Abstract
Alzheimer disease (AD) is the most common neurodegenerative disorder that affects millions of ageing people worldwide. AD is characterized by extensive synaptic and neuronal loss which lead to impaired memory and cognitive decline. The cause of pathology in AD is not completely understood and no effective therapy so far has been developed. The accumulation of toxic amyloid-beta 42 (Aβ42) peptide oligomers and aggregates in AD brain has been proposed to be primarily responsible for the pathology of the disease, an idea dubbed ‘amyloid hypothesis’ of AD etiology. In addition to increase in Aβ42 levels, disturbances in neuronal calcium (Ca2+) signaling and alterations in expression levels of Ca2+ signaling proteins have been observed in animal models of familial AD and in studies of postmortem brain samples from sporadic AD patients. Based on these evidence ‘Ca2+ hypothesis of AD’ has been proposed. In particular, familal AD has been linked with enhanced Ca2+ release from the endoplasmic reticulum (ER) and elevated cytosolic Ca2+ levels. The augmented cytosolic Ca2+ levels can trigger signaling cascades that affect synaptic stability and function and can be detrimental to neuronal health, such as Ca2+-dependent phosphatase calcineurin and Ca2+-dependent proteases calpains. Here we review the latest results supporting ‘Ca2+ hypothesis’ of AD pathogenesis. We further argue that over long period of time supranormal cytosolic Ca2+ signaling can impaire mitochondrial function in AD neurons. We conclude that inhibitors and stablizers of neuronal Ca2+ signaling and mitochondrial function may have a therapeutic potential for treatment of AD. We discuss latest and planned AD therapeutic trials of agents targeting Ca2+ channels and mitochodria.
Keywords: Alzheimers, calcium, mitochondria, endoplasmic reticulum, excitotoxicity, Dimebon
The role of intracellular Ca2+ dysregulation in Alzheimer’s disease
The most prevalent idea of Alzheimer’s disease (AD) pathogenesis is based on the “amyloid cascade hypothesis”, first penned in 1992 by Hardy and Higgins (Hardy and Higgins, 1992), which states that accumulation of amyloid β (Aβ) peptide, altered processing or lack of clearance, is the initiating molecular event that ultimately leads to amyloid plaques, neurofibrillary tangles, inflammation, synaptic loss and neurodegeneration in both sporadic (late-onset AD, LOAD) and familial (genetically-linked) AD (FAD) (Hardy and Selkoe, 2002). In patients, evidence to support the “amyloid cascade hypothesis” is demonstrated by the accumulation of amyloid plaques in the AD brain; the FAD cases resulting from missense mutations in Aβ-precursor protein (APP) or presenilin (PSEN1/2), transmembrane proteins which comprise the catalytic subunit of the APP-cleaving enzyme γ-secretase (De Strooper, 2003). All mutations responsible for FAD affect the proteolysis of the type 1 transmembrane glycoprotein APP and result in the overproduction of the hydrophobic, aggregation-prone 1-42 Aβ fragment, increased Aβ42/40 ratio and plaque deposition. Thus, “amyloid-targeting” therapies have been the main focus of AD drug development for the past 30 years. They include immunotherapies that target Aβ, γ-/β-secretase inhibitors to block Aβ production, selective Aβ42-lowering agents, statins to enhance α-secretase activity, anti-Aβ aggregation agents and others. However, the considerable failure rate of Aβ-targeting drugs in clinical trials (Seabrook et al., 2007) suggest that reduction of Aβ alone might be insufficient to significantly modify AD outcomes and that alternative therapeutic targets must be considered.
Accumulating data suggests that neuronal Ca2+ dysregulation plays an important role in AD pathogenesis. Given the ubiquitous nature of intraneuronal Ca2+ signalling it is necessary to maintain strict regulation of cellular [Ca2+] but at the same time, to do so is energetically expensive. In aged neurons, ATP-generating mechanisms are less efficient and Ca2+ handling mechanisms become compromised, leading to excessive free Ca2+, increased intracellular [Ca2+] over time (or Ca2+ overload), activation of Ca2+-dependent proteases, reactive oxygen/nitrogen species formation (ROS/RNS), mitochondrial dysfunction, oxidative damage and apoptosis/necrosis. This is the basis for the “Ca2+ hypothesis of brain aging and AD” first proposed by Khatchaturian in 1989 (Khachaturian, 1989), which states that age-dependent, subtle changes to Ca2+ homeostasis would account for the age-related changes in neuronal function (Gant et al., 2006; Toescu and Verkhratsky, 2007; Toescu and Vreugdenhil, 2009) and that the accumulation of these changes to Ca2+ handling could account for the neuronal damage and cognitive decline in AD. The common features of ageing neurons is increased Ca2+ release from intracellular stores via IP3R and RyanR, increased Ca2+ influx via L-type VGCC, increased slow afterhyperpolarization due to activation of Ca2+-dependent K+ channels, reduced contribution of NMDAR-mediated Ca2+ influx, reduced cytosolic Ca2+ buffering capacity and activation of calcineurin and calpains. Resulting changes in neuronal Ca2+ dynamics lead to augmented susceptibility to induction of long-term depression (LTD) and an increase in the threshold frequency for induction of long-term potentiation (LTP) in ageing neurons, which may contribute to age-related memory decline (Foster, 2007).
