Abstract
Background
Primary sclerosing cholangitis (PSC) recurs in 15%–25% of patients transplanted for PSC. In the United States, PSC transplant patients are more likely to receive an organ from a living donor (LD) than patients without PSC. Our aims were to: 1) compare risk of PSC recurrence in LD versus deceased donor (DD) recipients; and 2) identify risk factors for PSC recurrence.
Methods
241 LD liver transplant (LT) and 65 DDLT subjects transplanted between 1998 and 2013 enrolled in the Adult to Adult Living Donor Liver Transplantation Cohort Study were evaluated. PSC recurrence risk for LDLT and DDLT recipients was compared using Kaplan-Meier survival curves and log-rank test. Cox models were used to evaluate PSC Risk factors.
Results
Overall PSC recurrence probabilities were 8.7% and 22.4% at 5 and 10 years post-LT, respectively. The risk of PSC recurrence was not significantly different for DDLT versus LDLT recipients (p=0.36). For DDLT versus LDLT recipients, unadjusted 5- and 10-year PSC recurrence was 9.4% versus 9.5% and 36.9% versus 21.1%. Higher lab Model for End-Stage Liver Disease (MELD) at LT, onset of a biliary complication, cholangiocarcinoma, and higher donor age were associated with increased risk of PSC recurrence (hazard ratio [HR]=1.06, 95% confidence interval [CI] 1.02–1.10 per MELD point, p=0.002; HR=2.82, CI 1.28–6.25 for biliary complication, p=0.01; HR=3.98, CI 1.43–11.09 for cholangiocarcinoma, p=0.008; HR=1.17, CI 1.02–1.35 per 5-years donor age, p=0.02). Factors not significantly associated with PSC recurrence: First-degree relative donor (p=0.11), post-LT cytomegalovirus infection (p=0.29), and acute rejection (p=0.37).
Conclusion
Risk of recurrent PSC was not significantly different for DDLT and LDLT recipients. Biliary complications, cholangiocarcinoma, MELD, and donor age were significantly associated with risk of PSC recurrence.
Keywords: end-stage liver disease, living donor liver transplant, biliary complications, cholangiocarcinoma, MELD
INTRODUCTION
Since the initiation of Model for End-Stage Liver Disease (MELD)-based allocation of liver organs in 2002, patients with primary sclerosing cholangitis (PSC) have been significantly more likely to receive a living donor liver transplant (LDLT) when compared with patients without PSC (1). In fact, since 2002, nearly 15% of transplant recipients with PSC in the United States received an LDLT, compared with 4% of non-PSC patients, despite PSC patients comprising only 6% of the population receiving a deceased donor liver transplant (DDLT) (1).
Recent data from Japan demonstrated a 25% risk of recurrent PSC in LDLT recipients, with a significantly increased risk when the living donor (LD) was a first-degree relative of the recipient (2). Additional risk factors for recurrence included higher MELD scores at transplant, postoperative cytomegalovirus (CMV) infections, and early biliary anastomotic complications. Importantly, in this cohort, recurrent PSC was associated with a higher risk of graft loss. Despite the potential risk of recurrent PSC, a recent analysis of all transplant recipients in the United States showed that, among transplant recipients with cholestatic liver disease (PSC or primary biliary cirrhosis), recipients of an LDLT had significantly higher post-transplant graft and patient survival compared with those who received DDLT (3). Analysis utilizing data from the national Organ Procurement and Transplantation Network (OPTN), which does not collect data on recurrent PSC, showed superior graft and patient survival among LDLT recipients with PSC, in contrast to the Japanese report that revealed high PSC recurrence rates and graft loss in LDLT recipients.
It is of great importance to validate these findings in a North American cohort, as donor-relatedness is a potentially modifiable risk factor in two ways. First, if the relationship applies to North American patients, LDLT recipients with more than one potential LD could be counseled to preferentially accept a non-related donor in order to reduce the risk of post-transplant PSC recurrence. Second, for those whose only potential donor is a first-degree relative, consideration of deferring LDLT and waiting for a DDLT and/or a potential donor swap might be options. In contrast, if the risk of recurrent PSC in those who receive a liver from a first-degree related donor in North America is much lower, then this issue should not affect donor selection. Thus, we used data from the only large multi-center prospective cohort of LDLT in North America (Adult to Adult Living Donor Liver Transplantation Cohort Study [A2ALL]) to estimate the risk of recurrent PSC in North American LDLT recipients and to identify risk factors for recurrence.
PATIENTS and METHODS
Study Population
Subjects in this study were transplanted for PSC between January 1, 1998 and July 31, 2013 at all centers participating in the A2ALL study. A2ALL is an observational cohort study designed to investigate outcomes in both donors and recipients of adult-to-adult LDLT, with comparison to DDLT recipients who had an LD evaluated. A2ALL was carried out in two phases: A2ALL-1 (1/1/1998 – 8/31/2009, which included both LDLT and DDLT recipients) and A2ALL-2 (9/1/2009 – 1/31/2014, which continued follow-up of A2ALL-1 LDLT and DDLT recipients and included new LDLT recipients). Nine North American centers were involved in each phase, with six centers spanning both phases. This analysis includes subjects transplanted for PSC at one of the nine A2ALL-2 centers (eight US, one Canadian). At the three new A2ALL-2 centers, non-A2ALL recipients (both LDLT and DDLT) transplanted for PSC during the A2ALL-1 time period were included in the PSC study by waiver of consent if they met the A2ALL-1 entry criteria of having had at least one potential LD evaluated. Patients in the three A2ALL-1 centers that were not part of A2ALL-2 were not included.
