Abstract
Carotenoids are ubiquitous pigments that play key roles in photosynthesis and also accumulate to high levels in fruit and flowers. Specific carotenoids play essential roles in human health as these compounds are precursors for Vitamin A; other specific carotenoids are important sources of macular pigments and all carotenoids are important anti-oxidants. Accurate determination of the composition and concentration of this complex set of natural products is therefore important in many different scientific areas. One of the richest sources of these compounds is the fruit of Capsicum; these red, yellow and orange fruit accumulate multiple carotenes and xanthophylls. This report describes the detailed method for the extraction and quantification of specific carotenes and xanthophylls.
Materials and Reagents
Fresh Capsicum fruit (dissected into pericarp tissue, frozen at -80 °C or lyophilized pericarp tissue)
N2 gas
CHCl3 (HPLC grade) (Sigma-Aldrich, catalog number: 472476)
2-propanol (HPLC grade) (Sigma-Aldrich, catalog number: 34863)
Methanol (HPLC grade) (Sigma-Aldrich, catalog number: 179337)
KOH (Sigma-Aldrich, catalog number: P-1767)
Methyl-t-butyl ether (MTBE) (HPLC grade) (Sigma-Aldrich, catalog number: 34875)
β-carotene (Sigma-Aldrich, catalog number: C4582)
Lutein (Sigma-Aldrich, catalog number: 07168)
Lycopene (Sigma-Aldrich, catalog number: 75051)
Antheraxanthin (CaroteNature, catalog number: 0231)
Capsanthin (CaroteNature, catalog number: 0335)
Capsorubin (CaroteNature, catalog number: 0413)
Zeaxanthin (CaroteNature, catalog number: 0119)
Violaxanthin (CaroteNature, catalog number: 0259)
β-cryptoxanthin (CaroteNature, catalog number: 0055)
Methanolic KOH (add solid KOH crystals to methanol until the solution is saturated)
Equipment
Farberware soft grips (Food Chopper, model: 83427-93)
Polytron generator (Polytron Technologies, model: PT 10-35)
Temperature block (with 1.8 ml microfuge tube rack) (Thermolyne Dri-Bath, model: DB-17615)
HPLC system equipped with a photodiode array detector and YMC carotenoid column (4.6 × 250 mm) (Waters)
Bath sonicator (Branson Ultrasonic Cleaner, model: 2510)
Centrifuge (capable of 1,000 × g, 5 min at 4 °C)
Microcentrifuge
UV/Vis spectrophotometer
Vortex mixer
Fume hood
Nitrogen evaporator (Organomation Associates, model: N-EVAP-112)
Procedure
- Extraction
- If using dried (lyophilized) pericarp samples use ∼0.25 to 0.5 g for each extraction, grind samples to a powder in a mortar and pestle. If using fresh frozen pericarp samples, use ∼2.5 to 5 g for each extraction. Chop the frozen pericarp into small pieces measuring ∼4 mm2 using a manual food chopper. See Figure 1.
- Place samples in a 50 ml plastic tube, add ∼25 ml CHCl3 and use the polytron to thoroughly homogenize the sample, ∼20 to 30 sec. See Figure 2.
- Allow the homogenized tissue to sit in the CHCl3 for 30 min with occasional mixing by either a vortex mixer or by a bath sonicator.
- If using frozen fresh samples, centrifuge the sample to separate the CHCl3 phase from the aqueous phase (upper layer); 1,000 × g, 5 min at 4 °C. Keep the CHCl3 phase. If using dried samples, this step is not performed.
- Filter the CHCl3 extract using Whatman 1 (or equivalent) filter paper and a vacuum filter.
- Transfer filtrate to a ∼40 ml glass amber vial and evaporate the solvent using a stream of N2 gas and gentle heating (50 °C). A nitrogen evaporator works well for this step.
- Resuspend the dried sample in 1.1 ml of 2-propanol. Bath sonication is advised to help resuspend the material.
- Material can be stored at -20 or -80 °C for several weeks.
- We keep the extracted sample out of bright light as much as possible. During the time the sample is on the nitrogen evaporator in the fume hood, we keep the lights off in the hood; we store the samples in amber vials and we keep the vials in the dark as much as possible.
- Saponification
- In a standard microfuge tube, combine 0.5 ml of the 2-propanol extract with 0.1 ml of methanolic KOH, in a 1.8 ml microfuge tube.
- Mix well by pipetting, then incubate for 30 min at 50 °C.
- Cool the sample by immersion in an ice bath for ∼2 min, then add 0.5 ml of water.
- Add 0.4 ml of CHCl3. Vortex vigorously.
- Centrifuge for 1-2 min at top speed (10,000 to 12,000 × g) in a microcentrifuge to resolve the phases. Lower centrifugal forces will also resolve the CHCl3 and aqueous phases.
- Remove (discard) the upper phase; recover the lower phase.
