidney was performed after renal IR injury was induced. A higher cell retention rate was detected in the preischemic group. Preischemic administration of SVF showed stronger functional and morphologic protection from renal IR injury than postischemic administration, through enhancing tubular cell proliferation and reducing apoptosis. Progression of kidney fibrosis was also significantly delayed by preischemic administration of SVF, which exhibited stronger inhibition of transforming growth factor-β1-induced epithelia-mesenchymal transition and microvascular rarefaction. In addition, in vitro study showed that prehypoxic administration of SVF could significantly promote the proliferation, migration, and survival of hypoxic renal tubular epithelial cells. In conclusion, our study demonstrated that preischemic administration of nonexpanded adipose SVF protected the kidney from both acute IR injury and long-term risk of developing CKD.
Significance
Renal ischemia/reperfusion (IR) injury is a common clinical syndrome. Cell-based therapy provides a promising option to promote renal repair after IR injury. However, several challenges still remain because of the potential risks during cell culture, low retention rate after transplantation, and unclear effect on the progression of chronic kidney disease. Stromal vascular fraction (SVF) is considered as an attractive cell source. This study demonstrated that preischemic administration of uncultured SVF could increase cell retention and then improve renal function and structure at both early and long-term stage after IR, which may provide a novel therapeutic approach for IR injury.
Introduction
During the process of surgery (e.g., kidney transplantation, nephron-sparing surgery, cardiovascular surgery, etc.), transient ischemia and subsequent reperfusion occur commonly in the kidney. However, the reduction or interruption of renal perfusion and subsequent reflow often results in kidney injury, termed renal ischemia/reperfusion (IR) injury, which is a common cause of acute kidney injury (AKI) [1]. One of the important cellular pathophysiologies of AKI is tubular epithelial cell (TEC) injury and even death by apoptosis and necrosis, which is associated with renal functional impairment involving cell metabolism perturbations and reduced excretion of metabolic waste products [2, 3]. Although the kidney has intrinsic capacity to recover from IR-induced TEC injury or death, the repair is frequently maladaptive and incomplete, associating with the proliferation of fibroblasts and excessive deposition of extracellular matrix, which may contribute to a high risk of developing chronic kidney disease (CKD) with progressive renal fibrosis [2, 4]. Despite the fact that the mechanism underlying the pathogenesis of renal fibrosis is incompletely understood, tubular epithelia-mesenchymal transition (EMT) has been suggested as one of the main pathways toward fibrosis [5]. Transforming growth factor-β (TGF-β)/Smad signaling has been shown to play a pivotal role in mediating EMT [6]. Furthermore, sustained loss of peritubular capillary, leading to long-term hypoxia of TEC, has been thought to be another major contributor to postischemic fibrosis [7].
The application of stem/progenitor cells is viewed as a promising option to foster the intrinsic capacity for kidney repair and regeneration [8]. Adipose-derived stem cells (ADSCs) have been considered as an attractive cell source for cell-based therapy, because they can be easily obtained through a minimally invasive method and possess similar characteristics to bone marrow-derived stem cells [9]. ADSCs have been shown to restore renal structure and function in an IR-induced animal model of AKI through reducing apoptosis and promoting proliferation of TEC [10]. Studies also showed that ADSCs were capable of preventing renal fibrosis progression by reducing the tubular EMT [11] or improving revascularization outcomes in injured kidney [12]. The adipose stromal vascular fraction (SVF) is a rich source of ADSCs [13]. Recent evidence has shown that administration of freshly isolated SVF could also protect the kidney from IR or cisplatin-induced acute injury [14, 15]. Because it might be time-consuming for in vitro cell culture, a process that may face the potential risk of microbial contamination [16], nonexpanded SVF has emerged as a more attractive cell source for cell-based therapy.
Even though functional and structural recovery of injured kidney has been promoted because of the application of both cultured and uncultured stem/progenitor cells in animal models of AKI, there are still several problems that need to be resolved. Previously, studies mainly focused on the therapeutic effects of stem/progenitor cell treatment to ameliorate acute injury of kidney [14, 15, 17, 18], whereas few studies addressed the preventive effects of stem/progenitor cell administration against the development of CKD [19, 20]. It has been reported that, although stem/progenitor cells could reduce interstitial fibrosis after AKI, they were still insufficient to delay the progression of CKD [21]. In addition, low retention and unexpected extensive acute death of engrafted stem/progenitor cells have been found after transplantation [22–24]. The low engraftment and survival of transplanted cells in injured kidney will lead to an unsatisfactory improvement of renal function [24].
Preconditioning with ischemia has been suggested to be a useful method to induce a delay of lethal cell injury and enhance cellular tolerance to injurious effects of IR [25, 26]. Plenty of studies from both our team and other groups have shown that preconditioning with ischemia enhanced the mobilization and recruitment of stem/progenitor cells to target organs, promoted cell retention and survival, and then contributed to the functional rescue of injured organs [27–31]. Therefore, we speculated that preconditioning with ischemia through preischemic injection of stem/progenitor cells into the kidney may improve the retention rate of grafted cells and then enhance therapeutic effects on IR-induced kidney injury. Because SVF is regarded as an attractive cell source, as described above, the present study was performed to investigate the effect and mechanism of nonexpanded adipose SVF against IR-induced AKI via preischemic administration in a rat model. In addition, preventing the effect of nonexpanded SVF on the progression of CKD was also studied.
Materials and Methods
Study Subjects
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Nanjing First Hospital, Nanjing Medical University. The investigation was performed in strict conformity with the institutional and national guidelines for laboratory animals. A total of 96 8-week-old male Sprague-Dawley rats with initial weight of 220∼250 g were used in this study and bred in the animal house of the Experimental Animal Center affiliated with Nanjing First Hospital. Animals were housed in a standard room under controlled conditions of humidity and temperature, with a 12-h light/dark cycle, and ad libitum access to food and water.
Isolation of SVF
SVF was isolated from rat epididymal adipose tissue as described in our previous protocol [32]. Briefly, adipose tissue was rinsed with ice-cold sterile phosphate-buffered saline (PBS) thrice and minced into small pieces, followed by enzymatic digesting with 0.075% type I collagenase at 37°C for 40 minutes on an orbital shaker. After being filtered with 200-μm nylon mesh, collected, and centrifuged at 400g for 5 minutes, the cell pellet was treated with Red Blood Cell Lysis Buffer for 1 minute and washed twice with ice-cold PBS. Then the nucleated cells from the SVF pellet ere resuspended in PBS, counted with an automated cell counter, and diluted to 5 × 103 cells per microliter in PBS.