Recent evidence in supporting the “Ca2+ hypothesis of AD” was compiled in several recent reviews (Bezprozvanny, 2009; Bezprozvanny and Mattson, 2008; LaFerla, 2002; Smith et al., 2005a; Stutzmann, 2007; Supnet and Bezprozvanny, 2010). Changes to intracellular Ca2+ signalling in patients were first described in fibroblasts isolated from those at risk for AD. Cells from familial AD patients harboring PSEN1 mutations displayed enhanced inositol(1,4,5)-trisphosphate (IP3)-induced Ca2+ responses when compared to cells from healthy subjects (Ito et al., 1994). Expression of Ca2+-handling genes was significantly altered in brain tissues from AD patients (Emilsson et al., 2006). The only genetic factor that consistently influences risk or onset age in sporadic AD is the apolipoprotein E ε4 allele (ApoE4), with the risk increasing as the number of copies an individual carries increases (Saunders et al., 1993). Studies in cell lines and primary cortical neurons expressing recombinant ApoE4 showed elevated cytosolic Ca2+ levels by efflux through plasma membrane Ca2+ channels (Tolar et al., 1999; Veinbergs et al., 2002), however it is not known if such changes are present in ApoE4 carriers. Finally, a single nucleotide polymorphism in CALHM1, a newly identified plasma membrane Ca2+ channel, interferes with Ca2+ permeability and slightly increases susceptibility to sporadic, late-onset AD [Dreses-Werringloer, 2008 #4858;Cui, 2009 #5316]. The role of CALHM1 in AD is controversial, with recent studies showing no association between the two (Beecham et al., 2009; Bertram et al., 2008; Minster et al., 2009), therefore further study of CALHM1 function is required. Recenty, other genes that increase the risk of developing sporadic AD have been identified (Harold et al., 2009; Lambert et al., 2009; Roses et al., 2009) but their effect(s) on intracellular Ca2+ are unknown. However, Ca2+ dysregulation in neurons appears to be a genuine consequence AD pathology and further investigations regarding drugs or drug targets that can modulate intraneuronal Ca2+ are warranted. The goal of this review is to discuss if the ER and mitochondria, two organelles intimately involved in both intracellular Ca2+ signalling (see Figure 1) and AD pathogenesis, could offer new opportunities for the modulation of intracellular Ca2+ and design of disease-modifying therapies.
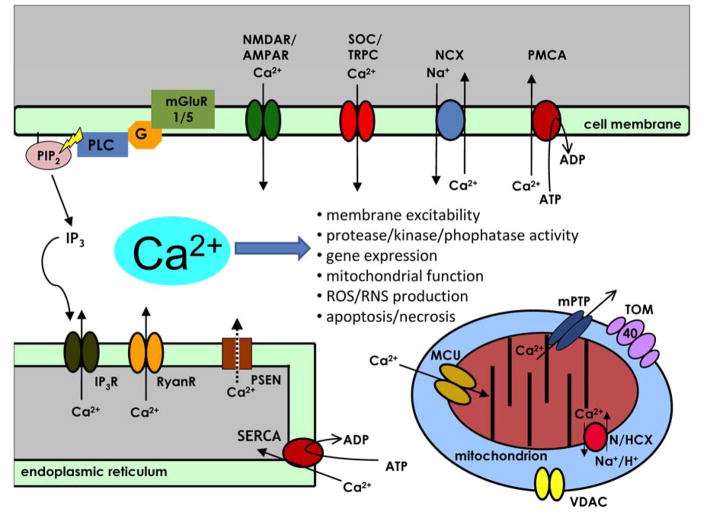
Figure 1. The compartmentalization of intracellular Ca2+ signalling in neurons and AD pathogenesis.
Calcium (Ca2+) is a key regulator of many neuronal processes and serves as the critical link between environmental stimuli and the intracellular effectors that result in a physiological response (Berridge, 1998). Gene expression, protein processing, ATP production, neurotransmitter release, action potential generation, modulation of membrane excitability, short-term and long-term synaptic plasticity, neurite outgrowth and control of cell death mechanisms are Ca2+-regulated processes that are imperative for neuronal function. The proteins that bind free Ca2+, such as calmodulin (CaM), and activate Ca2+-dependent cellular processes are expressed in membrane enclosed compartments such as the cytoplasm, the endoplasmic reticulum (ER) or the mitochondria (mt). The concentration of free Ca2+ ([Ca2+]) and the spatio-temporal pattern of Ca2+ microdomains determines the activation of particular cellular processes (Berridge, 2006). Thus, the [Ca2+] in each compartment is tightly regulated. Plasma membrane Ca2+ ATPases (PMCA), sodium/calcium exchangers (NCX) and sarco-/endoplasmic reticulum Ca2+ ATPases (SERCA) set up an electrochemical gradient which, upon neuronal activation, Ca2+ ions can passively move between cellular compartments through voltage- and/or ligand-gated channels. Calcium influx from the extracellular matrix can happen through voltage-gated Ca2+ channels (VGCC), N-methyl-D-aspartate receptors (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), store-operated channels (SOC, eg. transent receptor potential channels (TRPC)). Calcium efflux from intracellular ER stores is mediated by inositol 1,4,5-trisphosphate receptors (IP3R), ryanodine receptors (RyanR) and presenilins (PSEN) which facilitate “ER Ca2+ leak” (Tu et al., 2006). Mitochondria participate in Ca2+ signaling by taking up Ca2+ from cytosolic or ER microdomains across the outer mitochondrial membrane (OMM) through unknown mechanisms, likely through the voltage-dependent anion channel (VDAC) into the inner mitochondrial membrane (IMM) lumen through the mitochondrial Ca2+ uniporter (MCU). Recently, a Ca2+/H+ anti-porter (leucine zipper EF-hand–containing transmembrane protein 1, Letm1) that transports Ca2+ from the cytosol into the IMM lumen in was identified in HeLa cells (Jiang et al., 2009) but its function in neurons is unknown. Polymorphisms in TOMM40 gene encoding outer mitochondrial membrane component of the TOM complex have been linked with the probability of developing late-onset AD (Potkin et al., 2009; Roses et al., 2009; Shen et al., 2010; Takei et al., 2009). Calcium equilibrium is maintained along the IMM by NCX or hydrogen/calcium exchangers (HCX). Opening of the mitochondrial permeability transition pore (mtPTP) allows large efflux of Ca2+ from the IMM lumen and is often a trigger for the cell death signalling cascade (Giacomello et al., 2007). Under normal circumstances following neuronal stimulation, active Ca2+ transport returns [Ca2+] in each compartment to homeostatic levels. Both active and passive Ca2+ handling mechanisms are subject to regulation, in fact, Ca2+ itself is an important regulator of Ca2+ channel activity.
