Bone marrow-derived mesenchymal stromal cells (BM-MSCs) and induced pluripotent stem cells (iPSCs) from patients with familial osteochondritis dissecans (FOCD) were studied. Chondrogenic pellets had poor structural integrity but were rich in glycosaminoglycan. Large amounts of aggrecan accumulated within the endoplasmic reticulum of chondrocytes, with a marked absence of aggrecan in the extracellular matrix. Matrix synthesis and assembly were globally dysregulated. These results suggest that FOCD is a chondrocyte aggrecanosis with associated matrix dysregulation.
Keywords: Osteoarthritis, Familial osteochondritis dissecans, Mesenchymal stromal cells, Induced pluripotent stem cells, Stem cell disease models, Cellular pathology, Aggrecan mutation, Endoplasmic reticulum stress
Abstract
Familial osteochondritis dissecans (FOCD) is an inherited skeletal defect characterized by the development of large cartilage lesions in multiple joints, short stature, and early onset of severe osteoarthritis. It is associated with a heterozygous mutation in the ACAN gene, resulting in a Val-Met replacement in the C-type lectin domain of aggrecan. To understand the cellular pathogenesis of this condition, we studied the chondrogenic differentiation of patient bone marrow mesenchymal stromal cells (BM-MSCs). We also looked at cartilage derived from induced pluripotent stem cells (iPSCs) generated from patient fibroblasts. Our results revealed several characteristics of the differentiated chondrocytes that help to explain the disease phenotype and susceptibility to cartilage injury. First, patient chondrogenic pellets had poor structural integrity but were rich in glycosaminoglycan. Second, it was evident that large amounts of aggrecan accumulated within the endoplasmic reticulum of chondrocytes differentiated from both BM-MSCs and iPSCs. In turn, there was a marked absence of aggrecan in the extracellular matrix. Third, it was evident that matrix synthesis and assembly were globally dysregulated. These results highlight some of the abnormal aspects of chondrogenesis in these patient cells and help to explain the underlying cellular pathology. The results suggest that FOCD is a chondrocyte aggrecanosis with associated matrix dysregulation. The work provides a new in vitro model of osteoarthritis and cartilage degeneration based on the use of iPSCs and highlights how insights into disease phenotype and pathogenesis can be uncovered by studying differentiation of patient stem cells.
Significance
The isolation and study of patient stem cells and the development of methods for the generation of iPSCs have opened up exciting opportunities in understanding causes and exploring new treatments for major diseases. This technology was used to unravel the cellular phenotype in a severe form of inherited osteoarthritis, termed familial osteochondritis dissecans. The phenotypic abnormalities that give rise to cartilage lesions in these patients were able to be described via the generation of chondrocytes from bone marrow-derived mesenchymal stromal cells and iPSCs, illustrating the extraordinary value of these approaches in disease modeling.
Introduction
Mesenchymal stromal cells (MSCs) from bone marrow and other tissues have the capacity to differentiate into chondrocytes. The differentiated cell phenotype has been well characterized and shares many cellular and extracellular matrix (ECM) features of primary chondrocytes isolated from articular cartilage. MSCs can also be prepared from induced pluripotent stem cells (iPSCs) generated from dermal fibroblasts or other cells and can form chondrocytes. Our objective in this study was to assess whether analysis of the differentiated phenotype in cells from patients with osteoarthritis (OA) would shed light on the cellular pathology of the disease. Idiopathic OA occurs widely and has a broad spectrum of symptoms and probably a broad spectrum of causation, making interpretation of results in studies such as this potentially difficult. We therefore selected a monogenic disease, familial osteochondritis dissecans (FOCD), associated with a known single mutation and characterized by the development of early and severe OA. We isolated MSCs and generated iPSCs from FOCD patients and assessed their chondrogenic differentiation in an attempt to shed light on the cellular pathology that gives to the unstable and rapidly degenerating articular cartilage in these patients.
Osteochondritis dissecans is a serious disease of joints characterized by separation of articular cartilage from subchondral bone [1, 2]. The most commonly affected joints are the knee, elbow, and ankle, but other joints may be involved [3–7]. Symptoms include pain on activity, effusion, crepitation, and limited range of movement [8]. Development of degenerative osteoarthritis (OA) is common in patients with osteochondritis dissecans [9]. On a number of occasions, FOCD has been reported with an underlying hereditary factor [10–13]. This study focuses on a family with FOCD from northern Sweden (referred to as FOCD-NS), first reported by Stougaard in 1964 [13], in which the disease is inherited in an autosomal dominant pattern [12]. The results of a genome-wide scan and DNA sequencing showed that all affected family members bear a heterozygous G–A mutation in aggrecan exon 17, which results in a Val-Met amino acid replacement (V2303M) in the G3 aggrecan C-type lectin domain (CLD) [14].
Aggrecan, a major proteoglycan of articular cartilage produced by chondrocytes, has a large protein core richly substituted with sulfated glycosaminoglycan (GAG) chains. The aggrecan protein core follows the secretory pathway within the chondrocyte; it is made in the rough endoplasmic reticulum (rER) and then translocated to the Golgi for GAG chain synthesis and sulfation, before secretion to the ECM [15]. Its unique structure, its high concentration within the ECM, and its ability to form a supermolecular complex with hyaluronan and bind to other matrix proteins all profoundly influence the biomechanical properties of the tissue [16]. Deletion of aggrecan G3 domain in a mutant chicken (nanomelia) was found to block aggrecan secretion from chondrocytes [17]. Further evidence using aggrecan synthesized with no naturally folded G3 also indicated a role for this domain in intracellular trafficking and secretion [18]. An important function of the G3 CLD is binding to tenascin-C, tenascin-R, fibulin-1, and fibulin-2 in cartilage ECM [19], and human aggrecan constructs carrying the V2303M mutation were shown to have diminished interactions with these binding partners [14].
We cultured MSCs from bone marrow (BM) of an FOCD-NS patient and induced them to differentiate into chondrocytes [20]. Analysis of the phenotype of differentiated cells revealed an abnormal intracellular processing pathway and limited extracellular distribution of aggrecan. Furthermore, we showed that the mutated aggrecan caused morphologic changes in the rER within the chondrocytes. Our experiments suggested a cellular pathology of FOCD-NS with misfolded aggrecan accumulating inside the rER, resulting in ER stress and leading to a deficiency of cell function in protein secretion. As a consequence, there was defective development of the cartilaginous ECM, with a distinctly abnormal composition of key proteins and proteoglycans. This suggested that, in addition to defective aggrecan processing, synthesis and assembly of the entire ECM is impaired.
To further extend our observations, we prepared patient-specific iPSCs with successful reprogramming of skin fibroblasts from two FOCD-NS patients. By exploiting the potential of iPSCs when implanted in vivo to form teratomas with tissues from all three germ layers, including cartilage, we were able to show that the same disease phenotype was recapitulated in the iPSC-derived cartilage within teratomas. These results provide new insight into the cellular phenotype of FOCD and demonstrate for the first time a disease model of a monogenic OA based on patient-specific iPSCs.
Materials and Methods
Ethical Approval
All animal experimentation complied with institutional guidelines and had ethical approval from the Animal Care Research Ethics Committee at the National University of Ireland Galway (June 5, 2003). MSCs were isolated from BM harvested from the iliac crest of a healthy donor with approval from both the National University of Ireland Galway Research Ethical Committee (May 8, 2014) and Galway University Hospitals Clinical Research Ethics Committee (February 2008). The ethical approval for harvesting skin tissue and BM from FOCD-NS patients for primary cell culture and stem cell study were issued by the Regional Ethical Review Board in Umeå, Sweden, Dnr 2010/408-31M and 2007/109M (01-244).
