Abstract
BACKGROUND
In a phase 3, randomized, open-label trial (COMPARZ; NCT00720941), pazopanib was found to be non-inferior to sunitinib in terms of progression-free survival in patients with metastatic renal cell carcinoma with no prior therapy. Overall treatment differences were evaluated in a post hoc analysis using a quality-adjusted time without symptoms of disease or toxicity of treatment (Q-TWiST) methodology.
METHODS
Each patient’s overall survival was partitioned into 3 mutually exclusive health states: grade 3 or 4 toxicity (TOX), time without symptoms of disease or grade 3/4 toxicity (TWiST), and time after progression or relapse (REL). Time spent in each state was weighted by a health-state utility associated with that state and summed to calculate the Q-TWiST. A threshold utility analysis was used, applying utilities across the range of 0 (similar to death) to 1 (perfect health).
RESULTS
A total of 1,110 patients were enrolled (557 pazopanib, 553 sunitinib). The mean time spent with TOX was 31 days (95% confidence interval, 13–49) higher for sunitinib compared with pazopanib. In the threshold utility analysis, the difference in Q-TWiST ranged from -11 days (utility TOX=1, REL=0) to 43 days (TOX=0, REL=1), in favor of pazopanib across most utility combinations. Differences were significant in less than half of the utility combinations examined, typically when the utility for TOX was lower than the utility for REL.
CONCLUSIONS
Patients randomized to pazopanib had slightly longer Q-TWiST compared with sunitinib patients, primarily due to a reduced length of time spent with grade 3/4 toxicities.
Keywords: renal cell carcinomas, pazopanib, sunitinib, drug toxicity, angiogenesis inhibitors
INTRODUCTION
Pazopanib and sunitinib are oral angiogenesis inhibitors approved for the treatment of patients with advanced/metastatic renal cell carcinoma (mRCC).1,2 COMPARZ was a phase 3 randomized, open-label trial to assess non-inferiority of pazopanib compared with sunitinib in patients with mRCC without prior systemic therapy (NCT00720941 and NCT01147822).3,4 The primary non-inferiority endpoint was progression-free survival (PFS). Median PFS was found to be similar between the 2 arms,3 and median overall survival (OS) was 28.3 months for pazopanib and 29.1 months for sunitinib (hazard ratio [HR] for death with pazopanib vs sunitinib: 0.92; stratified log-rank P = .24).4
While it was anticipated that the 2 targeted drugs would have similar efficacy, data were collected to evaluate potential differences in secondary endpoints of safety and quality of life (QOL). In comparison to PFS findings, the safety and QOL profiles favored pazopanib. Adverse events (any grade) were reported more frequently (>5%) with sunitinib versus pazopanib and were significant for hand-foot syndrome, mucosal inflammation, stomatitis, hypothyroidism, dysgeusia, dyspepsia, epistaxis, and fatigue. Similarly, QOL analyses suggested a benefit to pazopanib for many of the QOL domains, including fatigue and soreness of the mouth, throat, hands, and feet.3
A further step to examine the differences between pazopanib and sunitinib is to conduct a quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis. The Q-TWiST method allows for a broad estimate of treatment difference that incorporates OS, progression, toxicities, and health-related QOL.5–7 The survival time can be partitioned into different health states: time with grade 3 or 4 toxicity (TOX), time without symptoms of disease or grade 3/4 toxicity of treatment (TWiST), and time after tumor progression or relapse (REL). The time spent in each state is then weighted by a health-state utility associated with that state and summed to calculate the Q-TWiST. If data are not available to estimate the health utility for each state, a sensitivity-threshold analysis can be conducted where the utilities for each non-TWiST state are varied across a range of values.
Although comparisons between pazopanib and sunitinib have been conducted on survival, safety, and QOL as distinct endpoints, little is known about the health utility of each state for pazopanib compared with sunitinib. Therefore, the aim of this study was to use Q-TWiST analysis to evaluate the overall effect of pazopanib and sunitinib treatment differences on the quality of survival in patients with mRCC.
METHODS
Patients and Procedures
This phase 3, open-label, non-inferiority trial randomly assigned patients with clear-cell mRCC to treatment with pazopanib or sunitinib. Patients were randomized in a 1:1 ratio to receive a continuous dose of pazopanib (800 mg/day with no days off) or sunitinib in 6-week cycles (50 mg/day for 4 weeks, then 2 weeks without treatment). The primary non-inferiority endpoint of PFS was met3 and OS was similar between both arms.4 The CONSORT diagram for this trial is provided in Figure 1. Additional details about the study procedures, including ethics review, are described elsewhere.3
Figure 1.
