Abstract
Background
An objective definition of clinically relevant extracapsular nodal spread (ECS) in head and neck squamous cell carcinoma (SCC) is unavailable.
Methods
Pathologic review of 245 pathologically positive oral cavity SCC neck dissection specimens was performed. The presence/absence of ECS, its extent (in millimeters), and multiple nodal and primary tumor risk factors were related to disease-specific survival (DSS) at a follow-up of 73 months.
Results
ECS was detected in 109 patients (44%). DSS was significantly better for patients without ECS than patients with ECS. Time-dependent receiver operator curve (ROC) analysis identified a prognostic cutoff for ECS extent at 1.7 mm. In multivariate analyses, DSS was significantly lower for patients with major ECS compared with patients with minor ECS, but not significantly different between patients with minor ECS and patients without ECS.
Conclusion
ECS is clinically relevant in oral cavity SCC when it has extended more than 1.7 mm beyond the nodal capsule.
Keywords: lymphatic metastasis/pathology, neoplasm invasiveness, neck dissection, prognosis, head and neck neoplasms/pathology/surgery
INTRODUCTION
Metastasis to cervical lymph nodes occurs in almost 50% of patients with oral cavity squamous cell carcinomas (SCC) and is a major determinant of prognosis in this disease.1,2 In patients with metastatic cervical lymph nodes, prognosis is further affected by size and number of meta-static lymph nodes, anatomic location of nodal metastases, and presence or absence of extracapsular spread (ECS).3,4 Retrospective studies have reported that ECS is one of the most important nodal characteristics that influences prognosis3,5–7 and subset analysis of randomized data suggests that chemotherapy-based intensification of postoperative radiotherapy (RT) may improve the adverse prognosis of some patients with ECS.8–11 However, the ECS phenomenon is fundamentally ill-defined, which affects the inclusion criteria of randomized studies, and may ultimately contribute to undertreatment of patients at high risk, or exposure of patients to the harmful side-effects of chemotherapy-based RT without apparent benefit.
Although most experts agree that overt, macroscopically visible ECS is a clinically relevant adverse prognostic factor,7,12 much less consensus exists about the interpretation of microscopic ECS.7,13,14 From a diagnostic perspective, the differentiation of microscopic ECS from intracapsular tumor containment is complicated by ambiguous pathological criteria, which result in significant intraobserver and interobserver variability.5,6,15 In addition, the prognostic implications of microscopic ECS are ill-defined, as limited evidence confirms that the adverse implications associated with this diagnosis are independent from other accepted nodal prognostic factors.16,17 Essentially, the current ECS debate is sustained by unavailability of an objective and widely accepted definition of clinically relevant ECS.
The purpose of this report was to improve the definition of clinically relevant ECS through an empiric appraisal of its microscopic extent and associated prognostic implications.
PATIENTS AND METHODS
Patients
The present study was approved by the Institutional Review Board at our institution. All patients undergoing primary surgical treatment for oral cavity SCC at a tertiary care cancer center between 1985 and 2009 were identified from an institutional database (n=1617). Five hundred thirty-three patients with oral cavity SCC had pathologically confirmed cervical metastasis in their neck dissection specimen. Only patients with histopathological slides available for review were included in this study (n = 245). When compared to the excluded patients (n = 288), the included patients were more likely to have pT4 disease (39.0% vs 27.9%; p = .007), had thicker tumors (1.5 cm vs 1.2 cm; p = .001), were less likely to have unreported ECS status (16.7% vs 29.2%; p = .001), and were less likely to have negative margins (51.0% vs 62.7%; p = .007). There were more patients in the exclusion group with primary tumors of the oral tongue; however, comparison of sex, vascular invasion (VI), perineural invasion (PNI), bone invasion, grade, tumor size, and treatment showed that there were no differences between the 2 groups (data not shown). On univariate analysis, the study group had worse disease-specific survival (DSS) compared to the excluded group (5-year DSS = 45.2% vs 57.1%; p = .015); however, on multivariate analysis, factoring these differences in patient populations, there was no inherent difference in DSS between the study group and the exclusion group (p = NS).
Neck dissections were classified according to the American Head and Neck Society nomenclature.18 In patients with a clinically negative neck (n = 99), elective neck dissection consisted of selective neck dissection of levels I to III/IV and was used as standard policy based on accepted clinical criteria of the preoperative risk of occult metastasis.19,20 In patients with clinically evident neck metastasis (n = 146), the type of therapeutic neck dissection was chosen based on the clinical N classification following accepted clinical criteria.21 Seventy patients (29%) underwent unilateral selective neck dissection (levels I–III or I–IV), 129 patients (53%) underwent unilateral comprehensive neck dissection (levels I–V), and 15 patients (6%) underwent bilateral selective neck dissection (levels I–III). Seventeen patients (7%) underwent bilateral comprehensive neck dissection and 14 patients (6%) underwent an ipsilateral comprehensive neck dissection with a contralateral selective neck dissection. Neck dissection specimens were submitted for pathologic evaluation after the surgeon had designated the levels in the operating room. Postoperative radiotherapy (PORT) was used in 183 patients (75%). Twenty-five patients had addition of cisplatin-based chemotherapy to their PORT (10%). Median follow-up of living patients (n = 62) was 73 months.
