The induction of mesenchymal stem cells (MSCs) toward the osteoblastic lineage using osteogenic supplements prior to implantation is one approach under examination to enhance their bone-forming potential. Spheroids formed from induced cells exhibited improved retention of osteogenic markers as a function of integrin binding to cell-secreted extracellular matrix (ECM). These results demonstrate the capacity of spheroidal culture to sustain the mineral-producing phenotype of MSCs, thus enhancing their contribution toward bone formation and repair.
Keywords: Spheroid, Extracellular matrix, Osteogenesis, Bone, Collagen
Abstract
The induction of mesenchymal stem cells (MSCs) toward the osteoblastic lineage using osteogenic supplements prior to implantation is one approach under examination to enhance their bone-forming potential. MSCs rapidly lose their induced phenotype upon removal of the soluble stimuli; however, their bone-forming potential can be sustained when provided with continued instruction via extracellular matrix (ECM) cues. In comparison with dissociated cells, MSC spheroids exhibit improved survival and secretion of trophic factors while maintaining their osteogenic potential. We hypothesized that entrapment of MSC spheroids formed from osteogenically induced cells would exhibit better preservation of their bone-forming potential than would dissociated cells from monolayer culture. Spheroids exhibited comparable osteogenic potential and increased proangiogenic potential with or without osteogenic preconditioning versus monolayer-cultured MSCs. Spheroids were then entrapped in collagen hydrogels, and the osteogenic stimulus was removed. In comparison with entrapped dissociated MSCs, spheroids exhibited significantly increased markers of osteogenic differentiation. The capacity of MSC spheroids to retain their osteogenic phenotype upon withdrawal of inductive cues was mediated by α2β1 integrin binding to cell-secreted ECM. These results demonstrate the capacity of spheroidal culture to sustain the mineral-producing phenotype of MSCs, thus enhancing their contribution toward bone formation and repair.
Significance
Despite the promise of mesenchymal stem cells (MSCs) for cell-based therapies for tissue repair and regeneration, there is little evidence that transplanted MSCs directly contribute to new bone formation, suggesting that induced cells rapidly lose their osteogenic phenotype or undergo apoptosis. In comparison with dissociated cells, MSC spheroids exhibit increased trophic factor secretion and improved cell survival. The loss of phenotype represents a significant clinical challenge for cell therapies, yet there is no evidence for whether MSC spheroids retain their osteogenic phenotype upon entrapment in a clinically relevant biomaterial. These findings demonstrate that MSC spheroids retain their osteogenic phenotype better than do dissociated MSCs, and this is due to integrin engagement with the cell-secreted extracellular matrix. These data provide evidence for a novel approach for potentiating the use of MSCs in bone repair.
Introduction
Cell-based therapies represent an exciting strategy for driving nonhealing bone repair and addressing concerns regarding bone tissue availability and donor site morbidity associated with the gold standard autograft. Mesenchymal stem cells (MSCs) are a promising cell population for use in bone tissue engineering because of their proliferative, osteogenic, and proangiogenic potential. MSCs are typically differentiated in an osteogenic cocktail prior to transplantation to enhance their mineral deposition and integration into the surrounding bone. Although it is commonly assumed that this pretreatment regimen translates to phenotype preservation once delivered in vivo, we demonstrated that human MSCs rapidly dedifferentiate upon removal of the stimulus [1]. However, changes in the cell phenotype were sustained when MSCs were deployed with a biologically relevant extracellular matrix (ECM), providing continued instruction and enhancing bone formation in vivo.
MSC spheroids, multicellular aggregates formed through cell-cell interactions, are under widespread investigation for tissue repair. MSC spheroids exhibit enhanced prosurvival [2, 3], proangiogenic [4–6], and anti-inflammatory [7] properties in comparison with dissociated cells expanded in monolayer culture. Additionally, spheroid formation does not hinder their osteogenic potential [8]. These advantages originate from their unique culture geometry, inducing a complex microenvironment within the spheroid that better reflects the complex in vivo milieu [3, 9, 10]. Furthermore, delivering MSCs as spheroids bypasses the deleterious trypsinization step required to free cells from traditional monolayer culture, preserving their intact ECM and cell-cell contacts formed during culture. Successful bone repair requires prolonged transplanted cell survival, angiogenesis, and mineral deposition [11–14]. Thus the inherent properties of MSC spheroids make them uniquely suited for bone tissue engineering applications.
Although others have transplanted spheroids in vivo in the absence of a biomaterial carrier to stimulate neovascularization [3], the deployment of cells in a hydrogel can promote cell persistence and provide continuous biophysical cues. The successful deployment of MSC spheroids in a functional biomaterial may promote their tissue regenerative potential. Type I collagen is the predominant organic matrix found in bone, and collagen I hydrogels are well established as an osteogenic biomaterial [15]. MSCs engage with the collagen fibrils through an integrin-signaling cascade leading to upregulation of osteoblast-related genes [16]. Thus, collagen gels represent an ideal platform for investigating the role of cell culture geometry on persistence of an induced osteoblastic phenotype.
We hypothesized that osteogenically induced MSCs formed into three-dimensional (3D) spheroids would exhibit a more sustained osteoblastic phenotype than dissociated MSCs when encapsulated in a collagen hydrogel. To explore this hypothesis, we examined the osteogenic and proangiogenic potential of MSC spheroids in comparison with cells expanded in monolayer culture before and after entrapment in a collagen hydrogel. In an effort to model deploying cells to a defect without exogenous soluble osteogenic cues, we entrapped cells in a collagen gel and removed the stimulus. Although cadherins have been implicated as mediators in spheroid formation [17], we examined alternative mechanisms by which MSC spheroids were able to retain their osteoblastic phenotype by interrogating integrin signaling. Here we show that α2β1 signaling to cell-secreted collagen facilitates MSC spheroid’s retention of their osteoblastic phenotype. The results of this study demonstrate the capacity of 3D culture to enhance the clinical translation of MSCs for bone tissue repair.