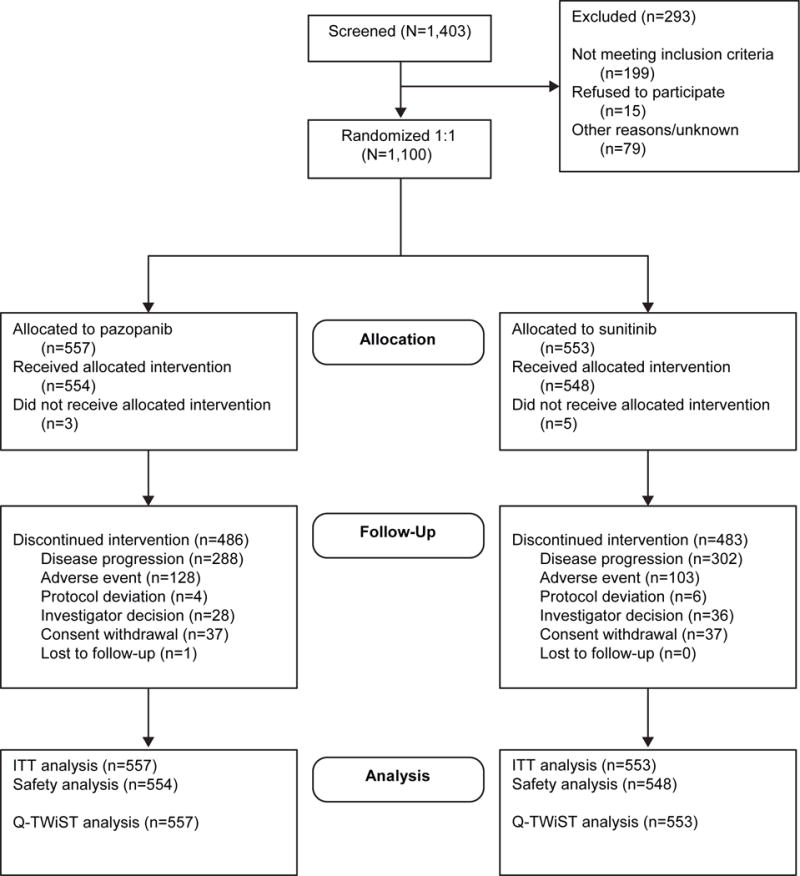
CONSORT diagram. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; ITT, intent to treat; Q-TWiST, quality-adjusted time without symptoms or toxicity.
Measures
Health States
The OS time was partitioned into 3 health states: TOX, TWiST, and REL. Time with toxicities (TOX) was defined as the sum of all time spent with grade 3 or 4 adverse events (AEs), excluding gaps and AEs that occurred after progression. The toxicity time periods for an individual patient need not be contiguous. For example, if patient A experiences a toxicity for 8 days in cycle 1, 3 days in cycle 2, and 12 days in cycle 5—then their TOX = 23 days. Furthermore, TOX counts calendar time—so if a patient is experiencing grade 3 nausea and grade 3 fatigue for the same 4-day period, it counts as 4 days toward TOX, not 8. Time after progression or relapse (REL) was defined as the OS less the PFS. All remaining time is part of the TWiST period—before progression or death and not experiencing grade 3 or 4 AEs. For OS, the date for data cutoff was May 2012. Progression-free survival was assessed by independent review. Exploratory analyses included lower-grade toxicities in the toxicity state definition.
Safety
All AEs were graded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.8 Any AEs occurring after progression were not included. Cardiac function was monitored on electrocardiograms or multigated acquisition scans every 3 cycles. Time with grade 5 AEs before death were included in the analysis; however, these only made up 1% of the AEs analyzed.
Statistical Analyses
Event and censoring times for OS and PFS followed the definitions of the main trial statistical analysis plan. Estimates were restricted to the first 600 days of follow-up (ie, the approximate median follow-up time) and mean estimates are therefore referred to as restricted means.
The time spent in each health state was weighted by the health-state utility associated with that state and then summed to calculate the Q-TWiST. Health-state utility values range from 0 (equivalent to death) to 1 (perfect health). It is common practice to assume a weight of 1.0 for the TWiST state, with other states discounted from 1.0. Although not all patients in the TWiST state value their health as perfect, anchoring health states to a perfect TWiST score is a useful way to compare treatment arms with regard to differences in time living with toxicity and disease progression.
Because the COMPARZ study did not administer a health utility measure, a sensitivity-threshold analysis was conducted in which the utilities for each non-TWiST state were varied across the entire range of 0 to 1 to show the full range of possible results depending on the utility values assigned to TOX and REL. Health-state utility values from the literature were used to interpret the threshold analysis by identifying the values that were most consistent with the literature. Finally, the difference between treatment arms in mean Q-TWiST was calculated and the bootstrap used to calculate P values and 95% confidence intervals (CIs).
RESULTS
A total of 1,110 patients were enrolled (557 pazopanib, 553 sunitinib) in the COMPARZ trial. Patients were predominantly male (71% pazopanib, 75% sunitinib), had lung metastases at baseline (76% pazopanib, 77% sunitinib), and had Karnofsky performance status scores of 90 or 100 (75% pazopanib, 76% sunitinib). Additional baseline demographic and disease characteristics are presented in Table 1.6
Table 1.