- HPLC Analysis
- Sample volume for the HPLC is typically 10 to 30 μl. The volume used depends on the concentration of the carotenoid preparation. Determine the OD 450 nm for a 1: 100 dilution of the carotenoid sample. If the A450 of the diluted sample is 0.5 to 1.0, then inject 10 μl of concentrated sample onto the HPLC. If the A450 is 0.25 to 0.5, then inject 20 μl. If the A450 is 0.1 to 0.25, then inject 30 μl. If the absorbance is above 1.0, then dilute the sample appropriately. If the absorbance is below 0.1, then concentrate the sample appropriately (e.g. evaporate some of the solvent under a stream of N2 gas).
- Chromatography is conducted on an HPLC system with a photodiode array detector using the full visible spectrum (400 - 700 nm) with monitoring employed at 450 nm. Column is a YMC carotenoid column, 250 mm × 4.6 mm, S = 5 μm.
- Flow rate, 2 ml/min, with a linear gradient for 0 to 30 min as below:
- Solvent A: methanol: MTBE: water∷ 81:15:4 (vol: vol: vol)
-
Solvent B: MTBE: methanol: water∷ 88:8:4 (vol: vol: vol)
Time (min) A B
0 100% 0% 30 33% 67% At 31 min we start washing/reconditioning the column:31 0% 100 34 0% 100 35 100% 0 39 100% 0
- Calibration curve
- Calibration curves were generated at 450 nm with the reference standards: β-carotene, lutein, lycopene, capsanthin, capsorubin, zeaxanthin, antheraxanthin, violaxanthin and β-cryptoxanthin.
- Stock solutions at 1 mg/ml are prepared in CHCl3 (hexane can also be used).
- Samples representing the range of 10 ng to 3 μg are prepared by dilution of the stock solution and independently analyzed by HPLC as described above. The retention time of the single peak and the area under the curve is recorded and plotted against the amount of the carotenoid injected.
- In our lab most carotenoids have a linear response at 450 nm for detection between 10 ng to 3 μg, with R2 values of 0.999.
-
The formulas we used to convert HPLC peak area for the specific carotenoids into concentrations expressed as ng/g tissue are:Antheraxanthin (ng/g tissue): (peak area - 1316.8)/5,816.9/ injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)β-carotene (ng/g tissue): (peak area - 55,271)/4,253.5/ injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)β-cryptoxanthin (ng/g tissue): (41,437 + peak area)/6,758.4/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Capsanthin (ng/g tissue): (28,266 + peak area)/4,489.5 injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Capsorubin (ng/g tissue): (26,864 + peak area)/5,541.4/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Lutein (ng/g tissue): (peak area - 1,428.9)/6312/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Lycopene (ng/g tissue): (28,266 + peak area)/4,489.5/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Violaxanthin (ng/g tissue): (28,616 + peak area)/7,453.4/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Zeaxanthin (ng/g tissue): (92,586 + peak area)/7,220.6/injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)Total carotenoids (ng/g tissue): (peak area of entire chromatogram - 55,271)/4,253.5/ injected volume (μl) × sample dilution × undiluted sample volume (μl)/tissue mass (grams)
- For plant sample characterization, triplicate independent extractions are performed on each genotype and the average concentration for each carotenoid is calculated following independent HPLC analyses using the specific calibration curve to determine the abundance of each respective peak (e.g. peaks 1-7 in Figure 3). We use the calibration curve for β-carotene to estimate the abundance of unassigned carotenoid peaks, those that do not elute at the retention time of a reference standard (e.g. peaks a-g in Figure 3).
Figure 1. Processing of fresh Capsicum pericarp.

A. Pericarp samples are placed in lower chamber of food chopper. B. Samples are chopped to very small pieces C.
Figure 2. Homogenization of fresh Capsicum pericarp.
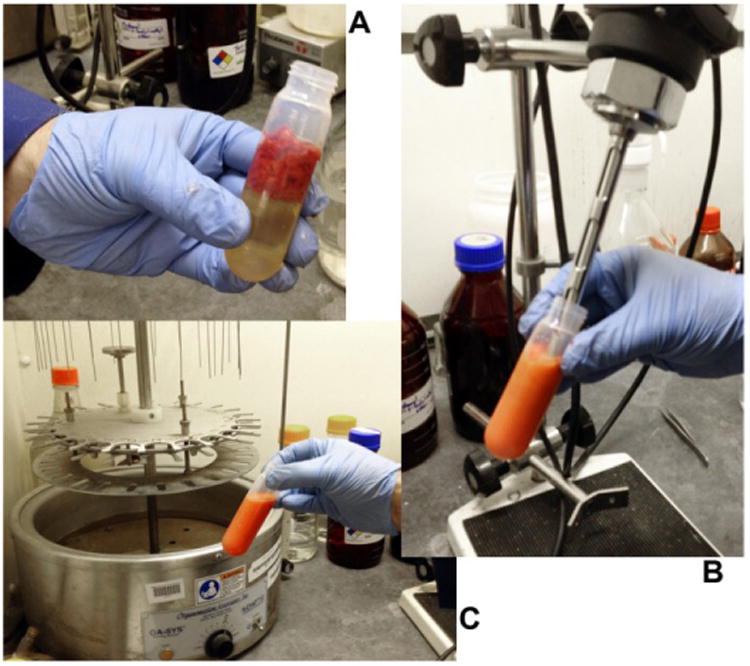
A. Chopped pericarp in a 40 or 50 ml tube with 25 ml CHCl3. B. Sample is homogenized with polytron for 20 to 30 sec. C. Fresh fruit sample is completely dispersed in CHCl3.