Tissue Samples
A bone marrow sample was obtained from a 49-year-old male FOCD-NS patient during joint replacement surgery, and normal human BM-MSCs (control) were isolated from a 33-year-old male healthy donor. Dermal fibroblasts were obtained from a 25-year-old patient (FOCD-NS1) and his 49-year-old mother (FOCD-NS2) for generation of patient-derived iPSCs. An iPSC line (33D-6) from a healthy donor (56-year-old male) was obtained from G. Sullivan (University of Edinburgh, Edinburgh, UK) [21].
Isolation and Characterization of MSCs From Patient and Control Marrow
BM-MSCs were isolated and characterized (adipogenesis and osteogenesis) as previously described [20]. To assess positive and negative surface markers of BM-MSCs, the Human MSC Analysis Kit (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) was used according to the manufacturer’s protocol. Data were analyzed by FlowJo 6.0 (FlowJo, LLC, Ashland, OR, http://www.flowjo.com).
Chondrogenesis of BM-MSCs
To detect disease phenotypes, chondrogenesis of BM-MSCs was performed using micropellet culture as described previously [20]. Because the brief centrifugation step associated with micropellet formation affected the structure of pellets derived from patient cells, a modified high-cell-density micromass method was performed as previously described [22, 23]. Briefly, 2 × 105 BM-MSCs in 10 µl were incubated at 37°C for 2 hours in low-adherence round-bottom 96-well plates (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). MSC maintenance medium (200 µl) was added to each well, followed by overnight incubation. Medium was replaced by chondrogenic medium with 10 ng/ml transforming growth factor-β3 (Peprotech, London, U.K., https://www.peprotech.com) [20] at day 2 and was changed every second day. Micropellets and micromass cultures were harvested at different time points.
Sulfated Glycosaminoglycan and DNA Quantification
For quantification of GAG, dimethylmethylene blue (DMMB) staining (Sigma-Aldrich) was performed as described previously [20]. DNA was measured by Picogreen dsDNA assay kit (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com), according to the manufacturer’s protocol. To assess the chondrogenic capability of control and FOCD-NS cells, the ratio of GAG to DNA was calculated.
Histologic and Immunohistochemical Analysis of Chondrogenic Pellets
Samples were processed as previously described [20]. For detection of GAG, sections were stained with 0.5% toluidine blue (pH 8) (Sigma-Aldrich) for 5 minutes at 60°C. Immunostaining was performed to investigate the distribution of ECM proteins (collagen type II and aggrecan) and colocalization of aggrecan and glucose regulated protein 78 (GRP78) in chondrogenic pellets and cultures. Detailed experimental procedures can be found in supplemental online data and supplemental online Table 1.
Bright-field and confocal microscopy were used to examine the immunohistochemical and immunofluorescence stained specimens. The magnified images for detection of cell morphology and ECM distribution were created using ImageJ.
Transmission Electron Microscopy
To analyze ECM formation and ultrastructure of ER, day-56 micromass cultures were processed for transmission electron microscopy (TEM) examination as previously described [24]. Samples were examined using the Hitachi H7000 transmission electron microscope (Hitachi, Tokyo, Japan, http://www.hitachi.com).
To estimate the volume fraction of rER to cell cytoplasm, a systematic random sampling protocol was adopted. This consisted of a series of images (10) taken across the pellet at an initial magnification of ×3,000. Three further images were taken from each field at magnification ×10,000. Simple point-counting methods were used to estimate cellular composition [25].
Mass Spectrometry
For sample preparation, a modified protocol for extracting peptides from chondrogenic pellets derived from BM-MSCs was performed based on a previous description [26]. Detailed experimental procedures can be found in supplemental online data. Each sample was run in technical duplicate. The measured peptide transitions are listed in supplemental online Table 3. Three to six transitions for each peptide were measured in a scheduled multiple reaction monitoring method [26]. Briefly, the amount of each protein was normalized by the pellet volume. The mean ratios of ECM proteins between FOCD and control BM-MSC derived pellets were calculated.
iPSC Generation and Characterization
For reprogramming, isolated skin fibroblasts from two patients (FOCD-NS1 and FOCD-NS2) were transfected by retrovirus containing the four Yamanaka reprogramming factors as described previously [27]. Resulting iPSC colonies were expanded. To assess the pluripotency of these iPSCs, semiquantitative reverse-transcription polymerase chain reaction (RT-PCR), immunocytochemistry, flow cytometry, teratoma formation in vivo, karyotype analysis, and mutation verification were performed. See supplemental online data and supplemental online Table 2 for detailed protocols.
Verification of Disease Phenotype in iPSC-Derived Cartilage
Cartilaginous nodules formed in teratoma derived from one control and four FOCD-NS iPSC lines were studied. To identify cartilage tissues in teratoma sections, samples were stained with 0.02% fast green solution (Sigma-Aldrich) followed by 0.1% safranin-O solution (Sigma-Aldrich). Cartilage tissue showed red, and noncartilage tissues showed green. To study the distribution of aggrecan protein in these tissue, immunohistochemical staining was performed. Detailed experimental procedures can be found in supplemental online data.
Results
FOCD-NS MSC Characterization
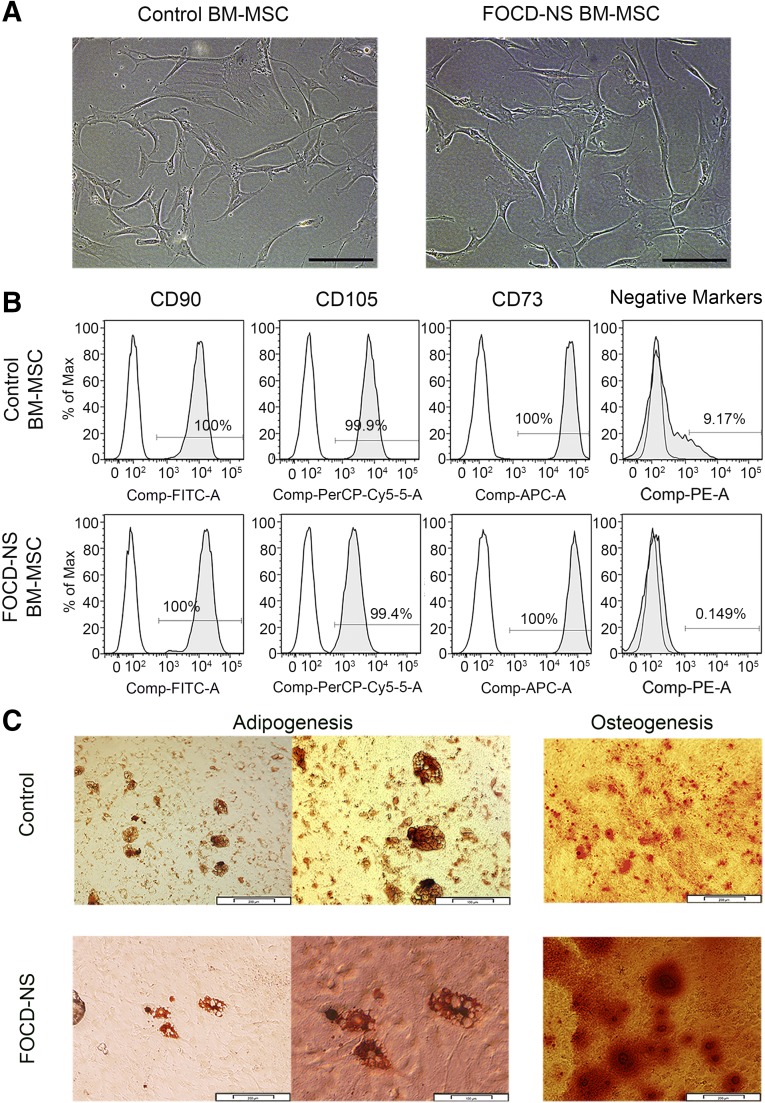
MSCs isolated from bone marrow of a FOCD-NS patient were morphologically similar to cells from a healthy donor (Fig. 1A), with similar cell surface phenotype. Both control and FOCD-NS BM-MSCs were positive for CD90, CD73, and CD105 (Fig. 1B). Combined levels of hematopoietic cell markers CD11b, CD34, CD19, CD45, and human leukocyte antigen (HLA)-DR were substantially lower in patient cells (0.149%) compared with controls (9.17%) (Fig. 1B). The patient marrow appeared to have a high fat content and yielded low numbers of mononuclear cells. Patient and control MSCs were assessed for multipotency using trilineage differentiation tests. Both populations showed a capacity to differentiate into osteoblasts and adipocytes (Fig. 1C). In contrast, there were marked differences between patient and control cells in chondrogenic pellet cultures. Both MSC populations readily formed white cartilaginous pellets, but patient samples had an irregular discoid morphology (Fig. 2A, 2B). Patient pellets (diameter 1.5–2 mm) were larger with a rough surface compared with the smooth glistening surface of control pellets (diameter 1–1.5 mm). In addition, there was greater GAG staining in the FOCD-NS pellets (Fig. 2B).