Histopathologic analysis
Postoperative histopathologic analysis of the neck dissection specimens was performed as per institutional protocol. Lymph nodes were identified by inspection and palpation during gross pathologic evaluation. All lymph nodes identified by the pathologist were sampled in toto for microscopic analysis, except for large grossly positive nodes (>2 cm in size). For the latter, 2 slices of the lymph node were examined. Only 1 hematoxylin-eosin section from each lymph node tissue block irrespective of the node size was examined histologically. Foci of perinodal vascular invasion were not categorized as ECS. Standard pathological factors related to the primary tumor were extracted from our institutional database. For the purpose of the present study, all microscopic slides from the neck dissection specimens were re-reviewed by at least 2 of 3 dedicated head and neck pathologists (R.A.G., N.K., or D.C.), who were blinded to clinical outcomes. The presence of ECS was defined as complete breach of the lymph node capsule (Figures 1–3). In all positive nodes, including matted nodes and those completely replaced by tumor, extent of ECS was defined as the maximal distance in millimeters between the outer aspect of the intact or reconstructed nodal capsule and the invasive front of the extracapsular tumor deposit. This measurement was accomplished using an ocular micrometer (Figures 1 and 2). In cases in which ECS was very minimal or questionable, the study pathologists arrived at a consensus after examining the entire node/s and were able to reach an agreement in all instances. In cases in which more than 1 node harbored ECS, the largest extent of ECS was used for the analysis. Involvement of the perinodal adipose tissue in the area of the hilum was considered as ECS. Pathological features that were the focus of this pathologic review included features related to ECS and features related to nodal metastatic volume (ie, number of metastatic nodes, greatest diameter of largest node with metastasis, and greatest size of largest metastatic focus).
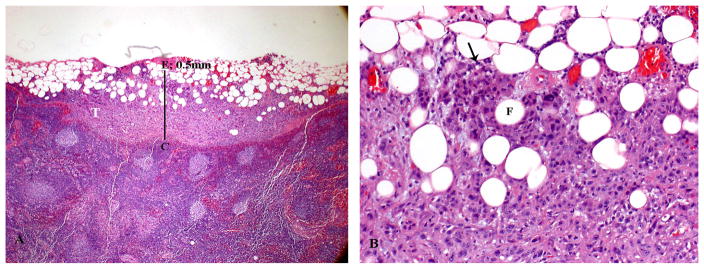
FIGURE 1.
Histologic appearance of minor extracapsular spread (ECS). (A) Lymph node with metastatic tumor (T) invading perinodal fat. The extent of ECS (0.5 mm) is measured (solid bar) from the outer aspect of the lymph node capsule (C) to the more distant point of perinodal invasion (E). A 1-mm bar is inserted for size comparison. (B) Higher power showing the squamous cell carcinoma (arrow) infiltrating in between adipose cells (F).
FIGURE 3.
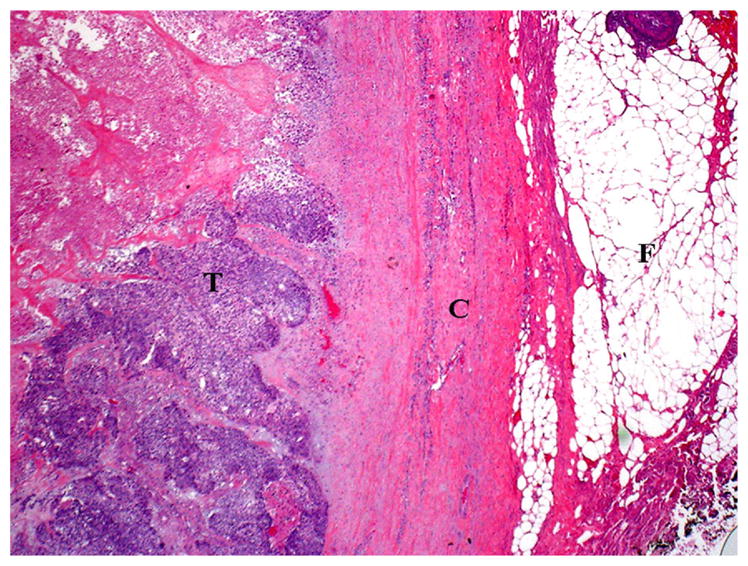
Histologic appearance of a metastatic lymph node without extracapsular spread (ECS). The metastatic carcinoma (T) does not invade perinodal fat (F). Invasion of the lymph node capsule (C) is also lacking in this case.