Both retrospective and prospective data were collected as part of A2ALL. The current study required data in addition to that collected as part of A2ALL. These data, as well as imaging reports documenting PSC recurrence and biliary complications, were obtained through detailed chart review under a waiver of consent. Data fields specific to the PSC sub-study included: PSC type, inflammatory bowel disease, colectomy, cholangiocarcinoma, CMV status, immunosuppression, rejection, surgical technique, and PSC recurrence. Immunosuppression was at the discretion of each center. Each center and the Data Coordinating Center had study protocols and consents approved by the Institutional Review Board (IRB) prior to collection and analysis of data.
Recurrent PSC, the primary outcome of interest, was defined using the Graziadei criteria, which require: confirmed diagnosis of PSC prior to transplantation and either cholangiography demonstrating multiple biliary strictures >90 days after transplantation or biopsy findings of fibrous cholangitis and/or fibro-obliterative lesions, in the absence of hepatic artery thrombosis/stenosis, chronic ductopenic rejection, isolated anastomotic strictures, non-anastomotic strictures prior to post-transplant day 90, and donor-recipient blood-type incompatibility (4). “Biliary complication” was defined as anastomotic stricture, bile leak, or biliary cast after transplantation. All data needed to define recurrent PSC were captured from each center using a standardized data form, with the case definition being adjudicated by each site’s primary investigator. Secondary outcomes were graft failure and death. Graft failure was defined as retransplantation or death.
Statistical Methods
Study subjects were followed from the time of transplant to the earlier of death or chart review. Descriptive statistics are given as means and standard deviations for continuous variables or as frequencies and percentages for categorical variables. Differences between DDLT and LDLT were compared using t-tests for continuous characteristics and chi-squared tests for categorical characteristics. Probabilities of PSC recurrence, retransplant, or death as the first event were displayed with a cumulative incidence graph using competing risks methods. To specifically examine PSC recurrence and risk factors associated with recurrence, we estimated cause-specific outcome, censoring at retransplant and death.
Potential factors associated with PSC recurrence were examined with multivariable Cox regression models stratified by donor type (DDLT, LDLT). The best subsets selection method was used to find parsimonious models. The following covariates were included in the model selection process: degree of relatedness between donor and LDLT recipient (first-degree relative vs. all others), time-dependent biliary complication, immunosuppression, time-dependent rejection and rejection treatment, recipient age, sex, race, ethnicity, blood type, MELD at transplant, PSC type, pre-transplant colectomy, ulcerative colitis, cholangiocarcinoma, cold ischemia time, warm ischemia time, presence of Roux-en-Y anastomosis, number of biliary anastomoses, and donor age, sex, race, ethnicity, and blood type. A history of a biliary complication was a time-dependent covariate, as the complication itself may have led to an inflammatory cascade, and/or discovery of recurrent PSC. Since first-degree relative has been previously shown to be associated with PSC recurrence, we forced inclusion of that covariate in one model. To examine the association of PSC recurrence with the outcomes of graft failure and death, Cox regression models with time-dependent covariates for PSC recurrence were used.
Statistical analyses were carried out using SAS version 9.4 (SAS Institute; Cary, NC). The cumulative incidence function was calculated using the comprisk macro available from the Mayo Clinic (http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros). Results with a two-sided p-value ≤0.05 were considered statistically significant.
RESULTS
Subject Characteristics
The characteristics of the PSC study sample are shown in Table 1 separately for recipients of DD (n=65) and LD (n=242) transplants. The distributions of donor and recipient race and ethnicity were significantly different between DDLT and LDLT, with higher percentages of African Americans (9.2% vs. 4.1%) and Hispanics/Latinos (12.3% vs. 2.4%) for DDLT compared with LDLT. DDLT recipients had a higher mean laboratory MELD score at transplant than LDLT recipients 20 versus 15, respectively (p<0.001). Mean cold and warm ischemia times were significantly longer for DDLT than LDLT grafts. The use of Roux-en-Y type biliary anastomoses were more common in LDLT procedures than DDLT (90.4% vs. 75.3%, p=0.001).
