Abstract
We conducted a group randomized trial to assess the feasibility and effectiveness of a multi-component, clinic-based HIV prevention intervention for HIV-positive patients attending clinical care in Namibia, Kenya, and Tanzania. Eighteen HIV care and treatment clinics (six per country) were randomly assigned to intervention or control arms. Approximately 200 sexually active clients from each clinic were enrolled and interviewed at baseline and 6- and 12-months post-intervention. Mixed model logistic regression with random effects for clinic and participant was used to assess the effectiveness of the intervention. Of 3522 HIV-positive patients enrolled, 3034 (86 %) completed a 12-month follow-up interview. Intervention participants were significantly more likely to report receiving provider-delivered messages on disclosure, partner testing, family planning, alcohol reduction, and consistent condom use compared to participants in comparison clinics. Participants in intervention clinics were less likely to report unprotected sex in the past 2 weeks (OR = 0.56, 95 % CI 0.32, 0.99) compared to participants in comparison clinics. In Tanzania, a higher percentage of participants in intervention clinics (17 %) reported using a highly effective method of contraception compared to participants in comparison clinics (10 %, OR = 2.25, 95 % CI 1.24, 4.10). This effect was not observed in Kenya or Namibia. HIV prevention services are feasible to implement as part of routine care and are associated with a self-reported decrease in unprotected sex. Further operational research is needed to identify strategies to address common operational challenges including staff turnover and large patient volumes.
Keywords: HIV/AIDS, Sub-Saharan Africa, HIV prevention, People living with HIV
Introduction
Over the past 30 years, remarkable progress has been made in curbing the HIV epidemic. From 2001 to 2013, annual incidence of new HIV infections decreased by 38 % and AIDS-related deaths decreased by 35 % [1]. Despite this progress, HIV remains a significant public health burden in many countries, with 2.1 million individuals newly infected with HIV in 2013 [1]. Sub-Saharan Africa remains the most heavily affected region, accounting for 70 % of all new infections and 71 % of the estimated 36.9 million people living with HIV (PLHIV) globally [1].
The Joint United Nations Programme on HIV/AIDS (UNAIDS) has released several ambitious goals to help end the global HIV epidemic. The first set of goals, known as the 90–90–90 goals, aim to strengthen the HIV care continuum [2]. These goals state that by 2020: 90 % of all PLHIV will know their HIV status; 90 % of all people with diagnosed HIV infection will receive sustained antiretroviral therapy (ART); and 90 % of all people receiving ART will achieve viral suppression [2]. Similarly, an HIV prevention goal aims to reduce the number of new HIV infections worldwide to less than 500,000 by 2020 and to less than 200,000 by 2030 [3]. Achieving these goals will require scaling up both treatment and prevention efforts as well as focusing on key and priority populations including PLHIV.
Prevention interventions for PLHIV integrated into facility and community-based ART programs can dramatically reduce sexual transmission of HIV and effectively engage PLHIV as equal partners in efforts to curb the HIV epidemic [4]. Evidence-based prevention interventions for PLHIV include: adherence counseling and support, risk reduction counselling and condom provision, partner HIV testing and counseling, family planning counseling and services, and STI treatment and management [5]. These interventions also provide a supportive framework for achieving the 90–90–90 goals by facilitating diagnosis of PLHIV through a focus on testing the partner(s) and children of HIV-positive individuals (first 90); retention in HIV care and treatment programs by addressing the sexual and reproductive health needs of PLHIV (second 90); and viral suppression through adherence counselling and support (third 90).
Evidence from U.S. settings indicates that provider delivered HIV prevention services can effectively reduce sexual risk behavior [6, 7], STI incidence [8, 9], and unintended pregnancy among PLHIV [10]. However, data from sub-Saharan Africa are limited. Clinics in these regions face operational challenges not experienced in the U.S. including inadequate staffing, large patient volumes, and poor recordkeeping [11]. To address this knowledge gap, this manuscript reports the results of a group randomized trial examining the feasibility and effectiveness of a clinic-based package of HIV prevention interventions delivered to PLHIV attending clinical care in three sub-Saharan African countries.
Methods
This study was conducted in 18 HIV clinics in Kenya, Namibia, and Tanzania (six per country). Clinics were matched on clinic-level factors (e.g. provider/patient ratio, number of patients enrolled, and clinical services provided) and then randomly assigned to either the intervention or a wait-list comparison arm. In the comparison arm, providers delivered services according to their usual standard of care. Following final study data collection, providers in the comparison clinics were trained to deliver the HIV prevention services as described below.
Objectives and Hypotheses
The main objective of this study was to evaluate the effectiveness of integrating HIV prevention interventions into routine HIV care and treatment services in resource-limited settings. Project hypotheses focused on the effectiveness of these prevention interventions at impacting behavioral outcomes (i.e. decreased unprotected vaginal sex, decreased alcohol use, increased HIV serostatus disclosure and partner testing, and increased adherence to anti-retroviral (ARV) medications), biologic outcomes (i.e. decreased unintended pregnancy), and service delivery outcomes (i.e. provision of family planning counseling and services) comparing intervention and comparison clinics, with intervention participants hypothesized to report lower risk behavior than comparison participants after 12-months of follow-up. A secondary objective was to assess the feasibility and acceptability of integrating the prevention interventions into HIV clinical settings.