Figure 1.
Characterization of control and FOCD-NS BM-MSCs. (A): Morphology of cultured mesenchymal cells isolated from control and FOCD-NS bone marrow. Scale bars, 200 µm. (B): Flow cytometric analysis of control and FOCD-NS BM-MSCs for positive antigens CD90, CD73, and CD105 and negative markers CD11b, CD34, CD19, CD45, and HLA-DR. White histograms represent isotype controls, and the gray overlay represents each antigen; percentage positive cells shown within histograms. (C): Adipogenic and osteogenic differentiation of control and FOCD-NS BM-MSCs. In adipogenic culture, phase-contrast microscopy images show Oil Red O-positive cells. Scale bars, 200 µm (left) and 100 µm (right). In osteogenic differentiation culture, mineralized nodules produced by osteoblasts were stained with Alizarin red. Scale bars, 100 µm. Abbreviations: APC, allophycocyanin; BM-MSC, bone marrow mesenchymal stem cell; Comp, compensated; Cy, cyanine; FITC, fluorescein isothiocyanate; FOCD-NS, familial osteochondritis dissecans from northern Sweden; HLA, human leukocyte antigen; PE, phycoerythrin; PerCP, peridinin chlorophyll.
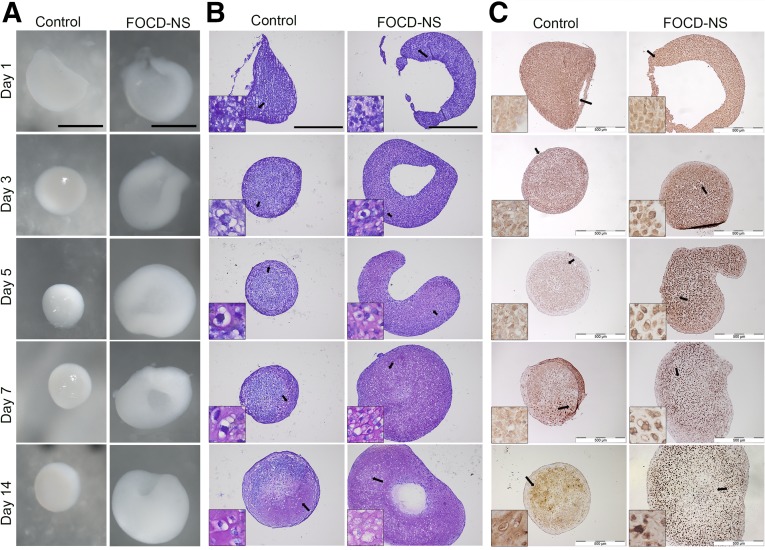
Figure 2.
Morphologic and immunohistochemical analysis of in vitro chondrogenic differentiation using micropellet culture. Pellets were harvested at different time points (days 1, 3, 5, 7, and 14). (A): Representative stereomicroscope images of overall morphology of chondrogenic pellets differentiated from control and FOCD-NS BM-MSCs. Scale bars, 1 mm. (B): Toluidine blue staining on the sections of these pellets. Scale bars, 500 µm. (C): Representative images of immunostained sections for aggrecan distribution. Scale bars, 500 µm. Insets are magnified images of the areas indicated by black arrows. Abbreviations: BM-MSC, bone marrow mesenchymal stem cell; FOCD-NS, familial osteochondritis dissecans from northern Sweden.
Characterization of Chondrogenic Cultures
Immunohistochemical staining of chondrogenic pellets was performed using an antibody specific for the interglobular domain of aggrecan. In control pellets, aggrecan was clearly discernible within the chondrocytes and throughout the matrix; staining was primarily intracellular at early time points and progressed through pericellular localization evident from day 5 to intraterritorial distribution by day 14 (Fig. 2C, left). In contrast, in the patient pellets aggrecan staining was predominantly cellular or pericellular with minimal territorial or intraterritorial matrix staining at 14 days. At earlier times, some aggrecan was detected in the extracellular matrix, perhaps reflecting the heterozygous nature of the FOCD-NS mutation, but this diminished by day 5 in culture (Fig. 2C, right). This result suggested that the effect of the mutation within the CLD was to impede normal processing and stabilization of aggrecan within the ECM.
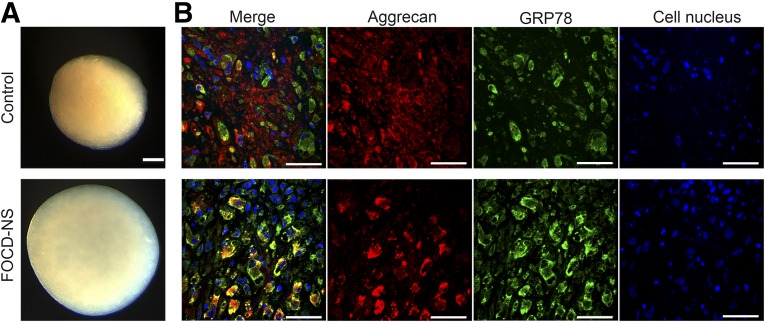
To allow careful comparison between patient and control cells, we used micromass cultures without centrifugation. Micromass culture allows self-assembly of cells at high density into organized aggregates and may allow for better cell–cell contact, thus facilitating chondrogenic differentiation more typically seen during development [22]. To reflect the adult onset of the condition, micromass cultures were maintained for periods between 14 and 100 days. Histologic analysis showed that patient cells were able to form regular spherical micromass cultures of larger dimension than those derived from control cells (Fig. 3A). We carried out costaining for aggrecan protein and GRP78, an rER chaperone [28]. GRP78 resides within the rER and its functions include control of new protein folding and recognition of unfolded or misfolded proteins. Analysis of long-term chondrogenic micromass cultures of control cells (Fig. 3B) revealed large amounts of aggrecan in the ECM with some intracellular GRP78 staining, indicating aggrecan processing through the ER. There was also some evidence of nascent aggrecan within cells. In stark contrast, patient cultures showed no aggrecan staining in the ECM but rather a distinctly intracellular pattern with a clear colocalization with GRP78. This dramatic difference in patient cells indicated an accumulation of aggrecan within the rER and an inability to process and secrete to the ECM (Fig. 3B).