Table 1.
Characteristics of study population
| DDLT (n=65) Mean (s.d.) or n (%) |
LDLT (n=242) Mean (s.d.) or n (%) |
p-value | |
|---|---|---|---|
| Recipient Characteristics | |||
| Recipient age | 45.7 (13.8) | 44.4 (13.1) | 0.45 |
| Female recipient | 15 (23.0%) | 77 (31.8%) | 0.17 |
| Recipient race | 0.01 | ||
| Asian | 1 (1.5%) | 5 (2.0%) | |
| African American | 6 (9.2%) | 10 (4.1%) | |
| Native American | 0 (0.0%) | 1 (0.4%) | |
| White | 47 (72.3%) | 195 (80.5%) | |
| Multiracial | 3 (4.6%) | 0 (0.0%) | |
| Unknown | 8 (12.3%) | 31 (12.8%) | |
| Recipient ethnicity | 0.002 | ||
| Hispanic/Latino | 8 (12.3%) | 6 (2.4%) | |
| Non-Hispanic/non-Latino | 43 (66.1%) | 194 (80.1%) | |
| Unknown | 14 (21.5%) | 42 (17.3%) | |
| PSC type | 0.11 | ||
| Intrahepatic | 25 (38.4%) | 127 (52.4%) | |
| Extrahepatic | 1 (1.5%) | 12 (4.9%) | |
| Intra- and extrahepatic | 36 (55.3%) | 96 (39.6%) | |
| Small duct | 2 (3.0%) | 3 (1.2%) | |
| Unknown | 1 (1.5%) | 4 (1.6%) | |
| Lab MELD at transplant | 20.2 (9.0) | 14.7 (7.2) | <0.001 |
| Inflammatory bowel disease | 0.32 | ||
| Ulcerative colitis | 33 (50.7%) | 134 (55.3%) | |
| Crohn's disease | 8 (12.3%) | 37 (15.2%) | |
| Indeterminate | 0 (0.0%) | 5 (2.0%) | |
| None | 24 (36.9%) | 66 (27.2%) | |
|
Cholangiocarcinoma found at or before transplant |
4 (6.1%) | 22 (9.0%) | 0.45 |
| Transplant and Donor Characteristics | |||
| Donor age | 42.5 (19.7) | 38.0 (10.2) | 0.12 |
| Female donor | 26 (40.0%) | 113 (46.6%) | 0.34 |
| Donor race | <0.001 | ||
| Asian | 1 (1.5%) | 4 (1.6%) | |
| Black | 4 (6.1%) | 9 (3.7%) | |
| White | 43 (66.1%) | 214 (88.4%) | |
| Unknown | 17 (26.1%) | 15 (6.1%) | |
| Donor ethnicity | <0.001 | ||
| Hispanic/Latino | 3 (4.6%) | 5 (2.0%) | |
| Non-Hispanic/non-Latino | 19 (29.2%) | 215 (88.8%) | |
| Unknown | 43 (66.1%) | 22 (9.0%) | |
| LDLT graft type | |||
| Right lobe | N/A | 227 (93.8%) | |
| Left lobe | 12 (4.9%) | ||
| Left lateral segment | 2 (0.8%) | ||
| Unknown | 1 (0.4%) | ||
| DDLT donor type | |||
| Missing | 3 (4.6%) | N/A | |
| DBD | 60 (92.3%) | ||
| DCD | 2 (3.0%) | ||
| Donor relationship to recipient | |||
| First-degree LDLT | N/A | 120 (49.5%) | |
| Other blood relative LDLT | 23 (9.5%) | ||
| Other LDLT | 99 (40.9%) | ||
| Cold ischemia time (minutes) | 489.3 (170.9) | 80.8 (81.2) | <0.001 |
| Warm ischemia time (minutes) | 45.8 (32.8) | 36.2 (13.6) | 0.03 |
| Type of biliary anastomosis | 0.001 | ||
| All duct-to-duct | 16 (24.6%) | 23 (9.5%) | |
| At least one Roux-en-Y | 49 (75.3%) | 219 (90.4%) | |
Abbreviations: DBD, donation after brain death; DCD, donation after cardiac death; DDLT, deceased donor liver transplant; LDLT, living donor liver transplant; MELD, model for end-stage liver disease; PSC, primary sclerosing cholangitis.
There were 121 subjects treated for 181 episodes of acute cellular rejection (ACR): 87 LDLT recipients were treated for 127 episodes, and 34 DDLT recipients were treated for 54 episodes of ACR. The proportions of patients with ACR were similar between LDLT and DDLT recipients. Chronic rejection was also similar between LDLT and DDLT recipients (4.6% in each group).
There were 26 subjects diagnosed with cholangiocarcinoma at (n=6) or before (n=20) transplant. Of these, five (19.2%) had recurrence of cholangiocarcinoma. Among these five, one had PSC recurrence 2.25 years after transplant, and all five died at a median of 2.4 years.
Overall Outcomes
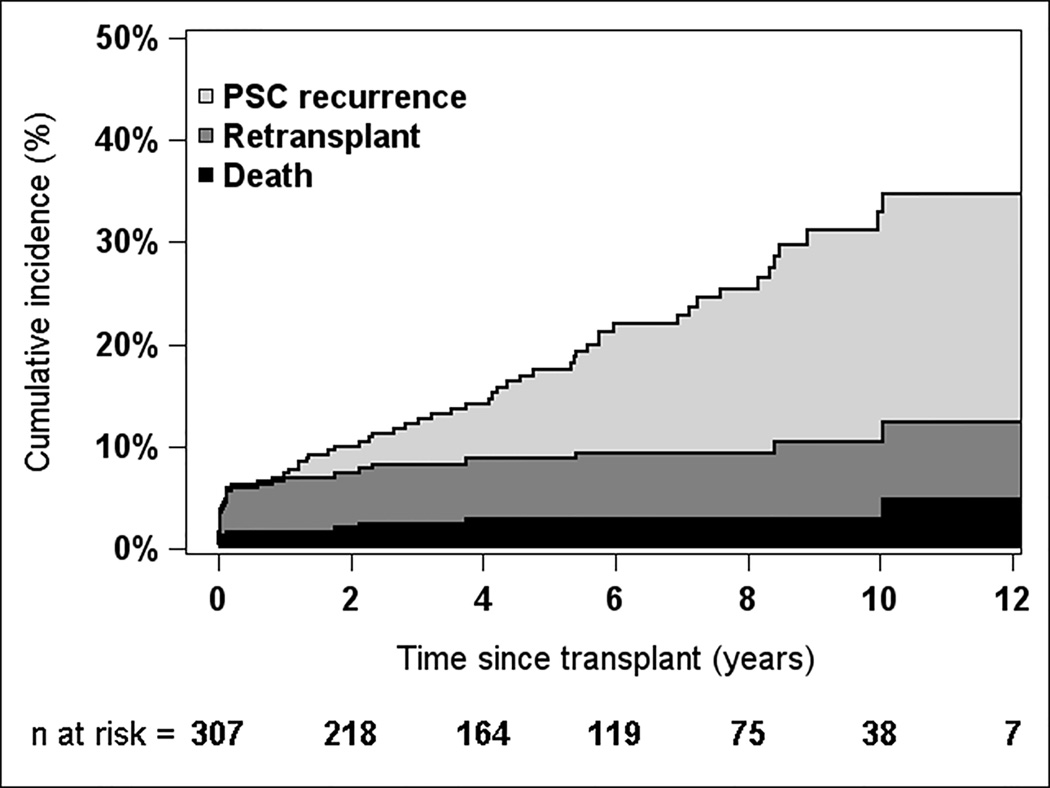
Median follow-up time was 5 years, and maximum was 15 years. The cumulative incidence of death, retransplant, and PSC recurrence is shown in Figure 1. Fifty-four percent of retransplants were performed within 3 months of the primary transplant. PSC recurrence tended to occur later post-transplant; only one recurrence was observed within 1 year after transplant. The respective cumulative incidences at 1, 3, 5, and 10 years were 1.6%, 2.5%, 3.0%, and 3.0% for death; 5.4%, 5.8%, 5.8%, and 7.5% for retransplant; and 0.3%, 4.0%, 8.7%, and 22.4% for PSC recurrence. Recurrence-free survival probabilities (alive with original graft and no PSC recurrence) at 1, 3, 5, and 10 years were 92.7%, 87.7%, 82.5%, and 67.0%, respectively.
