Summary
A primary rat choroidal epithelial cell culture system was developed to investigate mechanisms of heavy metal toxicity on the blood-cerebrospinal fluid (CSF) barrier. Epithelial cells were dissociated from choroidal tissue by pronase digestion and cultured in standard DMEM culture media supplemented with 10% fetal bovine serum and 10 ng epithelial growth factor per ml. The procedure yielded 2–5 × 104 cells from pooled plexuses of three to four rats, and a viability of 77–85%. The cultures displayed a dominant polygonal type of epithelial cells, with a population doubling time of 2–3 d. The cultures were of distinct choroidal epithelial origins. For example, immunocytochemical studies using monospecific rabbit anti-rat TTR polyclonal antibody revealed a strong positive stain of transthyretin (TTR), a thyroxine transport protein exclusively produced by the choroidal epithelia. Also, reverse-transcriptase polymerase chain reaction (PCR) confirmed the presence of specific TTR mRNA in the cultures. The cultures were further adapted to grow on a freely permeable membrane sandwiched between two culture chambers. The formation of an impermeable confluent monolayer occurred within 5 d after seeding and was verified by the presence of a steady electrical resistance across the membrane (80 ± 10 ohm per cm2). The epithelial barriers appeared to actively transport [125I]-thyroxine from the basal to apical chamber. These results suggest that this primary cell culture system possesses typical choroidal epithelial characteristics and appears to be a suitable model for in vitro mechanistic investigations of blood–CSF barrier.
Keywords: choroid plexus, blood–cerebrospinal fluid barrier, blood–CSF barrier, choroidal epithelial cells, transthyretin, transport, primary cell culture
Introduction
The choroid plexus is a highly vascularized tissue localized within the brain ventricles. Tight junctions between the choroidal epithelial cells seal one epithelium to another, constituting an important barrier between the blood and cerebrospinal fluid (CSF) (13,22). Heavy metals and metalloids such as lead (Pb), mercury (Hg), manganese (Mn), and arsenic (As) accumulate in the choroid plexus at concentrations much greater than those found in the CSF and elsewhere in brain tissues (5,26,27). Our recent in vivo study in rats revealed that long-term, low-dose Pb exposure resulted in a significant decrease in CSF concentration of transthyretin (TTR), which was inversely associated with Pb deposition in the choroid plexus (28). These findings suggest that the choroid plexus may be a target for toxicities associated with environmental exposure to heavy metals. However, it is not known why toxic metals accumulate in the choroid plexus, how plexus dysfunction may affect brain development, or what subcellular functions are influenced by heavy metal ions.
We concluded that in order to study the mechanistic aspects of metal toxicity in the blood–CSF barrier, it was necessary to develop an in vitro primary cell culture system for the choroid plexus. Several procedures have been described for the establishment of primary culture of murine choroid plexus; some of these cultures were apparently contaminated with other cell types such as fibroblasts and endothelial cells (6,19), whereas the others lacked well-defined culture parameters (23,24).
The purpose of this study was to establish a simple, reproducible, and less-contaminated procedure for the primary culture of choroidal epithelial cells from rats. We characterized choroidal epithelial cells using TTR as a unique marker, because TTR is exclusively localized, produced, and secreted by the choroidal epithelia in mammalian brain (1,11,17). In addition, the culture was adapted onto a two-chamber system in order to evaluate the transport properties of the epithelial barrier.
Materials and Methods
Materials
Chemicals were obtained from the following sources: Dulbecco's modified essential medium (DMEM), Hanks’ balanced salt solution (HBSS), fetal bovine serum, and antibiotic-antimycin from GIBCO (Grand Island, NY); pronase and cis-hydroxyproline from CalBiochem-Novabiochem (La Jolla, CA); mouse epidermal growth factor (EGF), bovine fibroblast growth factor (FGF), gentamycin, diethylpyrocarbonate (DEPC), and laminin from Sigma Chemical Co. (St. Louis, MO); RNA STAT-60™ from Tel-Test “B” (Friendswood, TX); Transwell-COL culture wells from Costar (Cambridge, MA); [125I]-thyroxine (specific activity: 4.4 Ci/μmol) from Du Pont (Boston, MA). All reagents were of analytical grade, HPLC grade or the best available pharmaceutical grade. Purified rat plasma TTR and monospecific rabbit anti-rat TTR polyclonal antibody were the gifts of Dr. W Blaner at the Institute of Human Nutrition, Columbia University.