Figure 3.
Morphologic and immunohistochemical analysis of micromass cultures derived from control and FOCD-NS BM-MSCs. (A): Images showing the morphology of day-35 micromass cultures. Scale bar, 200 µm. (B): Confocal microscopy images showing double staining of aggrecan (red), GRP78 (green), and overlapping areas (yellow) on day-35 cultures. Cell nuclei stained with DAPI (blue). Scale bar, 50 µm. Abbreviations: BM-MSC, bone marrow mesenchymal stem cell; DAPI, 4′,6-diamidino-2-phenylindole; FOCD-NS, familial osteochondritis dissecans from northern Sweden.
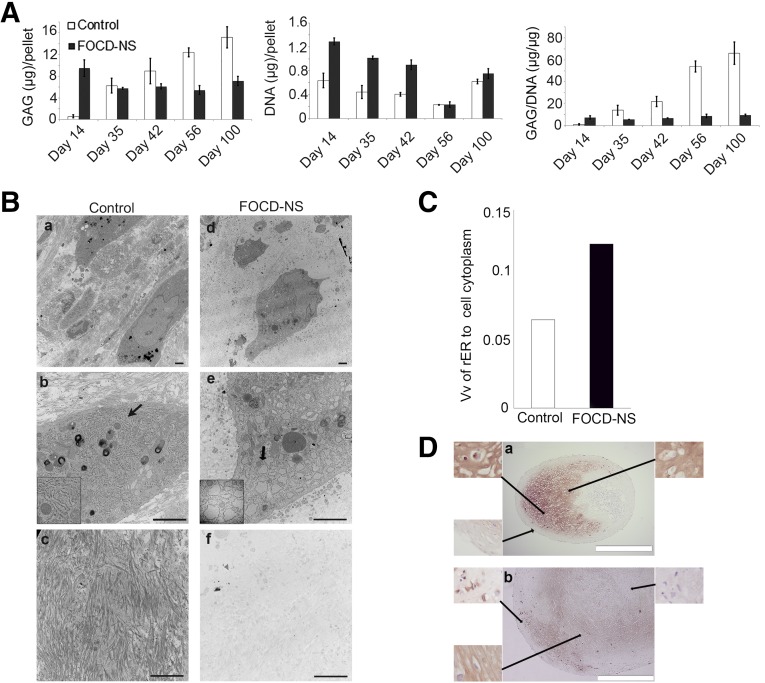
Analysis of GAG accumulation in long-term chondrogenic cultures of patient cells was carried out. At early time points, there was 15-fold higher GAG accumulation in FOCD-NS cultures compared with controls (9.5 vs. 0.6 µg GAG/pellet) (Fig. 4A). However, in these cells, longer incubation resulted in decreased GAG content, whereas control cells demonstrated a progressive increase. Measurement of DNA content per pellet showed a higher level in patient cells, suggesting a greater degree of mitotic activity. When GAG was measured as a function of DNA content, patient cells only produced a substantially higher GAG per cell at day 14, and a low ratio of GAG/DNA remained constant throughout the long-term culture of patient cells. Whereas this ratio increased greatly with time in control samples and exceeded that in patient samples at day 35. These results suggested that GAG synthesis in the patient cells is inhibited in long-term cultures, that there is a higher degradative activity, or that chondrogenic differentiation is otherwise delayed or arrested in FOCD-NS cultures (Fig. 4A).
Figure 4.
Examination of ECM formation and chondrocyte status in micromass cultures derived from control and FOCD-NS BM-MSCs. (A): Quantification of GAG and DNA amounts in micromass cultures harvested at different time points. Average (± SD) ratios of GAG to DNA are shown. (B): Day-56 TEM images showed the distribution of protein fibers in ECM (a, c, d, and f) and the ultrastructure of chondrocytes (b and e). The structure of rER is indicated by black arrows (b and e), with the corresponding magnified region shown bottom left in each case. The components of ECM in each group are showed in c and f. Scale bar, 2 µm. (C): Volume fractions of rER to cell cytoplasm in control and FOCD-NS sections. (D): Immunostaining of collagen type II (brown) in control (a) and FOCD-NS (b) sections from the day-42 cultures. Cell nuclei were stained with hematoxylin (blue). Scale bar, 500 µm. Abbreviations: BM-MSC, bone marrow mesenchymal stem cell; ECM, extracellular matrix; FOCD-NS, familial osteochondritis dissecans from northern Sweden; GAG, glycosaminoglycan; rER, rough endoplasmic reticulum; TEM, transmission electron microscopy; Vv, volume fraction.
Analysis by TEM of control pellets showed evidence of long fibrillar bundles in the ECM of day 56 micromass cultures, but little evidence of these in patient samples (Fig. 4B and supplemental online Fig. 1). A characteristic of the FOCD chondrocytes was rER with physically enlarged surface area (Fig. 4Be). In contrast, the rER in control chondrocytes had a normal appearance (Fig. 4Bb). The volume fractions of rER to cell cytoplasm in control and FOCD-NS samples indicated that the surface area of rER in patient samples was twice that of control samples (Fig. 4C). Immunostaining of micromass pellets from day 42 showed that the patient sample had a lower density of collagen type II fibers than found in the control sample (Fig. 4D).