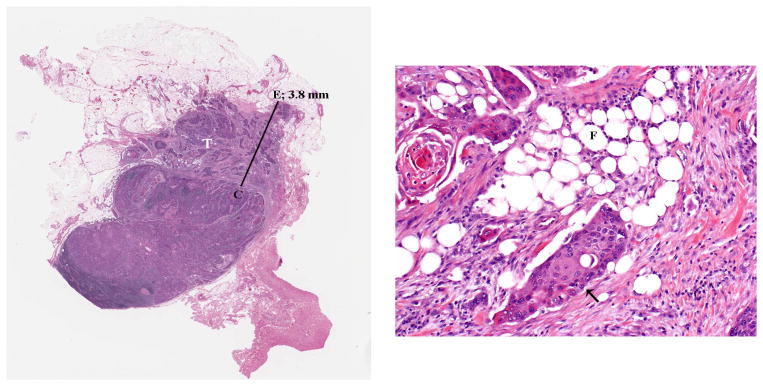
FIGURE 2.
Histologic appearance of major extracapsular spread (ECS). (A) Lymph node with metastatic tumor (T) invading perinodal fat. The extent of ECS (3.8 mm) is measured (solid bar) from the outer aspect of the lymph node capsule (C) to the more distant point of perinodal invasion (E) A 1-mm bar is inserted for size comparison. (B) Higher power showing the squamous cell carcinoma (arrow) infiltrating in between adipose cells (F).
Statistical analysis
Associations between variables were assessed by Fisher’s exact or Pearson’s chi-square statistics. Comparisons of continuous variables were performed using the independent sample t test. The primary endpoint of interest was 5-year DSS. A DSS event was recorded only for patients who died with active primary oral cavity SCC. DSS time to event was calculated from the surgery date to the date of death. Patients who did not die of their primary oral cavity SCC were censored at the time of last their follow-up. A prognostic cutoff for ECS extent was defined using time-dependent receiver-operator curve (ROC) analysis.22 The optimal cutoff was calculated by maximizing the sensitivity and specificity by taking the largest difference between true positives and false positives.23 Survival outcomes for each prognostic variable were calculated by the Kaplan–Meier method and groups were compared by univariate analysis using the log-rank test. Prognostic factors that were significant on univariate analysis were analyzed by multivariate analysis using a Cox proportional hazards model to assess for independent significance, with a p value of .05 as significance threshold. Models were chosen using the concordance statistic, which measures the model’s ability to predict.24 If necessary, restricted cubic splines were used to relax the linearity assumption of the Cox regression model. All analyses were performed with SPSS (IBM, Armonk, NY) and R software (Vienna, Austria), specifically with packages RMS (Regression Modeling Strategies, version 42-1) and survival ROC (Paramita survival ROC: time-dependent ROC curve estimation from censored survival data, version 103).
RESULTS
Overall analysis of all patients with metastatic nodal disease
Demographic, clinical, and pathological data are summarized in Tables 1 and 2. It is of note that 99 patients (40%) presented with clinically occult metastasis that were identified on histopathologic examination of their elective neck dissection specimens, whereas the remainder had clinically and/or radiographically positive metastasis. A majority of the patients (64%) were pathologically classified N2 (70% of these were N2b; 27% were N2c; and only 2.5% were N2a), whereas only 4 (1.6%) were pN3. For all patients with nodal involvement, the median size of the largest positive lymph node was 17 mm (range, 1.9–80 mm). ECS was identified in 109 patients (44%); 36.4% of cN0pN+ and 50.0% of cN+pN+ patients. PORT was given in 77 patients with ECS (71%), and 106 patients without ECS (78%; p = NS). Adjuvant chemotherapy-based RT was given in 15 patients with ECS and 10 patients without ECS (p = NS). There were 101 cancer-related deaths. The 5-year DSS for patients with pathologically demonstrated nodal disease was 45.2%. DSS was significantly higher for patients without ECS than patients with ECS on univariate analysis (5-year DSS = 57.8% vs 25.0%; p < .001). T classification (p < .001), PNI (p = .010), VI (p = .034), nodal status (p = .001), number of metastatic nodes (p < .001), size of the largest metastatic node (p < .001), size of the largest metastatic focus (p < .001), and ECS were all associated with significantly decreased DSS. Multivariate models were used in order to test for the effect of multiple predictors. When adjusting for multiple factors, such as tumor and nodal presentation, the presence of ECS remained a significant predictor. The final model indicated that the presence of ECS (hazard ratio [HR] = 2.44; p < .001), size of the largest metastatic node (HR = 1.30; p = .005), and T classification greater than T1 (HR = 2.51; p = .004) were significant predictors of DSS (data not shown). The concordance index was 0.713.