Figure 1.
Overall risk of PSC recurrence, death, and retransplant for liver transplant recipients with PSC, based on the cumulative incidence function (n=307)
PSC Recurrence by Donor Type
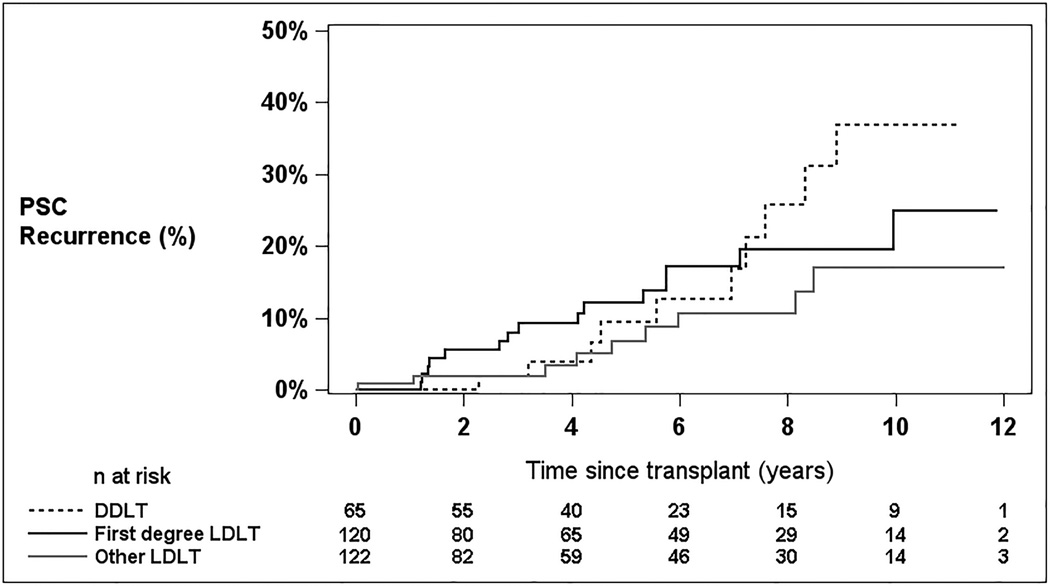
Overall, there were 34 recorded cases of recurrent PSC in the study group, 24 in LDLT recipients, and 10 in DDLT recipients. The diagnosis of recurrence was made by contrast cholangiography in eight, by computed tomography or magnetic resonance imaging in 14, by histology in 15, and by more than one diagnostic method in three subjects. Time to PSC recurrence, censored at retransplant or death, is shown by group in Figure 2 for DDLT, first-degree relative LDLT, and other LDLT. There was no significant difference in recurrence rate among the three groups (log rank p=0.36), between all LDLT recipients compared with DDLT recipients (p=0.36), or between first-degree relative and other LDLT (p=0.25).
Figure 2.
Probability of PSC recurrence by DDLT, first-degree relative donors, and non-first-degree relative donors (censored at graft failure)
Risk Factors for PSC Recurrence (Univariable Models)
In univariable models, PSC recurrence was significantly associated with the time-dependent onset of a biliary complication, including stricture, leak, or biliary cast (hazard ratio [HR] 2.69, 95% confidence interval [CI] 1.23–5.85, p=0.01), the presence of cholangiocarcinoma (HR 3.27, 95% CI 1.24–8.62, p=0.02), higher MELD at transplantation (HR 1.04 per point, 95% CI 1.00–1.08, p=0.04), and donor age (HR 1.16 per 5 years, 95% CI 1.02–1.32, p=0.02) (Table 2).
Table 2.
Cox models of time to PSC recurrence, stratified by donor type (living vs. deceased)
| Single Factor Models | Multivariable Model* | |||||
|---|---|---|---|---|---|---|
| Factor | Hazard Ratio |
95% CI | p-value | Hazard Ratio |
95% CI | p-value |
| Time-dependent biliary complication |
2.69 | 1.23–5.85 | 0.01 | 2.82 | 1.28–6.25 | 0.01 |
| Cholangiocarcinoma diagnosed at or before transplant |
3.27 | 1.24–8.62 | 0.02 | 3.98 | 1.43–11.09 | 0.008 |
| Time-dependent biliary complication |
2.69 | 1.23–5.85 | 0.01 | 2.82 | 1.28–6.25 | 0.01 |
| Cholangiocarcinoma diagnosed at or before transplant |
3.27 | 1.24–8.62 | 0.02 | 3.98 | 1.43–11.09 | 0.008 |
| Lab MELD at transplant (per point higher) |
1.04 | 1.00–1.08 | 0.04 | 1.06 | 1.02–1.10 | 0.002 |
| Donor age (per 5 years older) | 1.16 | 1.02–1.32 | 0.02 | 1.17 | 1.02–1.35 | 0.02 |
| First-degree related donor | 1.62 | 0.71–3.71 | 0.25 | |||
| Recipient age (per 5 years older) | 0.95 | 0.82–1.10 | 0.49 | |||
| Female donor | 0.82 | 0.41–1.65 | 0.58 | |||
| Female recipient | 0.49 | 0.20–1.20 | 0.12 | |||
| Donor race non-white | 1.72 | 0.41–7.27 | 0.46 | |||
| Recipient race non-white | 1.02 | 0.24–4.29 | 0.98 | |||
| Recipient Hispanic/Latino | 0.49 | 0.07–3.60 | 0.48 | |||
| Time-dependent rejection | 1.54 | 0.78–3.05 | 0.22 | |||
| Post-transplant CMV | 0.39 | 0.05–2.88 | 0.38 | |||
| >1 biliary anastomosis | 1.56 | 0.70–3.46 | 0.28 | |||
| Roux-en-Y anastomosis | 1.42 | 0.48–4.21 | 0.53 | |||
| Cold ischemia time (per hour) | 0.92 | 0.76–1.11 | 0.39 | |||
| Ulcerative colitis or Crohn’s disease | 1.00 | 0.49–2.07 | >0.99 | |||
| Pre-transplant colectomy performed | 1.02 | 0.40–2.66 | 0.96 | |||
| Steroid | 0.60 | 0.29–1.24 | 0.17 | |||
| Sirolimus or everolimus | 0.70 | 0.17–2.93 | 0.63 | |||
| AZA or MMF or mycophenolic acid | 1.51 | 0.77–2.96 | 0.23 | |||
Abbreviations: CI, confidence interval; MELD, model for end-stage liver disease; PSC, primary sclerosing cholangitis
Covariates for multivariable model were selected based on best subset selection method.