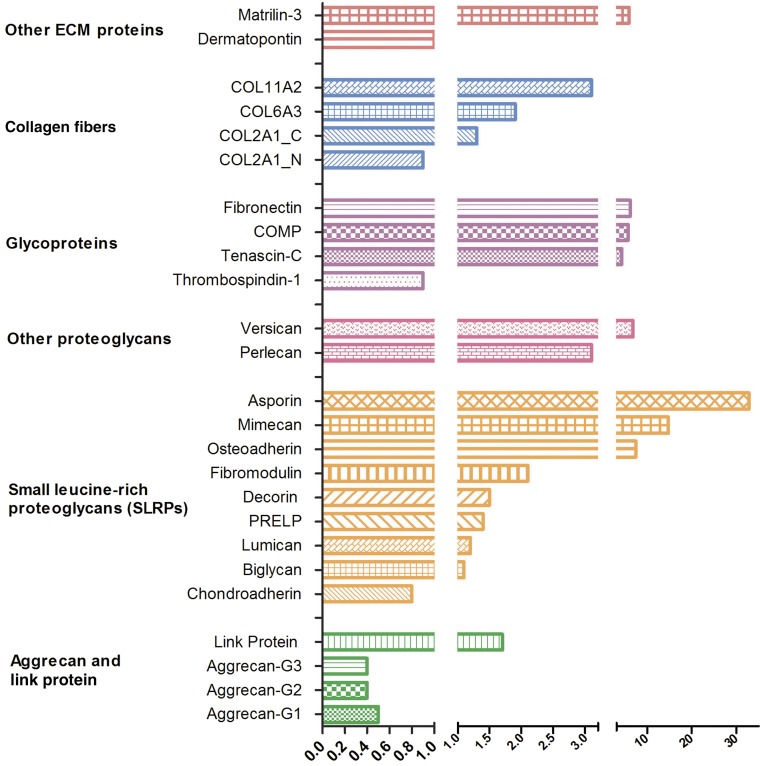
Quantitative Analysis of ECM Proteins
We analyzed the protein, proteoglycan, and collagen composition of ECM generated by chondrocytes differentiated from patient marrow-derived MSCs compared with controls. These analyses were performed using guanidinium hydrochloride extracts of pellets and included both intracellular and extracellular components. There were marked differences that pointed to a highly dysregulated matrix assembly in patient cells (Fig. 5 and supplemental online Table 3). First, we noted a reduction of approximately 50% in aggrecan content in patient cells, whereas the hyaluronan proteoglycan link protein was increased approximately 1.7-fold. The peptide analysis used in these measurements did not include the mutated region of the patient aggrecan G3 domain. Nine common small leucine-rich proteoglycans (SLRPs) were analyzed. Of these, asporin (class 1 SLRP), osteoadherin (class 2 SLRP), and mimecan (class 3 SLRP) were dramatically upregulated in patient cells. These three proteins have all been linked to mineralization [29, 30] often seen in OA [31]. The proteoglycans versican and perlecan also clearly increased in patient cultures. Among the glycoproteins analyzed, cartilage oligomeric matrix protein (COMP), fibronectin, and tenascin-C were all upregulated dramatically in FOCD-NS, whereas thrombospondin-1 was slightly increased in controls. Examination of collagen fibers contained in the ECM revealed that patient samples had two- to threefold higher extractable collagen α-3 (VI) and collagen α-2(XI) than controls. However, the amounts of collagen α-1(II) chain chondrocalcin and collagen α-1(II) chain N-propeptide were similar between the two groups. Among other ECM proteins, we found that cartilage intermediate-layer protein 2 was uniquely present in the patient samples (supplemental online Table 3), matrilin 3 was expression was increased sixfold in FOCD-NS, and there was no difference between the two groups in the level of dermatopontin.
Figure 5.
Quantitative analysis of ECM proteins. ECM proteins in day-100 chondrogenic micromass cultures derived from healthy and FOCD-NS BM-MSCs were quantified using mass spectrometry. Three to six transitions for each protein were measured. The average amount of transition was calculated and normalized to the representative pellet volume to represent the corresponded protein. The mean ratio of protein expression of FOCD-NS to control was calculated (n = 3). A value lower than 1 means protein is down-regulated in the patient sample. Abbreviations: BM-MSC, bone marrow mesenchymal stem cell; COMP, cartilage oligomeric matrix protein; ECM, extracellular matrix; FOCD-NS, familial osteochondritis dissecans from northern Sweden.
Generation and Characterization of FOCD-NS-Specific iPSCs
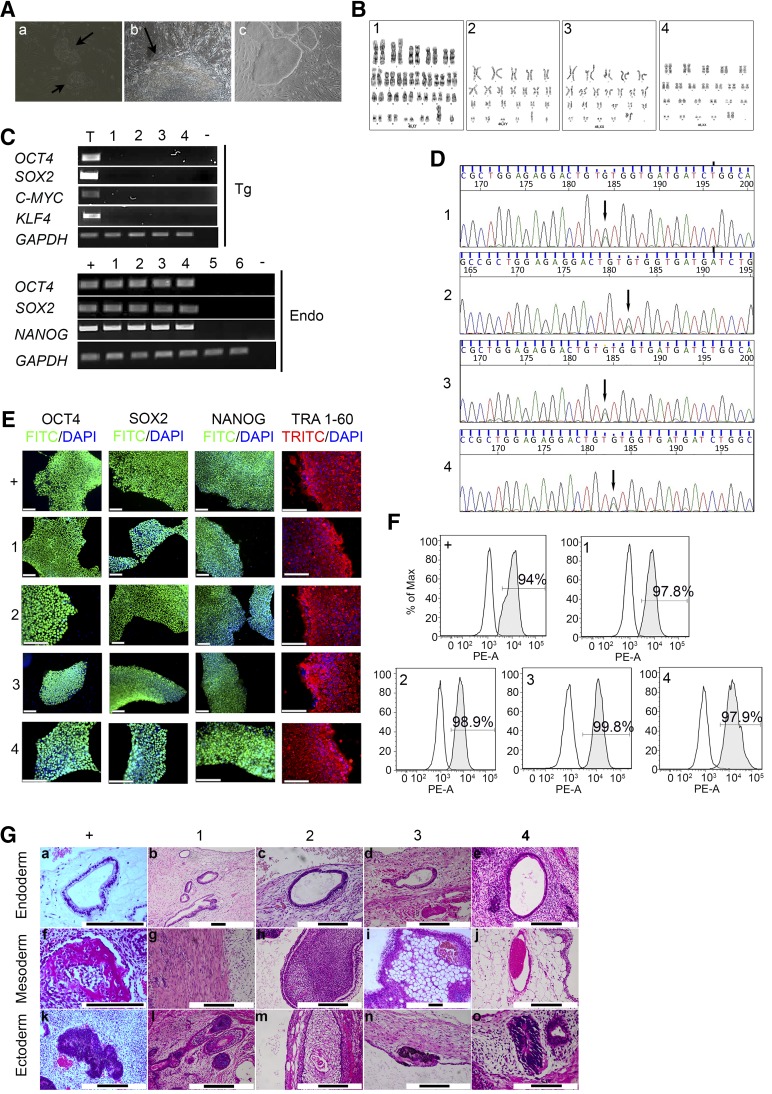
We generated patient-specific iPSCs from dermal fibroblasts from two patients, a son aged 25 (FOCD-NS1) and his mother aged 49 (FOCD-NS2). Both patients had the heterozygous G–A transition in exon 17 of the ACAN gene. The fibroblasts were transfected by retrovirus encoding OCD4, SOX2, C-MYC, and KLF4 and cultured in iPSC maintenance medium supplemented with valproic acid. Mesenchymal-to-epithelial transition was detected in transfected fibroblasts 6 days after infection (Fig. 6Aa), and colonies with morphology typical of human embryonic stem cells (ESCs) appeared at approximately day 14 (Fig. 6Ab). These colonies were picked for cell line establishment from day 21 to day 30 (Fig. 6Ac). Seven human ESC-like colonies were obtained from FOCD-NS1 fibroblasts and three from FOCD-NS2 fibroblasts. All were successfully expanded in culture. Once the iPSC lines were established, cells were adapted from feeder-dependent to feeder-free culture conditions.