Flow Cytometric Analysis
Flow cytometric analysis was performed to determine cell surface marker expression of freshly isolated SVF cells. A panel of cell surface markers was examined by immunostaining with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD45 (1:200; all from BioLegend, San Diego, CA, http://www.biolegend.com, unless otherwise indicated), FITC-conjugated anti-CD90 (1:200), phycoerythrin (PE)-conjugated anti-CD11b/c (1:100), PE-conjugated anti-CD29 (1:100), PE-conjugated anti-CD106 (1:20), PE-conjugated anti-CD31 (1:50, Bioss Antibodies, Woburn, MA, http://www.biossusa.com), PE-conjugated anti-CD34 (1:50, Bioss Antibodies), and PE-conjugated anti-vascular endothelial growth factor receptor 2 (anti-VEGFR-2) (1:50; Bioss Antibodies). The labeled SVF cells were washed twice, resuspended, and analyzed with FACSCalibur (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). An isotype-matched IgG was used as a negative control for each primary antibody.
Cell Coculture in Hypoxic Environment
The Milllicell hanging Cell Culture Inserts (8-μm pore size, EMD Millipore, Billerica, MA, http://www.emdmillipore.com) were used for coculture [33]. The rat renal tubular epithelial cell line (NRK-52E) and freshly isolated SVF resuspended with serum-free Dulbecco’s modified Eagle’s medium (DMEM) were cocultured in different compartments (NRK-52E cells in the bottom chambers and SVF [105 cells in 200 μl of serum-free DMEM] in the upper chambers) for physically separated, while communication could be maintained because of the transduction of paracrine signaling through the polyethylene terephthalate (PET) membrane. Cells were cocultured in Thermo 3131 incubator (Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) for 24 hours set at 37°C, 1% O2, and 5% CO2. NRK-52E cells cultured in 24- or 96-well without the inserts were also plated in the hypoxic environment for 24 hours. All the hypoxic cultured cells were used in the following cellular biological experiments, which were performed in triplicate.
Cell Proliferation Assay
Cell proliferation assay was performed according to our previous protocol, but with some modifications [34]. Briefly, NRK-52E cells (1.2 × 103 per well) cocultured with SVF or independently cultured in 96-well plates in the above-described hypoxic environment were used. Cells were divided into three groups: NRK-52E cells cocultured with SVF in hypoxic environment (prehypoxic group), NRK-52E cells independently cultured in hypoxic environment for 24 hours and then the inserts seeded with freshly isolated SVF were placed into the wells (posthypoxic group), and NRK-52E cells independently cultured in hypoxic environment (control group). After 24 hours of hypoxic culture, the plates of the three groups were transferred into the normoxic humidified incubator at 37°C with 5% CO2 and cultured for another 24 hours. Subsequently, 10 μl of Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Rockville, MD, http://www.dojindo.com) was added into each well, and the plates were incubated for 3 hours. Finally, the absorbance was measured at 450/620 nm on a microplate reader (Tecan, Männedorf, Switzerland, http://www.tecan.com).
Cell Scratch Wound Healing Assay
Cell scratch wound healing assay was also performed as described in our previous protocol with some modifications [34]. Briefly, NRK-52E cells (50%–60% confluence) were cocultured with SVF or cultured independently in 24-well plates under hypoxic environment for 24 hours. Then, cells were divided into three groups as described above. The wounded areas of NRK-52E cells in the three groups were made artificially with a 200-μl pipette tip, washed twice with PBS, and recorded under an inverted microscope. Then, the plates were transferred into the normoxic humidified incubator and cultured for another 24 hours, after which the remaining wound areas were recorded. The image analysis for the measurements of wound areas was determined by using Image-Pro Plus software (Media Cybernetics, Rockville MD, http://www.mediacy.com). The percent of wound closure was calculated as: wound closure (%) = (total wound areas − remaining wound areas)/total wound areas.
Annexin V/Propidium Iodide Apoptosis Assay
NRK-52E cells (50%–60% confluence) cocultured with SVF or cultured independently in 24-well plates in a hypoxic environment were used for this assay. After the hypoxic culture was accomplished, cells were divided into three groups as described above. Then, the plates were transferred into the normoxic humidified incubator and cultured for another 24 hours. NRK-52E cells were collected by using trypsin, stained with Annexin V Apoptosis Detection Kit FITC (eBioscience, San Diego, CA, http://www.ebioscience.com) according to the manufacturer’s instructions and then measured by flow cytometry.
Renal IR Injury Model and SVF Transplantation
Before the establishment of the renal IR injury model, autologous SVF was isolated, labeled with CM-DiI as described in our previous protocol [32], and transplanted in vivo within 2 hours after the isolation. Renal IR injury model was conducted as previously described with some modifications [35]. Briefly, the rats were anesthetized by inducting with isoflurane, followed by intraperitoneal administration of ketamine (100 mg/kg). The right kidney was removed through a midline abdominal incision, followed by the establishment of IR injury model through blocking the left renal pedicle for 45 minutes by using a nontraumatic vascular clamp. Animals were divided into four groups: (a) sham group (right nephrectomy); (b) IR + PBS (right nephrectomy + PBS administration after IR); (c) IR + SVF pre-I (right nephrectomy + preischemic administration of SVF before IR); and (d) IR + SVF post-I (right nephrectomy + postischemic administration of SVF after IR). A total of 2 × 106 SVF cells suspended in 100 μl of PBS were used for cell transplantation. In the pre-I group, SVF/PBS was administrated before ischemia through intraparenchymal injection to the left kidney, whereas it was injected after IR injury in the post-I group.
Renal Function
To assess the renal function, blood urea nitrogen (BUN) and serum creatinine (SCr) values were measured at 12, 24, and 72 hours and 6 months after IR injury in all four groups of rats (n = 6 for each group). Quantification of BUN and SCr concentrations was performed by using standard laboratory equipment in our hospital.
Cell Tracking
Cell tracking of SVF was performed by using a previously described protocol with minor modifications [36]. Briefly, kidney specimens were retrieved at 24 and 72 hours after IR. Frozen kidney tissue was cut into 5-μm sections and mounted with 4′-6-diamidino-2-phenylindole (DAPI). To calculate the retention rate of SVF in the injured kidney, CM-DiI-labeled cells were detected by red labeling and recorded under a fluorescence microscope. The CM-DiI-labeled red area in 10–15 fields of each section was determined by using Image-Pro Plus software (Media Cybernetics).
Histological and Immunohistochemical Analysis
Kidney specimens retrieved at 12, 24, and 72 hours and 6 months after IR were fixed, dehydrated, and embedded in paraffin. Tissue sections (5 µm) were stained with hematoxylin and eosin (H&E) to evaluate the degree of tubular injury. Proliferation of tubular cell was detected by using anti-proliferating cell nuclear antigen (anti-PCNA) antibody (Abcam, Cambridge, U.K., http://www.abcam.com). Apoptosis was evaluated with terminal transferase-mediated deoxyuridine triphosphate nick-end-labeling (TUNEL) assay (Roche, Basel, Switzerland, http://www.roche.com) according to the manufacturer’s protocol. Retention of TEC was detected with anti-E-cadherin antibody (Abcam). Peritubular capillaries were assessed by staining with anti-rat endothelial cell antigen-1 (anti-RECA-1) antibody (Abcam) antibody [35]. For analysis of renal fibrosis, sections were stained with Masson’s trichrome and anti-α-smooth muscle actin (anti-α-SMA) antibody (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Immunohistochemical assay for the staining of anti-PCNA, anti-E-cadherin, anti-RECA-1, and anti-α-SMA was performed according to our previous protocol [37].