Materials and Methods
Cell Culture
Human bone marrow-derived MSCs (Lonza, Walkersville, MD, http://www.lonza.com) were used without further characterization and passaged prior to confluence in growth medium: α-modified minimum essential medium (α-MEM; Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) supplemented with 10% fetal bovine serum (JR Scientific, Woodland, CA, http://www.jrscientific.com), 1% penicillin (10,000 U/ml), and streptomycin (10 mg/ml) (Gemini Bio-Products, Sacramento, CA, http://www.gembio.com). MSCs were expanded under standard culture conditions (37°C, 5% CO2, 21% O2).
MSC Spheroid Formation
MSCs were seeded at 2,000 cells per cm2 in 225-cm2 flasks (Corning, Corning, NY, https://www.corning.com) and cultured for 12 days prior to passage in growth media (GM) or osteogenic media (OM). Osteogenic media consisted of growth media supplemented with 50 µg/ml ascorbate-2-phosphate, 10 mM β-glycerophosphate, and 100 nM dexamethasone (all from Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com). Medium changes were performed every third day. To recover cells, we incubated MSCs with 0.3% collagenase II (Worthington Biochemical Corp., Lakewood, NJ, http://www.worthington-biochem.com) to disrupt matrix prior to adding 0.05% trypsin/0.53 mM EDTA (Corning). MSCs were formed into spheroids using the hanging-drop technique with 15,000 cells per 25-μl droplet [4]. The resulting spheroid previously exhibited increased metabolic activity, reduced apoptosis, and uniform distribution of proliferating cells in comparison with larger spheroids. As a control, MSCs were plated as adherent monolayers at 10,000 cells per cm2 and to avoid the confounding variable of endogenous matrix accumulation during the first 12 days in culture. MSCs were maintained in spheroids or monolayer for an additional 2 days for a total of 2 weeks of osteogenic induction.
Entrapment of MSCs Within Collagen Hydrogels
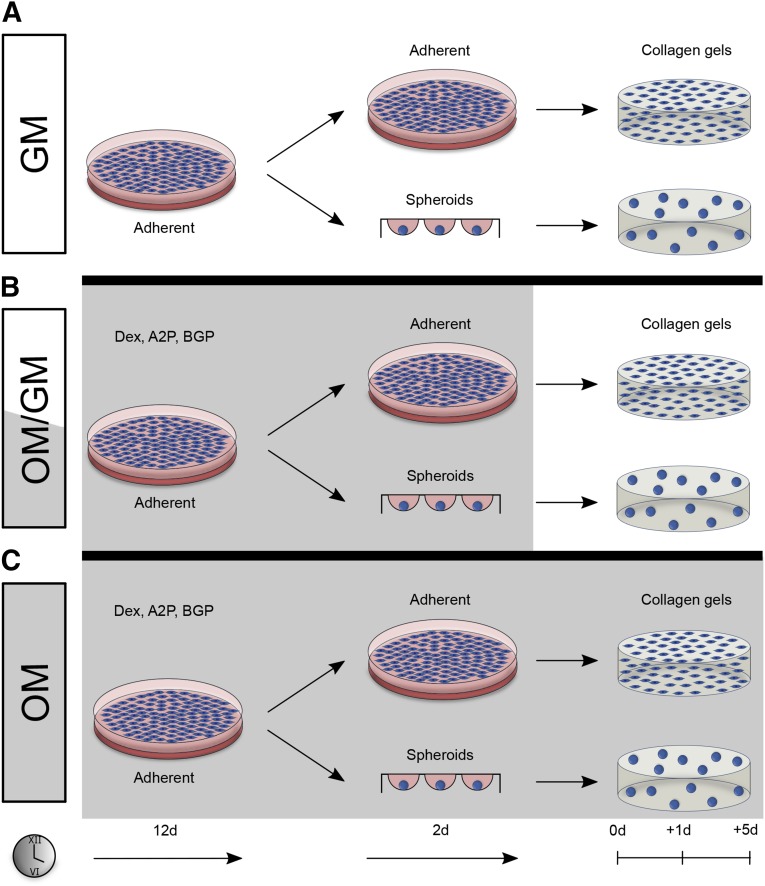
Collagen hydrogels were prepared by mixing MSCs with the following reagents: 10× α-MEM (10%), 5 N NaOH (0.4%), deionized water (39.6%), and rat tail collagen I (50%; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) for a final collagen concentration of 4 mg/ml. We formed 100-µl collagen hydrogels containing 4.5 × 106 MSCs per milliliter in polydimethylsiloxane (PDMS) molds as previously described [18]. Collagen hydrogels containing osteogenically induced MSCs were incubated at 37°C for 3 hours, released from the PDMS molds, and transferred to 24-well plates containing growth media for an additional 5 days (OM/GM). As a positive control, osteogenically induced MSCs remained in OM. MSCs cultured in GM for the full duration served as the negative control. Osteogenic markers were assessed at +1 day (+1d) and at +5 days (+5d), where 0d marks the day cells were entrapped in hydrogels and osteogenic media were switched to growth media (OM/GM). Gels were maintained in standard cell culture conditions with medium changes performed every 3 days (Fig. 1).
Figure 1.
Adherent and spheroidal mesenchymal stem cells (MSCs) were encapsulated within collagen I hydrogels and cultured in three medium regimens. After 12 days of culture in growth or osteogenic media, cells were trypsinized and formed into spheroids or replated in adherent monolayer. After 2 days of spheroidal or adherent culture in the same media, cells were encapsulated within collagen gels. (A): MSCs cultured in growth media for the full duration (GM) served as the negative control. (B): Osteogenic media were replaced with growth media for an additional 5 days (+5d) (OM/GM). (C): As a positive control, osteogenically induced MSCs remained in OM. Osteogenic markers were assessed at +1d and +5d, where 0d marks the day cells were entrapped in hydrogels and osteogenic media were switched to growth media (OM/GM). Abbreviations: ASP, ascorbate-2-phosphate; BGP, β-glycerophosphate; d, day; Dex, dexamethasone; GM, growth media; OM, osteogenic media.