Baseline Patient Demographics and Disease Characteristics
| Pazopanib (n = 557) | Sunitinib (n = 553) | |
|---|---|---|
| Median age, years (range) | 61 (18-88) | 62 (23–86) |
| Male sex, n (%) | 398 (71) | 415 (75) |
| Prior nephrectomy, n (%) | 459 (82) | 465 (84) |
| Karnofsky performance status, n (%) | ||
| 70 or 80 | 141 (25) | 130 (24) |
| 90 or 100 | 416 (75) | 423 (76) |
| Lactate dehydrogenase, n (%) | ||
| > 1.5 × ULN | 40 (7) | 29 (5) |
| ≤ 1.5 × ULN | 517 (93) | 524 (95) |
| Most common metastatic sites, n (%) | ||
| Lung | 424 (76) | 425 (77) |
| Lymph node | 223 (40) | 247 (45) |
| Bone | 110 (20) | 85 (15) |
| Liver | 86 (15) | 110 (20) |
| MSKCC risk category,* n (%) | ||
| Favorable | 151 (27) | 152 (27) |
| Intermediate | 322 (58) | 328 (59) |
| Poor | 67 (12) | 52 (9) |
| Unknown | 17 (3) | 21 (4) |
Abbreviations: MSKCC, Memorial Sloan-Kettering Cancer Center; ULN, upper limit of normal.
Revicki DA, et al.6
The median overall follow-up time was approximately 600 days; therefore, the restricted Q-TWiST estimates were calculated using this cutoff. The restricted mean health-state durations, prior to utility weighting, are listed in Table 2. The mean number of days in TOX was 31 days longer in the sunitinib arm versus the pazopanib arm. This difference was offset by reduced time in TWiST and REL. The partitioned survival plots are presented in Figure 2.
Table 2.
Restricted Mean Health-State Durations (days)
| TOX | TWiST | REL | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SE) | 95% CI | Mean (SE) | 95% CI | Mean (SE) | 95% CI | |
| Pazopanib | 68 (6) | 57 to 78 | 248 (10) | 229 to 267 | 169 (9) | 152 to 186 |
| Sunitinib | 98 (7) | 84 to 112 | 228 (10) | 210 to 245 | 145 (9) | 128 to 162 |
| Difference (pazopanib – sunitinib) | −31 (9) | −48 to −13 | 20 (14) | −7 to 46 | 24 (12) | 0 to 48 |
Abbreviations: CI, confidence interval; REL, time after tumor progression or relapse; SE, standard error; TOX, time with grade 3 or 4 toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
Figure 2.
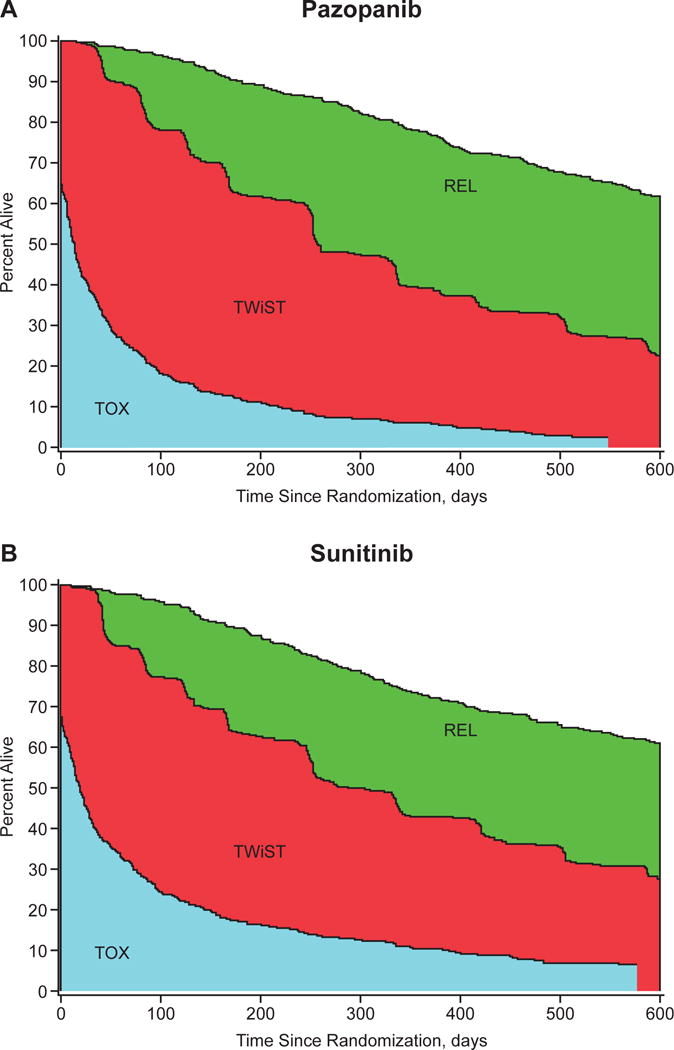
Partitioned survival curves for (A) the pazopanib group and (B) the sunitinib group. Abbreviations: REL, time after tumor progression or relapse (bounded by the overall survival and progression-free survival curves); TOX, total time with grade 3 or 4 toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
The threshold analysis is presented in Table 3 and Figure 3. The difference in Q-TWiST ranged from −11 days to 43 days, nearly always in favor of pazopanib. For example, when TOX was weighted at 0.5 relative to TWiST and REL was also weighted at 0.5, the Q-TWiST difference between the arms was 16 days (95% CI, −4 to 36), in favor of pazopanib but not significantly different from zero. When TOX was weighted at 0.75 and REL 0.5, the Q-TWiST difference was 9 days (95% CI, −12 to 29), again in favor of pazopanib but not significantly different from zero. Only 3 of the 25 utility combinations examined favored sunitinib (nonsignificantly): TOX = 1 with REL = 0 or 0.25, and TOX = 0.75 with REL = 0. Differences were statistically significant for fewer than half the utility combinations examined; significant differences were typically observed when the utility weight for toxicity was less than the utility weight for REL, as demonstrated by the darker shading in the lower right corner of the threshold plot (Figure 3). For example, when TOX was weighted at 0.5 and REL 1.0, the Q-TWiST difference was 28 days (95% CI, 8 to 48), in favor of pazopanib.