Cell preparation and culturing
Sprague-Dawley rats of both sexes were purchased from Harlan Inc. (Indianapolis, IN), and were (unless otherwise indicated) 4–6 wk old (80–90 g) at experiment. Rabbits (New Zealand White, 2–3 kg) were obtained from Hare Marland (Hewitt, NJ). Animals were euthanized with pentobarbital and brains removed from the skull. The whole brain was placed in ice-cold phosphate-buffered saline (PBS) solution to chill the tissues and wash off excess blood. The choroid plexuses were dissected from both lateral and third ventricles in a cell culture hood. A pool of tissues usually from 10–15 rats was rinsed in 2 ml of HBSS and transferred to another beaker containing 0.5 ml of HBSS. The tissues were chopped with a fine ophthalmologic scissors to about 1-mm cubes, and the volume was brought to 1 ml.
The minced tissue preparation was mixed with 1 ml of digestion solution (0.4% pronase in HBSS). The digestion continued at 37° C for 5 min in an incubator and was stopped when 5 ml of PBS solution was added to the digestion mixture. After centrifugation at 300 × g for 5 min, the supernatant containing primarily nonepithelial cells was decanted. The pellet of epithelial clumps was washed with 5 ml of PBS once and resuspended in 4 ml of growth medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units penicillin per ml, 100 μg streptomycin per ml, 100 μg gentamycin per ml, 2.5 μg amphotericin B per ml, and 10 ng EGF per ml. The cells were further mechanically dissociated by seven to eight forced passages through a 20-gauge needle. This procedure is necessary and must be done with care in order to triturate the cell clumps and to produce the maximal yield of epithelia. An aliquot (0.1 ml) of cell suspension was then removed and mixed with 0.1 ml of 0.4% Trypan blue to count cell numbers and to assess the viability.
Following dilution to approximately 1–2 × 105/ml with the culture medium, the cells were plated in 35-mm petri dishes (2–3 × 105 cells per dish) and cultured in a humidified incubator with 95% air–5% CO2 at 37°C. The cell culture continued without disturbance for at least 48 h following initial seeding to ensure good attachment. The medium was changed every 2 d thereafter for the duration of the culture.
To study the growth dynamics, the culture was incubated with 0.02% trypsin and 0.02% EDTA in PBS at 37°C for 5 min to bring about cell detachment. An aliquot (0.1 ml) of cell suspension was mixed with 0.1 ml of 0.4% Trypan blue in an Eppendorf tube. Both viable and dead cells were recorded.
In some cases, cis-hydroxyproline was added to the culture medium (final concentration: 25–100 μg/ml) to control the growth of fibroblastic cells. In other cases, the cultures were allowed to grow in the dishes precoated with collagen.
Culture on two-chamber system
Permeable membranes attached to the Transwell-COL culture wells were pretreated with laminin (14 μg/ml) for 10 min and allowed to air dry for at least 45 min prior to cell seeding. Aliquots (0.5 ml) of cell suspension were plated in 12-mm laminin-coated culture wells (2 × 105 cells per well). This was designated as the inner (apical) chamber. The inner chambers were then inserted into the outer (basal) chambers which contained 1 ml of culture medium. The cultures continued for 48 h and the medium was changed every 2 d thereafter.
Transepithelial electrical resistance was measured with an epithelial voltohmmeter (EVOM, World Precision Instruments, Sarasota, FL) after culture in the bicameral chambers for at least 4 d. The net value of electrical resistance was computed by subtracting the background, which was measured on laminin-coated, control cell-free chambers, from values of epithelial cell-seeded chambers. The formation of confluent impermeable cell monolayers was judged by two criteria: (1) the height of the culture medium in the inner chamber had to be at least 2 mm higher than that in the outer chamber for at least 24 h; and (2) the electrical resistance across the cell layer had to fall into the range of 80 ± 10 ohm per cm2 (mean ± SD, n = 6).
For thyroxine (T4) transport studies, the cells, upon the formation of an impermeable monolayer, were washed with and cultured for 2 h in a serum-free DMEM medium supplemented with 5 μg insulin per ml, 5 μg transferrin per ml, 5 ng sodium selenite per ml, and 5 ng fibroblast growth factor per ml. [125I]-T4 was added into the outer chamber to the final concentration of 40 pM (0.18 μCi/ml). Aliquots (5 μl) of media in both chambers were removed at various times and counted with a Packard model Cobra-II gamma counter.
Immunocytochemical studies
The cells to be stained were cultured on a glass coverslip for 5–7 d. The cells were fixed in 4% paraformaldehyde in PBS solution followed by washing three times with PBS. The cell monolayers that attached to the glass coverslips were permeabilized by washing with 0.1% Triton X-100 in PBS three times. For immunohistochemical staining, the cells were incubated with rabbit anti-rat TTR antiserum (1:250 dilution) in PBS at room temperature for 30 min and rinsed with 0.05% Triton X-100 to reduce the background. The cultures were then incubated for 30 min with biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) diluted 1:1000 in PBS. The sections were further stained with ABC reagent (VECTASTAIN®, Vector Laboratories) to form avidin-biotin-horseradish peroxidase complex.