Measurement of Osteogenic and Proangiogenic Potential of MSCs
We limited the osteogenic induction period to 2 weeks because longer induction durations do not significantly preserve the osteoblastic phenotype upon soluble stimulus withdrawal [1]. The baseline osteogenic and proangiogenic potentials of spheroids and adherent cells were quantified using biochemical assays and quantitative polymerase chain reaction (qPCR) prior to entrapment within collagen hydrogels. Intracellular alkaline phosphatase (ALP) activity was quantified as previously described [19] and normalized to total DNA content from the same lysate quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific Life Sciences). Calcium deposition was quantified as previously described [20] and normalized to DNA content from the same lysate. After +1d and +5d (days following medium change), collagen hydrogels were collected within microcentrifuge tubes containing 100 μl of passive lysis buffer (Promega, Madison, WI, http://www.promega.com) and sonicated on ice prior to analysis. ALP activity and calcium content were then quantified.
Gene expression was assessed by quantitative PCR as previously described [12, 19]. Total RNA was collected using the RNeasy Mini kit (Qiagen, Valencia, CA, https://www.qiagen.com) and 480 ng of total RNA was reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen). The qPCR was performed using TaqMan1 Universal PCR Master Mix (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) on a Mastercycler1 realplex2 (Eppendorf, Hamburg, Germany, https://www.eppendorf.com). Primers and probes consisted of COL1A1 (Hs01076777_m1), VEGFA (Hs00900055_m1), and RPL13 (Hs00204173_m1) (Thermo Fisher Scientific Life Sciences). Quantitative PCR results were normalized to housekeeping transcript level (RPL13) to yield ΔCt.
To measure vascular endothelial growth factor (VEGF) secretion, we refreshed the media 24 hours before collection, and VEGF concentration was determined using a human-specific VEGF ELISA kit (R&D Systems, Minneapolis, MN, https://www.rndsystems.com/) according to the manufacturer’s instructions [4, 21].
Characterization of ECM Components Within MSC Spheroids
Spheroids that received osteogenic media or growth media for 2 weeks were collected prior to hydrogel encapsulation to assess the effect of osteogenic induction on matrix deposition within the spheroid. Spheroids were collected and fixed in 10% formalin, washed with phosphate-buffered saline (PBS), and then embedded in HistoGel (Thermo Fisher Scientific Life Sciences). Collagen gels were fixed in 10% formalin, washed with PBS, and soaked in O.C.T. compound (Sakura Finetek, Torrance, CA, http://www.sakura-americas.com) overnight. Samples were encased in O.C.T. compound, and 10-µm sections were cut on a Leica CM1850 Cryostat (Leica Microsystems, Bannockburn, IL, http://www.leica-microsystems.com) and mounted onto microscope slides (VWR Superfrost Plus Micro Slide; VWR International, Radnor, PA, https://us.vwr.com) for analysis. Immunohistochemistry was performed using a mouse-specific horseradish peroxidase (HRP)/3,3′-diaminobenzidine (DAB) detection kit (Abcam, Cambridge, U.K., http://www.abcam.com) in conjunction with primary antibodies for type 1 collagen (1:1000, Abcam 90395, Abcam), fibronectin (1:50, 2Q604; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), and α2β1 integrin (1:30, AB24697; Abcam). Additionally, we performed a Picrosirius Red (PSR; Abcam) stain, counterstained with hematoxylin, to further elucidate differences in collagen deposition. Lastly, collagen gel sections were stained with hematoxylin and eosin. Spheroid sections were imaged using a Nikon Eclipse TE2000U microscope (Nikon, Melville, NY, http://www.nikon.com) and Andor Zyla digital camera (Oxford Instruments, Abingdon, U.K., http://www.oxford-instruments.com).
Measurement of Mechanical Properties of Collagen Hydrogels
The storage modulus of acellular and cellular collagen hydrogels was measured using a Discovery HR2 Hybrid Rheometer (TA Instruments, New Castle, DE, http://www.tainstruments.com) as we described [22]. An oscillatory sweep of 0.004%–4% strain was performed on each hydrogel using a cone and plate (1° angle). Storage moduli in the linear viscoelastic region were averaged from n ≥ 5 points per gel, and resultant averages were calculated from n = 4 gels.
Integrin-Mediated Contribution Toward Preservation of Osteoblastic Markers
We investigated the contribution of an integrin-mediated mechanism by adding 5 μg/ml α2β1 blocking antibody (AB24697, Abcam) to every medium change starting from the first passage following 12 days of culture. Control groups were maintained in media supplemented with an equal volume of PBS. The distribution of cells expressing collagen was determined by immunohistochemistry. At +5d, hydrogels were fixed in phosphate-buffered formalin for 24 hours, after which point they were soaked in O.C.T. overnight. They were then cryosectioned at 5 μm thickness. Sections were stained for Picrosirius Red, H&E, and immunohistochemistry (IHC) using a primary antibody against type 1 collagen in conjugation with a mouse-specific HRP/DAB detection kit as described above.
Statistical Analysis
Data are presented as means ± SD from at least four independent experiments. Statistical analysis was performed using a two-way analysis of variance with Bonferroni correction for multiple comparisons or paired t tests when appropriate. All statistical analyses were performed using Prism 6 software (GraphPad, San Diego, CA, http://www.graphpad.com); p values less than .05 were considered statistically significant. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, whereas groups with significance do not share the same letters.
Results
MSC Spheroids Are Responsive to Osteogenic Induction
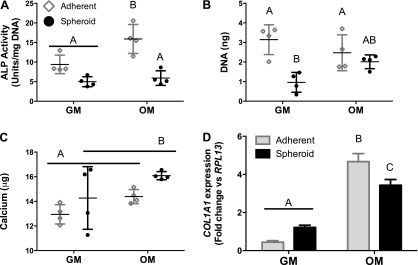
Adherent cells exhibited greater baseline ALP activity than did spheroids after culture in osteogenic media (OM) (Fig. 2A); however, total calcium was comparable (Fig. 2C). As expected, adherent cells cultured in OM exhibited greater ALP activity than did adherent cells in growth media (GM). Differences in ALP activity and calcium content for GM cultures may be attributed to reduced DNA content in MSC spheroids in comparison with adherent MSCs (Fig. 2B), perhaps because of contact inhibition in spheroids. We further probed the baseline osteogenic status via gene expression of spheroids and adherent cells. MSCs cultured as spheroids or adherent cells in OM exhibited greater COL1A1 expression than did their GM counterparts (Fig. 2D). COL1A1 expression of adherent MSCs was significantly higher than that of spheroids when both groups were cultured in OM.