Two colonies with human ESC-like morphology were randomly selected from each patient and named FOCD-NS1-iPSC-2/30 and FOCD-NS2-iPSC-9/13. As a control, a healthy donor iPSC line (33D-6) was generated by the same methodology. Standard G-banding chromosome analysis was performed and showed that the selected FOCD-NS-iPSC lines had a normal karyotype (Fig. 6B). The expression of retroviral transgenes (Tg-OCD4/SOX2/KLF4/C-MYC) and endogenous pluripotency markers was assessed by semiquantitative RT-PCR, which confirmed that transgenic transcripts were silenced and endogenous OCT4, SOX2, and NANOG were detected in all FOCD-NS-iPSC lines (Fig. 6C). It was confirmed that the heterozygous G–A transition in exon 17 of the ACAN gene persisted after reprogramming by DNA sequence analysis (Fig. 6D). Moreover, immunostaining confirmed that FOCD-NS-iPSCs expressed OCT4, SOX2, NANOG, and the pluripotent-specific surface antigen TRA1-60 (Fig. 6E). Flow cytometry indicated that 97.8%–99.8% of the cell population in the FOCD-NS-iPSC lines expressed the surface antigen stage-specific embryonic antigen 4 (SSEA4), in levels somewhat higher than in the control iPSC line (94%) (Fig. 6F). To determine pluripotency in vivo, four FOCD-NS-iPSC lines and 33D-6 were injected subcutaneously into SCID-Bergin mice. Formed teratomas were observed from week 6 postinjection and were harvested after 2–3 months. All cell lines showed the ability to differentiate into different tissues from three germ layers (Fig. 6G): endoderm (glands a–e), mesoderm (bone [f], muscle [g], gut-associated lymphoid tissue [h], fat [i], blood vascular [j]), and ectoderm (neural rosette [k, o], hair bulb hair follicle [l], squamous epithelium cell [m], pigmented cell [n]).
Figure 6.
Generation and characterization of iPSCs: FOCD-NS1-iPSC-2 (1), FOCD-NS1-iPSC-30 (2), FOCD-NS2-iPSC-9 (3), and FOCD-NS2-iPSC-13 (4). Normal iPSC line 33D-6 was the positive control (+). Skin fibroblasts obtained from FOCD-NS1 (5) and FOCD-NS2 (6) were included. (A): Reprogrammed fibroblasts underwent mesenchymal–epithelial transition at day 6 (a) and formed an early iPSC-like colony at day 14 (b) (arrows; magnification ×20). Morphology of iPSC colonies (magnification ×10) (c). (B): G-banding analysis showed normal karyotype in FOCD-NS iPSCs. (C): RT-PCR data showing expression of retroviral transgenes (Tg) and endogenous pluripotent genes (Endo) in FOCD-NS iPSCs. Nontemplate controls (−) were included in each PCR reaction. RNA extracted from retroviral-infected 293T cells acted as positive controls for detection of Tg factors (T). (D): DNA sequencing confirming the aggrecan mutation in FOCD-NS iPSCs (arrows). (E): Expression of pluripotency markers in FOCD-NS iPSCs by immunostaining. Scale bar, 120 µm. (F): Flow cytometry analysis of the expression of SSEA4. White histograms represent isotype controls, and gray overlays represent SSEA4; percentage positive cells shown within histograms. (G): Histologic analysis of hematoxylin and eosin-stained teratoma sections derived from iPSCs. Detected tissue types included endoderm (top row), mesoderm (middle row), and ectoderm (bottom row). Black scale bar, 200 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Endo, endogenous pluripotent genes; FITC, fluorescein isothiocyanate; FOCD-NS, familial osteochondritis dissecans from northern Sweden; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iPSC, induced pluripotent stem cell; PE-A: phycoerythrin-labeled antibody; RT-PCR, reverse-transcription polymerase chain reaction; SSEA, stage-specific embryonic antigen; Tg, retroviral transgenes; TRITC, tetramethylrhodamine isothiocyanate.
The iPSC Model Reproduces the Disease Phenotype
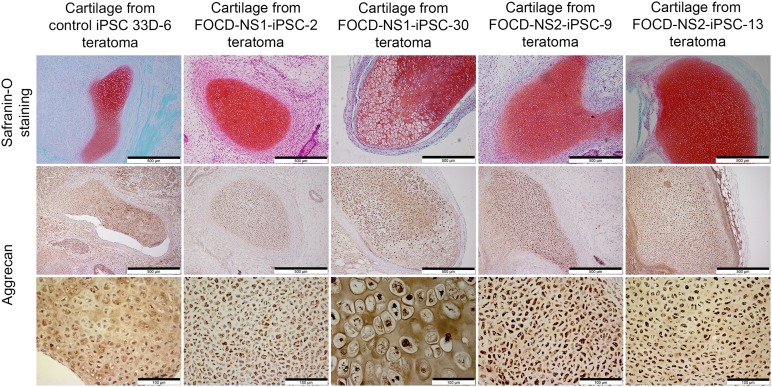
Cartilage tissues in teratomas generated from the FOCD-NS-iPSC lines were compared with those from control iPSCs. The cartilage was visualized by Safranin-O staining (Fig. 7, top). To verify whether the FOCD-NS iPSC-derived cartilage reproduced the disease phenotype, immunohistochemical staining for aggrecan was performed. In control teratoma cartilage, aggrecan was extracellular and distributed evenly in the ECM. In teratoma cartilage from the four FOCD-NS-iPSC lines, aggrecan was found dominantly within chondrocytes and the ECM was largely depleted (Fig. 7, middle and bottom). Furthermore, cells were densely packed, perhaps reflecting decreased matrix production or delayed differentiation. Histologic analysis showed that intercellular accumulation of aggrecan in patient lines occurred at different stages of chondrocytic differentiation, from early resting or proliferating phase to hypertrophic phase (Fig. 7, bottom).
Figure 7.
Verification of disease phenotype in iPSC-derived cartilage. Cartilage tissues in teratoma were positively stained with Safranin-O, shown as red (top row; scale bar, 500 µm). Aggrecan stained brown in teratoma cartilage from each iPSC line (middle row; scale bar, 500 µm). Corresponding magnified images of aggrecan staining in teratoma cartilage (bottom row; scale bar, 100 µm). Abbreviations: FOCD-NS, familial osteochondritis dissecans from northern Sweden; iPSC, induced pluripotent stem cell.
Discussion
We have characterized MSCs taken from the bone marrow of patients with FOCD-NS, carrying a V2303M mutation compared with MSCs from healthy controls. In terms of analysis of cell-surface markers and osteogenic and adipogenic differentiation, MSCs from the two groups appeared identical (Fig. 1A-C) [32]. As expected, substantial differences were observed in chondrocytes derived from patients compared with control MSCs. Chondrogenic pellets from patient MSCs were irregular, with a donut shape rather than spherical (Fig. 2A). It was evident that patient chondrocytes rapidly produced abundant GAG chains attached to aggrecan during processing in the Golgi (Fig. 2B) [15]. This may have resulted from expression of the nonmutated allele. In micromass cultures, the FOCD-NS chondrocytes formed uniform spheres (Fig. 3A), whereas in conventional pellet cultures, they were irregular discoids. This may be a consequence of the inability of the patient cells to resist the mechanical load associated with centrifugation.
Immunostaining showed that aggrecan was clearly distributed throughout the ECM in control chondrocytes but had a sharply cellular distribution in patient chondrocytes, suggesting that the processing, assembly, and secretion of aggrecan were defective (Fig. 2C). Confocal microscopy confirmed this and also showed that the ECM of patient chondrocytes was devoid of aggrecan (Fig. 3B). Furthermore, we found that intracellular aggrecan was clearly associated with the ER, indicated by costaining with the ER marker protein GRP78 (Fig. 3B). This confirmed a defect in processing of aggrecan associated with retention within the ER and failure to translocate to Golgi and be secreted. Quantification of GAG and DNA in micromass cultures derived from controls and FOCD-NS patients suggested that early intracellular accumulation of aggrecan proteins resulted in enhanced cell proliferation and also reduced chondrogenic capacity (Fig. 4A). Further evaluation using TEM confirmed the absence of a fibrillar matrix and the presence of an atypical, diffuse ultrastructure within patient chondrocytes (Fig. 4B and supplemental online Fig. 1). Physically enlarged rER in patient cells further proved that it was packed with protein products during chondrogenesis (Fig. 4B). All these observations pointed toward a failure of FOCD-NS mesenchymal progenitors to assemble aggrecan within the ER and secrete it from the cell after differentiation.