Microscopy and Image Analysis
Microscopic assessment was performed in a blinded manner, and at least 20 random fields from each slide section were evaluated at ×400 magnification for all the staining except the staining of anti-E-cadherin, which was examined in 10 random fields of each section at ×200 magnification. The degree of tubular injury was scored ranging from grade 0 to 5 as previously described [38]. The severity of tubular injury was semiquantified by using the following scoring system: 0, normal kidney; 1, ≤10%; 2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, ≥76%.
Western Blot Analysis
Total protein was extracted from kidney specimens retrieved at 6 months after IR with RIPA lysis buffer containing a cocktail of protease inhibitor (Roche). Equal amounts of proteins were separated by using SDS-polyacrylamide gel electrophoresis on 10% polyacrylamide gels and then transferred electrophoretically to polyvinylidene difluoride membranes (EMD Millipore). The membranes were blocked with 10% skimmed milk for 1 hour and incubated at 4°C overnight with anti-α-SMA, anti-E-cadherin, or anti-glyceraldehyde-3-phosphate dehydrogenase (EMD Millipore), followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (Cell Signaling, Danvers, MA, http://www.cellsignal.com). Immunoreactive proteins were visualized by using an enhanced chemiluminescence detection kit (Bio-Rad, Hercules, CA, http://www.bio-rad.com) and then quantitatively analyzed by densitometry using the ImageJ software (National Institutes of Health, Bethesda, MD, https://imagej.nih.gov). This experiment was performed in triplicate.
Enzyme-Linked Immunosorbent Assay
Levels of TGF-β1 in the serum and kidney tissues at 6 months after IR in the four groups was determined by enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science, Inc., Wuhan, China, http://www.uscnk.com) according to the manufacturer’s instructions. The absorbance was evaluated on a microplate reader (Tecan), and the concentration was calculated on the basis of a standard curve. The level of TGF-β1 in kidney tissue was determined as previously described [39]. Protein content was collected from the supernatant of kidney homogenate and determined by Coomassie blue assay. The level of TGF-β1 in kidney sample was corrected per microgram of protein. This experiment was performed in triplicate.
Statistical Analysis
All data were expressed as mean ± SEM. Statistical comparisons of the data among the groups were evaluated by analysis of variance (ANOVA) tests. When ANOVA showed a significant difference, further comparison between groups was conducted by using the post hoc Tukey test. Statistical significance was set at a value of p < .05.
Results
Characterization of SVF
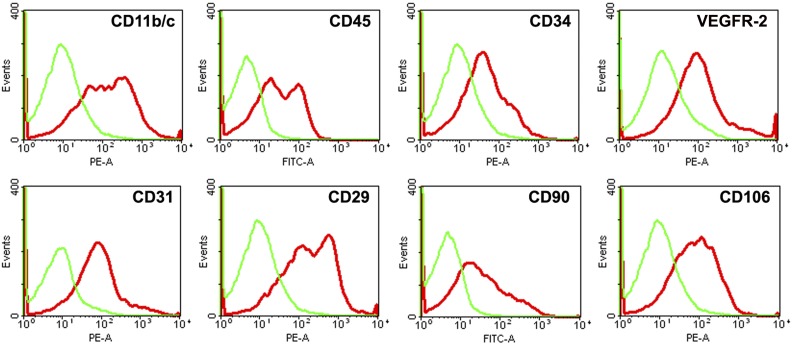
By flow cytometric analysis, freshly isolated SVF expressed hematopoietic (CD11b/c [42.9% ± 11.05%], CD34 [22.8% ± 10.3%], and CD45 [31.3% ± 9.6%]), mesenchymal (CD29 [58.7% ± 10.4%], CD90 [31.0% ± 6.1%], and CD106 [37.4 ± 7.8%]), and endothelial (CD31 [18.8% ± 5.8%], CD34 [22.8% ± 10.3%], and VEGFR-2 [16.9% ± 5.2%]) markers, which indicated that SVF cells were a heterogeneous population (Fig. 1).
Figure 1.
Characterization of freshly isolated stromal vascular fraction (SVF) by flow cytometric analysis. Representative flow cytometry histograms of SVF showed that freshly isolated SVF could express hematopoietic, mesenchymal, and endothelial markers. Abbreviations: FITC-A, fluorescein isothiocyanate area; PE-A, phycoerythrin area; VEGFR-2, vascular endothelial growth factor 2.
In Vitro Effects of SVF Prehypoxic Administration on Renal Tubular Epithelial Cells in Hypoxic Environment
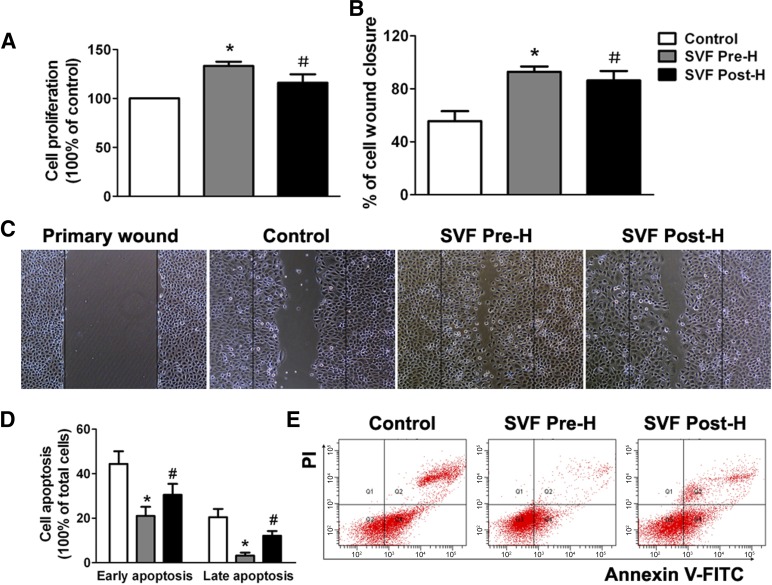
The results of cell proliferation assay showed that SVF administration either before hypoxia or after hypoxia could significantly enhance the proliferation activity of NRK-52E cells. However, the proliferation of NRK-52E cells was significantly promoted in the prehypoxic group compared with that in the posthypoxic group (Fig. 2A). Cell scratch wound healing assay demonstrated similar findings to those in the cell proliferation assay. Wound closure was significantly enhanced in both prehypoxic and posthypoxic groups, whereas stronger enhancement could be found in prehypoxic group (Fig. 2B, 2C). Therefore, prehypoxic administration of SVF could significantly promote the proliferation and migration of hypoxic renal TEC in vitro. Additionally, cell apoptosis was induced and assessed by Annexin V/propidium iodide (PI) apoptosis assay. Both early apoptotic cells (Annexin V-positive, PI-negative) and late apoptotic cells (Annexin V-positive, PI-positive) were increased when NRK-52E cells were cultured in a hypoxic condition followed with a normoxic condition. SVF administration significantly reduced the proportion of apoptotic cells. Furthermore, a significant reduction was observed in the prehypoxic group, which indicated that cell survival was further improved by prehypoxic administration of SVF compared with posthypoxic treatment (Fig. 2D, 2E).