For immunofluorescent staining, the sections were treated in the same way as described above except that the secondary antibody was fluorescein-conjugated goat anti-rabbit antibody (Amersham, Arlington Heights, IL). The stained sections were examined with a Nikon Model HB-1010 microscope with FITC-fluorescence and phase-contrast optics.
Reverse PCR analysis
We extracted total RNA from the cultured cells, plexus tissue, cerebral cortex, or liver of rats according to the procedure described by Sambrook et al. (21) using an RNA isolation kit (RNA STAT-60™, Tel-Test “B”). In short, the cells or tissues were homogenized in RNA Stat-60™ solution, followed by chloroform extraction to remove DNA and proteins. RNA was then precipitated by isopropanol, washed with 75% ethanol, and reconstructed in DEPC-treated RNase-free solution.
One microgram of total RNA was reverse transcribed with MuLv Reverse Transcriptase (GeneAmp) with random hexamers (2.5 μM) or selected antisense primers (2.5 μM) in a 20-μl reaction mixture containing 10 mM Tris (pH 8.3), 50 mM KCl, 5 mM MgCl2, 1 mM each dATP, deoxyribosylthymine triphosphate (dTTP), deoxycytidine triphosphate (dCTP), and deoxyguanosine triphosphate (dGTP), and 20 units of RNase inhibitor (GeneAmp). The reaction was continued at 42° C for 45 min. For PCR amplification, one set of specific oligonucleotide pairs (0.15 μM) was incubated with the above reaction mixture and 2.5 units of Taq DNA polymerase (Perkin-Elmer) in a 100-μl reaction mixture containing 10 mM Tris (pH 8.3), 50 mM KCl, and 2 mM MgCl2. The first cycle was 2 min at 94° C for initial denaturation, 0.5 at 55° C for annealing, 0.5 min at 72° C for extension. The subsequent cycle parameters were 0.5 min at 94° C, 0.5 min at 55° C, and 0.5 min at 72° C for 35 cycles, followed by a final 5-min incubation at 72° C. An aliquot (10 μl) of each reaction mixture was analyzed by electrophoresis on 1.5% agarose gels containing 0.5 μg ethidium bromide per ml. The primers (synthesized, as requested, by KeyStone, Menlo Park, CA) designed specifically for rat TTR consist of 5′ primer: 5′-CCTGGGGGTGCTGGAGAAT-3′, and 3′ primer: 5′-ATGGTGTAGTGGCGATGAC-3′, which produce a product of 317 bp covering most of the TTR mature peptide region from rats (4).
Determination of protein content
Total protein content was measured by a Bio-Rad Protein Assay Kit (Bio-Rad Lab, Richmond, CA) with bovine serum albumin (range: 12.5–100 μg/ml) as the standard.
Results
Epithelial culture
The procedure for cell isolation described above yielded 0.8 to 1 × 105 epithelial cells per rat. Attempts to increase the cell yield by extending the pronase digestion period for 10 min appeared to slightly increase the total cell counts by 1.2-fold, but lessened cell viability and weakened cell attachment. We therefore adopted the procedure of mechanically forcing the digested preparation through a 20-gauge needle to triturate the epithelial clumps. This step compromises both cell yield and viability; mean cell viability was 82% with a range of 77–85%.
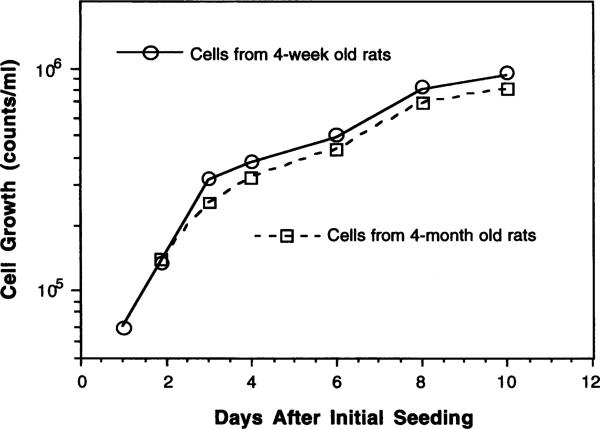
Cell attachment typically occurred within 24–36 h and was nearly completed by 48 h following initial plating. Culturing the cells on collagen-coated dishes enhanced cell attachment but augmented the growth of fibroblasts in the later stage of the culture, and was therefore abandoned. The epithelial cells grew rapidly during the first 4–5 d with a population doubling time of 2–3 d (Fig. 1). The cultures derived from 4-mo.-old rats yielded slightly lower (about 16%) total cell counts at the plateau as compared to cultures from 4-wk-old rats. Thus, the cell growth rate seemed to depend, to a small extent, on the age of the animals from which the choroid plexuses were harvested.