Neuronal Ca2+ dysfunction in AD
The ER is the largest intracellular organelle which functions to regulate post-translational protein processing. In addition, the ER participates in intraneurononal Ca2+ signalling and serves as a dynamic store and source of Ca2+ ions (Berridge, 2002) (Fig 1). Upon generation of inositol 1,4,5 triphosphate (IP3), Ca2+ is released from the ER via IP3-gated receptors (IP3R) and amplification of the Ca2+ signal is be medicated by the RyanRs, termed Ca2+ -induced Ca2+ release (CICR). To refill depleted stores, SERCA sequesters cytosolic Ca2+ into the ER where it is bound by Ca2+ binding proteins such as calreticulin and calnexin (Milner et al., 1991; Wada et al., October 15, 1991). Disruption of ER Ca2+ homeostasis can affect protein folding by intra-ER chaperones, cellular function and can initiate cell survival and/or death programs (Paschen, 2001). Because ER Ca2+ signalling is essential to intracellular Ca2+ homeostasis, the involvement of aberrant ER Ca2+ signalling in AD has received much attention and has been recently reviewed (Supnet and Bezprozvanny, 2010).
Interest in the role of aberrant ER Ca2+ signalling in AD began when it was discovered that mutations responsible for FAD also affected ER Ca2+ signalling, which predominantly resulted in exaggerated release of Ca2+ from overloaded ER stores. Skin fibroblasts from human patients that harbour a mutation in PSEN1-A246E showed exaggerated Ca2+ release from IP3-gated stores compared to controls after treatment with bombesin and bradykinin (Ito et al., 1994). Alterations in Ca2+ signalling were detected before the development of overt clinical symptoms and such changes were not present in cells from subjects that failed to develop AD (Etcheberrigaray et al., 1998). These initial results were recapitulated experimentally in various model systems expressing FAD-related mutations in PSEN and the data suggested that in addition to contributing to altered γ-secretase function, PSEN mutations had a significant impact on Ca2+ signalling in AD models. The PSEN1-M146V mutation augmented Ca2+ release from IP3- and caffeine- gated stores in hippocampal and cortical neurons in 3XTg-AD mice (Stutzmann et al., 2004; Stutzmann et al., 2006). In human post-mortem tissue, ryanodine binding (indicative of increased RyanR protein) was elevated in hippocampal regions (subiculum, CA2 and CA1) of AD brain in the early stages of the disease prior to extensive neurodegeneration and overt Aβ plaque deposition (Kelliher et al., 1999). RyanR protein levels and channel function are increased in mouse models of containing PSEN mutations PSEN1-M146V and PSEN2-N141I (Chan et al., 2000; Lee et al., 2006; Smith et al., 2005b). Clinical mutations of PSEN2 also enhanced Ca2+ release from IP3R-gated ER stores (Leissring et al., 1999). PSEN1 mutations or genetic deletion attenuate capacitative Ca2+ entry (CCE), a refilling mechanism for depleted ER Ca2+ stores (Giacomello et al., 2005; Leissring et al., 2000; Yoo et al., 2000). To explain these data, it has been hypothesized that PSENs able to function as ER Ca2+ leak channels and that FAD mutations in PSEN1 and PSEN2 disrupt this function (Tu et al., 2006). This idea was supported by demonstration of overloaded ER Ca2+ stores and exaggerated ER Ca2+ release in double PSEN knock-out mouse fibroblasts and in fibroblasts transfected with PSEN1 and PSEN2 FAD mutant constructs (Nelson et al., 2007; Tu et al., 2006). Additional mechanisms which may contribute to abnormal Ca2+ signaling in PSEN FAD cells may include increased RyanR expression and recruitment (Chakroborty et al., 2009; Chan et al., 2000; Stutzmann et al., 2006), directly affect gating of RyanR (Rybalchenko et al., 2008) or IP3R (Cai et al., 2006; Cheung et al., 2008), or affect function of the SERCA pump (Green et al., 2008). Taken together, these results indicate that presenilins play a direct role in Ca2+ signaling and affect activity and/or expression of many proteins involved in ER Ca2+ signalling. Thus, it is not surprising that many PSEN FAD mutations have major effects on intracellular Ca2+ homeostasis (Smith et al., 2005a).
In contrast, few studies have identified changes to ER Ca2+ signalling in cells expressing APP mutations. It has been documented that fibroblasts from AD patients harbouring the Swedish double mutation, APP-K670N/M671L, showed reduced bombesin-induced intracellular Ca2+ elevations compared to controls while all other pools of Ca2+ were unaffected (Gibson et al., 1997). Primary cortical neurons from TgCRND8 mice that express both APP-KM670/671NL and APP-V717F (Indiana) demonstrated elevated release of Ca2+ from up-regulated RyanR type 3 (Supnet et al., 2006) while global Ca2+ handling was unaffected (Supnet et al., 2010). The effects of APP mutations on ER Ca2+ signalling appear to be more subtle compared to the effects of PSEN mutations. The cumulative result of these small changes over time could have a significant effect on neuronal function that may contribute to cognitive decline. For example, in vivo Ca2+ imaging experiments revealed that neurons of aged APP-expressing Tg2567 and APP/PS1ΔE9 mice that were in close proximity to Aβ plaques were overloaded with Ca2+ when compared to neurons in the young mice prior to plaque formation and to neurons from the PSEN1-M146V and wild-type mice (Kuchibhotla et al., 2008). The augmented cytosolic Ca2+ leads to a loss of Ca2+ compartmentalization in dendritic spines and distorted neurite morphologies mediated by activation of Ca2+-dependent phosphatise calcineurin (Kuchibhotla et al., 2008; Reese et al., 2008; Wu et al., 2010). Resulting functional and structural modifications of synaptic connections could negatively impact neuronal networks and memory function. Consistent with this idea, inhibitors of calciunerin exerted positive effects in memory tests with Tg2567 mice (Dineley et al., 2007; Taglialatela et al., 2009). Activation of calciunerin and resulting changes in synaptic connectivity can be induced by relatively modest Ca2+ elevations. More pronounced cytosolic Ca2+ increase may lead to activation of calpains, Ca2+-dependent proteases which can degrade cellular signalling proteins that are involved in learning and memory (Trinchese et al., 2008; Vosler et al., 2008).