Figure 2.
Mesenchymal stem cells spheroids are responsive to osteogenic induction prior to encapsulation in collagen gels. Cells were cultured in growth media or osteogenic media for 12 days prior to being formed into spheroids or replated in adherent monolayers and analyzed for alkaline phosphatase activity (A), DNA content (B), total calcium (C), and COL1A1 gene expression (D). Chart values represent mean ± SD for n = 4. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, and groups with significance do not share the same letters. Abbreviations: ALP, alkaline phosphatase; GM, growth media; OM, osteogenic media.
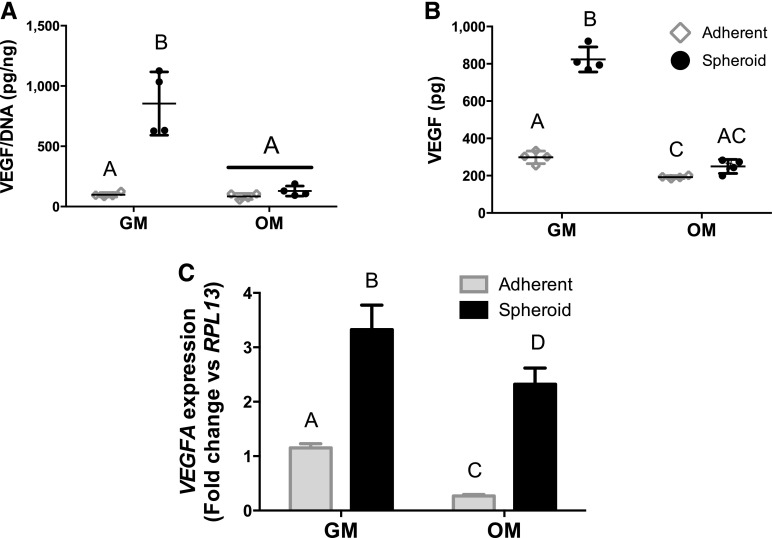
When quantifying baseline proangiogenic potential, we detected a significant increase in normalized and total VEGF protein secretion for MSC spheroids in comparison with adherent MSCs cultured in GM. However, this advantage was abrogated when the MSCs were cultured in OM (Fig. 3A, 3B). Unlike VEGF secretion, VEGFA expression was greater for spheroids in both media formulations (Fig. 3C). Overall, VEGF production by MSCs in OM was reduced in comparison with MSCs in GM, and this reduction is consistent with our previous findings with adherent MSCs in that the proangiogenic potential of MSCs is impaired when osteogenically induced [19].
Figure 3.
The proangiogenic potential of mesenchymal stem cell spheroids is dependent on osteogenic induction prior to encapsulation in collagen gels. Cells were cultured in growth media or osteogenic media for 12 days prior to being formed into spheroids or replated in adherent monolayers and analyzed for normalized VEGF secretion (A), total vascular endothelial growth factor secretion (B), and VEGFA gene expression (C). Chart values represent mean ± SD for n = 4. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, and groups with significance do not share the same letters. Abbreviations: GM, growth media; OM, osteogenic media; VEGF, vascular endothelial growth factor.
ECM Deposition in MSC Spheroids Is Enhanced by Osteogenic Differentiation
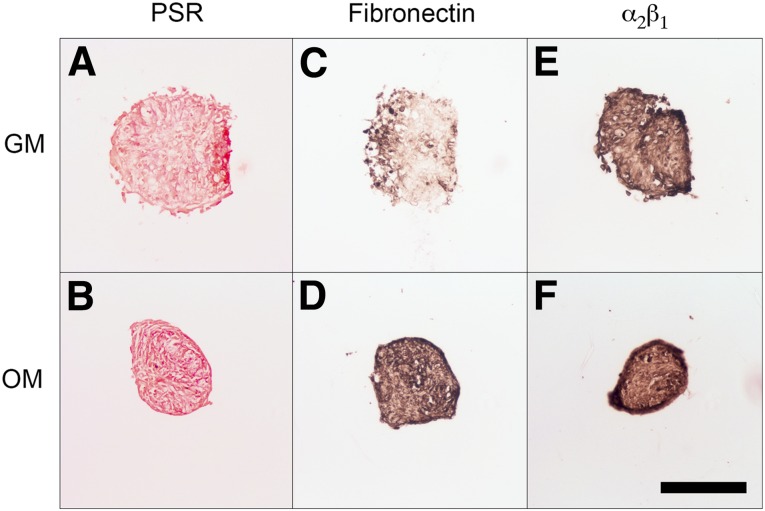
To visualize critical ECM components within aggregates, we sectioned MSC spheroids and stained for total collagen (Fig. 4A, 4B), fibronectin (Fig. 4C, 4D), and α2β1 expression (Fig. 4E, 4F). MSC spheroids cultured in osteogenic media (OM) stained more intensely for these ECM components than did their growth media (GM) counterparts. The α2β1 activity was more evident in spheroids cultured in OM. Spheroids cultured in GM appeared less compact and had less punctate staining.
Figure 4.
Characterization of extracellular matrix components within mesenchymal stem cell spheroids preconditioned in either growth media or osteogenic media. (A, B): Picrosirius Red. (C, D): Fibronectin. (E, F): α2β1. Scale bar = 200 µm. Abbreviations: GM, growth media; OM, osteogenic media; PSR, Picrosirius Red.