Figure 2.
In vitro effects of SVF prehypoxic administration on renal tubular epithelial cell (TEC) in hypoxic environment. (A): Prehypoxic administration of SVF could significantly promote the proliferation of hypoxic renal TEC, compared with the control and posthypoxic administration groups. (B): Prehypoxic administration of SVF could significantly promote the scratch wound healing of hypoxic TEC, compared with the control and posthypoxic administration groups. (C): Representative images of cell scratch wound healing (magnification, ×100). (D): Prehypoxic administration of SVF could significantly reduce the apoptosis of hypoxic TEC, compared with the control and posthypoxic administration groups. (E): Representative flow cytometry histograms of Annexin V/PI apoptosis assay in different groups. ∗, p < .05 (vs. control and SVF post-H); #, p < .05 (vs. control). Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide; SVF, stromal vascular fraction; SVF Post-H, posthypoxic administration of stromal vascular fraction; SVF Pre-H, prehypoxic administration of stromal vascular fraction.
Effects of SVF Preischemic Administration on Renal Function and Tubular Injury
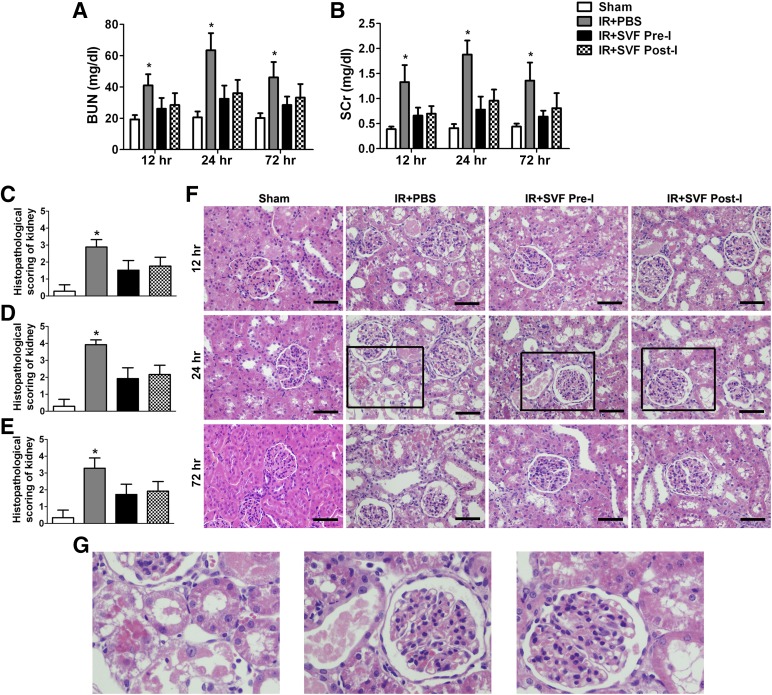
Compared with sham-operated animals, animals subjected to renal IR injury showed a significant rise in serum levels of SCr and BUN at 12, 24, and 72 hours, associated with a marked tubular injury such as cast formation, vacuolization, tubular dilatation, and necrosis. When animals were treated with SVF, a significant decrease in SCr and BUN in parallel with the reduction of tubular injury was observed at each time point after IR. Although there was no significant difference in renal function and histopathological score between the preischemic and postischemic groups, preischemic administration of SVF still contributed to lower BUN and SCr values, as well as reduced tubular injury, compared with postischemic administration (Fig. 3).
Figure 3.
Early protective effect of stromal vascular fraction (SVF) preischemic administration on acute renal IR injury. (A, B): Rats that received SVF administration showed significantly lower BUN (A) and SCr (B) values at 12, 24, and 72 hours after IR compared with control group. (C–E): SVF administration could also significantly reduce tubular injury score of kidneys in rats at 12 (C), 24 (D), and 72 (E) hours after IR compared with the control group. Although there was no significant difference in renal function and histopathological score between preischemic and postischemic groups, preischemic administration of SVF still contributed to lower BUN and SCr values, as well as reduced tubular injury, compared with postischemic administration. (F, G): Representative images of H&E staining preformed on sections of kidneys at 12, 24, and 72 hours after IR in different groups (magnification, ×400). Scale bars = 50 μm. ∗, p < .05 (vs. sham, IR+SVF pre-I, and IR+SVF post-I). Abbreviations: BUN, blood urea nitrogen; IR, ischemia/reperfusion; PBS, phosphate-buffered saline; SCr, serum creatinine; SVF Post-I, postischemic administration of stromal vascular fraction; SVF Pre-I, preischemic administration of stromal vascular fraction.
In Vivo Retention of SVF
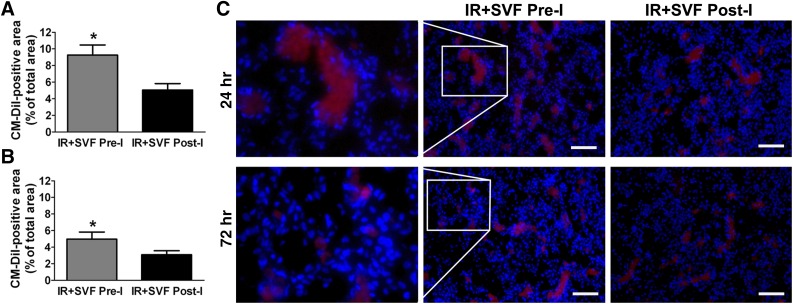
At 24 and 72 hours after IR, retention of SVF was detected by the presence of CM-DiI, which demonstrated the engraftment of SVF in the injured kidney. Red labeling of CM-DiI was mostly detected at the glomeruli, whereas little staining was detectable in the tubular region (Fig. 4). At 24 hours after IR, CM-DiI-positive SVF was significantly increased in the kidney that received preischemic administration of SVF compared with the kidney that underwent postischemic treatment. Although the fluorescent intensity of CM-DiI decreased at 72 hours after IR in both groups, preischemic administration of SVF still contributed to a stronger fluorescent intensity, suggesting an improved retention of SVF in injured kidney.
Figure 4.