Using peptide-specific mass spectrometric analysis, we analyzed ECM proteins to determine how they are differently modulated in patient chondrocytes derived from primary MSCs. There were several striking differences between the patient chondrocytes and those from healthy controls. We found a 50% reduction in aggrecan in patient samples (Fig. 5 and supplemental online Table 3), which suggested that the CLD mutation results in attenuated production of aggrecan during the chondrogenic process. Interestingly, versican expression was 6.8-fold higher in patient samples (Fig. 5 and supplemental online Table 3). We speculate that this up-regulation may act to compensate for the lack of aggrecan in the ECM or could reflect delayed chondrogenic differentiation. Second, a group of proteins associated with cartilage degeneration was highly upregulated in FOCD-NS samples: asporin, mimecan, fibronectin, matrilin-3, COMP, tenascin-C, and perlecan (Fig. 5 and supplemental online Table 3) [30, 33–39]. This composition of the ECM in FOCD-NS chondrocytes reflects the changes seen in advanced OA. Interestingly, thrombospondin 1, whose function relates to the restoration of homeostasis in degenerated joints [40], was slightly reduced in FOCD-NS cultures. In addition, comparing other essential components of the ECM between healthy and FOCD-NS samples revealed wide variations in production levels (Fig. 5 and supplemental online Table 3). In summary, these results outlined the consequences of early intracellular accumulation of mutated aggrecan and indicated cartilaginous dysplasia in later chondrogenesis of FOCD-NS samples.
There are two difficulties associated with examination of primary MSCs from patients with FOCD-NS: the limited availability of marrow and its abnormal cellularity. Marrow aspirates taken from the patients were white, fatty, and different from healthy marrow. Upon plating, the yield of MSCs was low, although sufficient for the characterization described here. Further characterization required the generation of patient-specific iPSCs. We successfully generated iPSCs from patient fibroblasts (Fig. 6) and confirmed that the mutation in exon 17 of the aggrecan gene was preserved (Fig. 6D).
We looked in detail at cartilage nodules within teratomas derived from FOCD-NS iPSCs. We found the same disease phenotype observed in chondrogenic cultures of primary MSCs (i.e., rich in GAG but with a distinct lack of aggrecan in the ECM and a pronounced intracellular localization of aggrecan within early and late chondrocytes) (Fig. 7). This suggested that the cartilage derived from reprogrammed patient fibroblasts preserved the disease phenotype and showed the same aberrant aggrecan processing.
Aggrecan G3 domain plays a role in intracellular processing of the core protein and in facilitating its secretion [41]. Our observations of the chondrogenic behavior of progenitor cells from FOCD-NS patients allow us to draw a number of conclusions about the cellular abnormalities associated with this mutation. It leads to retention of the aggrecan core within the ER of chondrocytes and induces continued ER stress associated with an enlarged structure. This results in abnormal processing and assembly of the ECM, leading to rapid joint destruction and development of OA. This suggests that the mutation promotes a nonreversible cellular pathology during cartilage development.
ER dilation and ER stress, caused by mutations in ECM genes such as COMP, MATN3, and COL2A1, have been associated with the pathology of osteochondrodysplasias [42]. In the future, FOCD-NS iPSCs will be used to study the pathological roles associated with this G3 mutation in inducing ER stress, affecting cell behavior, and disturbing matrix regulation. In terms of development of therapy for FOCD-NS, these cells may be used in the development of drugs to restore cellular function or retard OA development. Furthermore, correction of the mutation in FOCD-NS iPSCs using gene-editing strategies may lead to a corrective cellular therapy for these patients.
Conclusion
In our current study, we have conducted research to investigate the molecular pathologies of FOCD, which results in disproportionate growth, disturbed chondroskeletal development, and early-onset OA.
Studying chondrocytes differentiated from patients’ BM-MSCs and iPSCs, we found large dysregulation and aberration in assembled extracellular matrix and dysregulated cell fate, as consequences of ER stress, which was caused by an abnormal accumulation of mutated aggrecan protein.
Our observations reveal that cartilaginous tissue is the initial location where FOCD-NS develops and suggest that the short stature in FOCD-NS patients would be the sequel of abnormal growth plate formation and regulation. Moreover, we present further evidence about the association between FOCD-NS and early-onset OA.
These studies have provided us with new insights into the cellular pathology of FOCD, a new model for studying the molecular mechanisms of osteochondrodysplasias and OA at early stages based on stem cell differentiation, and a new understanding for developing therapeutic approaches.
Supplementary Material
Acknowledgments
We sincerely thank the family members who participated in the study and Dr. Yelverton Tegner, Luleå Technical University, Luleå, Sweden, for providing skin samples. We acknowledge the facilities and technical assistance of the Flow Cytometry Facility at the National University of Ireland Galway, a facility that is funded by National University of Ireland Galway and the Irish Government’s Programme for Research in Third Level Institutions, Cycle5, National Development Plan 2007–2013. We thank Pierce Lalor, Dr. Kerry Thompson, and the Centre for Microscopy and Imaging (http://www.imaging.nuigalway.ie) for assistance with TEM and confocal imaging, and Jingqiu Zhang (A*STAR Institute of Medical Biology, Singapore) for assistance with the iPSC reprogramming technique. We also thank Maggie Hall for helpful editing of the manuscript. This study was funded by Science Foundation Ireland Grant SFI 09/SRC.B1794 and Irish Research Council Grant GOIPG/2014/96. This project has also received funding from the European Union’s Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no. 223298.
Footnotes
Deceased.
Author Contributions
M.X. and G. Shaw: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; E.-L.S.: provision of study material or patients, manuscript writing; D.H.: conception and design, data analysis and interpretation; G. Sullivan and I.W.: provision of study material or patients; A.C.: conception and design; P.Ö. and A.K.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; A.A., P.D., and T.H.: data analysis and interpretation, manuscript writing; M.M.: conception and design, data analysis and interpretation, manuscript writing; F.B.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.M.’s spouse is a compensated advisor for Orbsen and has compensated shares in Osiris Therapeutics. F.B. is an uncompensated shareholder of Orbsen Therapeutics Ltd. and Osiris Therapeutics Inc. The other authors indicated no potential conflicts of interest.