Risk Factors for PSC Recurrence (Multivariable Models)
Multivariable Cox regression using best subset selection was performed to identify risk factors for post-transplant PSC recurrence in liver transplant recipients. A parsimonious four-variable model demonstrated that time-dependent onset of a biliary complication, higher MELDS at transplant, higher donor age, and the presence of pre-transplant cholangiocarcinoma had statistically significant associations with higher risk of PSC recurrence (Table 2). To test whether the degree of donor-relatedness was associated with PSC recurrence, we added a covariate for first-degree related donor to the model specified above. The estimated HR for first-degree relative was 1.99 (95% CI 0.86–4.64) compared with other LDLT recipients, but this result was not statistically significant (p=0.11). Adding the first-degree related donor term to the model resulted in only very small changes to the parameter estimates for the other factors in the model.
Risk Factors for Graft Failure and Mortality (Multivariable Models)
There were 43 deaths and 59 graft failures (26 retransplants and 33 deaths) recorded during post-transplant follow-up. Multivariable models using time-dependent PSC recurrence as predictor of graft failure and death with covariate selection informed by the best subset method demonstrated the following risk factors for graft failure: time-dependent PSC recurrence (HR 4.16, 95% CI 1.75–9.86, p=0.001), time-dependent biliary complication (HR 4.00, 95% CI 2.17–7.36, p<0.001), cholangiocarcinoma diagnosed at or before transplantation (HR 4.01, 95% CI 2.12–7.57, p<0.001), laboratory MELD at transplant (HR 1.03 per point increase, 95% CI 1.00–1.054, p=0.05), and donor age (1.15 per 5 years older, 95% CI 1.03–1.27, p=0.01). Risk factors for death included time-dependent PSC recurrence (HR 2.70, 95% CI 1.01–7.25, p=0.049), time-dependent biliary complication (HR 3.21 95% CI 1.59–6.50, p<0.001), cholangiocarcinoma diagnosed at or before transplantation (HR 6.85, 95% CI 3.44–13.62, p<0.001), laboratory MELD at transplant (HR 1.03 per point increase, 95% CI 1.00–1.06, p=0.08), and donor age (1.10 per 5 years older, 95% CI 0.97–1.24, p=0.15).
Patient and Graft Survival after PSC Recurrence
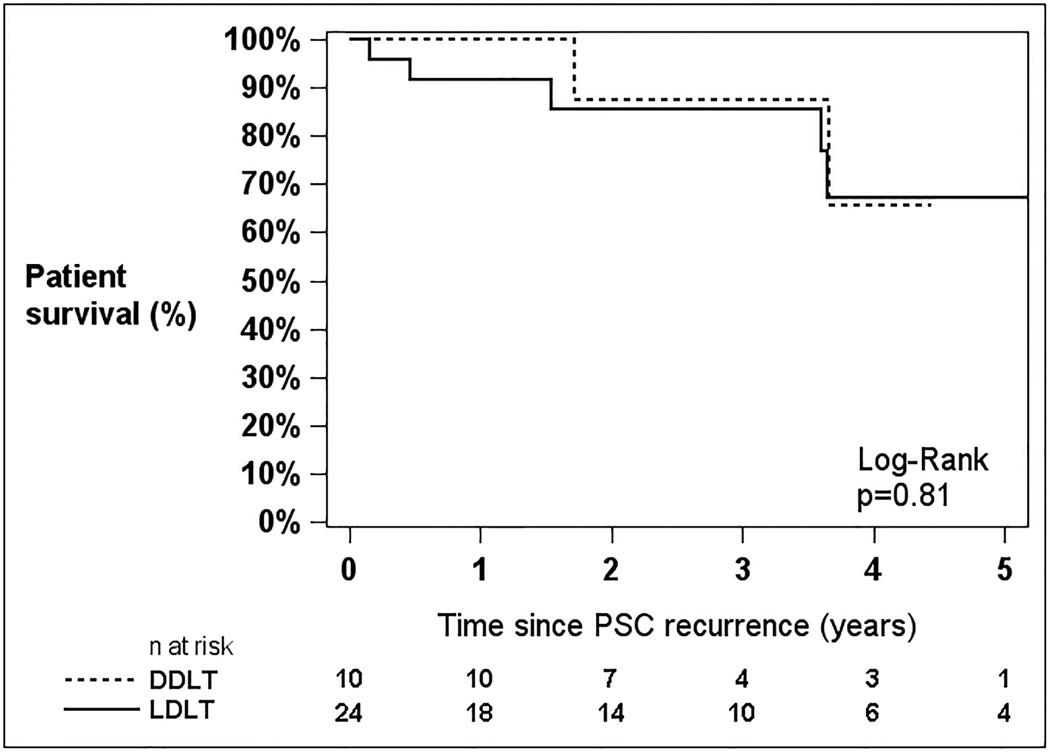
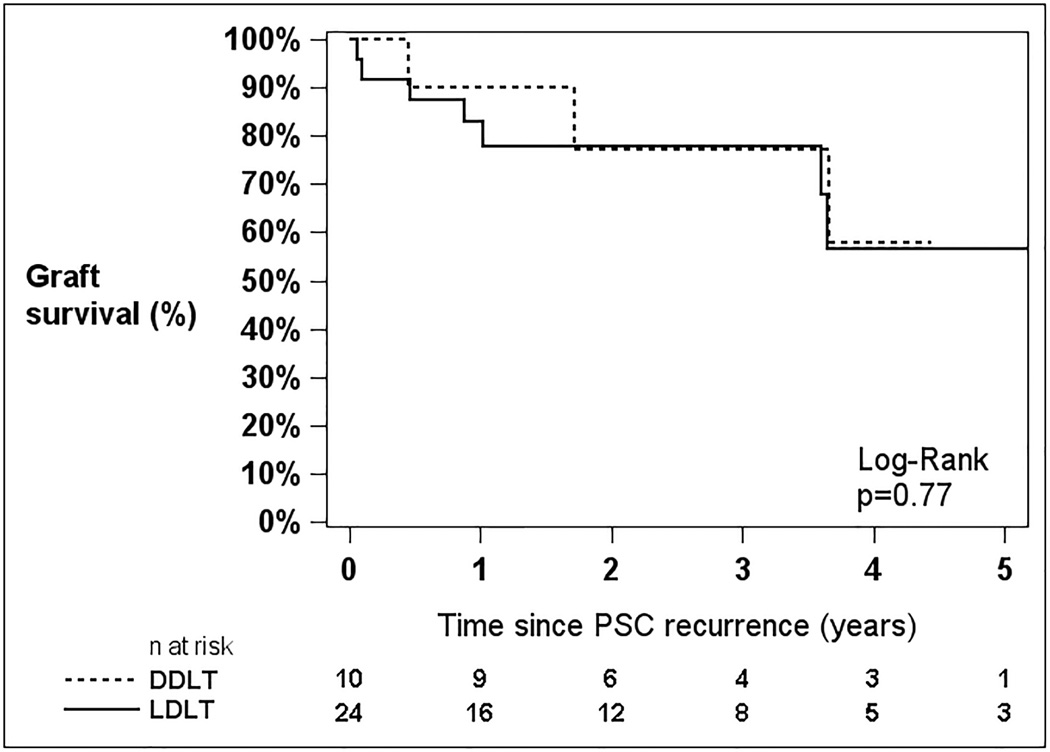
For those patients with documented PSC recurrence, the 5-year unadjusted patient and graft survival probabilities were 66.5% and 56.8%, respectively. There were no statistically significant survival differences after PSC recurrence between LD and DD recipients (Figure 3) for patient survival (p=0.81) or graft survival (p=0.77).
Figure 3.
Patient (A) and Graft (B) survival following recurrence of PSC after liver transplantation
DISCUSSION