MSC Spheroids Promote Continued Osteogenic Differentiation in Collagen Hydrogels
A minimally viscous hydrogel was a design criterion to study the response of MSCs in a biomaterial following removal of osteoinductive cues. Materials with enhanced mechanical properties facilitate osteogenic differentiation [21, 23] and may act as a confounding variable. Acellular collagen hydrogels exhibited a shear storage modulus, G′ = 2.34 ± 0.62 Pa. We did not detect significant differences in shear storage moduli between acellular and cellular collagen hydrogels (data not shown).
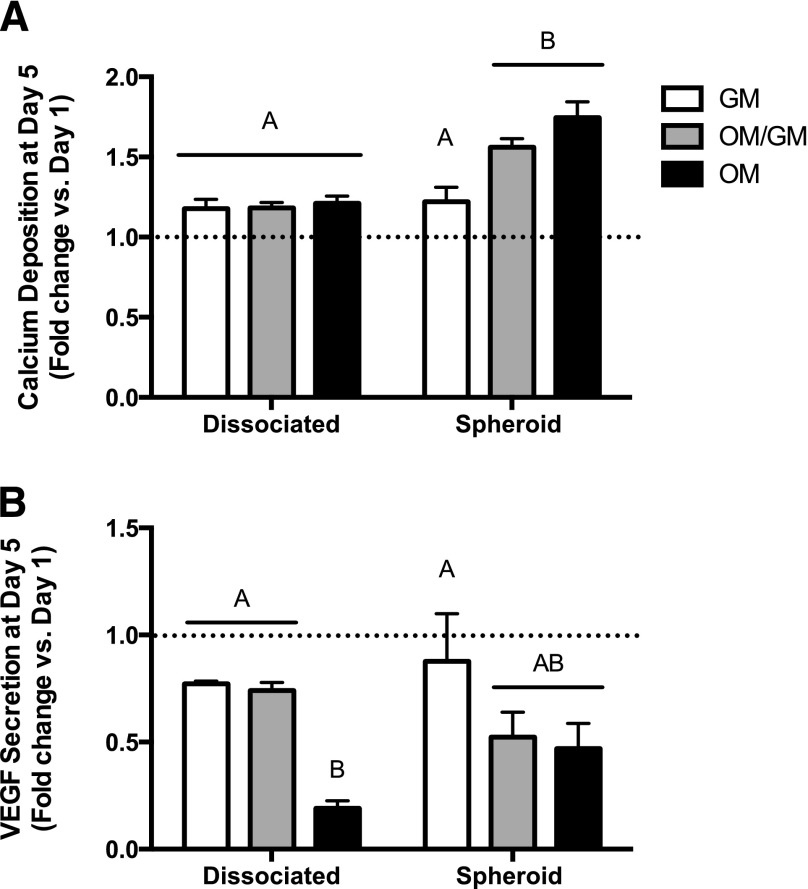
After cells were encapsulated in collagen hydrogels, we measured osteogenic and proangiogenic markers as a function of stimulus withdrawal. Because there were no statistical differences between groups at +1d, we normalized values from +5d to those from the same group at +1d to evaluate osteoblastic phenotype retention. Overall calcium secretion increased over time for all gels containing dissociated MSCs, yet there were no significant differences between media groups (Fig. 5A). Spheroids cultured in the presence of osteogenic cues significantly outperformed dissociated MSCs under the same culture conditions, as well as spheroids cultured in GM. Although spheroids cultured in OM for the entire duration had slightly more calcium deposition, there were no statistical differences in calcium levels for MSC spheroids cultured in hydrogels with or without continuous osteogenic cues (OM/GM and OM). In contrast to calcium content, VEGF secretion decreased from +1d to +5d, regardless of culture geometry (Fig. 5B). There was a general trend for reduced VEGF secretion by osteogenically induced MSCs. These data are in contrast to those observed in MSCs prior to entrapment in collagen gels, because MSC spheroids had greater VEGF secretion in GM.
Figure 5.
Mesenchymal stem cell (MSC) spheroids exhibit sustained persistence of osteogenic markers in the absence of cues when encapsulated within collagen I hydrogels. MSC-loaded hydrogels were subjected to stimulus withdrawal (osteogenic media [OM]/growth media [GM]) and included a positive (OM) and negative control (GM). Hydrogels were analyzed for calcium deposition (A) and secreted vascular endothelial growth factor (B). Chart values represent mean day 5 values normalized to the mean day 1 values ± SD for n = 4. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, whereas groups with significance do not share the same letters. Abbreviations: GM, growth media; OM, osteogenic media; VEGF, vascular endothelial growth factor.