Fig. 1.
Growth curves of choroidal epithelial cells in primary culture. Cells were initially seeded at 3 × 105 cells per dish.
To minimize the fibroblast contamination, cis-hydroxyproline was introduced into the culture medium. The presence of cis-hydroxyproline effectively inhibited the growth of fibroblasts, but it also affected the normal growth of epithelia. Table 1 indicates that the concentration and the time of addition of cis-hydroxyproline are critical to the overall growth of the culture. The early addition of cis-hydroxyproline at 6 h delayed the cell attachment and severely interfered with epithelial growth, even at a low concentration (25 μg/ml). Higher concentrations of cis-hydroxyproline (100 μg/ml), though not affecting the cell attachment, suppressed the epithelial growth when added into 4-d-old culture (Table 1). Thus, the optimal condition for cis-hydroxyproline treatment appeared to be 25 μg/ml of culture medium added at 48 h after initial seeding.
TABLE 1.
EFFECT OF cis-HYDROXYPROLINE ON 5-DAY CHOROIDAL CELL CULTUREa
| Time and amount of cis-HPO addition | Cell attachment | Fibroblasts | Epithelial cells |
|---|---|---|---|
| 6 h | |||
| 25 μg/ml | ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ |
| 50 μg/ml | ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ |
| 100 μg/ml | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ |
| 24 h | |||
| 25 μg/ml | +/− | ↓ ↓ | ↓ |
| 50 μg/ml | +/− | ↓ ↓ | ↓ ↓ |
| 48 h | |||
| 25 μg/ml | +/− | ↓ ↓ | +/− |
| 96 h | |||
| 25 μg/ml | +/− | ↓ | +/− |
| 100 μg/ml | +/− | ↓ ↓ | ↓ |
cis-Hydroxyproline (cis-HP) (25-100 μg/ml) was added into the culture medium at the time indicated. The cultures were examined 5 days after initial seeding. The outcomes in a specified viewing field were scored as follows. +/−: no effect, ↓ : minor inhibitory effect; ↓↓ : moderate inhibitory effect; ↓↓↓ : severe inhibitory effect.
Characterization
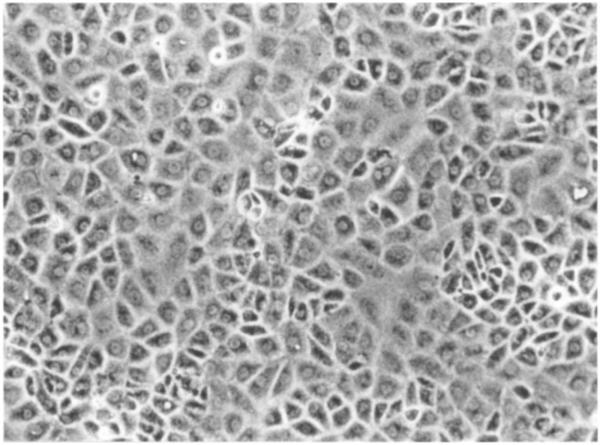
Under the microscope, polygonal epithelial cells dominated the culture for at least 2 wk (Fig. 2). Following cell attachment, the epithelial cells formed many dispersing clusters and grew in a reach-forth manner into their surroundings. The other cell type identified under light microscopy was the fibroblasts whose nuclei were condensed and elongated in the direction of cell stretch. The fibroblasts typically spread in the space between epithelial clusters. In aged cultures (i.e., > 3 wk after seeding), the epithelial cells became shrunken, the size reduced, and the nuclei condensed, and the fibroblasts overgrew. Cultures obtained from the young animals (4–5 wk) showed much less fibroblast contamination than those from the old animals (4–5 mo.), although the total cell counts did not seem to differ greatly (Fig. 1).
Fig. 2.
Primary culture of choroidal epithelial cells after 4 d in culture. Note the confluent layer of the cells with predominant polygonal cell type (× 100). The choroid plexuses were obtained from 5-wk-old Sprague-Dawley rats.
The same culture procedure was applied to tissues collected from rabbits and dogs. The morphology of the cultures from rabbit choroid plexus highly resembled that of rats (data not shown). However, in the case of dogs, fibroblasts outgrew the epithelia even at the early stage of the culture, and only a few epithelial cells survived during a 7-d culture (data not shown). Therefore, the current procedure may not be suitable for primary cultures of canine choroid plexus.