TABLE 1.
Categorical demographics, treatment characteristics, and histopathologic characteristics of patients with negative, minor, and major extracapsular spread.
| Categorical variables | Negative ECS (n = 136) No. of patients (%) |
Minor ECS (n = 52) No. of patients (%) |
Major ECS (n = 57) No. of patients (%) |
p value Neg vs min |
p value Min vs major |
|---|---|---|---|---|---|
| Sex, male | 84 (55) | 28 (18) | 40 (26) | .322 | .079 |
| Tobacco, never | 35 (52) | 20 (30) | 12 (18) | .086 | .076 |
| Alcohol, never | 37 (73) | 1 (2) | 13 (25) | .457 | .248 |
| cN status, N0 | 63 (64) | 25 (25) | 11 (11) | .829 | .001 |
| Subsite | |||||
| Buccal mucosa | 13 (62) | 5 (24) | 3 (14) | 1.000* | .475* |
| Tongue | 58 (58) | 15 (15) | 27 (27) | .082 | .047 |
| Floor of mouth | 21 (45) | 17 (36) | 9 (19) | .008 | .045* |
| Hard palate/upper gum | 5 (63) | 1 (12.5) | 2 (2.5) | 1.000* | 1.000* |
| Lower gum | 24 (62) | 9 (23) | 6 (15) | .956 | .305 |
| Retromolar trigone | 15 (54) | 5 (18) | 8 (29) | .778 | .477 |
| VI, present | 19 (35) | 16 (29) | 20 (36) | .002 | .984 |
| PNI, present | 46 (46) | 21 (21) | 33 (33) | .132 | .196 |
| Margin status, close/positive | 63 (52.5) | 27 (22.5) | 30 (25) | .492 | .941 |
| pT status | |||||
| T1 | 45 (67) | 16 (24) | 6 (9) | .751 | .007 |
| T2 | 36 (57) | 11 (17) | 16 (25) | .444 | .436 |
| T3 | 8 (47) | 5 (29) | 4 (24) | .354* | .732* |
| T4 | 44 (47) | 19 (20) | 31 (33) | .593 | .075 |
| pN status | |||||
| N1 | 72 (86) | 11 (13) | 1 (1) | < .001 | .001 |
| N2 | 64 (41) | 40 (25) | 53 (34) | < .001 | .018 |
| N3 | 0 (0) | 1 (25) | 3 (75) | .277* | .354* |
| Muscle invasion, present | NA | 1 (12.5) | 7 (87.5) | NA | .063* |
| Desmoplasia, present | NA | 48 (47) | 55 (53) | NA | .422* |
| PORT, yes | 96 (61) | 32 (20) | 30 (19) | .234 | .348 |
| PORT + chemotherapy, yes | 10 (40) | 6 (24) | 9 (36) | .386* | .520 |
Abbreviations: ECS, extracapsular spread; Neg, negative; Min, minimum; VI, vascular invasion; PNI, perineural invasion; NA, not applicable; PORT, postoperative radiotherapy.
Denotes Fisher’s exact test. All other tests with categorical variables were performed using Pearson’s Chi-square test. All tests with continuous variables were performed using an independent sample t test.
TABLE 2.
Continuous demographics, treatment characteristics, and histopathologic characteristics of patients with negative, minor, and major extracapsular spread.
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) |
p value Neg vs min |
p value Min vs Major |
|---|---|---|---|---|---|
| Age, y | 61.24 (14.69) | 64.49 (11.93) | 61.26 (13.63) | .156 | .193 |
| Tumor thickness, mm | 13.71 (10.05) | 13.27 (8.11) | 19.05 (121.74) | .790 | .07 |
| Tumor size, mm | 27.83 (14.64) | 26.90 (12.28) | 35.45 (16.06) | .690 | .003 |
| No. of metastatic nodes | 1.84 (1.50) | 3.67 (3.68) | 5.60 (4.80) | .001 | .022 |
| Size largest metastatic node, mm | 16.19 (8.01) | 18.86 (9.37) | 25.5 (14.57) | .053 | .005 |
| Size largest metastatic focus, mm | 9.88 (8.92) | 14.26 (9.37) | 23.55 (15.29) | .002 | < .001 |
Abbreviations: Neg, negative; min, minimum.