Osteogenic Commitment of MSC Spheroids Is α2β1-Dependent
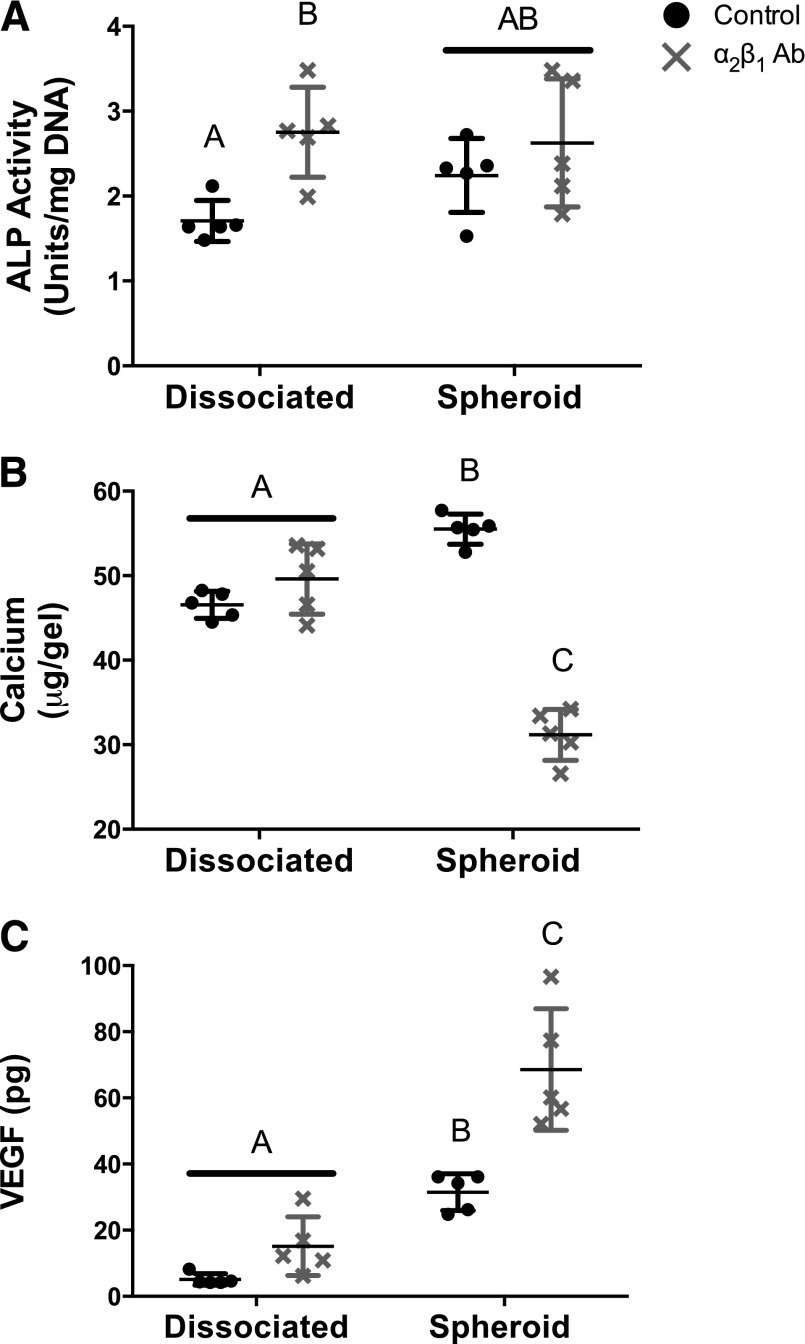
Previous reports confirm spheroidal culture upregulates the transcription of numerous collagen matrix proteins in comparison with adherent monolayer culture [24]. Our laboratory has shown that cell-secreted matrices predominantly consist of collagen type I [25] and drive bone formation in vivo by MSCs [1]. The α2β1 integrin regulates the survival and osteogenic differentiation of MSCs on collagen type I [26, 27]. Thus, we investigated whether blocking the α2β1 integrin on MSCs would abrogate the osteogenic response. Neither spheroid formation nor spheroid diameter was affected by the presence of the α2β1 antibody (data not shown). We analyzed preservation of osteogenic markers only at +5d, because we did not detect discernable differences in osteogenic potential of MSC spheroids or dissociated cells at +1d when entrapped in collagen gels. Addition of the α2β1 antibody increased ALP activity in dissociated MSCs (Fig. 6A), whereas ALP activity in MSC spheroids remained relatively constant and comparable in magnitude to levels in antibody-treated dissociated MSCs. Cell-secreted calcium was examined as a late-stage marker of osteogenesis. Dissociated MSCs exhibited equal levels of calcium secretion in the presence of the α2β1 antibody, yet calcium secretion in MSC spheroids was abrogated by antibody treatment (Fig. 6B). VEGF secretion followed the inverse trend of calcium deposition; MSC spheroids secreted more VEGF in the presence of the α2β1 antibody, though there was no significant effect in antibody-treated dissociated MSCs (Fig. 6C). Taken together, these data provide evidence that VEGF secretion may serve as a negative indicator of MSC osteodifferentiation state in this model.
Figure 6.
Markers of osteogenic differentiation in mesenchymal stem cell (MSC) spheroids are sustained in the absence of osteoinductive cues through an α2β1 integrin-mediated mechanism. Osteogenically induced MSCs were dissociated or formed as spheroids and encapsulated within collagen I hydrogels before stimulus withdrawal (OM/GM). Medium changes were performed regularly with culture media (control) or media supplemented with 5 ng/ml α2β1 blocking antibody. Analysis was performed at +5 days for alkaline phosphatase activity (A), calcium deposition (B), and vascular endothelial growth factor secretion (C). Chart values represent mean ± SD for n = 5. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, whereas groups with significance do not share the same letters. Abbreviations: α2β1 Ab, α2β1 blocking antibody; ALP, alkaline phosphatase activity; VEGF, vascular endothelial growth factor.
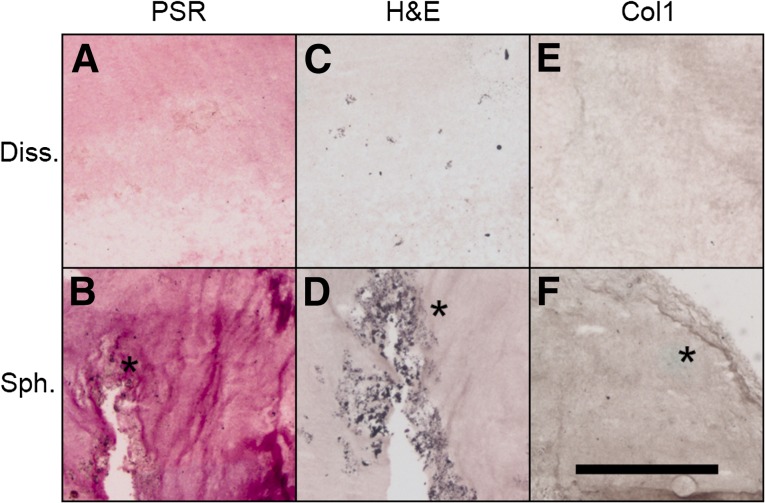
To further examine the role of the cell-secreted ECM on MSC osteogenic potential within collagen hydrogels, we visualized total collagen (Fig. 7A, 7B), general morphology via H&E (Fig. 7C, 7D), and type 1 collagen (Fig. 7E, 7F) at +5d. MSC spheroids entrapped in collagen gels secreted more collagen and connective tissue constituents than did dissociated MSCs and demonstrated areas of clustered cells. In contrast, collagen gels containing dissociated cells had lower levels of only diffuse staining throughout. These stains indicate that spheroids retain their ECM when entrapped in a collagen gel and that the dissociated cells are unable to produce the same quantity of self-secreted collagen in the 5-day time span.
