Dimethyl fumarate (DMF) is an oral medication for multiple sclerosis (MS) that is prescribed in part for its favorable safety profile. There have been 5 reported cases of progressive multifocal leukoencephalopathy (PML) with the use of fumaric acid esters in patients with psoriasis.1 Some have been on concomitant or prior immunosuppression and most had absolute lymphocyte counts (ALC) below 500. To date, 4 cases of PML (one published) have been reported in patients with MS on DMF (personal communication, Biogen, 2016).2 Herein, we report the details of one of these 4 cases: nonfatal PML in a patient with MS on DMF.
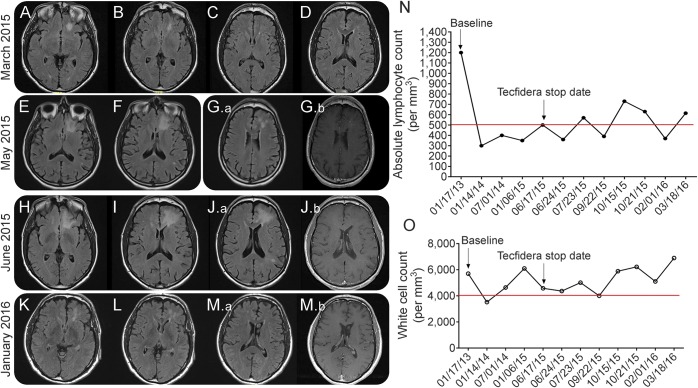
A 64-year-old man with primary progressive MS (PPMS) with no exposure to immunosuppressant or natalizumab started DMF 240 mg twice daily on May 2013. He had progressive leg numbness, spasticity, and imbalance. DMF was offered as an “off-label” treatment because of the reported antioxidative and neuroprotective effects in the midst of a treatment landscape with no known effective treatment for PPMS. He was seen in clinic for follow-up in June 2015. The first pertinent MRI on March 2015 showed a new nonenhancing left frontal lesion interpreted by radiology as a new MS lesion (figure, A and B). His brain MRI was repeated on May 2015 after he complained of disorientation. It showed expansion of the frontal lesion, again interpreted as MS progression (figure, E–G). Close examination revealed some patchy enhancement (figure, G.b). He had no new symptoms and his examination was unchanged. DMF was discontinued and MRI was repeated (figure, H–J.b). Grade 3 lymphopenia was present over the course of treatment (figure, N). Both CD4 and CD8 counts were reduced: CD4 was 96 (441–2,156) and CD8 was 14 (125–1,312); reduction in CD8 count was more profound leading to increase in CD4/CD8 ratio: 6.77 (1.2–5.3). Anti-JCV (John Cunningham virus) serum antibody was 1.31. HIV testing was negative. JCV DNA from CSF PCR, 2013 (CSF) PCR, was detectable at 12 copies/mL in 2 separate occasions (Focus and Quest diagnostics), and definite PML was diagnosed by 2013 consensus criteria.3 White blood count remained normal except one count (figure, O). He was started on mirtazapine 30 mg daily for a short period. He did not receive steroid treatment. In October, he noticed retro-orbital headache, a symptom that has persisted. An MRI on July 23 demonstrated an interval decrease in the size of the frontal lobe lesion and enhancement was no longer present. At his last appointment in January 2016, the patient reported constant retro-orbital headache but his neurologic examination was stable. Repeat MRI showed continued reduction in lesion size (figure, K–M.b). His lymphopenia persisted for a few months after discontinuation of DMF. His last ALC was 610 (March 2016) and his white blood count was normal (figure, N). CD4 and CD8 counts remained low (CD4:182; CD8:82) and the CD4/CD8 ratio was 5.8. We were unable to obtain repeat CSF testing because the patient did not consent.
Figure. MRI appearance of PML, graphs representing cell counts at baseline, on DMF and after discontinuation.
The initial MRI, axial fluid-attenuated inversion recovery, in March 2015 shows a T2 lesion in the left frontal white matter resulting from PML (A–D). Two subsequent follow-up MRIs, in May 2015 and June 2015, illustrate progression of PML with some infiltration to the adjacent white matter structures (E–J.b). There is evidence of patchy enhancement within the lesion in the May 2015 MRI (G.a, G.b). Diffusion-weighted imaging did not reveal evidence of restriction (not shown). Improvement was seen initially on brain MRI on July 23 (not shown). Last follow-up MRI in January 2016, 7 months after stopping DMF, shows significant decrease in the size of the PML lesion (K–M.a) and complete resolution of the enhancement previously noted (M.b). Absolute lymphocyte (N) and white blood cell (O) counts in the patient while on treatment with DMF and after stopping treatment. The lower limit of the normal range is indicated with a red line for leukocytes and lymphocytes. DMF = dimethyl fumarate; PML = progressive multifocal encephalopathy.