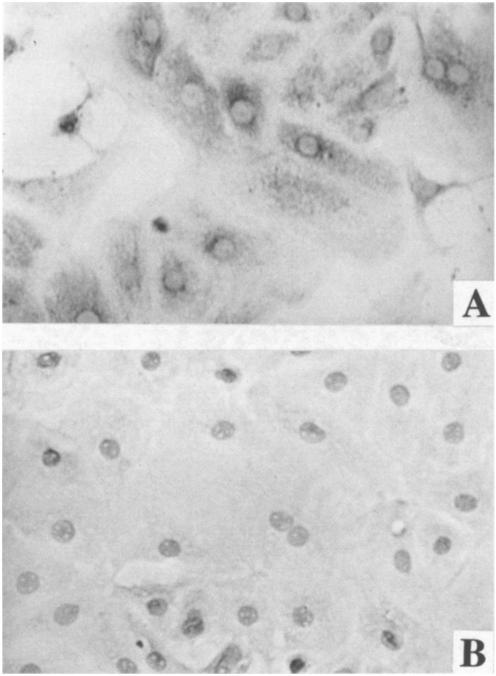
Immunohistochemical staining with anti-transthyretin (TTR) antibody showed that most of the rat epithelial cells in the primary cultures had positively stained cytosolic TTR (Fig. 3 a), whereas the cultures treated either with TTR-presaturated antibodies or with secondary antibodies alone failed to show such a positive reaction (Fig. 3 b). Immunofluorescence staining with the same primary antibody displayed similar results (data not shown).
Fig. 3.
Cultured choroidal epithelial cells possess cytosolic TTR by immunocytochemical staining. a, Cells were treated with anti-TTR primary antibody followed by secondary antibody conjugated with ABC reagent. Note the positive staining in cytosol (× 250). b, Cells were treated with TTR-presaturated primary antibody followed by ABC staining procedure (× 250).
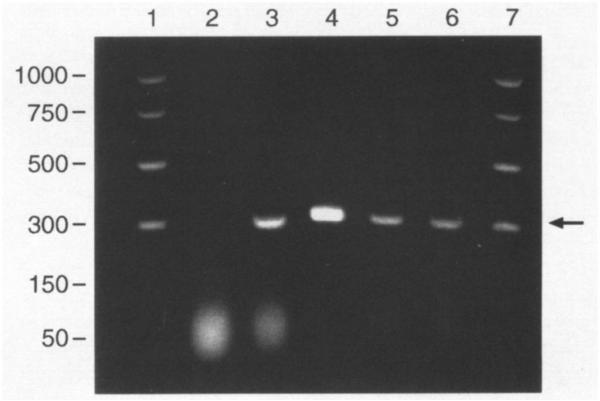
We further investigated the expression of TTR mRNA in the cultured cells. The selective primers designed by Gee et al. (7) have been used to identify TTR mRNA in choroid plexus from sheep, cow, and rat. However, the study employing this primer pair was not reproducible in our hands. Thus, we redesigned the sequence of the primer pairs according to Dickson et al. (4). Reverse PCR analysis clearly demonstrated that the cultured cells expressed TTR mRNA, as did freshly isolated choroid plexus tissue and liver cytosolic preparations (Fig. 4). Taken together, these observations strongly suggested that the primary cultures were of choroidal epithelial origin.
Fig. 4.
Choroid plexus tissues and the cultured choroidal epithelial cells express TTR mRNA by reverse transcriptase-PCR analysis. All samples underwent DNase digestion and RT-PCR unless otherwise stated. Arrow indicates bands corresponding to TTR mRNA. Lane I.D.: (1) base pair markers; (2) plexus tissue, total RNA, without RT-PCR; (3) plexus tissue, mRNA with selected primer; (4) liver, mRNA with selected primer; (5) and (6) cultured plexus cells, mRNA with selected primer; (7) same as lane (1).