Table 3.
Threshold Analysis: Q-TWiST Difference Between Treatment Groups
| Utilities | Q-TWiST (SE) | Difference in Q-TWiST | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| TOX | TWiST | REL | Pazopanib | Sunitinib | Diff (SE) | 95 % CI |
| 0 | 1 | 0 | 248 (10) | 228 (10) | 20 (14) | −7 to 46 |
| 0.25 | 1 | 0 | 265 (9) | 253 (9) | 12 (13) | −14 to 38 |
| 0.5 | 1 | 0 | 282 (9) | 278 (9) | 4 (13) | −22 to 30 |
| 0.75 | 1 | 0 | 299 (10) | 302 (10) | −3 (14) | −30 to 23 |
| 1 | 1 | 0 | 316 (10) | 327 (10) | −11 (14) | −39 to 17 |
|
| ||||||
| 0 | 1 | 0.25 | 290 (8) | 265 (9) | 26 (12) | 2 to 49 |
| 0.25 | 1 | 0.25 | 307 (8) | 289 (8) | 18 (12) | −5 to 40 |
| 0.5 | 1 | 0.25 | 324 (8) | 314 (8) | 10 (11) | −12 to 32 |
| 0.75 | 1 | 0.25 | 341 (8) | 339 (8) | 3 (12) | −20 to 25 |
| 1 | 1 | 0.25 | 358 (8) | 363 (9) | −5 (12) | −29 to 19 |
|
| ||||||
| 0 | 1 | 0.5 | 333 (8) | 301 (8) | 32 (11) | 10 to 53 |
| 0.25 | 1 | 0.5 | 350 (7) | 326 (7) | 24 (10) | 4 to 44 |
| 0.5 | 1 | 0.5 | 366 (7) | 350 (7) | 16 (10) | −4 to 36 |
| 0.75 | 1 | 0.5 | 383 (7) | 375 (7) | 9 (10) | −12 to 29 |
| 1 | 1 | 0.5 | 400 (8) | 399 (8) | 1 (11) | −20 to 22 |
|
| ||||||
| 0 | 1 | 0.75 | 375 (8) | 337 (8) | 38 (11) | 16 to 59 |
| 0.25 | 1 | 0.75 | 392 (7) | 362 (7) | 30 (10) | 10 to 50 |
| 0.5 | 1 | 0.75 | 409 (7) | 386 (7) | 22 (10) | 3 to 41 |
| 0.75 | 1 | 0.75 | 426 (7) | 411 (7) | 14 (10) | −4 to 33 |
| 1 | 1 | 0.75 | 442 (7) | 436 (7) | 7 (10) | −13 to 27 |
|
| ||||||
| 0 | 1 | 1 | 417 (8) | 374 (9) | 43 (12) | 20 to 66 |
| 0.25 | 1 | 1 | 434 (7) | 398 (8) | 36 (11) | 15 to 57 |
| 0.5 | 1 | 1 | 451 (7) | 423 (7) | 28 (10) | 8 to 48 |
| 0.75 | 1 | 1 | 468 (7) | 447 (7) | 20 (10) | 1 to 40 |
| 1 | 1 | 1 | 485 (7) | 472 (8) | 13 (10) | −8 to 33 |
Rows in bold indicate differences that are significantly different from zero.
Abbreviations: CI, confidence interval; Q-TWiST, quality-adjusted time without symptoms or toxicity; REL, time after tumor progression or relapse; SE, standard error; TOX, time with grade 3 or 4 toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
Figure 3.