As an immune-mediated liver disease, PSC may be affected by liver transplantation in several ways. The transplanted organ brings with it innumerable immune features such as cellular antigens, passenger lymphocytes, DNA, and infectious agents. The recipient immune system, including the presence of inflammatory bowel disease, prior surgery, prior infection exposure, and sex, may play a role in PSC recurrence (5). Transplantation issues, including ischemic time, immunosuppression, type of biliary anastomosis, and hepatic artery thrombosis, may potentially affect the liver allograft (6). Weismuller et al (5) postulated that the etiopathogenesis of PSC was multifactorial and included genetics (HLA), autoimmunity, and inflammation caused by infectious agents. Recipients of first-degree relative donor organs may have increased exposure to these factors. Differences in these characteristics may exist between LDLT and DDLT recipients. In this study, we were able to investigate these characteristics in a large North American cohort of LDLT and DDLT recipients transplanted for PSC.
In 2007, the first case series examining LDLT outcomes in eight PSC patients, as part of a larger report on LDLT from Tokyo, was published. All received a related-donor allograft, and 4/8 (50%) had recurrent PSC after a mean 3.3 years (7). In a retrospective, single center North American cohort, Kashyap et al (8) compared outcomes in 14 LDLT and 44 DDLT patients transplanted for PSC. The LDLT recurrence rate was 28% with a mean follow up of time of 41.5.±24.8 months. The DDLT recurrence rate was 16% with a mean follow up of 57.2±35.9 months (p=0.29). Among biologically-related donors, the rate of recurrence was 37%. Retransplantation rates for LDLT and DDLT were similar (7% vs. 20%, p=0.25). One-, 3-, and 5-year patient and graft survivals were also similar in the two groups (96%, 90%, 88% and 89%, 83%, 81%, respectively). A 2010 analysis of the United Network for Organ Sharing (UNOS) database, including 972 recipients of DDLT and 185 recipients of LDLT for PSC, demonstrated similarly high 1- and 5-year patient and graft survivals, but did not assess for PSC recurrence (9).
In our study, we observed 5- and 10-year PSC recurrence rates of 9.5% and 21.2%, respectively, for LDLT recipients. This compares favorably to the Japanese cohort, which had recurrence rates of 32% at 5 years and 52% at 10 years (2). This may be attributable to differing baseline population characteristics, surgical technique, or application of the definition of recurrent PSC to the data set. The risk of PSC recurrence was not significantly different in LDLT and DDLT recipients, and we observed excellent 1-, 3-, and 5-year recurrence-free survival rates (92.7%, 87.7%, and 82.5%, respectively). In patients with PSC recurrence, 1-, 3-, and 5-year patient and graft survivals were 94.1%, 85.9%, and 66.5%; and 85.0%, 77.3%, and 56.8%, respectively. These rates also compare favorably to the Japanese cohort with recurrent PSC, who had graft survivals of 54% at 3 years and 39% at 5 years (2).