Transport function
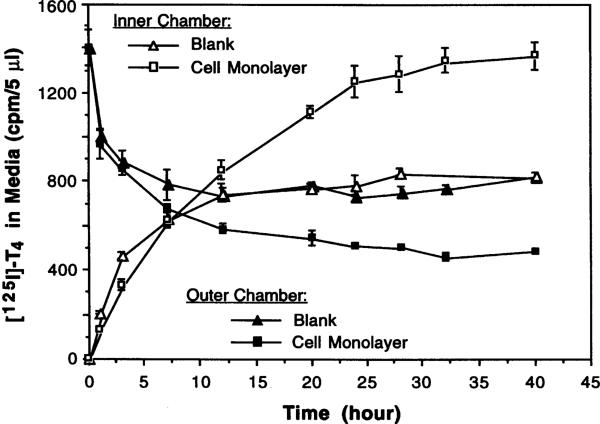
Cells grown on the membrane of the inner chambers displayed similar morphology to those observed in the culture dishes and survived for at least 2 wk. An 8-d culture was used to study T4 transport in order to evaluate the ability of the epithelial barrier to actively transport hormone between the chambers. Following addition of [125I]-T4 to the outer (basal) chamber, in the absence of cells, the radioactivity in the inner (apical) chamber rose while that in the outer chamber declined, both reaching equilibrium at about 12 h (Fig. 5). In the presence of cells, however, the radioactivity migrated from the basal to apical chamber and far exceeded the equilibrium (Fig. 5). Our study confirmed the results reported by Southwell et al. (23), suggesting that radiolabeled T4 was actively transported across the impermeable “cell barrier” formed of choroidal epithelia.
Fig. 5.
Choroidal epithelial cells cultured on Transwell-COL membrane actively transport [125I]-thyroxine across a confluent epithelial monolayer. Data represent means ± SD (n = 3).
Discussion
This study demonstrates that rat choroidal epithelial cells can be successfully cultured for at least 2 wk in DMEM supplemented with FBS and EGF. The cultures possess relatively uniform epithelial cell type with minor contamination from fibroblasts. The epithelial cells can also grow on a permeable membrane to form a confluent cell monolayer for transepithelial transport studies. The procedure is simple and highly reproducible.
The establishment of choroidal epithelial cell culture has been described in the literature for the cells from various sources, including mouse (2,6,19), rat (2,6,19,24), rabbit (15), sheep (10), cow (3,25), and human (8). Some of these reports have used transferrin (24), Na+, K+-ATPase (19,25), G proteins (19), ciliary rootlet proteins (6), and the absence of antihemophilic factor (AHF) antigen (3) as the cellular markers to characterize the origin of the cultures. In the current studies, we chose TTR because choroid plexus epithelium is the only cell type capable of manufacturing TTR in the mammalian brain. TTR is a 55 000-dalton protein consisting of four identical subunits in a tetrahedral symmetry (12). Per unit of weight, rat choroid plexus contains 10 times more TTR mRNA than liver, which produces TTR for serum (4). The presence of TTR per se and/or TTR mRNA in the culture, therefore, should exclusively reflect the origin of the cells from the choroidal epithelia. Using two different staining techniques, our immunocytochemical results revealed that the epithelial cells in the cultures indeed possessed TTR polypeptides (Fig. 3). Reverse PCR with selective primers encoding TTR also confirmed the presence of TTR mRNA in these cells (Fig. 4). Thus, these studies assure that a high proportion of the cultured cells were of choroidal epithelial origin.
A number of factors affect the quality of our cultures, notably the proteolytic enzymes. Tsutsumi et al. (24) reported that the cultures produced using collagenase and DNase maintained a polygonal epithelial-like morphology, but did not address whether the enzyme digestion affected cell yield and attachment. In a separate experiment using bovine choroid plexus, Crook et al. (3) compared the effect of collagenase, dispase, pronase, and the combination of collagenase and dispase on the early stage of cell culture. It was found that tissue treated with collagenase yielded the lowest cell counts and viability, and the poorest cell attachment. These authors further reported that digestion with pronase generated the highest number of viable cells and a more effective cell attachment. In this study, we found that pronase digestion of rat choroid plexus effectively released epithelial cells. However, the duration and the concentration of pronase treatment must be well controlled, since prolonged digestion (≥ 10 min) reduced cell attachment.
Cultured choroidal epithelial cells have limited ability to differentiate, as evidenced by our failure to subculture via passage. The weak differentiation ability limits the life span of the culture. We have compared the growth dynamics of the cells from the young and elderly animals. While the total cell counts in the cultures from the older animals were slightly lower than those from the young ones (16% less at the plateau phase), the population doubling times did not seem to differ greatly among these two groups (Fig. 1). In another attempt to harvest the cells from 21-d-old weanling rats, we found that the tissue mass from these rats was too small (about 1–2 mg per rat) to provide sufficient cells unless many animals were pooled. However, we indeed noticed that cell differentiation and proliferation in cultures established from weanling rats were much better than those from the older rats. We believe that using the rats aged 4–5 wk seems to be a good compromise of adequate growth dynamics, reasonable animal cost, and convenient technical procedure.
Cell growth dynamics was also species-dependent. We observed that the growth properties of cells from rats and rabbits shared much similarity, whereas cultures from dogs were problematic. Canine plexus cells appeared to be very sensitive to pronase treatment, as few attached epithelial clusters could be seen after digestion. Our procedure was successful only for cultures derived from rats and rabbits. From the economic point of view, culturing one dish of rat plexus cells (usually requiring three rats) costs the dollar equivalent to that of rabbit (one per dish). Thus, the option as to which species is chosen is solely dependent upon the purpose of the study.