Cell tracking in rats treated with stromal vascular fraction (SVF). (A, B): Percentages of CM-DiI-positive areas in kidney sections of rats at 24 (A) and 72 (B) hours after IR were calculated. CM-DiI-positive SVF was significantly increased in the kidneys that received preischemic administration of SVF compared with those that underwent postischemic treatment. (C): Representative images of CM-DiI-labeled SVF in the kidneys at 24 and 72 hours after IR at different groups (magnification, ×200). Scale bars = 100 μm. ∗, p < .05 (vs. IR+SVF post-I). Abbreviations: IR, ischemia/reperfusion; SVF Post-I, postischemic administration of stromal vascular fraction; SVF Pre-I, preischemic administration of stromal vascular fraction.
In Vivo Effects of SVF Preischemic Administration on Proliferation and Apoptosis of Renal Tubular Epithelial Cells After IR Injury
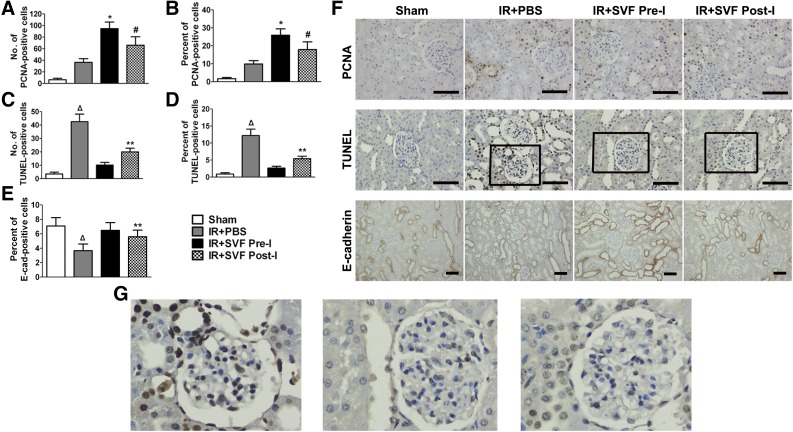
The effect of SVF preischemic administration on tubular cell proliferation was observed by evaluating PCNA expression in the kidney at 72 hours after IR. Rats subjected to renal IR injury exhibited a slight increase in the number and proportion of PCNA-positive cells compared with sham-operated ones, whereas the increase was significantly higher in the rats that received SVF treatment. Additionally, a significant increase in the number and proportion of PCNA-positive cells was detected in the preischemic group compared with the postischemic treatment ones. Consistent results were observed by evaluating apoptosis in the kidney that partly contributed to tubular cell loss. The number and proportion of TUNEL-positive cells in renal sections were significantly increased in animals that suffered from renal IR injury. Treatment with SVF could significantly reduce the number and proportion of TUNEL-positive cells, whereas the reduction was significantly enhanced in the preischemic group. For the overall evaluation of tubular cell proliferation and apoptosis in injured kidneys, renal sections were staining with E-cadherin, a typical marker of TEC. Lower expression of E-cadherin was observed in rats after IR compared with sham-operated animals. SVF treatment could restore the expression of E-cadherin, which was more obvious in rats that underwent preischemic treatment of SVF (Fig. 5).
Figure 5.
Immunohistochemical staining of PCNA, E-cadherin, and TUNEL staining in kidney sections at 72 hours after IR in different groups. (A–E): Quantitative analysis of PCNA-positive cells (A, B), TUNEL positive cells (C, D), and E-cadherin-positive cells (E) was performed by using Image-Pro Plus software. (F, G): Representative images of PCNA, E-cadherin, and TUNEL staining in the kidneys at 72 hours after IR at different groups (magnification, ×400 [PCNA and TUNEL staining], ×200 [E-cadherin staining]). Scale bars = 50 μm. ∗, p < .05 (vs. sham, IR+PBS, and IR+SVF post-I); #, p < .05 (vs. IR+PBS); Δ, p < .05 (vs. sham, IR+SVF pre-I, and IR+SVF post-I); ∗∗, p < .05 (vs. IR+SVF pre-I). Abbreviations: E-cad, E-cadherin; IR, ischemia/reperfurion; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; SVF Post-I, postischemic administration of stromal vascular fraction; SVF Pre-I, preischemic administration of stromal vascular fraction; TUNEL, terminal transferase-mediated deoxyuridine triphosphate nick-end-labeling.
Preischemic Administration of SVF Delayed the Development of CKD
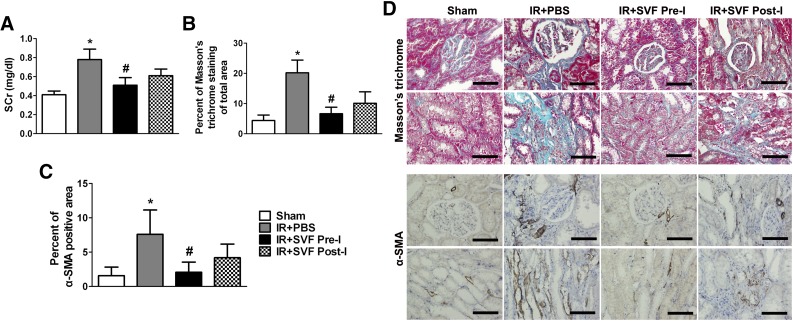
Six months after IR, rats that received SVF administration showed reduced levels of SCr. Furthermore, significant reduction was found in rats that received preischemic administration of SVF. Masson’s trichrome and α-SMA staining was performed to explore the degree of renal fibrosis. More fibrotic lesions were observed by Masson’s trichrome staining in the tubulointerstitial areas in the rats that underwent renal IR injury compared with sham-operated ones, whereas SVF-treated rats kidneys showed significant reduction of fibrosis. Animals received preischemic administration of SVF showed a more significant reduction of fibrosis in tubulointerstitial areas than the ones that underwent postischemic treatment with SVF. Immunostaining for α-SMA demonstrated similar findings to those for Masson’s trichrome staining. Preischemic administration of SVF resulted in a significantly declined expression of α-SMA compared with postischemic administration. Both Masson’s trichrome and α-SMA staining indicated that preischemic administration of SVF exhibited an inhibiting effect on renal fibrosis and then delayed the progression of CKD (Fig. 6).
Figure 6.
Long-term preservation of renal function and inhibition of fibrosis in tubulointerstitial areas by preischemic administration of stromal vascular fraction (SVF). (A): SCr value at 6 months after IR in different groups. (B, C): Quantitative analysis of Masson’s trichrome staining (B) and α-SMA positive area (C) was performed by using Image-Pro Plus software. (D): Representative images of Masson’s trichrome and α-SMA staining in the kidneys at 6 months after IR at different groups (magnification, ×400). Scale bars = 50 μm. ∗, p < .05 (vs. sham, IR+SVF pre-I, and IR+SVF post-I); #, p < .05 (vs. IR+SVF post-I). Abbreviations: IR, ischemia/reperfusion; SVF, stromal vascular fraction; PBS, phosphate-buffered saline; SCr, serum creatinine; α-SMA, α-smooth muscle actin; SVF Post-I, postischemic administration of stromal vascular fraction; SVF Pre-I, preischemic administration of stromal vascular fraction.