The dysregulation of cytosolic Ca2+ by aberrant ER Ca2+ signalling is an important aspect of AD pathogenesis, yet ER Ca2+ handling machinery are underutilized targets for AD therapeutics. The only exceptions are PSENs, which are targeted for their γ-secretase activity rather than their ER Ca2+ signalling function (Bergmans and De Strooper, 2010). The inherent problems associated with targeting ER Ca2+ channels and pumps are drug specificity, subtype selectivity and maintenance of biological function. For example, blockade of PSEN via γ-secretase inhibitors could hinder their ability to process Notch, which is important for neurodevelopment, and they may also affect their ER Ca2+ leak function. In addition, some of the observed Ca2+ signalling changes may in fact be compensatory and exert beneficial effect in AD (reviewed in (Supnet and Bezprozvanny, 2010)). For example, blockade of RyanR in dantrolene-fed APPPS1 mice actually attenuated Aβ plaque deposition and promoted synaptic loss in these mice (Zhang et al., 2010). Moreover, the up-regulation of RyanR type 3 is protective in cultured primary cortical neurons from TgCRND8 mice (Supnet et al., 2010) and up-regulation of RyanR2 maintains synaptic function in 3XTg-AD mice (Chakroborty et al., 2009). Therefore, efficacious targeting of ER Ca2+ signalling may require further understanding of these changes and sophisticated drug design to achieve required specificity.
Mitochondrial dysfunction in AD
Mitochondria (mt) are dynamic ATP-generating organelles which contribute to many cellular functions including intracellular Ca2+ regulation, alteration of redox-oxidation potential of cells, free radical scavenging and activation of caspase-mediated programmed cell death (Fig 1). ATP generation is accomplished through oxidative phosphorylation and because the activity of rate-limiting mitochondrial dehydrogenases (pyruvate, isocitrate, oxoglutarate) located in the inner mitochondrial matrix (IMM) are regulated by Ca2+, increases in mitochondrial Ca2+ correlate to enhanced ATP generation (Walter and Hajnoczky, 2005). The Ca2+ dependency of mitochondrial bioenergetics enables mt to decode Ca2+ signals and thus, to tune ATP synthesis to the energetic requirements of cells, including neurons (Satrustegui et al., 2007). The driving force for Ca2+ entry into the mt is the mitochondrial membrane potential (Δψ) and Ca2+ can be taken up by the low affinity mitochondrial Ca2+ uniporter MCU) on the IMM (Santo-Domingo and Demaurex, 2010). Ca2+ equilibrium is maintained along the IMM by sodium or hydrogen/calcium exchangers (N/HCX). If intramitochondrial Ca2+ levels become too high, the Δψ can collapse and cause the opening of the mitochondrial permeability transition pore (mtPTP), which allows efflux of Ca2+ with high conductance from the IMM lumen and is often a trigger for the cell death signalling cascade (Giacomello et al., 2007), though brief openings could serve as a rapid Ca2+ release mechanism (Bernardi et al., 2006). The alterations to neuronal Ca2+ homeostasis in AD can therefore negatively affect mitochondrial Ca2+ signalling (or vice versa), trigger mitochondrial dysfunction and ultimately compromise neuronal function and health.
Mt can participate in intracellular Ca2+ signalling in several capacities. They can buffer changes to local changes to [Ca2+] near the plasma membrane or the ER, enhance or decrease Ca2+ flux and modulate the frequency of Ca2+ oscillations in many cell types (Giacomello et al., 2007). In neurons, mt Ca2+ uptake occurs in presynaptic terminals and during periods of high Ca2+ activity such as epileptiform discharges or excitotoxic episodes (Billups and Forsythe, 2002; Budd and Nicholls, 1996; Kovacs et al., 2005). Neurons employ mt efflux mechanisms to shape cytoplasmic Ca2+ kinetics in response to intense electrical stimulation by slowly releasing the accumulated Ca2+ (Colegrove et al., 2000) or by allowing the efficient refilling of the ER and therefore modulating Ca2+ oscillations (Vay et al., 2007). It was demonstrated recently using mitochondrial-targeted ratiometric pericam (2 mtRP) to monitor mitochondrial Ca2+ transients that hippocamal mt located at the synapse were more sensitive to synaptic activation compared to mt located in the soma that the mitochondrial Ca2+ transients were independent of ER cross talk (Young et al., 2008). Given the involvement of mt in intraneuronal Ca2+ signalling it is conceivable then that alterations in mitochondrial Ca2+ handling could contribute to Ca2+ dysregulation in AD. The extent to which this occurs and contributes to AD pathogenesis is currently not known. One notable observation is that non-steroidal anti-inflammatory drugs (NSAIDs) have the ability to reduce mitochondrial Ca2+ uptake, which may account for their apparent benefits in warding off AD (Sanz-Blasco et al., 2008).
Recently it has been demonstrated that certain aspects of AD pathology can significantly alter mitochondrial Ca2+ signalling and trigger mitochondrial dysfunction in experimental models. The Aβ peptide has been shown to inhibit mitochondrial respiration (Casley et al., 2002) and in the presence of Ca2+ cause the opening of the mtPTP in isolated mt (Reddy and Beal, 2008). Cyclophilin D (CypD) is a mt protein located in the IMM lumen that associates with the mtPTP and regulates its open probability (Bernardi et al., 2006). Recently, it has been shown in vitro that Aβ oligomers target and form a complex with CypD, resulting in increased vulnerability to mtPTP opening (Du et al., 2008). Moreover, mt from CypD knock-out mice were insensitive to cyclosporine A, a strong inhibitor of Ca2+-induced mtPTP opening, and displayed a higher Ca2+ threshold than wild-type mt (Du et al., 2008). Interestingly, Aβ-induced alterations to LTP were attenuated in CypD knock-out mice compared to wild-type, which provided further evidence that potentially links mitochondrial Ca2+ signalling to neuronal dysfunction in AD. Aβ42 oligomers can indirectly alter mitochondrial Ca2+ signalling by inducing massive Ca2+ entry into neurons and promote mitochonrial Ca2+ overload (Sanz-Blasco et al., 2008), which lead to opening of the mtPTP, Δψ collapse, cytochrome c release, apoptosis and cell death. Depolarization of mt by a series of NSAIDS (including salicylate, sulindac sulphide, indomethacin, ibuprofen and R-flurbiprofen) inhibited mitochondrial Ca2+ overload, release of cytochrome c and cell death induced by Aβ (Sanz-Blasco et al., 2008). Taken together, these findings suggest that mitochondrial Ca2+ signalling could be compromised and play a significant role in AD pathogenesis.