One challenge for successfully culturing choroidal epithelial cells is to control the contamination by fibroblasts. One way to block fibroblasts is to exclude serum, which contains fibroblast growth factor (FGF), from the culture medium. In our earlier experiments, we reduced the FBS to 5% in order to limit the growth of fibroblasts. Reduction of FBS, however, debased the overall growth of the culture. An alternative way is to use a specific fibroblast inhibitor. Fibroblast proliferation relies on collagen, which is rich in proline. A number of studies have reported that cis-hydroxyproline, a proline analogue, could effectively remove fibroblasts from the cultures of mixed cell population (3,14). Our studies indicate that both the concentration of cis-hydroxyproline and the time of its addition to the medium are critical. Higher concentration and earlier treatment, while effectively inhibiting the contamination, killed the epithelia as well (Table 1). Therefore, we recommend the regimen adding 25 μM cis-hydroxyproline to the culture medium at 48 h after initial seeding.
The choroid plexus is known to actively transport many substances bidirectionally between the blood and CSF (13,17,22). Culturing epithelial cells on a permeable, translucent support membrane has proved useful in assessment of transepithelial movement of substances in kidney epithelial cells (16), Sertoli cells (9,18), and choroidal ependymocytes (23). Because the epithelial cells are connected by tight junctions when they grow to a confluent monolayer, the cells actually form an impermeable barrier between the media in the inner and outer chambers. The net electrical resistance across this barrier in our study (80 ± 10 ohm/cm2) is comparable to that reported by others (99 ± 15 ohm/cm2) (23). Saito and Wright (20), however, reported a transmural resistance of 170 ohm/cm2 in an intact plexus tissue from the IVth-ventricle of the bullfrog. It should be noted that determination of electrical resistance could be influenced by the differences in preparations (tissue vs cultured cells), temperature, pH (physiological solutions vs culture media), age of tissues or cultures, and the freshness of culture medium.
Our studies also demonstrated that this in vitro barrier system did maintain the ability to actively transport thyroxine from the basal exterior (in contact with the fluid in the outer chamber) to the apical surface (in contact with the fluid in the inner chamber) of the cells (Fig. 5). By monitoring the kinetic properties of substances in both chambers, this system should facilitate the study of transport mechanisms at the blood-CSF barrier.
In summary, a simple and reproducible method has been developed to culture epithelial cells derived from mammalian choroid plexus. The method minimizes interference from fibroblasts and produces a high yield of choroidal epithelial cells. The cultured cells grown on a permeable membrane possess the normal cellular functions, such as transport of thyroxine. In particular, this method will enable one to study mechanisms of heavy metal uptake, storage and toxicity at the blood-CSF barrier.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Mr. Sean (Xiaojun) Ren and the gift of TTR from Dr. William S. Blaner in the Institute of Human Nutrition, Columbia University. This research was supported in part by grants P20-ES06831, R01-ES07042, and the Calderone Foundation.
REFERENCES
- 1.Aldred AR, Brack CM, Schreiber G. The cerebral expression of plasma protein genes in different species. Comp. Biochem. Physiol. 1995;111B:1–15. doi: 10.1016/0305-0491(94)00229-n. [DOI] [PubMed] [Google Scholar]
- 2.Bouille C, Mesnil M, Barriere, et al. Gap junctional intercellular communication between cultured ependymal cells, revealed by Lucifer yellow CH transfer and freeze-fracture. GLIA. 1991;4:25–36. doi: 10.1002/glia.440040104. [DOI] [PubMed] [Google Scholar]
- 3.Crook RB, Kasagami H, Prusiner SB. Culture and characterization of epithelial cells from bovine choroid plexus. J. Neurochem. 1981;37:845–854. doi: 10.1111/j.1471-4159.1981.tb04470.x. [DOI] [PubMed] [Google Scholar]
- 4.Dickson PW, Howlett GJ, Schreiber G. Rat transthyretin (prealbumin): molecular cloning, nucIeotide sequence, and gene expression in liver and brain. J. Biol. Chem. 1985;260:8214–8219. [PubMed] [Google Scholar]
- 5.Friedheim E, Corvi C, Graziano J, et al. Choroid plexus as protective sink for heavy metals? Lancet. 1983;i(8331):981–982. doi: 10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- 6.Gabrion J, Peraldi, Faivre-Bauman A, et al. Characterization of ependymal cells in hypothalamic and choroidal primary cultures. Neuroscience. 1988;24:993–1007. doi: 10.1016/0306-4522(88)90082-6. [DOI] [PubMed] [Google Scholar]