Factors associated with increased risk of recurrent PSC after LDLT shared some similarities with the Japanese study (2): MELD score at the time of transplantation and the occurrence of biliary complications. In addition, the present study demonstrated that donor age and cholangiocarcinoma were important recurrence risk factors. Pre-transplant treatment of cholangiocarcinoma may result in chemotherapy-induced changes in the native hepatic artery, resulting in secondary sclerosing cholangitis in the transplanted liver, making it difficult to differentiate from recurrent PSC (10,11).
Interestingly, Egawa et al (2) found that a first-degree relationship between donor and recipient statistically significantly predicted recurrence of PSC with an HR of 2.61. In the present study, although we noted a trend in the same direction, the HR of 1.99 for this association was not statistically significant. We investigated this further by excluding donor age from the model (LDLT donor age was 35 compared with DDLT donor age 42, p<0.0001) and still did not find a significant result (HR 1.60, p=0.27). Another possible explanation for the discrepant finding between the two studies may be a difference in definition of first-degree relative. The Japanese study limited first-degree relatives to the parent/child relationship, while the present study also included sibling donors. Application of the Japanese definition of first-degree relative to the present study data yielded an HR of 1.52 and p=0.39 in our patient population. Similarly, applying the present study’s definition of first-degree relative, but using the Japanese significant variables, the HR for first-degree relative was 1.68 and p=0.22. This suggests that expansion or limitation of the definition of first-degree relative did not alter the findings of either study. The broader implication is that the North American and Japanese population may indeed differ with regard to risk of PSC recurrence.
Limitations of this study include the modest absolute number of PSC recurrences despite a large cohort. This may have resulted in underpowering of tests of the association of first-degree relative with PSC recurrence. Additionally, the recipients of DD livers may not be truly representative of all transplant candidates on the waiting list. This possibility was explored by Berg et al., who found there was no significant difference in portal hypertensive complications between those in whom LD was seriously contemplated and those who did not have a potential LD (12). The presence of pre-transplant cholangiocarcinoma, if anything, may have biased toward the discovery of recurrence, due to more intensive monitoring of these patients. The diagnosis of recurrent PSC was made retrospectively by chart review using a standardized form, which created the potential for missing cases of recurrent PSC that were not identified in real-time. However, given that these LDLT recipients had frequent lab tests, an elevated alkaline phosphatase suggesting the potential for recurrent PSC would be easily identified, and it is likely that we captured most, if not all, cases of recurrent PSC. We were unable to collect adequate HLA data to assess its potential association with PSC recurrence.
In conclusion, our data do not support the notion that live donation from a first-degree relative increases the risk of recurrent PSC. When investigating the identified risk factors for recurrent PSC, no difference was seen between the LD and DD recipient groups. Attempts should be made by transplant centers to address modifiable pre-transplant variables. These would include transplantation at lower MELD scores and younger donor age, both of which may be controllable with LDLT, and limitation of transplantation for cholangiocarcinoma to only optimized candidates. In addition, future studies investigating the observed differences between North American and Asian patients may yield further insight into the pathophysiology of PSC.
Acknowledgments
This is publication number 34 of the Adult to Adult Living Donor Liver Transplantation Cohort Study.
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, U01-DK62536, U01-DK85515, U01-DK85563, and U01-DK85587). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Martin R. Prince, MD, PhD, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Taruna Chawla, MD, Scott Heese, MPH, Theresa Lukose, PharmD, Rudina Odeh-Ramadan, PharmD, Jonah Zaretsky, BS, Connie Kim, BS, Tarek Mansour, MB BCH, Joseph Pisa, BA
Lahey Hospital & Medical Center, Burlington, MA (DK85515): PI: Elizabeth A. Pomfret, MD, PhD, FACS; Co-Is: Christiane Ferran, MD, PhD, Fredric Gordon, MD, James J. Pomposelli, MD, PhD, FACS, Mary Ann Simpson, PhD; Study Coordinators: Erick Marangos, Agnes Trabucco, BS, MTASCP.
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-Is: Talia Baker, MD, Zeeshan Butt, PhD, Laura M. Kulik, MD, Daniela P. Ladner, MD, Donna M. Woods, PhD; Study Coordinator: Patrice Al-Saden, RN, CCRC, Tija Berzins, Amna Daud, MD, MPH, Elizabeth Rauch, BS, Teri Strenski, PhD, Jessica Thurk, BA, MA, Erin Wymore, BA, MS, CHES.
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-I: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN. University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinator: Alexandra Birch, BS, Dulce MacLeod, RN.
University of Colorado, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; Co-Is: Gregory T. Everson, MD, FACP, Igal Kam, MD, James Trotter, MD, Michael A. Zimmerman, MD; Study Coordinators: Carlos Garcia, RN, BS, Anastasia Krajec, RN, Jessica Fontenot, BS.
University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Yevgeniya Abramovich, BA, Mary Akagi, MS, CCRP, Douglas R. Armstrong, BSN, MS, Abby Brithinee, BA, Charlotte A. Beil, MPH, Carl L. Berg, MD, Brenda W. Gillespie, PhD, Beth Golden, BScN, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Tania C. Ghani, MS, Anna S.F. Lok, MD, Monique Lowe, MSI, Akinlolu O. Ojo, MD, PhD, Samia Shaw, AAIT, Abigail Smith, MS, Anna Nattie, BA, Gary Xia, BA.Robert A. Wolfe, PhD.
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA.
University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD, Kim M. Olthoff, MD; Co-Is: K. Rajender Reddy, MD, Mark A. Rosen, MD, PhD, Abraham Shaked, MD, PhD, David S. Goldberg, MD, Karen L. Krok, MD, Mark A. Rosen, MD, PhD, Robert M. Weinrieb, MD; Study Coordinators: Brian Conboy, PA, MBA, Mary Kaminski, PA-C, Debra McCorriston, RN, Mary Shaw, RN, BBA.