Figure 7.
Mesenchymal stem cell (MSC) spheroids exhibit differences in matrix protein content compared with dissociated MSCs when entrapped in collagen hydrogels. Osteogenically induced adherent and spheroidal MSCs encapsulated within collagen I hydrogels were subjected to stimulus withdrawal (osteogenic media/growth media). Immunohistochemistry was performed at +5 days for Picrosirius Red (A, B), H&E (C, D), and type 1 collagen (E, F). Asterisks indicate areas of interest. Scale bar represents 500 µm. Abbreviations: Col1, Type 1 collagen; Diss., dissociated mesenchymal stem cells; PSR, Picrosirius Red; Sph., mesenchymal stem cell spheroids.
Discussion
In an attempt to enhance bone formation in vivo, MSCs are typically osteogenically induced with a cocktail of common soluble supplements [28–30]. We recently demonstrated that even after 6 weeks of induction using these osteogenic supplements, human MSCs rapidly dedifferentiate as early as 24 hours following stimulus withdrawal. However, their osteogenic phenotype could be preserved if the induced MSCs were cultured on decellularized extracellular matrix (ECM) [1]. MSC spheroids represent an exciting alternative to the traditional cell-based therapeutic strategies using dissociated cells from monolayer culture [4]. Spheroidal culture allows for the MSCs to still be entrapped in a biomaterial yet retain their intact ECM and cell-cell contacts formed during spheroid formation [3]. Therefore, we hypothesized that forming MSCs into spheroids would preserve their phenotype through ECM-mediated interactions.
To explore this hypothesis, we formed MSC spheroids from osteoinduced cells, entrapped them in collagen gels, and analyzed their osteogenic and proangiogenic potential before and after encapsulation. In agreement with our previous data, MSC spheroids had similar calcium secretion but decreased ALP activity in comparison with MSCs in monolayer culture prior to encapsulation in collagen hydrogels in both growth medium and osteogenic medium [4]. The higher ALP activity and increased COL1A1 expression in adherent MSC monolayers may be derived from differences in mechanical stimuli, because adherent monolayers were on stiff tissue culture plastic (TCP), whereas spheroids were suspended in media with no outside mechanical stimulus. In standard culture conditions, MSC spheroids had increased proangiogenic potential in comparison with adherent monolayers, yet this advantage was lost once cultured in the presence of osteogenic supplements. Once entrapped in collagen hydrogels, MSC spheroids exhibited stronger and persistent osteogenic potential in the absence of soluble cues in comparison with osteogenically induced dissociated MSCs. Importantly, the osteogenic potential of MSC spheroids increased from +1d to +5d in the absence of soluble osteoinductive stimuli. Dissociated MSCs did not suffer from the same rapid decline in osteogenic potential that we previously observed in two dimensions (2D) [1]. This is most likely due to the fact that 3D collagen I culture enhances osteogenic differentiation of MSCs through signaling pathways such as extracellular signal-regulated kinases (ERK) and α2β1 more robustly than does 2D culture [31].
Bone is a highly vascularized tissue, and many studies have shown that increased angiogenesis directly correlates with increased bone formation [14, 32, 33]. However, the proangiogenic potential of human MSCs commonly decreases as the osteogenic potential increases in both 2D and 3D cultures [19, 21]. In agreement with our previous work, MSC spheroids cultured in GM were significantly more proangiogenic than were their adherent counterparts prior to encapsulation in collagen hydrogels [4]. Whereas VEGF gene expression was increased in spheroids regardless of media, VEGF secretion, measured in conditioned media, was diminished in both culture geometries when cultured in OM. This may be because spheroids are rich in ECM proteins, possibly sequestering the VEGF within the spheroid itself. However, because functional protein levels are more impactful on the surrounding endothelial cells, we chose to measure secreted VEGF instead of gene expression for the remaining studies. Once entrapped in collagen hydrogels, the spheroids maintained their osteogenic phenotype at the expense of their proangiogenic potential. This differs slightly from our prior work in fibrin hydrogels, in which MSC spheroids exhibited comparable osteogenic potential to dissociated cells when cultured in osteogenic media and up to a 100-fold increase in VEGF secretion when cultured in serum deprivation and hypoxia [4]. These discrepancies likely arise because of differences in media conditions and the choice of biomaterial. Collagen is a mature protein in homeostatic tissues and directly promotes osteogenic differentiation of MSCs [34], whereas fibrin is a reparative biomolecule serving as a scaffold for tissue regeneration [35]. Further work is merited to define the characteristics of a biomaterial that would simultaneously capitalize on the osteogenic and proangiogenic potential of MSC spheroids.
The pathways by which spheroids are able to enhance MSC function have yet to be fully elucidated, in part because of the complexity of the 3D microenvironment within the spheroid. Although many have explored the importance of cadherins on MSC spheroid formation and enhanced survival [17, 36, 37], this is the first study to show the importance of the cell-ECM interaction on the osteogenic commitment of MSC spheroids. Delivering MSCs as spheroids as opposed to the more commonly deployed dissociated cells avoids the deleterious trypsinization step, allowing the ECM to provide continued instruction via integrin-mediated cues. In addition to having a vast secretome [38], MSCs deposit a wide array of ECM proteins [39]. Here we show that MSC spheroids produce comparable levels of collagen type 1 compared with adherent monolayers on tissue culture plastic and that this production is further increased with the addition of osteogenic supplements. MSCs engage this ECM via integrins such as α2β1, a key mediator of cellular engagement with an underlying collagen matrix [16, 40]. We previously demonstrated that α2β1 signaling is the predominant integrin by which MSCs bind to their self-secreted ECM. Although other matrix components such as fibronectin, biglycan, and decorin are constitutively expressed, blocking of αvβ3 or α5β1 integrins does not affect cell adhesion [25]. However, it is most likely the complex milieu within the spheroid that strengthens their osteogenic commitment, because we recently demonstrated that collagen alone was unable to sustain osteoblastic phenotype following stimulus withdrawal [1]. Thus, the presence of fibronectin within the spheroid likely works in concert with the rich collagen matrix to instruct the MSCs to produce mineral.