There is extensive data to support an obligatory path to mitochondrial damage and dysfunction in AD, possibly triggered by dysregulated Ca2+ signalling, and that these changes can occur early in disease progression. Increased cytosolic cytochrome c oxidase, increased oxidative stress markers and reduced energy metabolism have been described in the brain of AD patients prior to Aβ plaque formation (Hirai et al., 2001; Lin and Beal, 2006). Electron microscopy studies of mt in various regions of AD brain showed significant morphological changes, such as reduced IMM cristae size (Baloyannis, 2006). Mitochondrial dynamics, such as fusion, fission and motility, may also be affected in AD. Neuronal cells treated with conditioned medium from cells expressing mutant APP lead to increased mitochondrial fission, loss of dendritic spines and cell death (Cho et al., 2009). The increased mitochondrial fission observed was mediated by elevated levels of S-nitrosylated dynamin-like protein 1 (SNO-Drp1). Drp1 is a cytosolic protein recruited to mitochondria during fission (Detmer and Chan, 2007) and SNO-Drp1 is suggested to have increased fission activity due to enhanced dimerization. Increased SNO-Drp1 protein levels were found in brain from AD patients and AD mouse models (Cho et al., 2009). In contrast, fibroblasts from sporadic AD patients expressed lower levels of Drp1 and displayed elongated mt (Wang et al., 2008a; Wang et al., 2009). However, the same group found that neuroblastoma cells over-expressing APP had predominately fragmented mt, decreased levels of Drp1 and a defect in neuronal differentiation (Wang et al., 2008b). Decreased levels of other mitochondrial enzymes, namely pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase complex, have also been reported in AD brain (Reddy, 2007). Changes in the mRNA expression of mt-encoded genes involved in oxidative phosphorylation were also confirmed in AD brain. The down-regulation of complex I and up-regulation of complex III and IV genes suggested a demand on energy production in brain tissue from early and definite cases of AD (Manczak et al., 2004), which is interpreted as compensatory to the decrease in mt numbers. Oxidative stress was reported in mt of brain, platelets and fibroblasts from AD patients (Manczak et al., 2006; Manczak et al., 2004). It is clear that mitochondrial dysfunction and oxidative stress are pathological changes observed in AD patients and that mitochondria constitute an attractive therapeutic target for developing AD treatment (Moreira et al., 2010).
Additional support for potential role of mt in AD pathogenesis has been provided by recent genetic analysis of LOAD patients. Several groups demonstrated that polymorphisms in the intron region of the TOMM40 gene (translocase of outer mitochondrial membrane 40 homolog) protein correlate with probability of developing AD (Potkin et al., 2009; Roses et al., 2009; Shen et al., 2010; Takei et al., 2009). The TOMM40 gene is located on chromosome 19 in the immediate proximity of APOE gene, and TOMM40 and APOE genes are in the linkage disequilibrium with each other. Some investigators argued that apparent linkage with TOMM40 can be explained by proximity to APOE allele (Yu et al., 2007) and that the main effect of polymorphism in TOMM40 gene is to affect APOE expression levels (Bekris et al., 2009; Bekris et al., 2008). It is also feasible that that polymorphism in TOMM40 gene affect expression levels or function of TOMM40 itself. The function of TOMM40 is to mediate protein transport into mt (Bolender et al., 2008) and mitochondrial import complex has been implicated in trafficking APP and Aβ into mt (Devi and Anandatheerthavarada, 2010; Hansson Petersen et al., 2008). It remains to be determined if TOMM40 polymorphisms affect mtPTP or the ability of mt to handle Ca2+ load.
The convergence of dysregulated Ca2+ and mitochondrial dysfunction in AD
The control of intraneuronal Ca2+ signalling and neuronal homeostasis requires the participation of both the ER and mt as their functions are interdependent and crucial for neuronal function. Evidence in the literature suggests that the ER and mt are both functionally and structurally coupled (Giorgi et al., 2009), therefore it would be logical to hypothesize that Ca2+ disturbances in one organelle would affect Ca2+ signalling and potentially alter the function of the other. The ER and mt are physically linked by ER-mitochondria-associated membranes (MAM) (Csordas and Hajnoczky, 2009; Hayashi et al., 2009) and MAM were initially thought to function as a compartment for the synthesis and transfer of phospholipids between the two organelles (Vance, 1990). In addition to lipid metabolism, MAM are a site for exchange of Ca2+ ions (Csordas and Hajnoczky, 2009; Hayashi et al., 2009). MAMs are enriched in chaperones that stabilize the association between the ER and mt membranes and prolongs Ca2+ signalling mediated by the IP3R type 3 receptor (Hayashi and Su, 2007). The mitochondrial chaperone glucose-regulated protein 75 (grp75) regulates IP3R mediated mt Ca2+ signalling by stabilizing the physical interaction of VDAC isoform 1 to IP3R (Szabadkai et al., 2006). These data suggest that under normal circumstances ER and mt Ca2+ signalling are closely linked. It would be interesting to determine how MAM function is compromised in aged neurons and in AD.
It was recently discovered that MAM carefully isolated from mouse brain were highly enriched with PSEN1/2 protein, along with the other components of the γ-secretase complex; APH1, nicastrin and presenilin enhancer protein 2 (Area-Gomez et al., 2009). Moreover, γ-secretase activity was enhanced in the MAM fraction. Studies in the past have localized PSENs and the γ-secretase complex to many subcellular organelles, including the mt (Hansson et al., 2004). The divergent results regarding PSEN localization to organelles other that the ER could be attributed to the technical difficulty of subcellular fractionation and a lack of accurate MAM antigenic markers (Area-Gomez et al., 2009). However, the possibility that the γ-secretase complex may reside at the ER/mt interface could explain how Aβ peptides could be transported to and accumulate in mt (Hansson Petersen et al., 2008) and offer a physical coupling of Aβ generation and mitochondrial dysfunction in AD. As discussed above, in addtion to γ-secretase PSENs also function as ER Ca2+ leak channels (Tu et al., 2006). One of the functions of MAM is mediate ER-mitochondria Ca2+ transport (Csordas and Hajnoczky, 2009; Hayashi et al., 2009) and it is possible that PSEN FAD mutations may have significant effects on local Ca2+ leak rates within MAM domain of the ER, leading to impaired Ca2+ coupling between ER and mitochondria.