University of Pittsburgh Medical Center, Pittsburgh, PA (DK85587): PI: Abhinav Humar, MD; Co-Is: Andrea F. DiMartini, MD, Mary Amanda Dew, PhD, Mark Sturdevent, MD; Study Coordinators: Megan Basch, RN, Sheila Fedorek, RN, CCRC, Leslie Mitrik, BS; Mary L. McNulty, MLS.
University of Toronto, Toronto, ON, CA (DK85563): PI: David Grant, MD, FRCSC; Co-Is: Oyedele Adeyi, MD, FCAP, FRCPC, Susan Abbey, MD, FRCPC, Hance Clarke, MSc, MD, FRCPC, Susan Holtzman, PhD, Joel Katz, CRC, PhD, Gary Levy, BSc, FRCPC, MD, Nazia Selzner, MD, PhD; Study Coordinators: Kimberly Castellano, BSc, Andrea Morillo, BM, BCh, Erin Winter, BSc.
University of Virginia, Charlottesville, VA (DK62484): PI: Carl L. Berg, MD; Co-I: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN.
Virginia Commonwealth University – Medical College of Virginia Campus, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-Is: Martha K. Behnke, PhD, Adrian H. Cotterell, MD, FACS, Ann S. Fulcher, MD, Pamela M. Kimball, PhD, HCLD, Mary E. Olbrisch, PhD, ABPP, Marc P. Posner, MD, FACS, Mark A. Reimers, PhD, Amit Sharma, MD, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Sarah Hubbard, Andrea Lassiter, BS, Luke Wolfe, MS.
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Leonard B. Seeff, MD, Averell H. Sherker, MD, FRCPC, Rebecca J. Torrance, RN, MS.
Heather Van Doren, MFA, coordinating senior editor with Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
List of Abbreviations
- A2ALL
Adult to Adult Living Donor Liver Transplantation Cohort Study
- HRSA
Health Resources and Services Administration
- ASTS
American Society of Transplant Surgeons
- PSC
Primary Sclerosing Cholangitis
- LD
Living Donor
- DD
Deceased Donor
- LT
Liver Transplant
- MELD
Model for End-Stage Liver Disease
- HR
Hazard Ratio
- LDLT
Living Donor Liver Transplant
- DDLT
Deceased Donor Liver Transplant
- CMV
Cytomegalovirus
- OPTN
Organ Procurement and Transplantation Network
- IRB
Institutional Review Board
- ACR
Acute Cellular Rejection
- CI
Confidence Interval
- UNOS
United Network for Organ Sharing
- DBD
Donation After Brain Death
- DCD
Donation After Cardiac Death
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Contributor Information
Fredric D. Gordon, Email: fredric_d_gordon@lahey.org.
David S. Goldberg, Email: david.goldberg@uphs.upenn.edu.
Nathan P. Goodrich, Email: nate.goodrich@arborresearch.org.
Anna S.F. Lok, Email: aslok@umich.edu.
Elizabeth C. Verna, Email: ev77@columbia.edu.
Nazia Selzner, Email: nazia.selzner@uhn.ca.
R. Todd Stravitz, Email: richard.stravitz@vcuhealth.org.
Robert M. Merion, Email: bob.merion@arborresearch.org.
REFERENCES
- 1.Goldberg DS, French B, Thomasson A, Reddy KR, Halpern SD. Current trends in living donor liver transplantation for primary sclerosing cholangitis. Transplantation. 2011;91(10):1148–1152. doi: 10.1097/TP.0b013e31821694b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egawa H, Ueda Y, Ichida T, Teramukai S, Nakanuma Y, Onishi S, et al. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation in Japanese registry. Am J Transplant. 2011;11(3):518–527. doi: 10.1111/j.1600-6143.2010.03402.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg DS, French B, Abt PL, Olthoff K, Shaked A. Superior survival using living donors and donor-recipient matching using a novel living donor risk index. Hepatology. 2014;60(5):1717–1726. doi: 10.1002/hep.27307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graziadei IW. Recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2002;8(7):575–581. doi: 10.1053/jlts.2002.33952. [DOI] [PubMed] [Google Scholar]
- 5.Weissmuller TJ, Wedenmeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis – aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48(Suppl1):S38–S57. doi: 10.1016/j.jhep.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Gordon F. Recurrent primary sclerosing cholangitis: Clinical diagnosis and long-term management issues. Liver Transpl. 2006;12(11Suppl2):S73–S75. doi: 10.1002/lt.20948. [DOI] [PubMed] [Google Scholar]
- 7.Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int. 2007;27(1):86–94. doi: 10.1111/j.1478-3231.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap R, Mantry P, Sharma R, Maloo MK, Safadjou S, Qi Y, et al. Comparative analysis of outcomes in living and deceased donor liver transplants for primary sclerosing cholangitis. J Gastrointest Surg. 2009;13(8):1480–1486. doi: 10.1007/s11605-009-0898-3. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap R, Safadjou S, Chen R, Mantry P, Sharma R, Patil V, et al. Living donor and deceased donor liver transplantation for autoimmune and cholestatic liver diseases – an analysis of the UNOS database. J Gastrointest Surg. 2010;14(9):1362–1369. doi: 10.1007/s11605-010-1256-1. [DOI] [PubMed] [Google Scholar]
- 10.Mantel HT, Rosen CB, Heimbach JK, Nyberg SL, Ishitani MB, Andrews JC, et al. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl. 2007;13(10):1372–1381. doi: 10.1002/lt.21107. [DOI] [PubMed] [Google Scholar]
- 11.Lupaæcu C, Lerut J. Allograft inflow – concern in liver transplantation after intraoperative radiotherapy for cholangiocarcinoma. Chirurgia (Bucur) 2014;109(6):837–842. [PubMed] [Google Scholar]
- 12.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Baker TB, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54:1313–1321. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]