- 7.Gee P, Rhodes CH, Fricker LD, et al. Expression of neuropeptide processing enzymes and neurosecretory proteins in ependyma and choroid plexus epithelium. Brain Res. 1993;617:238–248. doi: 10.1016/0006-8993(93)91091-6. [DOI] [PubMed] [Google Scholar]
- 8.Gilden DH, Devlin M, Wroblewska Z, et al. Human brain in tissue culture. L. Acquisition, initial processing, and establishment of brain cell cultures. J. Comp. Neurol. 1975;161:295–306. doi: 10.1002/cne.901610302. [DOI] [PubMed] [Google Scholar]
- 9.Hadley MA, Djakiew D, Byers SW, et al. Polarized secretion of androgen-binding protein and transferrin by Sertoli cells grown in a bicameral culture system. Endocrinology. 1987;120:1097–1103. doi: 10.1210/endo-120-3-1097. [DOI] [PubMed] [Google Scholar]
- 10.Harter DH, Hsu KC, Rose HM. Immunofluorescence and cytochemical studies ofvisna virus in cell culture. J. Virol. 1967;1:1265–1270. doi: 10.1128/jvi.1.6.1265-1270.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert J, Wilcox JN, Pham KC, et al. Transthyretin: a choroid plexus-specific transport protein in human brain. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- 12.Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Ann. Rev. Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- 13.Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in medicine. Lippincott; Philadelphia: 1995. pp. 171–196. [Google Scholar]
- 14.Kao W, Prokop D. Proline analogue removes fibroblasts from cultured mixed cell population. Science. 1977;266:63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- 15.Mayer SE, Sanders-Bush E. Sodium-dependent antiporters in choroid plexus epithelial cultures from rabbit. J. Neurochem. 1993;60:1304–1316. doi: 10.1111/j.1471-4159.1993.tb03291.x. [DOI] [PubMed] [Google Scholar]
- 16.Misfeldt DS, Hamamoto ST, Pitelka DR. Transepithelial transport in cell culture. Proc. Natl. Acad. Sci. USA. 1976;73:1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson C, Lindvall-Axelsson M, Owman C. Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res. Rev. 1992;17:109–138. doi: 10.1016/0165-0173(92)90011-a. [DOI] [PubMed] [Google Scholar]
- 18.Onoda M, Suarez-Quian CA, Djakiew D, et al. Characterization of Sertoli cells cultured in the bicameral chamber system: Relationship between formation of permeability barriers and polarized secretion of transferrin. Biol. Reprod. 1990;43:672–683. doi: 10.1095/biolreprod43.4.672. [DOI] [PubMed] [Google Scholar]
- 19.Peraldi-Roux S, Nguyen-Than Dao B, Hirn M, et al. Choroidal ependymocytes in culture: expression of markers of polarity and function. Int. J. Dev. Neurosci. 1990;8:575–588. doi: 10.1016/0736-5748(90)90050-c. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Wright EM. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J. Physiol. 1983;336:635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. Vol. 7. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1989. pp. 6–7.pp. 11 [Google Scholar]
- 22.Smith QR. The blood-brain barrier and the regulation of amino acid uptake and availability to brain. Adv. Exp. Med. Biol. 1991;291:55–71. doi: 10.1007/978-1-4684-5931-9_6. [DOI] [PubMed] [Google Scholar]
- 23.Southwell BR, Duan W, Alcorn D, et al. Thyroxine transport to the brain: role of protein synthesis by the choroid plexus. Endocrinology. 1993;133:2116–2126. doi: 10.1210/endo.133.5.8404661. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi M, Skinner MK, Sanders-Bush E. Transferrin gene expression and synthesis by cultured choroid plexus epithelial cells. J. Biol. Chem. 1989;264:9626–9631. [PubMed] [Google Scholar]
- 25.Whittico MT, Hui AC, Giacomini KM. Preparation of brush border membrane vesicles from bovine choroid plexus. J. Pharmacol. Methods. 1991;25:215–227. doi: 10.1016/0160-5402(91)90012-t. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W. The choroid plexus and metal toxicities. In: Chang W, Magos L, Suzuki T, editors. Toxicology of metals. CRC Press; New York: 1996. pp. 609–626. [Google Scholar]
- 27.Zheng W, Perry DF, Nelson DL, et al. Protection of cerebrospinal fluid against toxic metals by the choroid plexus. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- 28.Zheng W, Shen H, Blaner SB, et al. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: the role of the choroid plexus. Toxicol. Appl. Pharmacol. 1996;139:445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]