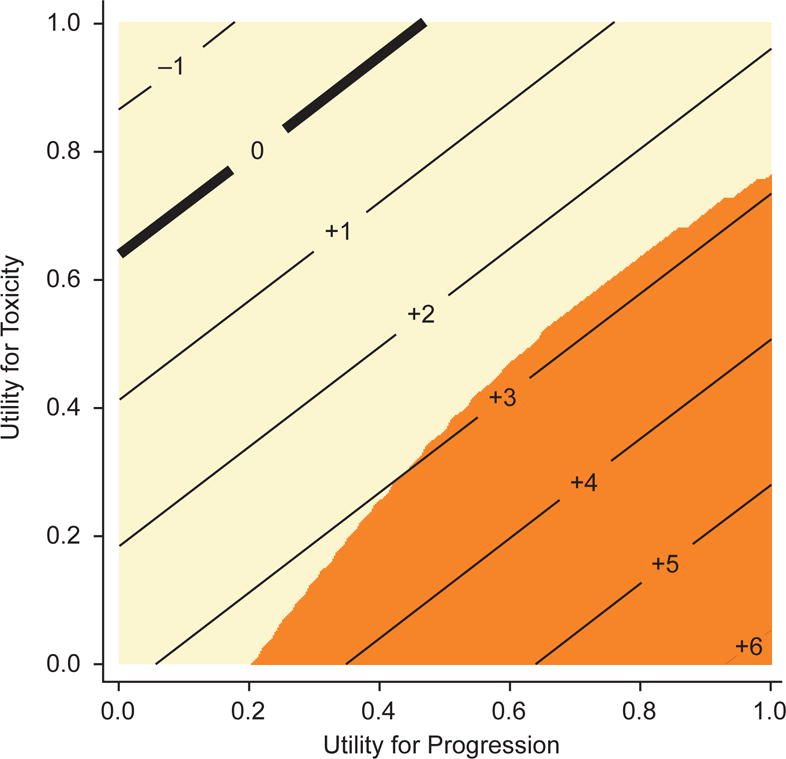
Threshold plot. Integers represent the estimated difference in Q-TWiST between arms (in weeks) for the corresponding combination of utilities for toxicity and progression; orange-shaded areas are significantly different from zero, in favor of pazopanib; tan-shaded areas indicate no significant difference. Abbreviation: Q-TWiST, quality-adjusted time without symptoms or toxicity.
The analyses were repeated with toxicities of grade 2 or higher in the TOX state. The restricted mean health-state durations are listed in Table 4. The mean number of days in TOX was now only 20 days longer in the sunitinib arm compared to the pazopanib arm. This difference was again offset by reduced time in TWiST and REL. The partitioned survival plots are presented in Figure 4.
Table 4.
Restricted Mean Health-State Durations (days)
| TOX (Grade 2+) | TWiST | REL | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SE) | 95% CI | Mean (SE) | 95% CI | Mean (SE) | 95% CI | |
| Pazopanib | 193 (9) | 175 to 211 | 123 (9) | 105 to 142 | 169 (9) | 152 to 186 |
| Sunitinib | 213 (9) | 194 to 231 | 114 (9) | 97 to 131 | 145 (9) | 128 to 162 |
| Difference (pazopanib – sunitinib) | −20 (13) | −46 to 6 | 9 (13) | −16 to 34 | 24 (12) | 0 to 48 |
Abbreviations: CI, confidence interval; REL, time after tumor progression or relapse; SE, standard error; TOX, time with grade 2 or higher toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
Figure 4.
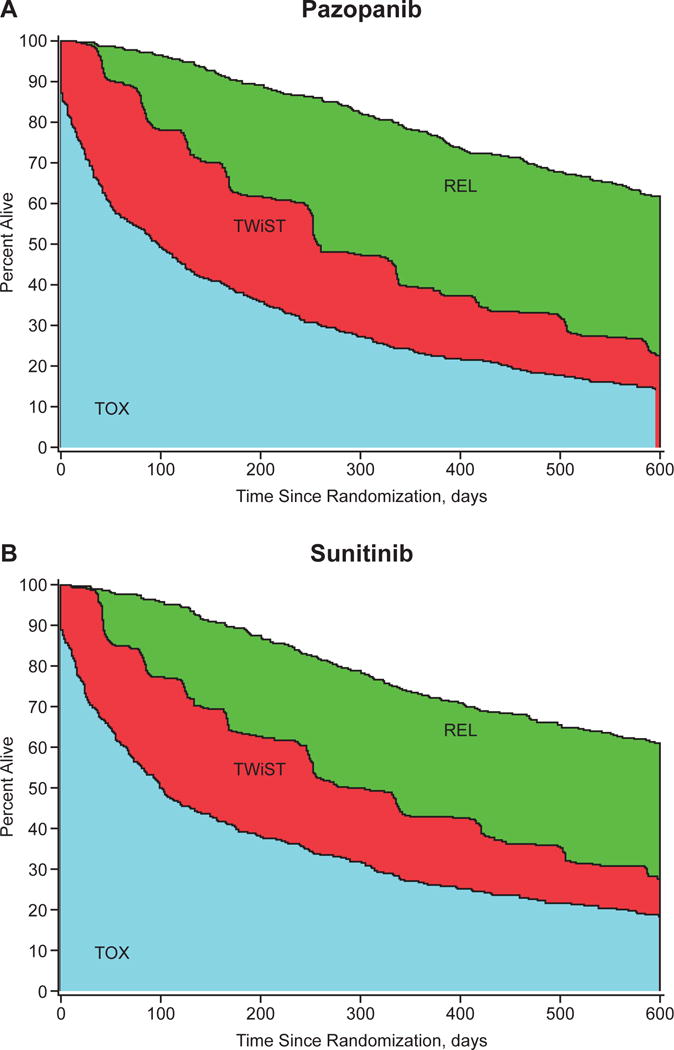
Partitioned survival curves for (A) the pazopanib group and (B) the sunitinib group, TOX defined as grade 2 or higher. Abbreviations: REL, time after tumor progression or relapse (bounded by the overall survival and progression-free survival curves); TOX, time with grade 2 or higher toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
The threshold analysis is presented in Table 5 and Figure 5. The difference in Q-TWiST ranged from −11 days to 33 days, nearly always in favor of pazopanib. Differences were statistically significant for very few utility combinations. For values of 0.5 for both the toxicity and progression states, the estimated Q-TWiST difference was 11 days (95% CI, −7 to 28), in favor of pazopanib but not statistically significant.