Prognostic threshold for extracapsular spread extent
The median extent of ECS was 2 mm (range, 0.025–20 mm). Time-dependent ROC analysis was used to identify the prognostic threshold of ECS associated with the most optimal sensitivity/specificity profile. This analysis identified ECS extent of 1.7 mm as the optimal prognostic threshold on the ROC curve with a sensitivity of 64% and a specificity of 82%. Fifty-two patients had ECS extent ≤1.7 mm (minor ECS; Figure 2) and 57 patients had ECS extent >1.7 mm (major ECS).
Patients without extracapsular spread versus patients with minor extracapsular spread (≤1.7 mm)
When compared with patients without ECS, patients with minor ECS were more likely to have vascular invasion (41% vs 17%; p = .002), a greater number of positive nodes (3.67 vs 1.84; p = .001), a greater size of the largest positive lymph node (1.89 vs 1.62 cm; p = .053), and a greater size of the largest metastatic focus within the involved node (1.42 vs 0.99 cm; p = .002). The 2 groups did not differ significantly in terms of sex (p = NS), age (p = NS), cN status (p = NS), T classification (p = NS), PNI (p = NS), surgical margin status (p = NS), grade (p = NS), adjuvant PORT (p = NS), and adjuvant chemotherapy-based RT (p = NS). In this subset of patient, there were 63 disease-related deaths. Figure 4 shows the Kaplan–Meier univariate analysis indicating that patients with minor ECS had similar DSS compared with patients without ECS (5-year DSS = 49.0% vs 57.8%; p = 0.197). In addition, positive margins (p = .020), T classification greater than T1 (p < .001), number of metastatic nodes (p = .025), thickness (p = .004), and tumor size (p = .008) were all associated with worse DSS on univariate analysis. On multivariate analysis, when controlling for other variables, minor ECS was not predictive of DSS (p = NS; data not shown).
FIGURE 4.
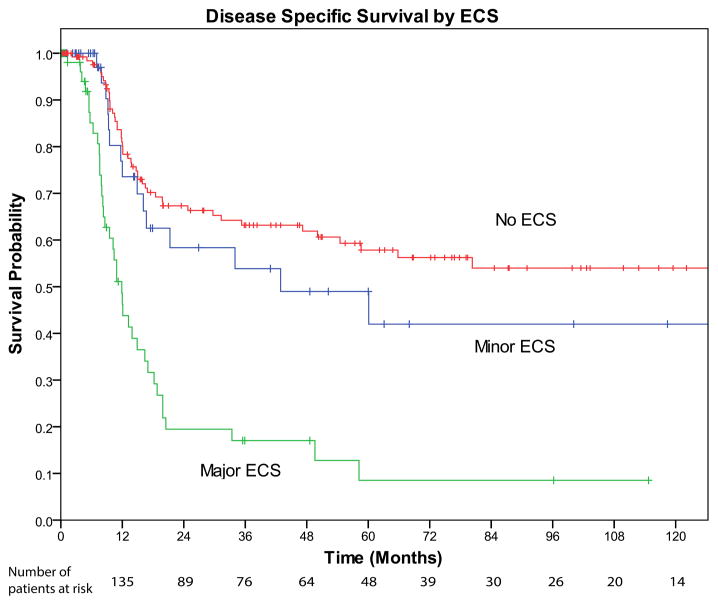
Kaplan–Meier disease-specific survival curve stratified by extracapsular spread (ECS). No ECS (red line) versus minor ECS (≤1.7 mm; blue line) are statistically similar (log-rank test p = .197). Minor ECS versus major ECS (>1.7 mm; green line) are statistically different (log-rank test p < .001).
Patients with minor extracapsular spread (≤1.7 mm) versus patients with major extracapsular spread (>1.7mm)
When compared with patients with minor ECS, patients with major ECS were more likely to have: a clinically positive neck (81% vs 52%; p = .001), larger primary tumors (3.54 vs 2.69 cm; p = .003), thicker tumors (1.9 vs 1.3; p = .007), T classification greater than T1 (89.5% vs 68.6%; p = .007), a greater number of metastatic lymph nodes (5.60 vs 3.64; p = .022), a greater size of the largest metastatic lymph node (2.55 vs 1.89 cm; p = .006), and a greater size of the largest metastatic focus (2.36 vs 1.43 cm; p < .001). There were no differences in sex (p = NS), age (p = NS), VI (p = NS), surgical margin status (p = NS), grade (p = NS), desmoplasia (p = NS), PORT (p = NS), and chemotherapy-based RT (p = NS). For this subset of patients with ECS, there were 54 patients who died of their disease. On univariate analysis, as shown in Figure 4, patients with major ECS had worse DSS than patients with minor ECS (5-year DSS = 8.5% vs 49.0%; p = .001). In addition, T classification greater than T1 (p = .035), tumor size (p = .002), number of metastatic nodes (p = .011), size of the largest metastatic node (p = .004), size of the largest metastatic focus (p = .012), and muscle invasion (p = .001), were all associated with decreased DSS on univariate analysis. Some of the variables found to be significant on univariate analysis were correlated with other factors, such as tumor size and T classification. Therefore, variables were carefully chosen for the multivariate model because of the small number of events that limited our number of variables. In the instance of tumor size and T classification, a continuous variable indicating the actual size of tumor was used rather than the binned groups that make up T classifications. On multivariate analysis, when controlling for other factors, major ECS remained a significant predictor of DSS, having an HR of 3.37 with a p value of .001. The concordance index of the model was 0.716. The results are shown in Tables 3 and 4.