References
- 1.Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471:1118–1126. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paget J. On the production of some of the loose bodies in joints. St Bartholomew’s Hospital Reports. 1870;6:1–4. [Google Scholar]
- 3.Schulz JF, Chambers HG. Juvenile osteochondritis dissecans of the knee: Current concepts in diagnosis and management. Instr Course Lect. 2013;62:455–467. [PubMed] [Google Scholar]
- 4.Kosaka M, Nakase J, Takahashi R, et al. Outcomes and failure factors in surgical treatment for osteochondritis dissecans of the capitellum. J Pediatr Orthop. 2013;33:719–724. doi: 10.1097/BPO.0b013e3182924662. [DOI] [PubMed] [Google Scholar]
- 5.Preiss A, Heitmann M, Frosch KH. Osteochondritis dissecans of the talus. Diagnosis and treatment [in German] Unfallchirurg. 2012;115:1099–1108. doi: 10.1007/s00113-012-2308-7. [DOI] [PubMed] [Google Scholar]
- 6.Lunden JB, Legrand AB. Osteochondritis dissecans of the humeral head. J Orthop Sports Phys Ther. 2012;42:886. doi: 10.2519/jospt.2012.0417. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda DK, Safran MR. Arthroscopic internal fixation of osteochondritis dissecans of the femoral head. Orthopedics. 2013;36:e683–e686. doi: 10.3928/01477447-20130426-37. [DOI] [PubMed] [Google Scholar]
- 8.Hixon AL, Gibbs LM. Osteochondritis dissecans: A diagnosis not to miss. Am Fam Physician. 2000;61:151–156, 158. [PubMed] [Google Scholar]
- 9.Detterline AJ, Goldstein JL, Rue JP, et al. Evaluation and treatment of osteochondritis dissecans lesions of the knee. J Knee Surg. 2008;21:106–115. doi: 10.1055/s-0030-1247804. [DOI] [PubMed] [Google Scholar]
- 10.Andrew TA, Spivey J, Lindebaum RH. Familial osteochondritis dissecans and dwarfism. Acta Orthop Scand. 1981;52:519–523. doi: 10.3109/17453678108992141. [DOI] [PubMed] [Google Scholar]
- 11.Phillips HO, Grubb SA. Familial multiple osteochondritis dissecans. Report of a kindred. J Bone Joint Surg Am. 1985;67:155–156. [PubMed] [Google Scholar]
- 12.Stattin EL, Tegner Y, Domellöf M, et al. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage. 2008;16:890–896. doi: 10.1016/j.joca.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Stougaard J. Familial occurrence of osteochondritis dissecans. J Bone Joint Surg Br. 1964;46:542–543. [PubMed] [Google Scholar]
- 14.Stattin EL, Wiklund F, Lindblom K, et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86:126–137. doi: 10.1016/j.ajhg.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong-Palms S, Plaas AH. Glycosaminoglycan addition to proteoglycans by articular chondrocytes—evidence for core protein-specific pathways. Arch Biochem Biophys. 1995;319:383–392. doi: 10.1006/abbi.1995.1308. [DOI] [PubMed] [Google Scholar]
- 16.Kiani C, Chen L, Wu YJ, et al. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 17.Vertel BM, Walters LM, Grier B, et al. Nanomelic chondrocytes synthesize, but fail to translocate, a truncated aggrecan precursor. J Cell Sci. 1993;104:939–948. doi: 10.1242/jcs.104.3.939. [DOI] [PubMed] [Google Scholar]
- 18.Day JM, Murdoch AD, Hardingham TE. The folded protein modules of the C-terminal G3 domain of aggrecan can each facilitate the translocation and secretion of the extended chondroitin sulfate attachment sequence. J Biol Chem. 1999;274:38107–38111. doi: 10.1074/jbc.274.53.38107. [DOI] [PubMed] [Google Scholar]
- 19.Day JM, Olin AI, Murdoch AD, et al. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem. 2004;279:12511–12518. doi: 10.1074/jbc.M400242200. [DOI] [PubMed] [Google Scholar]
- 20.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan GJ, Hay DC, Park IH, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Su P, Xu C, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32:1339–1346. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari D, Gong G, Kosher R, et al. Chondrogenic differentiation of hESC in micromass culture. In: Ye K, Jin S, editors. Human Embryonic and Induced Pluripotent Stem Cells. New York, NY: Humana Press; 2012. pp. 359–367. [Google Scholar]
- 24.Conroy PC, Saladino C, Dantas TJ, et al. C-NAP1 and rootletin restrain DNA damage-induced centriole splitting and facilitate ciliogenesis. Cell Cycle. 2012;11:3769–3778. doi: 10.4161/cc.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dockery P, Tidey RR, Li TC, et al. A morphometric study of the uterine glandular epithelium in women with premature ovarian failure undergoing hormone replacement therapy. Hum Reprod. 1991;6:1354–1364. doi: 10.1093/oxfordjournals.humrep.a137268. [DOI] [PubMed] [Google Scholar]
- 26.Gillette MA, Carr SA. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat Methods. 2013;10:28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Lian Q, Zhu G, et al. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Bonucci E. Bone mineralization. Front Biosci (Landmark Ed) 2012;17:100–128. doi: 10.2741/3918. [DOI] [PubMed] [Google Scholar]
- 30.Balakrishnan L, Nirujogi RS, Ahmad S, et al. Proteomic analysis of human osteoarthritis synovial fluid. Clin Proteomics. 2014;11:6. doi: 10.1186/1559-0275-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ea H-K, Nguyen C, Bazin D, et al. Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–18. doi: 10.1002/art.27761. [DOI] [PubMed] [Google Scholar]
- 32.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 33.Vincourt JB, Vignaud JM, Lionneton F, et al. Increased expression of matrilin-3 not only in osteoarthritic articular cartilage but also in cartilage-forming tumors, and down-regulation of SOX9 via epidermal growth factor domain 1-dependent signaling. Arthritis Rheum. 2008;58:2798–2808. doi: 10.1002/art.23761. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima M, Kizawa H, Saitoh M, et al. Mechanisms for asporin function and regulation in articular cartilage. J Biol Chem. 2007;282:32185–32192. doi: 10.1074/jbc.M700522200. [DOI] [PubMed] [Google Scholar]
- 35.Sakao K, Takahashi KA, Arai Y, et al. Asporin and transforming growth factor-beta gene expression in osteoblasts from subchondral bone and osteophytes in osteoarthritis. J Orthop Sci. 2009;14:738–747. doi: 10.1007/s00776-009-1401-4. [DOI] [PubMed] [Google Scholar]
- 36.Tesche F, Miosge N. Perlecan in late stages of osteoarthritis of the human knee joint. Osteoarthritis Cartilage. 2004;12:852–862. doi: 10.1016/j.joca.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Chockalingam PS, Glasson SS, Lohmander LS. Tenascin-C levels in synovial fluid are elevated after injury to the human and canine joint and correlate with markers of inflammation and matrix degradation. Osteoarthritis Cartilage. 2013;21:339–345. doi: 10.1016/j.joca.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Vilím V, Olejárová M, Machácek S, et al. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 39.Chevalier X. Fibronectin, cartilage, and osteoarthritis. Semin Arthritis Rheum. 1993;22:307–318. doi: 10.1016/s0049-0172(05)80010-1. [DOI] [PubMed] [Google Scholar]
- 40.McMorrow JP, Crean D, Gogarty M, et al. Tumor necrosis factor inhibition modulates thrombospondin-1 expression in human inflammatory joint disease through altered NR4A2 activity. Am J Pathol. 2013;183:1243–1257. doi: 10.1016/j.ajpath.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Luo W, Tanzer ML. Aggrecan synthesis and secretion. A paradigm for molecular and cellular coordination of multiglobular protein folding and intracellular trafficking. J Biol Chem. 1998;273:12999–13006. doi: 10.1074/jbc.273.21.12999. [DOI] [PubMed] [Google Scholar]
- 42.Tsang KY, Chan D, Bateman JF, et al. In vivo cellular adaptation to ER stress: Survival strategies with double-edged consequences. J Cell Sci. 2010;123:2145–2154. doi: 10.1242/jcs.068833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.