Table 5.
Threshold Analysis, TOX Defined as Grade 2 or Higher
| Utilities | Q-TWiST (SE) | Difference in Q-TWiST | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| TOX | TWiST | REL | Pazopanib | Sunitinib | Diff (SE) | 95% CI |
| 0 | 1 | 0 | 123 (9) | 114 (9) | 9 (13) | −16 to 34 |
| 0.25 | 1 | 0 | 171 (9) | 167 (8) | 4 (12) | −19 to 27 |
| 0.5 | 1 | 0 | 220 (8) | 221 (8) | −1 (12) | −24 to 22 |
| 0.75 | 1 | 0 | 268 (9) | 274 (9) | −6 (13) | −31 to 19 |
| 1 | 1 | 0 | 316 (10) | 327 (10) | −11 (14) | −39 to 17 |
|
| ||||||
| 0 | 1 | 0.25 | 165 (8) | 150 (8) | 15 (12) | −8 to 38 |
| 0.25 | 1 | 0.25 | 214 (8) | 204 (7) | 10 (10) | −11 to 30 |
| 0.5 | 1 | 0.25 | 262 (7) | 257 (7) | 5 (10) | −15 to 24 |
| 0.75 | 1 | 0.25 | 310 (8) | 310 (8) | −0 (11) | −21 to 21 |
| 1 | 1 | 0.25 | 358 (8) | 363 (9) | −5 (12) | −29 to 19 |
|
| ||||||
| 0 | 1 | 0.5 | 208 (8) | 187 (8) | 21 (11) | −2 to 43 |
| 0.25 | 1 | 0.5 | 256 (7) | 240 (7) | 16 (10) | −3 to 35 |
| 0.5 | 1 | 0.5 | 304 (6) | 293 (6) | 11 (9) | −7 to 28 |
| 0.75 | 1 | 0.5 | 352 (7) | 346 (7) | 6 (9) | −13 to 24 |
| 1 | 1 | 0.5 | 400 (8) | 399 (8) | 1 (11) | −20 to 22 |
|
| ||||||
| 0 | 1 | 0.75 | 250 (8) | 223 (8) | 27 (12) | 3 to 50 |
| 0.25 | 1 | 0.75 | 298 (7) | 276 (7) | 22 (10) | 2 to 41 |
| 0.5 | 1 | 0.75 | 346 (6) | 329 (6) | 17 (9) | −1 to 34 |
| 0.75 | 1 | 0.75 | 394 (6) | 382 (6) | 12 (9) | −6 to 29 |
| 1 | 1 | 0.75 | 442 (7) | 436 (7) | 7 (10) | −13 to 27 |
|
| ||||||
| 0 | 1 | 1 | 292 (9) | 259 (9) | 32 (13) | 6 to 59 |
| 0.25 | 1 | 1 | 340 (8) | 312 (8) | 28 (11) | 6 to 50 |
| 0.5 | 1 | 1 | 388 (7) | 366 (7) | 23 (10) | 3 to 42 |
| 0.75 | 1 | 1 | 436 (7) | 419 (7) | 18 (10) | −1 to 36 |
| 1 | 1 | 1 | 485 (7) | 472 (8) | 13 (10) | −8 to 33 |
Rows in bold indicate differences that are significantly different from zero.
Abbreviations: CI, confidence interval; Q-TWiST, quality-adjusted time without symptoms or toxicity; REL, time after tumor progression or relapse; SE, standard error; TOX, time with grade 2 or higher toxicity; TWiST, time without symptoms of disease or toxicity of treatment.
Figure 5.