TABLE 3.
Dichotomous prognostic predictors of disease-specific survival for patients with extracapsular spread.
| Dichotomous covariates | No. of patients | DSS 5-y survival | Univariate analysis p value |
Multivariate analysis
|
|
|---|---|---|---|---|---|
| HR (95% CI) | p value | ||||
| Sex | |||||
| Male | 68 | 22.6% | NS | NA | NA |
| Female | 41 | 29.3% | |||
| Tobacco use | |||||
| Never | 33 | 22.4% | NS | NA | NA |
| Ever | 76 | 25.8% | |||
| Alcohol use | |||||
| Never | 30 | 32.0% | NS | NA | NA |
| Ever | 79 | 20.8% | |||
| Perivascular invasion | |||||
| No | 52 | 17% | NS | NA | NA |
| Yes | 36 | 25.5% | |||
| PNI | |||||
| No | 34 | 25.1% | NS | NA | NA |
| Yes | 54 | 17.3% | |||
| Muscle invasion | |||||
| No | 101 | 27.1% | .001 | NA | NA |
| Yes | 8 | 0% | |||
| T classification | |||||
| T1 | 22 | 50.8% | .035 | NA | NA |
| T2, T3, or T4 | 86 | 19.0% | |||
| ECS | |||||
| Minor | 52 | 49.0% | < .001 | 3.366 (1.624–6.980) | .001 |
| Major | 57 | 8.5% | |||
Abbreviations: DSS, disease-specific survival; HR, hazard ratio; 95% CI, 95% confidence interval; NS, not significant; NA, not applicable; PNI, perineural invasion; ECS, extracapsular spread.
TABLE 4.
Continuous prognostic predictors of disease specific survival for patients with extracapsular spread.
| Continuous covariates | Median | Range | Univariate analysis p value |
Multivariate analysis
|
|
|---|---|---|---|---|---|
| HR (95% CI) | p value | ||||
| Age | 64 | 24–87 | NS | NA | NA |
| Size of largest node, mm | 20 | 5–80 | .004 | NA | NA |
| Size of tumor, mm | 30 | 10–85 | .002 | 1.026 (1.006–1.047) | .011 |
| Size of largest focus, mm, nonlinear* | 16 | 2–80 | .012 | 0.90 (0.38–2.12) | .014 |
| No. of metastatic nodes | 3 | 1–30 | .011 | 1.07 (0.996–1.142) | .064 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; NS, not significant; NA, not applicable.
HR was reported for the contrast of Q3 vs Q1.
DISCUSSION
Adverse implications associated with the presence of ECS in SCC of the head and neck are well described in multiple studies.3,4,6,7 Moreover, analysis of randomized data suggests that patients with ECS have better survival when treated with postoperative chemotherapy-based RT compared to PORT alone, despite the higher toxicity associated with the former.8–11 However, these studies have been hampered significantly by lack of specificity to patients with oral cancer and ambiguity pertaining to the definition of clinically relevant ECS. For example, the pathologic criteria for the diagnosis of ECS are poorly defined, making the pathological assessment of ECS subject to significant intraobserver and interobserver variability.15 This may create the potential for a degree of “overdiagnosis” among true borderline cases, fueled by concerns over the putative implications of a missed ECS diagnosis among pathologists. Although this is reasonably based upon reports suggesting that microscopic ECS is a significant prognostic factor in head and neck SCC,5,6 the prognostic implications of ECS are far from consensual, evidenced by the lack of convincing data showing that the prognostic value of ECS is independent from other nodal and primary tumor risk factors.4,9,10,14,25–27 Uncertainty regarding the definition of clinically relevant ECS may contribute to undertreatment of patients at risk, or exposure of patients to potentially severe side effects and toxicities of adjuvant treatment in the absence of definite benefit.