Many questions remain regarding the interplay between ER and mt and their contribution to dysregulated intracellular Ca2+ in AD. How would mutations in PSEN affect this functional and physical coupling? Does the compromised Ca2+ leak function of mutant PSEN affect mitochondrial Ca2+ uptake at MAM? If so, how? Are the proteins essential for the association of ER and mt compromised in AD? Are other Ca2+ handling proteins, such as RyanR and SERCA, important for ER to mitochondrial Ca2+ transport? Is Ca2+ transport unidirectional? Further studies of MAM, in cellular and animal AD models and using tissue from AD patients, are required to determine their functional significance in AD pathogenesis. However, it is clear that the functional coupling of ER and mitochondrial Ca2+ handling could link the effects of dysregulated intracellular Ca2+ signalling to Aβ generation and mitochondrial dysfunction in AD. Drugs designed to modulate ER and mitochondrial Ca2+ signalling could increase the chances of efficacy as they would have the potential to modify many aspects of AD pathology.
Ca2+ blockers and mitochondrial stabilizers as potential AD therapeutics
The experimental results discussed above lead to conclusion that Ca2+ blockers and mitochondrial stabilizers are potential AD treatments. Similar conclusions have been also reached previously for AD as well as other neurodegenerative disorders (Bezprozvanny, 2009; Moreira et al., 2010). However, there are only a few drugs targeting these pathways that have been evaluated in AD clinical trials so far. Memantine is an non-competitive NMDAR inhibitor which is already approved by FDA for AD treatment and sold under brand name Namenda. Potentially more specific NMDAR inhibitors such as nitromemantines can be developed (Lipton, 2006). Evotec Inc has developed orally-active NR2B subtype selective NMDA antagonists, EVT101 and EVT103. EVT101 has been determined to be safe based on Phase I trial sponsored by Evotec (NCT00526968). In collaboration with Roche, Evotec is developing EVT101 for treatment-resistant depression and Phase II trial for this condition is planned. EVT101 and EVT103 are also very promising candidates for treatment of AD. Nimodipine, a dihydropyridine derivative and L-VGCC antagonist, has beneficial effects in AD patients and slows the progression of the disease (Lopez-Arrieta and Birks, 2002). The L-VGCC inhibitor MEM-1003 with better brain permeability has been developed by Memory Pharmaceuticals and tested in Phase II AD clinical trial (NCT00257673), but the patients failed to show significant improvement in cognitive function after 12 weeks of treatment and development of MEM-1003 for AD has been discontinued. Another L-type VGCC antagonist isradipine (Dynacirc CR) is being tested in on-going Phase II clinical trial in PD (NCT00753636) and may have potential utility for treatment of AD as well.
Perhaps the most promising and also most controversial compound from this class is Dimebon (Latrepirdine). Dimebon is a drug that has been developed and used as an antihistamine in Russia since 1983. Recently Dimebon has been proposed to be useful for treating neurodegenerative disorders (Bachurin et al., 2001) and licensed by Medivation for this application. Dimebon demonstrated significant positive effects in six-month randomized, double-blinded, placebo-controlled phase II trial of 183 patients with mild to moderate Alzheimer’s disease (AD) sponsored by Medivation and conducted in Russia (NCT00377715). At conclusion of the trial it was reported that after 12 weeks of taking Dimebon, patients significantly improved over baseline for ADAS-cog score (mean drug-placebo difference -4.0; p<0.0001) (Doody et al., 2008). More recently Phase II trial for cognitive effects in Huntington’s disease was completed in USA (NCT00497159). The results of the study with 91 patients has been recently reported – after 90 days treatment with Dimebon there was no significant difference in ADAS-cog, but there was an increase in MMSE score (0.97 points difference, p = 0.03) for the treatment group at conclusion of the trial (Kieburtz et al., 2010). The large Phase III 26 weeks long clinical trial of Dimebon in AD patients sponsored by Medivation and Pfizer has been recently completed (NCT00838110, CONNECTION trial) and the results were disappointing as treatment with Dimebon did not significantly improve ADAS-cog (p=0.86) or met any other primary or secondary efficiacy endpoints (http://investors.medivation.com/releasedetail.cfm?ReleaseID=448818). The Phase III study of Dimebon in Huntington’s disease (NCT00920946, HORIZON trial) sponsored by Medivation and Pfizer is currently recruiting. It is not clear at the moment if development of Dimebon for AD and HD by Medivation and Pfizer will be continued.