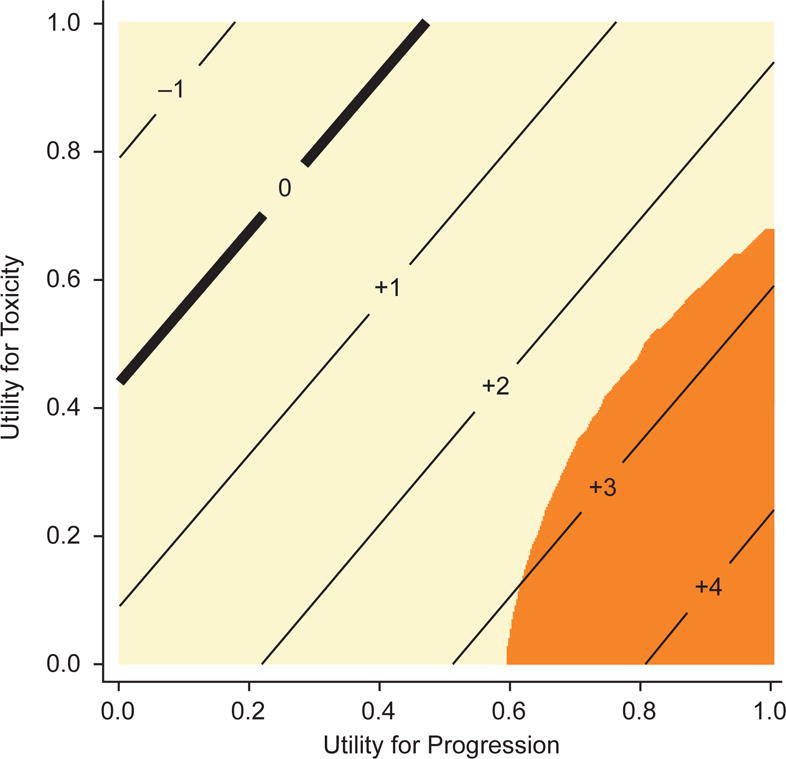
Threshold plot, TOX defined as grade 2 or higher. Integers represent the estimated difference in Q-TWiST between arms (in weeks) for the corresponding combination of utilities for toxicity and progression; orange-shaded areas are significantly different from zero, in favor of pazopanib; tan-shaded areas indicate no significant difference. Abbreviations: Q-TWiST, quality-adjusted time without symptoms or toxicity; TOX, time with grade 2 or higher toxicity.
DISCUSSION
The Q-TWiST approach with threshold analysis evaluated all possible combinations of health-state utility weights applied to toxicity or progression. This report is the first study to use a Q-TWiST analysis to examine the survival quality for pazopanib versus sunitinib. Several combinations of weights for toxicity or progression significantly favored pazopanib. In contrast, no combination of weights significantly favored sunitinib.
Pazopanib-treated patients had slightly longer clinical benefits based on Q‐TWiST scores versus sunitinib-treated patients, primarily due to a reduced length of time spent with grade 3/4 toxicities. This difference (−11 to 43 days, depending on utility combination) was <10% of OS (median, 28 months). Therefore, while there was a statistically significant benefit for pazopanib versus sunitinib in Q-TWiST analyses, the magnitude of that difference tended to be rather small.
Similarly, under certain conditions of the weight or value patients place on toxicity over progression, pazopanib demonstrated superior Q-TWiST versus sunitinib. A large proportion of the significant results occurred in combinations where REL was weighted similarly to TWiST, and these may not be clinically reasonable combinations. Scenarios where REL or TOX have a weight of 1.0, equivalent to TWiST, are similarly not likely to represent true patient experience but provide the boundaries for the comprehensive threshold analysis. Compared with patients on sunitinib, patients on pazopanib had longer PFS after accounting for the total time with significant toxicity. However, when toxicities included AEs that were grade 2 or higher, there were fewer significant Q-TWiST differences between pazopanib and sunitinib.
When conducting Q-TWiST analyses, it is important to identify the relevant health-state utility values in order to provide meaningful interpretations of the threshold analyses. In a previous study of mRCC in the general public, the following health-state utility ratings were obtained: stable disease = 0.795, progressive disease = 0.355, grade 1/2 fatigue = 0.751, and grade 3 hand-foot syndrome = 0.469.9 These values support an approximate relative utility of 0.5 for both the toxicity and progression states, which corresponds to an estimated Q-TWiST difference of 16 days (95% CI, −4 to 36) in favor of pazopanib.
Interpretation of the magnitude of Q-TWiST differences should consider OS and time to progression. In a scenario where OS or time to progression is shorter, smaller differences in Q‐TWiST may be meaningful. General guidelines relative to OS have been developed based on a review of the oncology clinical trial literature, suggesting that Q-TWiST differences of 10% to 15% of OS are clinically important.6 Differences of 5% may be meaningful in some settings. The difference seen in this study corresponds to <5% of OS. At present there are no guidelines for interpretation of Q-TWiST scores relative to PFS; however, such guidelines, if established, could potentially prove useful in settings such as this, where little survival difference is anticipated and the primary concerns lie with balancing disease symptoms and treatment toxicities.
A few limitations are worth noting. First, the AEs and related grades of toxicity used were obtained from the NCI CTCAE, which is a provider-driven assessment.10 A more patient-centric paradigm may be useful when assessing safety. For example, the NCI’s Patient-Reported Outcomes version of the CTCAE (PRO-CTCAE)10 would be a useful complement to physician ratings of safety and would help yield a more comprehensive rating of health-state utilities of pazopanib versus sunitinib. Second, the accuracy of AE start and end dates used to calculate duration of TOX is unknown. Finally, the utility weights used for toxicities in our analysis were obtained from a study that queried the general public. However, AEs such as fatigue and mucositis seem to have a profound and persistent effect on patients (ie, there is little adaptation over time).11,12 This effect could be missed or not reflected in the public assessments. On the other hand, utility values based on general population estimates tend to provide lower (ie, worse) ratings compared to those obtained from cancer patients.