The present study aimed to address these issues through an empiric and quantitative analysis of ECS extent and its relationship with outcome. Previous studies attempted to determine the prognostic implications of ECS extent through comparison of microscopic and macroscopic ECS.7,17 These studies suggested that ECS is only associated with prognostic significance when it is macroscopically identifiable, whereas microscopic ECS was rejected as a significant prognostic factor in these studies. Several other studies found no difference between microscopic and macroscopic ECS.5,14,28 Greenberg et al28 published a non-empirical univariate analysis of ECS >2 mm and ECS <2 mm in oral cavity SCC, and found no outcome differences between these groups. Other nonempirical microscopic analyses of ECS extent in laryngopharyngeal, oropharyngeal cancers, and oral cavity SCC suggested that only extensive ECS was a predictor of outcome.16,17,29 Our study reports, we believe for the first time, an empirical, quantitative, analysis of the extent of ECS and suggests that the optimal prognostic cutoff for the extent of ECS lies at the 1.7 mm mark. At this cutoff, identified by time-dependent ROC analysis, predictive power reaches the optimal tradeoff between sensitivity and specificity in our patients with oral cavity SCC.
We believe that our findings may help to decrease intraobserver and interobserver variability among pathologists by setting a threshold at which clinically relevant ECS can be conclusively diagnosed. The current diagnosis of ECS is based on criteria formulated by the College of American Pathologists, wherein the presence or absence of any full thickness extension through the nodal capsule with or without the presence of a surrounding desmoplastic stromal reaction is consistent with ECS.30 In cases in which the tumor has extended overtly outside of the confines of the lymph node, or in cases in which the tumor is clearly confined to the lymph node, the present criteria lead to minimal diagnostic controversy.15 However, diagnostic discrepancies often arise in early ECS cases in which the invasive front of the tumor is situated closely near the lymph node capsule, cases in which capsular rupture is incomplete, cases in which the tumor approaches the nodal hilum area that lacks a capsule, or cases in which a desmoplastic stromal reaction surrounds the lymph node and mimics the capsule.15,31 If ECS is indeed clinically relevant only when it has extended >1.7 mm outside the confines of the nodal capsule, this is consistent with overt ECS and we anticipate that most pathologists will have no difficulty to recognize, measure, and document its extent.31
Clinical relevance of ECS >1.7 mm is supported by our data showing its independent prognostic significance, in contrast to ECS <1.7 mm. Some recent studies have reported independent prognostic significance of any ECS, but the additional prognostic factors included were heterogeneous and incomplete, and all studies were based on information derived from pathologic reports without detailed blinded re-review of histopathologic slides.3,32,33 To the best of our knowledge, the present study is the first to perform a thorough prognostic analysis of ECS, in the context of all conceivable additional prognostic factors, especially focused on those nodal metastatic factors that are associated with ECS. The strength of this work lies in the fact that it is based upon a uniform review of pathologic slides rather than pathology reports, and the inclusion of a homogeneous cohort of previously untreated patients with oral cavity SCC who were treated with a uniform treatment approach at a major tertiary care cancer center. The prognostic value of extent of ECS in our patients is unlikely to be influenced by the effect of adjuvant treatment, as a low number of ECS-positive cases received chemotherapy (13%), and no significant differences existed between the analyzed groups in the frequency of PORT or chemotherapy-based RT use.
Several limitations are inherently associated with the retrospective nature of the present study. Potential limitations include the fact that measurement of ECS extent was based on microscopic slides derived from standard pathological processing, which only represent a sample of involved lymph nodes, the choice for a 2D ECS extent marker (distance between capsule and invasive tumor front in millimeters) as a surrogate for the in reality 3D ECS phenomenon, and the possibility for selection bias because of exclusion of cases without available slides (see Materials and Methods). In addition, the use of time-dependent ROC analysis to identify the optimal prognostic cutoff of ECS extent may be viewed as arbitrary by some, it being only one acceptable statistical approach to identify an optimal cutoff among many others. With the current standard for intensified adjuvant treatment in the case of an ECS diagnosis in mind, we chose the ROC for its equivalent appreciation of sensitivity and specificity, aiming to identify a threshold that would optimally balance the risk of under-treatment because of suboptimal sensitivity (increased risk for cancer recurrence) and the risk of overtreatment because of suboptimal specificity (increased risk for sequela of intensified adjuvant treatment). Clearly, our findings should be considered hypothesis generating and need validation in other independent datasets. In order for these validation studies to succeed, we advocate development of more precise guidelines for the processing of neck dissection specimens and reporting of ECS, as the current recommendations set by the College of American Pathologists are not sufficiently detailed. For example, no recommendation on the number of representative sections to be examined histologically from grossly positive nodes is included in these guidelines, nor do they contain sufficient recommendations on the optimal measurement and reporting of ECS and its extent. After these guidelines for definition and measurement of ECS have been established, it should then be examined in prospective studies to assess for possible evidence-based treatment implications.