Figure 3. HPLC separation of Capsicum annum pericarp extracts.
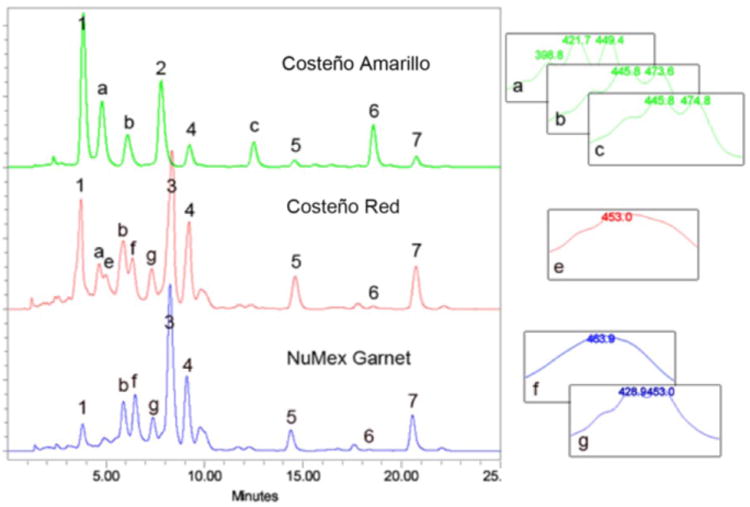
Saponifed carotenoid extracts from mature fruit pericarp from three Capsicum varieties, Costeño Amarillo, Costeño Red and NuMex Garnet. Known peaks are: 1, violaxanthin; 2, lutein; 3, capsanthin; 4, zeaxanthin; 5, β -cryptoxanthin; 6, -carotene; 7, β-carotene. Carotenoid peaks that are not identified are labeled with letters, a-g. Absorption spectra from the photodiode array for the unknown peaks are provided on the right. HPLC conditions as described.
Representative data
Examples of typical chromatograms are shown in Figure 3. The top sample (green line) is from an orange colored Capsicum fruit pericarp sample, while the two lower traces (red and blue lines) represent the carotenoids in red colored Capsicum fruit samples.
The coefficient of variation in our lab for this protocol is <5.0% when we are re-running samples from the same saponified fruit extract. The biological variability for carotenoid abundance in Capsicum fruit can be much higher, ∼20% variation. We routinely sample three to five independent samples of a particular fruit stage and/or genotype to capture the biological variability in these samples.
Notes
To help reduce the biological variability in the samples we prefer to use dried or lyophilized plant tissue. This reduces the variability in water weight that occurs when you allocate fruit samples for extraction. Depending on the fruit type, there can be quite a bit of difference in sample weight depending on fruit maturity stage and the thinness of the exocarp.
It is important to run calibration standards regularly, to ensure that the chromatography conditions are kept constant. The less expensive carotenoid standards, β-carotene and lutein, can be used to monitor the chromatography conditions. The frequency of these calibration runs depends on the work load of the research program, but checking that peaks are eluting at their appropriate retention times should be checked at least bimonthly or at the start of any new analysis project.
We have not tested a wide range of HPLC columns for the separation of the carotenoids, we do know that the C30 YMC carotenoid column works very well, while a C18 column will not resolve the carotenoids at least with our solvent system.
Recipes
All of the solutions are made up as described above using standard methods. There are no unusual aspects to the preparation of these solutions. All solvents are HPLC grade; the water is deionized water. We have not found it necessary to degas or to vacuum filter the solvents prior to use on the HPLC.
Solvent A: methanol: MTBE: water∷ 81:15:4 (vol: vol: vol)
Solvent B: MTBE: methanol: water∷ 88:8:4 (vol: vol: vol)
Acknowledgments
This protocol was adapted from previous work in our laboratory; see Rodriguez-Uribe et al. (2012). This work was supported in part by the NM Agricultural Experiment Station, USDA CSREES grant 2009-34604-19939 and USDA NIFA 2010-34604-20886.
References
- 1.Kilcrease J, Collins AM, Richins RD, Timlin JA, O'Connell MA. Multiple microscopic approaches demonstrate linkage between chromoplast architecture and carotenoid composition in diverse Capsicum annuum fruit. Plant J. 2013;76:1074–1083. doi: 10.1111/tpj.12351. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Uribe L, Guzman I, Rajapakse W, Richins RD, O'Connell MA. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. J Exp Bot. 2012;63(1):517–526. doi: 10.1093/jxb/err302. [DOI] [PMC free article] [PubMed] [Google Scholar]