Entrapment of MSCs in collagen hydrogels provided a platform in which the spheroids and dissociated cells were exposed to the same mechanical cues. Within the collagen gel, dissociated MSCs benefit by being surrounded by collagen fibers that directly promote osteogenic differentiation, whereas only the cells on the outside of the spheroid are engaging the hydrogel. However, the potent cues provided by the ECM within the spheroids allow them to have greater osteogenic potential than do their dissociated counterparts. Although entrapment in the collagen hydrogels was sufficient to prevent the rapid dedifferentiation we reported in monolayer culture [1], dissociated MSCs entrapped in collagen hydrogels may use very low levels of α2β1 integrin binding, because blocking integrin activity had no effect on their osteogenic or proangiogenic potential. However, abrogation of α2β1 activity in MSC spheroids prevented their capacity to effectively engage their endogenous matrix, leading the MSC spheroids to secrete less calcium and more VEGF, both indicators of reduced osteogenic potential. Although there was a slight increase in ALP activity for both dissociated cells and spheroids when treated with an α2β1 antibody, this may be due to disruption of the temporal and cyclical ALP activity upon incubation with the antibody. Furthermore, throughout these studies we observed a general trend of VEGF secretion serving as a negative indicator of MSC osteodifferentiation. Although we have published extensively on this phenomenon [19, 21, 22], this is the first work to directly link VEGF secretion to α2β1 activity within MSC spheroids. Moreover, we focused on VEGF as a key secreted molecule to characterize angiogenic potential of spheroids. However, compensatory proteins/pathways may contribute to the angiogenic potential of these induced spheroids, the concentration and identity of which are functions of the differentiation state and the surrounding ECM [41]. The role of additional cytokines and potential synergistic contributions to bone formation merits examination.
Conclusion
Our results demonstrate that MSCs formed as spheroids sustain their osteoblastic phenotype better than do dissociated MSCs when encapsulated within a collagen hydrogel, yet this is at the expense of their proangiogenic potential. Furthermore, the capacity of MSC spheroids to sustain the osteoblastic phenotype is at least partially mediated by an α2β1 integrin-mediated mechanism.
Acknowledgments
These studies were supported by NIH Grant R01 DE02547 to J.K.L. K.C.M. received financial support through the American Heart Association Western States Affiliate Predoctoral Fellowship (15PRE21920010). A.I.H. received financial support through the ARCS Foundation, Inc., Northern California Chapter, and an industry/campus-supported fellowship under the NIH Training Program in Biomolecular Technology (T32-GM008799) at the University of California, Davis. J.N.H. received financial support through a National Defense Science & Engineering Graduate Fellowship.
Author Contributions
K.C.M.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing; A.I.H.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation; J.N.H. and D.Z.: collection and/or assembly of data; J.K.L.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Hoch AI, Mittal V, Mitra D, et al. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–187. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhang SH, Lee S, Lee TJ, et al. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Part A. 2012;18:310–319. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhang SH, Lee S, Shin JY, et al. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhang SH, Cho SW, La WG, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos JM, Camões SP, Filipe E, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6:90. doi: 10.1186/s13287-015-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ylöstalo JH, Bartosh TJ, Coble K, et al. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2014;3:643–652. doi: 10.5966/sctm.2013-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520–1528. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaigler D, Wang Z, Horger K, et al. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 14.Leu A, Stieger SM, Dayton P, et al. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A. 2009;15:877–885. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita S, Finnegan M, Bucholz RW, et al. Three-dimensional collagen gel culture promotes osteoblastic phenotype in bone marrow derived cells. Kobe J Med Sci. 1999;45:201–211. [PubMed] [Google Scholar]
- 16.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129:133–138. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 17.Lee EJ, Park SJ, Kang SK, et al. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012;20:1424–1433. doi: 10.1038/mt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy KC, Leach JK. A reproducible, high throughput method for fabricating fibrin gels. BMC Res Notes. 2012;5:423. doi: 10.1186/1756-0500-5-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoch AI, Binder BY, Genetos DC, et al. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decaris ML, Leach JK. Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann Biomed Eng. 2011;39:1174–1185. doi: 10.1007/s10439-010-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy KC, Hughbanks ML, Binder BY, et al. Engineered fibrin gels for parallel stimulation of mesenchymal stem cell proangiogenic and osteogenic potential. Ann Biomed Eng. 2015;43:2010–2021. doi: 10.1007/s10439-014-1227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KC, Stilhano RS, Mitra D, et al. Hydrogel biophysical properties instruct coculture-mediated osteogenic potential. FASEB J. 2016;30:477–486. doi: 10.1096/fj.15-279984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 25.Decaris ML, Mojadedi A, Bhat A, et al. Transferable cell-secreted extracellular matrices enhance osteogenic differentiation. Acta Biomater. 2012;8:744–752. doi: 10.1016/j.actbio.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Popov C, Radic T, Haasters F, et al. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Lund AW, Stegemann JP, Plopper GE. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells Dev. 2009;18:331–341. doi: 10.1089/scd.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Genetos DC, Leach JK. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16:127–137. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung WK, Working DM, Galuppo LD, et al. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12:554–562. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 34.Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh HY, Liu BH, Hsu SH. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials. 2012;33:8943–8954. doi: 10.1016/j.biomaterials.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 37.Sart S, Tsai AC, Li Y, et al. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82-83:1–11. doi: 10.1016/j.addr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amos PJ, Kapur SK, Stapor PC, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai KS, Kao SY, Wang CY, et al. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res A. 2010;94:673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 41.Ho SS, Murphy KC, Binder BY, et al. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5:1–9. doi: 10.5966/sctm.2015-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]