Our patient was on DMF for 22 months before MRI scan showed evidence of PML. It is important to be wary of the diagnosis because early symptoms may be mild and overlooked, or MRI results may be misinterpreted as a new MS lesion. A good general maxim might be to consider PML as a potential cause of new brain lesions in patients with PPMS, given the relatively lower frequency of lesion development in PPMS. Low CD4+ T cells in patients with end-stage HIV increases the risk of PML development. There is a significant reduction in CD4+ and CD8+ T cells in the blood of patients on DMF, but reduction of CD8+ T cells is more pronounced. DMF causes significant reduction of memory T cells both in patients with and without lymphopenia. However, only lymphopenic patients had a disproportionate loss of CD8+ T cells.4,5 In clinical practice, about 6% of patients with MS on DMF are found to have grade 3 lymphopenia. Patients with significant lymphopenia took at least 6 months to achieve a normal ALC after DMF discontinuation.6 Our patient currently remains lymphopenic.
Because of the accumulating evidence, we believe that strong consideration should be given to discontinuation of DMF in patients with persistent lymphopenia. Other opportunistic infections may also be possible, given reported infectious complications that occurred with fumaric acid esters.7 Low CD4 counts might especially increase risk of PML, if HIV-related PML serves as any guide, although no monitoring guidelines for CD4/CD8 monitoring are available. The manufacturer estimates that 175,000 patients have been treated with DMF. Current estimates of PML risk to an individual receiving DMF are low but the safety profile for long-term treatment remains unknown and caution should be exercised when DMF is used as “off-label” treatment.
Footnotes
Author contributions: Dr. Moogeh Baharnoori: acquisition of data, preparing the manuscript, interpretation of clinical data. Dr. Jennifer Lyons: critical revision of the manuscript for important intellectual content. Dr. Akram Dastagir: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Igor Koralnik: critical revision of the manuscript for important intellectual content. Dr. James Stankiewicz: preparing the manuscript, interpretation of clinical data, critical revision of the manuscript for important intellectual content, study supervision.
Study funding: No targeted funding.
Disclosure: M. Baharnoori received research support from Canadian Network of MS Clinics. J.L. Lyons served as a guest editor for Current Infectious Disease Reports, and as a guest editor and on the editorial advisory board for The Neurohospitalist. A. Dastagir served on the advisory board for Aubagio, will receive travel funding from Novartis, received research support from Medscape Surveys. I. Koralnik served on the scientific advisory board for Hoffmann-La Roche, GlaxoSmithKline, Merck Serono, Johnson & Johnson, MedImmune, serves on the editorial board for Journal of NeuroVirology, Annals of Neurology, receives publishing royalties from UpToDate, consulted for Bristol-Myers Squibb, Ono Pharmaceutical, Merck Serono, Hoffmann-La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceuticals, Johnson & Johnson, received research support from Biogen Idec, NIH, National MS Society. J.M. Stankiewicz served on the scientific advisory board for Biogen Idec, Hoffmann-La Roche, Teva Neuroscience, Bayer Healthcare, EMD Serono, Genzyme, Novartis, consulted for Novartis, Teva Neuroscience, Genzyme, Hoffmann-La Roche. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Williamson EM, Berger JR. Central nervous system infections with immunomodulatory therapies. Continuum 2015;21:1577–1598. [DOI] [PubMed] [Google Scholar]
- 2.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 2015;372:1476–1478. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Aksamit AJ, Clifford DB, et al. . PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76. doi: 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longbrake EE, Ramsbottom MJ, Cantoni C, Ghezzi L, Cross AH, Piccio L. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler 2016;22:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 2015;21:796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipp S, Kokolakis G, Hund M, et al. . Immunological changes in psoriasis patients under long-term treatment with fumaric acid esters: risk of Kaposi sarcoma occurrence? Eur J Dermatol 2013;23:339–343. [DOI] [PubMed] [Google Scholar]