Perhaps the disappointing outcomes of the clinical trials involving Dimebon in AD and HD are a result of the fact that the mechanisms responsible for the postulated beneficial actions of Dimebon have never been clarified. It has been initially suggested that Dimebon may act as an inhibitor of NMDA receptors (Grigorev et al., 2003), blocker of voltage-gated Ca2+ channels (Lermontova et al., 2001) or as a blocker of the mitochondrial permeability transition pore (Bachurin et al., 2003). However, these initial experiments were performed with very high concentrations of Dimebon. For example, concentrations as high as 50 μM were required to inhibit mPTP opening of isolated mitochondria (Bachurin et al., 2003). Nevertheless, these potential targets indicated that Dimebon may act by stabilizing neuronal Ca2+ signaling and mPTP opening. More recent evaluation of Dimebon in experiments with primary neuronal cultures from an HD mouse model demonstrated that concentrations of at least 10 μM were required to inhibit VGCC and NMDAR (Wu et al., 2008). At least 50 μM of Dimebon was needed to exert neuroprotective effects in glutamate excitotoxicity model (Wu et al., 2008). The concentrations of Dimebon needed to affect Ca2+ signaling and mt in all published reports (Bachurin et al., 2003; Grigorev et al., 2003; Lermontova et al., 2001; Wu et al., 2008) were at least 10 μM, which is far above physiological range. In search for more physiologically relevant targets of Dimebon, an unbiased screen was performed (Wu et al., 2008). It was discovered that Dimebon very potently inhibits α1B adrenergic receptors, histamine H1 receptors and serotonin 5-HT6 receptors, as well as number of additional receptors (Wu et al., 2008). These findings were recently confirmed in an independent study (Okun et al., 2009). It is most likely that some cognitive effects of Dimebon observed in AD and HD clinical trials (Doody et al., 2008; Kieburtz et al., 2010) are due to interaction with these receptors. In particular, an ability of Dimebon to inhibit 5-HT6 serotonin receptors with high affinity (Ki=34 nM) (Okun et al., 2009) is of interest. Serotonin 5-HT6 receptors are known targets for cognitive enhancement which has been previously considered for AD treatment (Upton et al., 2008). A recently published evaluation of Dimebon in animal model confirmed the ability of Dimebon to interact with 5-HT6 receptors in vivo and to exert acute behavioral effects similar to specific 5-HT6 receptor antagonist SB-399885 (Schaffhauser et al., 2009). These studies support the hypothesis that cognitive effects of Dimebon are most likely due to its ability to inhibit 5-HT6 serotonin receptors. In addition, other potential effects of Dimebon such as its effects on amyloid metabolism (Steele et al., 2009) and protein aggregation (Yamashita et al., 2009) may have contributed to some of the results observed in the clinic.
Conclusion
There is much evidence to suggest that dysregulated Ca2+ signalling and mitochondrial dysfunction play a significant role in pathogenesis of AD. Evidence suggests that the various Ca2+ handling channels and pumps in the ER are prominent contributors to the alterations of intracellular Ca2+ signalling in AD. ER Ca2+ levels are increased in ageing neurons. Many AD-causing mutations in PSENs results in Ca2+ overload due to impaired ER leak function. Extracellular Aβ oligomers form Ca2+-permeable pores and destabilize neuronal Ca2+ signaling. Supranormnal cytosolic Ca2+ signals lead to activation of Ca2+-dependent phosphatase calcineurin, Ca2+-dependent protease calpain and changes in synaptic structure and function. Elevated Ca2+ signals lead to impaired mitochondrial function and eventually to cell death. Ca2+ and mitochondrial inhibitors and stabilizers have utility for AD treatment. Some of these compounds are already being evaluated in AD clinical trials. Additional compounds with increased potency and specificity need to be developed in the future.
Acknowledgments
IB is a holder of Carla Cooke Francis Professorship in Alzheimer’s Research and supported by the McKnight Neuroscience of Brain Disorders Award, Alzheimer’s Disease Drug Discovery Foundation, Carl and Florence King Foundation, and NIH grant R01AG030746.
References
- Area-Gomez E, de Groof AJ, Boldogh I, Bird TD, Gibson GE, Koehler CM, Yu WH, Duff KE, Yaffe MP, Pon LA, Schon EA. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am J Pathol. 2009;175:1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sablin S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Annals of the New York Academy of Sciences. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Annals of the New York Academy of Sciences. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 345-339. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis. 2006;9:119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- Beecham GW, Schnetz-Boutaud N, Haines JL, Pericak-Vance MA. CALHM1 polymorphism is not associated with late-onset Alzheimer disease. Ann Hum Genet. 2009;73:379–381. doi: 10.1111/j.1469-1809.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris LM, Galloway NM, Montine TJ, Schellenberg GD, Yu CE. APOE mRNA and protein expression in postmortem brain are modulated by an extended haplotype structure. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:409–417. doi: 10.1002/ajmg.b.30993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, Li G, Galasko DR, DeCarli C, Farlow MR, Clark CM, Quinn JF, Kaye JA, Schellenberg GD, Tsuang D, Peskind ER, Yu CE. Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers Dis. 2008;13:255–266. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans BA, De Strooper B. gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 2010;9:215–226. doi: 10.1016/S1474-4422(09)70332-1. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: organization and function. Cell calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bertram L, Schjeide BM, Hooli B, Mullin K, Hiltunen M, Soininen H, Ingelsson M, Lannfelt L, Blacker D, Tanzi RE. No association between CALHM1 and Alzheimer’s disease risk. Cell. 2008;135:993–994. doi: 10.1016/j.cell.2008.11.030. author reply 994-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, I, Forsythe D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 2008;9:42–49. doi: 10.1038/sj.embor.7401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. Journal of neurochemistry. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- Cai C, Lin P, Cheung KH, Li N, Levchook C, Pan Z, Ferrante C, Boulianne GL, Foskett JK, Danielpour D, Ma J. The presenilin-2 loop peptide perturbs intracellular Ca2+ homeostasis and accelerates apoptosis. The Journal of biological chemistry. 2006;281:16649–16655. doi: 10.1074/jbc.M512026200. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. Journal of neurochemistry. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. The Journal of biological chemistry. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrove SL, Albrecht MA, Friel DD. Dissection of mitochondrial Ca2+ uptake and release fluxes in situ after depolarization-evoked [Ca2+](i) elevations in sympathetic neurons. J Gen Physiol. 2000;115:351–370. doi: 10.1085/jgp.115.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Devi L, Anandatheerthavarada HK. Mitochondrial trafficking of APP and alpha synuclein: Relevance to mitochondrial dysfunction in Alzheimer’s and Parkinson’s diseases. Biochim Biophys Acta. 2010;1802:11–19. doi: 10.1016/j.bbadis.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilsson L, Saetre P, Jazin E. Alzheimer’s disease: mRNA expression profiles of multiple patients show alterations of genes involved with calcium signaling. Neurobiol Dis. 2006;21:618–625. doi: 10.1016/j.nbd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL. Calcium responses in fibroblasts from asymptomatic members of Alzheimer’s disease families. Neurobiol Dis. 1998;5:37–45. doi: 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Barbiero L, Zatti G, Squitti R, Binetti G, Pozzan T, Fasolato C, Ghidoni R, Pizzo P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol Dis. 2005;18:638–648. doi: 10.1016/j.nbd.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Vestling M, Zhang H, Szolosi S, Alkon D, Lannfelt L, Gandy S, Cowburn RF. Abnormalities in Alzheimer’s disease fibroblasts bearing the APP670/671 mutation. Neurobiol Aging. 1997;18:573–580. doi: 10.1016/s0197-4580(97)00149-8. [DOI] [PubMed] [Google Scholar]
- Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. The Journal of cell biology. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med. 2003;136:474–477. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. The Journal of biological chemistry. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M, Fastbom J, Cowburn RF, Bonkale W, Ohm TG, Ravid R, Sorrentino V, O’Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/s0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Calcium, membranes, aging, and Alzheimer’s disease. Introduction and overview. Annals of the New York Academy of Sciences. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, McDermott MP, Voss TS, Corey-Bloom J, Deuel LM, Dorsey ER, Factor S, Geschwind MD, Hodgeman K, Kayson E, Noonberg S, Pourfar M, Rabinowitz K, Ravina B, Sanchez-Ramos J, Seely L, Walker F, Feigin A. A randomized, placebo-controlled trial of latrepirdine in Huntington disease. Arch Neurol. 2010;67:154–160. doi: 10.1001/archneurol.2009.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs R, Kardos J, Heinemann U, Kann O. Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:4260–4269. doi: 10.1523/JNEUROSCI.4000-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lee SY, Hwang DY, Kim YK, Lee JW, Shin IC, Oh KW, Lee MK, Lim JS, Yoon DY, Hwang SJ, Hong JT. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. The Journal of cell biology. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Parker I, LaFerla FM. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1, 4,5-trisphosphate-mediated calcium signals. The Journal of biological chemistry. 1999;274:32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med. 2001;132:1079–1083. doi: 10.1023/a:1017972709652. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Lopez-Arrieta JM, Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev. 2002:CD000147. doi: 10.1002/14651858.CD000147. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Milner RE, Baksh S, Shemanko C, Carpenter MR, Smillie L, Vance JE, Opas M, Michalak M. Calreticulin, and not calseqestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J Biol Chem. 1991;266:7155–7165. [PubMed] [Google Scholar]
- Minster RL, Demirci FY, DeKosky ST, Kamboh MI. No association between CALHM1 variation and risk of Alzheimer disease. Hum Mutat. 2009;30:E566–569. doi: 10.1002/humu.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun I, Tkachenko ES, Khvat A, Mitkin O, Kazey V, Ivachtchenko VA. From Anti-allergic to Anti-Alzheimer’s: Molecular Pharmacology of Dimebon. Curr Alzheimer Res. 2009 doi: 10.2174/156720510790691100. [DOI] [PubMed] [Google Scholar]
- Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell calcium. 2001;29:1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, Weiner M, Macciardi F. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PloS one. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2009 doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PloS one. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Mathiasen JR, Dicamillo A, Huffman MJ, Lu LD, McKenna BA, Qian J, Marino MJ. Dimebolin is a 5-HT6 antagonist with acute cognition enhancing activities. Biochem Pharmacol. 2009;78:1035–1042. doi: 10.1016/j.bcp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Ray WJ, Shearman M, Hutton M. Beyond amyloid: the next generation of Alzheimer’s disease therapeutics. Mol Interv. 2007;7:261–270. doi: 10.1124/mi.7.5.8. [DOI] [PubMed] [Google Scholar]
- Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, Foroud T, Pankratz N, Moore JH, Sloan CD, Huentelman MJ, Craig DW, Dechairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Saykin AJ. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell calcium. 2005a;38:427–437. doi: 10.1016/j.ceca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. Journal of neurochemistry. 2005b;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- Steele JW, Kim SH, Cirrito JR, Verges DK, Restivo JL, Westaway D, Fraser P, Hyslop PS, Sano M, Bezprozvanny I, Ehrlich ME, Holtzman DM, Gandy S. Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol Neurodegener. 2009;4:51. doi: 10.1186/1750-1326-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE. The pathogenesis of Alzheimers disease is it a lifelong “calciumopathy”? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell calcium. 2010;47:183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. The Journal of biological chemistry. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- Supnet C, Noonan C, Richard K, Bradley J, Mayne M. Up-regulation of the type 3 ryanodine receptor is neuroprotective in the TgCRND8 mouse model of Alzheimer’s disease. Journal of neurochemistry. 2010;112:356–365. doi: 10.1111/j.1471-4159.2009.06487.x. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. The Journal of cell biology. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kimura H, Kakita A, Takahashi H, Tsuji S, Kanazawa I, Ihara Y, Odani S, Kuwano R. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell calcium. 2009 doi: 10.1016/j.ceca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Tolar M, Keller JN, Chan S, Mattson MP, Marques MA, Crutcher KA. Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:7100–7110. doi: 10.1523/JNEUROSCI.19-16-07100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee S-F, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer’s disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. The Journal of biological chemistry. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vay L, Hernandez-Sanmiguel E, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, Alvarez J. Modulation of Ca(2+) release and Ca(2+) oscillations in HeLa cells and fibroblasts by mitochondrial Ca(2+) uniporter stimulation. The Journal of physiology. 2007;580:39–49. doi: 10.1113/jphysiol.2006.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res. 2002;67:379–387. doi: 10.1002/jnr.10138. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJM. SSRalpha and Associated Calnexin Are Major Calcium Binding Proteins of the Endoplasmic Reticulum Membrane. Journal of Biological Chemistry. 1991 Oct 15;266:19599–19610. [PubMed] [Google Scholar]
- Walter L, Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008a;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Nonaka T, Arai T, Kametani F, Buchman VL, Ninkina N, Bachurin SO, Akiyama H, Goedert M, Hasegawa M. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett. 2009;583:2419–2424. doi: 10.1016/j.febslet.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Young KW, Bampton ET, Pinon L, Bano D, Nicotera P. Mitochondrial Ca2+ signalling in hippocampal neurons. Cell calcium. 2008;43:296–306. doi: 10.1016/j.ceca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, Rosenthal E, Wijsman EM, Tsuang DW, Devlin B, Schellenberg GD. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89:655–665. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]