Overall, the present study suggests that the prognostic implications of ECS are only independent from other risk factors when a certain threshold (1.7 mm in this study) beyond the capsule of the lymph node is exceeded. This observation indicates that patients with minimal ECS and absence of other tumor-related risk factors may not be at the same increased risk of adverse outcomes compared with similar patients with more extensive ECS. It remains to be ascertained whether such patients can be safely managed without addition of postoperative chemoradiation to their treatment.
References
- 1.Patel SG, Lydiatt WM. Staging of head and neck cancers: is it time to change the balance between the ideal and the practical? J Surg Oncol. 2008;97:653–657. doi: 10.1002/jso.21021. [DOI] [PubMed] [Google Scholar]
- 2.Montero PH, Yu C, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120:214–221. doi: 10.1002/cncr.28407. [DOI] [PubMed] [Google Scholar]
- 3.Mamelle G, Pampurik J, Luboinski B, Lancar R, Lusinchi A, Bosq J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am J Surg. 1994;168:494–498. doi: 10.1016/s0002-9610(05)80109-6. [DOI] [PubMed] [Google Scholar]
- 4.Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA. Prognostic factors of neck node metastasis. Clin Otolaryngol Allied Sci. 1982;7:185–192. doi: 10.1111/j.1365-2273.1982.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaw RJ, Lowe D, Woolgar JA, et al. Extracapsular spread in oral squamous cell carcinoma. Head Neck. 2010;32:714–722. doi: 10.1002/hed.21244. [DOI] [PubMed] [Google Scholar]
- 6.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92:3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Ferlito A, Rinaldo A, Devaney KO, et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncol. 2002;38:747–751. doi: 10.1016/s1368-8375(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 8.Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist. 2005;10:215–224. doi: 10.1634/theoncologist.10-3-215. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 11.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spector ME, Gallagher KK, Light E, et al. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–1733. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jose J, Coatesworth AP, Johnston C, MacLennan K. Cervical node metastases in squamous cell carcinoma of the upper aerodigestive tract: the significance of extracapsular spread and soft tissue deposits. Head Neck. 2003;25:451–456. doi: 10.1002/hed.10214. [DOI] [PubMed] [Google Scholar]
- 14.Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130–137. doi: 10.1016/s1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 15.van den Brekel MW, Lodder WL, Stel HV, Bloemena E, Leemans CR, van der Waal I. Observer variation in the histopathologic assessment of extra-nodal tumor spread in lymph node metastases in the neck. Head Neck. 2012;34:840–845. doi: 10.1002/hed.21823. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24:1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasilino de Carvalho M. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck. 1998;20:16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–538. doi: 10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 19.Spiro RH, Morgan GJ, Strong EW, Shah JP. Supraomohyoid neck dissection. Am J Surg. 1996;172:650–653. doi: 10.1016/s0002-9610(96)00300-5. [DOI] [PubMed] [Google Scholar]
- 20.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120:699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 21.Givi B, Linkov G, Ganly I, et al. Selective neck dissection in node-positive squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2012;147:707–715. doi: 10.1177/0194599812444852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48:277–287. doi: 10.1007/s13312-011-0055-4. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer; 2001. [Google Scholar]
- 25.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positiveoropharyngeal carcinoma. Cancer. 2012;118:3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 26.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(Suppl 2):S13–S33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell JH, Ferris RL, Gooding W, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013;119:3302–3308. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg JS, Fowler R, Gomez J, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–1470. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 29.Prabhu RS, Hanasoge S, Magliocca KR, et al. Extent of pathologic extracapsular extension and outcomes in patients with nonoropharyngeal head and neck cancer treated with initial surgical resection. Cancer. 2014;120:1499–1506. doi: 10.1002/cncr.28596. [DOI] [PubMed] [Google Scholar]
- 30.College of American Pathologists. [Accessed May 1, 2015];Protocol for the examination of specimens from patients with carcinomas of the lip and oral cavity. Available at: http://www.cap.org.
- 31.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–385. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Liao CT, Wang HM, Chang JT, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer. 2007;110:1501–1508. doi: 10.1002/cncr.22959. [DOI] [PubMed] [Google Scholar]
- 33.Jan JC, Hsu WH, Liu SA, et al. Prognostic factors in patients with buccal squamous cell carcinoma: 10-year experience. J Oral Maxillofac Surg. 2011;69:396–404. doi: 10.1016/j.joms.2010.05.017. [DOI] [PubMed] [Google Scholar]