Preischemic Administration of SVF Contributed to the Inhibition of EMT, TGF-β1 Production, and Microvascular Rarefaction
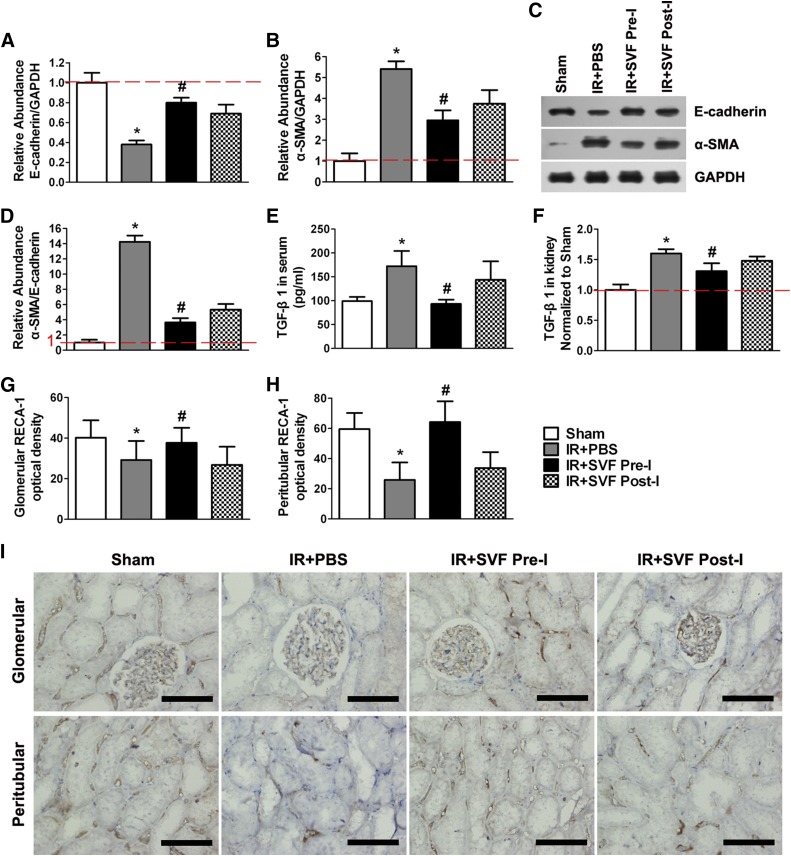
Western blot analysis showed that IR could induce the downregulation of E-cadherin and upregulation of α-SMA in injured kidney, which thus resulted in the upregulation of α-SMA/E-cadherin expression, an indicator for tubular EMT. However, the upregulation was significantly attenuated by the administration of SVF. Meanwhile, preischemic administration of SVF contributed to a stronger attenuating effect than postischemic administration (Fig. 7A–7D). In addition, we found that the expression of TGF-β1, a profibrogenic inducer for the initiation and completion of the entire EMT course, increased significantly in both serum and kidney at 6 months after IR. The increase of TGF-β1 expression was inhibited by the administration of SVF, and stronger inhibition was observed in the animals received preischemic treatment of SVF (Fig. 7E, 7F).
Figure 7.
Preischemic administration of stromal vascular fraction (SVF) inhibited TGF-β1-induced epithelia-mesenchymal transition (EMT) and microvascular rarefaction. (A–C): Relative abundance of E-cadherin/GAPDH (A) and α-SMA/GAPDH (B) was calculated by Western blot assay (C) at 6 months after IR in different groups. (D): Preischemic administration of SVF significantly attenuated the upregulation of α-SMA/E-cadherin expression compared with postischemic administration of SVF or PBS (p < .05). (E, F): The expression levels of TGF-β1 in serum (E) and injured kidney (F) in rats received preischemic administration of SVF were significantly lower than that received postischemic administration of SVF or PBS. (G, H): Mean optical density was evaluated by RECA-1 staining in glomerular (G) and peritubular (H) areas in kidney sections of rats at 6 months after IR. Preischemic administration of SVF significantly inhibited microvascular rarefaction in kidney sections compared with postischemic administration. (I): Representative microscopic images of RECA-1 staining preformed on sections of kidneys at 6 months after IR in different groups (×400). Scale bars = 50 μm. ∗, p < .05 (vs. sham, IR+SVF pre-I, and IR+SVF post-I); #, p < .05 (vs. IR+SVF post-I). Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IR, ischemia/reperfusion; PBS, phosphate-buffered saline; RECA-1, rat endothelial cell antigen-1; α-SMA, α-smooth muscle actin; SVF Post-I, postischemic administration of stromal vascular fraction; SVF Pre-I, preischemic administration of stromal vascular fraction; TGF-β1, transforming growth factor-β1.
Moreover, RECA-1 staining was performed to evaluate microvascular rarefaction that was proposed to be associated with accelerated progression toward CKD. We observed that induction of IR caused downregulation of RECA-1 expression, whereas SVF treatment contributed to a preserved expression of RECA-1 both in the peritubular structure and within the glomeruli. Additionally, preischemic administration of SVF reinforced RECA-1 expression compared with postischemic treatment, suggesting the stronger inhibition of microvascular rarefaction by preischemic treatment (Fig. 7G–7I).
Discussion
To the best of our knowledge, this is the first study demonstrating that preischemic administration of nonexpanded adipose SVF can improve renal function and structure at both the early and long-term stage after IR-induced kidney injury in an animal model. The underlying mechanisms in the SVF-mediated protection against IR injury through preischemic administration can be concluded as follows: (a) preischemic administration resulted in improved retention of SVF in injured kidney after IR; (b) preischemic administration of SVF promoted the proliferation and migration of renal TEC and reduced cell apoptosis; and (c) preischemic administration of SVF delayed the development of CKD through inhibiting TGF-β1-induced tubular EMT and microvascular rarefaction. Thus, the present study may provide a novel therapeutic approach to protect the kidney from both early and long-term damage induced by IR.
As is known, most types of stem/progenitor cells need a relatively long time of culturing in vitro to obtain adequate cell numbers before the administration in vivo [40], which is time-consuming and may result in the loss of the best opportunity for the treatment of acute organ injury, including AKI. Additionally, the potential risk of microbial contamination during cell culture has raised concerns regarding the safety of cultured stem/progenitor cells for clinical transformation [16]. SVF is isolated from adipose tissue and consists of heterogeneous cell populations, providing a rich source of stem/progenitor cells [13, 32, 41]. Nonexpanded SVF has been demonstrated to improve tissue regeneration and functional recovery of various organs, including the kidney [14, 15, 42, 43]. Furthermore, in an open-label phase I trial, autologous SVF was confirmed to be safe and effective during its application in patients with systemic sclerosis [44]. Because of the real-time isolation in a sufficient quantity without in vitro expansion and therapeutic effects on tissue injury, SVF is considered as an attractive cell source for cell-based therapy [16], especially for the treatment of acute organ injury, such as AKI. Therefore, in the present study, SVF was applied to protect the kidney from IR injury and stimulate renal tissue regeneration. Besides the early therapeutic efficacy of SVF administration against renal IR injury that was demonstrated as previous study [14], long-term protective effects of both preischemic and postischemic administration of SVF against the development of CKD after renal IR injury was also verified.