This study illustrates the usefulness of the Q-TWiST analytic approach as a quality-adjusted survival model when evaluating treatments for advanced cancer. For some utility weight combinations, patients randomized to pazopanib had slightly longer Q‐TWiST compared to sunitinib patients, primarily due to a reduced length of time spent with grade 3/4 toxicities. For many other utility weight combinations there were no differences, and for only a few, sunitinib was preferred. These findings underscore the competing benefits and risks of pazopanib versus sunitinib and may help guide treatment decision-making for patients with mRCC and their providers.
Acknowledgments
We thank the patients who participated in this study and their families. This study was supported by GlaxoSmithKline (GSK); pazopanib is an asset of Novartis AG as of March 1, 2015. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Nick Cianciola, PhD, ProEd Communications, Inc., for his medical editorial assistance with this manuscript.
Funding:
This study was supported by GlaxoSmithKline. Pazopanib is an asset of Novartis AG as of March 1, 2015. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. Dr. Robert Motzer received support through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Information Summary:
| Jennifer L. Beaumont | Received grants from GlaxoSmithKline for the conduct of the study and personal fees from GlaxoSmithKline, Gilead, and Ipsen outside the submitted work. |
| John M. Salsman | Reports other (support for manuscript writing) from GlaxoSmithKline during the conduct of the study. |
| Jose Diaz | Was employed by GlaxoSmithKline and Novartis, and owned stock in GlaxoSmithKline. |
| Keith Deen | Was employed by GlaxoSmithKline and owned stock in GlaxoSmithKline. |
| Lauren McCann | Was employed by GlaxoSmithKline and owned stock in GlaxoSmithKline. |
| Thomas Powles | Reports personal fees from Roche, Novartis and Pfizer outside the submitted work. |
| Michelle D. Hackshaw | Reports employment (former) by and stock ownership in GlaxoSmithKline. |
| Robert J. Motzer | Reports grants from GlaxoSmithKline during the conduct of the study; grants and personal fees from Novartis and Pfizer outside the submitted work. |
| David Cella | Reports personal fees from GlaxoSmithKline outside the submitted work. |
Supplemental Information = N/A
Contributor Information
Jennifer L. Beaumont, Email: j-beaumont@northwestern.edu.
John M. Salsman, Email: jsalsman@wakehealth.edu.
Jose Diaz, Email: dr.josediaz@outlook.com.
Keith Deen, Email: kcdsdl@msn.com.
Lauren McCann, Email: futed@alum.mit.edu.
Thomas Powles, Email: Thomas.Powles@bartsandthelondon.nhs.uk.
Michelle D. Hackshaw, Email: denise_hacks@yahoo.com.
Robert J. Motzer, Email: motzerr@mskcc.org.
David Cella, Email: d-cella@northwestern.edu.
References
- 1.Votrient [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2012. [Google Scholar]
- 2.Sutent [package insert] New York, NY: Pfizer Labs; 2015. [Google Scholar]
- 3.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370:1769–1770. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 5.Cole BF, Gelber RD, Gelber S, Mukhopadhyay P. A quality-adjusted survival (Q-TWiST) model for evaluating treatments for advanced stage cancer. J Biopharm Stat. 2004;14:111–124. doi: 10.1081/BIP-120028509. [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA, Feeny D, Hunt TL, Cole BF. Analyzing oncology clinical trial data using the Q-TWiST method: clinical importance and sources for health state preference data. Qual Life Res. 2006;15:411–423. doi: 10.1007/s11136-005-1579-7. [DOI] [PubMed] [Google Scholar]
- 7.Sherrill B, Amonkar MM, Stein S, Walker M, Geyer C, Cameron D. Q-TWiST analysis of lapatinib combined with capecitabine for the treatment of metastatic breast cancer. Br J Cancer. 2008;99:711–715. doi: 10.1038/sj.bjc.6604501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Common Terminology Criteria for Adverse Events, v3.0 (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed August 18, 2015.
- 9.Swinburn P, Lloyd A, Nathan P, Choueiri TK, Cella D, Neary MP. Elicitation of health state utilities in metastatic renal cell carcinoma. Current Medical Research and Opinion. 2010;26:1091–1096. doi: 10.1185/03007991003712258. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) National Institutes of Health; Website. Available at: http://healthcaredelivery.cancer.gov/pro-ctcae/. Updated August 5, 2015. Accessed August 18, 2015. [Google Scholar]
- 11.Mohamed AF, Hauber AB, Neary MP. Patient benefit-risk preferences for targeted agents in the treatment of renal cell carcinoma. Pharmacoeconomics. 2011;29:977–988. doi: 10.2165/11593370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15:1139–1148. doi: 10.3111/13696998.2012.708689. [DOI] [PubMed] [Google Scholar]