To deliver stem/progenitor cells for kidney repair after injury, numerous investigators have attempted many routes of cell administration, including intra-arterial [14], intravenous [10, 45], intrasubcapsular [15, 45], and intraparenchymal transplantation [24, 46]. No matter which route is used, cell recruitment and retention in injured kidney associated with systematic safety are the main considerations in cell-based therapy. Nevertheless, delivery of stem/progenitor cells via intra-arterial or intravenous transplantation often leads to an extremely low retention of administered cells in the injured kidney, which may result in unsatisfactory renal tissue repair [47]. Additionally, unexpected accumulation of injected cells in nontarget organs through intra-arterial or intravenous transplantation may cause adverse effects and diminish the therapeutic efficacy [48, 49]. Although stem/progenitor cells could be delivered directly to the kidney through intrarenal arterial injection to improve the amount of engrafted cells, the occlusion of renal microvasculature due to the infused cells is still a challenge for such a delivery strategy [10, 50]. Therefore, cell transplantation via intrasubcapsular or intraparenchymal injection has emerged as a preferred method; however, it has still ended up with low engraftment after cells were injected solely [15, 24, 45]. Because previous studies have demonstrated that preconditioning with hypoxia or ischemia could promote the retention and survival of transplanted cells in vivo [29, 51], in this study, preischemic administration of SVF through intraparenchymal injection was performed to induce an ischemic preconditioning in vivo. Based on the cell tracking, significantly improved cell retention and survival were observed when SVF was transplanted through preischemic administration compared with postischemic administration. These results indicated that preischemic administration through intraparenchymal injection might be an effective and targeted delivery method of stem/progenitor cells to injured kidney. Such a delivery strategy is especially preferred to a predictable injury that might be induced by kidney surgery, such as kidney transplantation, partial nephrectomy, etc.
During the early stage of AKI, tubular cell injury is one of the important cellular pathophysiologies [2]. Studies have demonstrated that transplantation of SVF could effectively improve renal function [14, 15], which was further confirmed in our study. The functional improvement of the injured kidney might be attributable to the protective effects of SVF against tubular cell injury through promoting cell proliferation and reducing apoptosis [14, 15]. In our experiments, renal IR injury resulted in serious apoptosis of TEC associated with low expression of E-cadherin, a marker of epithelial cells, including TEC [52, 53], due to the increase of TUNEL-positive TEC compared with PCNA-positive TEC in the injured kidney. SVF administration facilitated the reduction of cell apoptosis and improvement of E-cadherin expression. Additionally, a coculture system was applied to further investigate the relationship between SVF and TEC. Although cells may migrate through the pores because of the large pore size of the PET membrane we used, it has been reported that the migrated cells were mainly adherent to the underside of the PET membrane [54, 55], which might have little impact on the result of the experiment. Results showed that SVF administration could stimulate the proliferation and migration of TEC and reduce apoptosis under hypoxic condition because of the paracrine effects of SVF. It has been reported that SVF could secrete a list of growth factors including hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and so on, among which HGF has been reported to be a predominant mediator of renoprotection [15, 56]. HGF knockdown in vivo or neutralized in vitro resulted in a reduced attenuation of tubular injury or decreased antiapoptotic effect on tubular cells by stem/progenitor cells [15, 45]. In this study, we also found that significantly enhanced proliferation and reduced apoptosis of TEC were induced because of the administration of SVF through both preischemic treatment in vivo and prehypoxic treatment in vitro, compared with postischemic and posthypoxic administration. These results might be attributed to the increased secretion of growth factors by the stem/progenitor cells in SVF under hypoxic or ischemic conditioning [15].
Besides the acute damage of tubular cells associated with the impairment of renal function, adverse long-term outcome of AKI is an increasing concern because of the fibrosis development and progression to CKD [4]. TGF-β1 is considered as the main contributor that triggers EMT and leads to fibrogenesis through promoting fibroblast proliferation and collagen deposition [6]. In this study, animals at 6 months after IR injury showed interstitial fibrosis and high levels of TGF-β1, which was similar to previous studies [57]. The administration of SVF contributed to the attenuation of fibrosis through inhibiting the production of TGF-β1. Inhibition of TGF-β signaling could suppress the process of tubular EMT via a Smad-dependent mechanism [58]. In addition, HGF was reported to be a potent antifibrotic cytokine with the capability of blocking EMT through inhibition of TGF-β/Smad signaling [59]. A study showed that SVF cells could secrete high levels of HGF in vitro, and transplantation of SVF contributed to an enhanced expression level of HGF in vivo [15]. Therefore, administration of SVF could contribute to the upregulation of HGF and downregulation of TGF-β1, which might restore the biased balance of HGF/ TGF-β1 associated with the progression of renal fibrosis [60].
Furthermore, we also found that, compared with postischemic administration of SVF, the preischemic administration strategy caused a stronger inhibition of EMT in parallel with the progression of renal fibrosis. One of the reasons might be attributed to the stronger suppression of TGF-β1 production, whereas the promotion of HGF production was unknown. Although a previous study showed that hypoxia had no impact on the expression level of HGF in vitro [15], the expression level of HGF in vivo after preischemic administration of SVF needs further investigation. The other reason for SVF preischemic administration-induced attenuation of fibrosis might be the stronger inhibition of microvascular rarefaction. Sustained hypoxia because of the rarefaction of renal microvascular density could induce EMT and trigger fibrosis [61, 62], whereas inhibition of microvascular rarefaction contributed to reduced fibrosis and preserved renal function [35]. In addition, inhibition of microvascular rarefaction may also result in inhibited production of TGF-β1, because sustained hypoxia may trigger the expression of TGF-β1 [63].
Our study demonstrated that preischemic administration of SVF could protect the kidney from IR-induced injury, ascribing to the paracrine effect of SVF. However, to precisely evaluate the paracrine effect of SVF, further study is needed to identify or inhibit the growth factors released by SVF under normoxic and hypoxic conditions. In addition, whether SVF cells could survive and differentiate to tubular cells at 6 months after injection should be further investigated by long-term cell tracking, while the differentiation of stem/progenitor cells to renal TEC is important for the repair of AKI [64]. Finally, the present study showed that preischemic administration of SVF delayed the progression of renal fibrosis through the inhibition of TGF-β1-induced EMT and microvascular rarefaction. The exact molecular mechanisms and details of inhibiting effects on fibrosis should be explored in the future.
Conclusion
Together, our results demonstrated that preischemic administration of nonexpanded adipose SVF increased the retention of SVF in the injured kidney, which then contributed to the protective effects against IR-induced renal tubular cell injury and improvement of renal function, because of the paracrine effects of SVF. In addition, preischemic administration of SVF exhibited long-term protective effects against the development of CKD associated with the progression of renal fibrosis through inhibiting TGF-β1-induced EMT and suppressing microvascular rarefaction.
Acknowledgments
We thank Dr. Chen Yu (Tongji University, China) for kindly providing the NRK-52E cell line. This work was supported by National Natural Science Foundation of China Grants 81370853 and 81570613 and Nanjing Medical University Grant 2013NJMU081.
Author Contributions
L.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript; L.X.: data analysis and interpretation, manuscript writing, final approval of manuscript; J.S., Q.S., Y.G., and H.X.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; R.W., J.Z., and J.W.: data analysis and interpretation, final approval of manuscript; R.J.: conception and design, manuscript preparation, financial and administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Liaño F, Pascual J, Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 4.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 5.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 7.Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Moggio A, Bussolati B. Concise review: Stem/progenitor cells for renal tissue repair: Current knowledge and perspectives. Stem Cells Translational Medicine. 2013;2:1011–1019. doi: 10.5966/sctm.2013-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 10.Shih YC, Lee PY, Cheng H, et al. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plast Reconstr Surg. 2013;132:940e–951e. doi: 10.1097/PRS.0b013e3182a806ce. [DOI] [PubMed] [Google Scholar]
- 11.Donizetti-Oliveira C, Semedo P, Burgos-Silva M, et al. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant. 2012;21:1727–1741. doi: 10.3727/096368911X623925. [DOI] [PubMed] [Google Scholar]
- 12.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile P, Orlandi A, Scioli MG, et al. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Translational Medicine. 2012;1:230–236. doi: 10.5966/sctm.2011-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Z, Ting J, Alfonso Z, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25:3874–3884. doi: 10.1093/ndt/gfq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasuda K, Ozaki T, Saka Y, et al. Autologous cell therapy for cisplatin-induced acute kidney injury by using non-expanded adipose tissue-derived cells. Cytotherapy. 2012;14:1089–1100. doi: 10.3109/14653249.2012.693157. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Bunnell BA, Chiu ES, et al. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Cohen A, Hudson TE, et al. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 19.Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 20.Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 21.Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 23.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Liu R, Wu J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials. 2012;33:3673–3681. doi: 10.1016/j.biomaterials.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 26.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 28.Kamota T, Li TS, Morikage N, et al. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Kim HW, Haider HK, Jiang S, et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patschan D, Krupincza K, Patschan S, et al. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: Modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Wu R, Jia RP, et al. Ischemic preconditioning increases endothelial progenitor cell number to attenuate partial nephrectomy-induced ischemia/reperfusion injury. PLoS One. 2013;8:e55389. doi: 10.1371/journal.pone.0055389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Xia J, Qiu X, et al. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PLoS One. 2015;10:e0117644. doi: 10.1371/journal.pone.0117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou DB, Zeng D, Jin Y, et al. The long-term differentiation of embryonic stem cells into cardiomyocytes: An indirect co-culture model. PLoS One. 2013;8:e55233. doi: 10.1371/journal.pone.0055233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Zhou L, Peng B, et al. In vitro comparative evaluation of recombinant growth factors for tissue engineering of bladder in patients with neurogenic bladder. J Surg Res. 2014;186:63–72. doi: 10.1016/j.jss.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 36.Zhu XY, Urbieta-Caceres V, Krier JD, et al. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, Yang B, Sun C, et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A. 2013;19:264–276. doi: 10.1089/ten.tea.2011.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Zhang L, Wang N, et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84:521–531. doi: 10.1038/ki.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu X, Fu K, Zhao X, et al. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J Transl Med. 2015;13:28. doi: 10.1186/s12967-015-0388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinders ME, Rabelink TJ. Adipose tissue-derived stem cells: Can impure cell preparations give pure results? Nephrol Dial Transplant. 2010;25:3805–3807. doi: 10.1093/ndt/gfq651. [DOI] [PubMed] [Google Scholar]
- 41.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury [published correction appears in Eur Urol 2012;62:e106] Eur Urol. 2012;62:720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenke-Layland K, Strem BM, Jordan MC, et al. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153:217–223. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granel B, Daumas A, Jouve E, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann Rheum Dis. 2015;74:2175–2182. doi: 10.1136/annrheumdis-2014-205681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsuno T, Ozaki T, Saka Y, et al. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22:287–297. doi: 10.3727/096368912X655019. [DOI] [PubMed] [Google Scholar]
- 46.Mias C, Trouche E, Seguelas MH, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 47.Tögel F, Yang Y, Zhang P, et al. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F315–F321. doi: 10.1152/ajprenal.00098.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 49.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee PY, Chien Y, Chiou GY, et al. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012;21:2569–2585. doi: 10.3727/096368912X636902. [DOI] [PubMed] [Google Scholar]
- 51.Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818–1828. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mengaud J, Ohayon H, Gounon P, et al. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 53.Strutz FM. EMT and proteinuria as progression factors. Kidney Int. 2009;75:475–481. doi: 10.1038/ki.2008.425. [DOI] [PubMed] [Google Scholar]
- 54.Sharan A, Zhu H, Xie H, et al. Down-regulation of miR-206 is associated with Hirschsprung disease and suppresses cell migration and proliferation in cell models. Sci Rep. 2015;5:9302. doi: 10.1038/srep09302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang B, Zhou L, Sun Z, et al. In vitro evaluation of the bioactive factors preserved in porcine small intestinal submucosa through cellular biological approaches. J Biomed Mater Res A. 2010;93:1100–1109. doi: 10.1002/jbm.a.32534. [DOI] [PubMed] [Google Scholar]
- 56.Pallua N, Pulsfort AK, Suschek C, et al. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg. 2009;123:826–833. doi: 10.1097/PRS.0b013e318199ef31. [DOI] [PubMed] [Google Scholar]
- 57.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 58.Jia L, Ma X, Gui B, et al. Sorafenib ameliorates renal fibrosis through inhibition of TGF-β-induced epithelial-mesenchymal transition. PLoS One. 2015;10:e0117757. doi: 10.1371/journal.pone.0117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 60.Du T, Zou X, Cheng J, et al. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res Ther. 2013;4:59. doi: 10.1186/scrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Legrand M, Mik EG, Johannes T, et al. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502–516. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng R, Yao Y, Han M, et al. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol. 2008;19:380–387. doi: 10.1681/ASN.2006111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basile DP. The transforming growth factor beta system in kidney disease and repair: Recent progress and future directions. Curr Opin Nephrol Hypertens. 1999;8:21–30. doi: 10.1097/00041552-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Li K, Han Q, Yan X, et al. Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem Cells Dev. 2010;19:1267–1275. doi: 10.1089/scd.2009.0196. [DOI] [PubMed] [Google Scholar]