Intervention
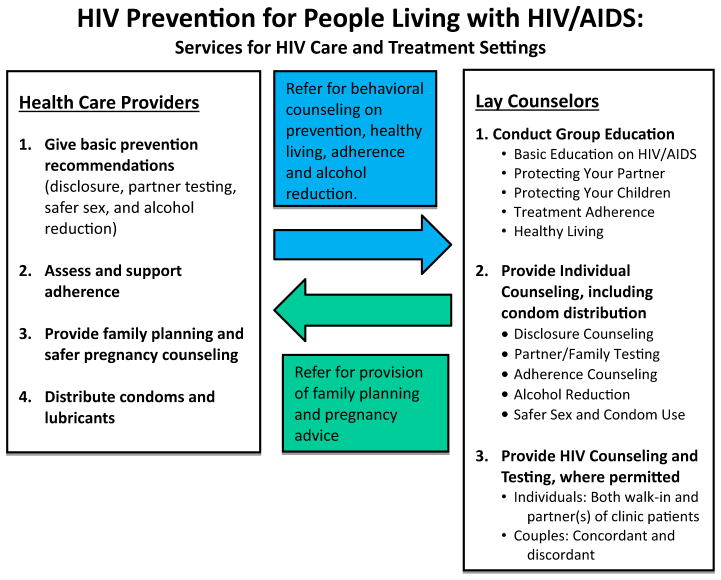
In the intervention arm, health care providers (HCPs) received a 1 week training on how to deliver prevention messages on HIV serostatus disclosure, alcohol and sexual risk reduction, partner HIV testing, medication adherence, and family planning (Fig. 1). Providers were also trained to distribute condoms to patients at every clinic visit. Research staff worked with HCPs to ensure a continuous supply of condoms to prevent condom stock-outs. Participants who did not desire pregnancy were encouraged to use condoms plus another form of highly effective contraception per current World Health Organization (WHO) guidelines [12]. To improve patients’ access to short-acting hormonal contraceptives, pills and injectables were offered at the HIV clinic. Providers referred patients desiring longer-acting contraceptive methods (e.g., intrauterine devices, implants) to family planning clinics.
Fig. 1.
Summary of model for integrating HIV prevention messages and services into the routine care offered to HIV-positive patients attending clinical care in Kenya, Tanzania, and Namibia
Lay counselors (LCs) received a 2-week training on how to support and reinforce provider-delivered HIV prevention services through group education (see Fig. 1) and individual counseling sessions. Counselors in Namibia and Kenya were also trained to conduct partner and couples HIV testing and counseling. In Tanzania, LCs only provided pre- and post-test counseling as they were not permitted by national guidelines to conduct HIV testing.
Trainings in all three countries were conducted by the same group of trainers using a written curriculum.
Procedures
At each of the 18 study clinics, a representative sample of approximately 200 sexually active patients was enrolled as part of an evaluation cohort to assess the effectiveness of the clinic-level intervention. The primary outcome variable for the study was self-report of any unprotected sex in the past 3 months. Sample size calculations assumed a rate of at least 50 % for any unprotected sex in the past 3 months for the comparison condition. Sample size estimates took into account expectations of attrition in the project cohort, which was expected to be moderate (at most 30 % at the 12-month follow-up). Sample size estimates were calculated using a formula developed by Murray for group-randomized trials [13]. Using this formula, it was determined that the project would have at least 80 % power to detect a 25 % difference between intervention and comparison arms (absolute difference of 12.5 %, with an intra-class coefficient of 0.02) with 9 clinics per condition, each with 200 patients enrolled.
Procedures for patient enrollment have been previously described [14]. In brief, study staff approached every third patient in the clinic waiting area and asked if he/she was interested in participating in the study. Interested patients were taken to a private area, told about the project, and screened for eligibility. Patients were eligible for inclusion if they were over 18 years of age, received HIV clinical care at the study clinic at least twice, reported being sexually active within the past 3 months, and planned to attend the clinic for at least a year. Women who knew they were pregnant and male partners of pregnant women were excluded.
Study staff administered questionnaires to patients at baseline and at 6- and 12-months post-intervention. Data collection start dates were staggered across countries with each data collection period taking approximately 3 months. Baseline data collection began in October 2009 and was completed by April 2010. All data collection was completed by December 2011. Participants who failed to keep a scheduled appointment were traced by study staff using phone calls, text messages or clinic defaulter staff.
Measures and Sources of Data
Observations of HCP and LC Patient Interactions
Study staff observed patient encounters with HCPs and LCs using a standardized observation form (see Table 2 for specific topics). Random days during the month were chosen to conduct the observations. Observations were conducted with patients who were and were not enrolled in the study since HCPs and LCs were trained to provide services to all patients in the clinic (not just those in the study). Approximately 3 % of clinic visits were observed throughout the intervention period.
Table 2.
Health care provider observations by time period and trial arm
| Variable | Intervention
|
Comparison
|
F statistic | p value | ||
|---|---|---|---|---|---|---|
| Baseline N (%) |
Follow-up N (%) |
Baseline N (%) |
Follow-up N (%) |
|||
| Sexual behavior and condoms | ||||||
| Assessed whether patient sexually active | 427 (61.6) | 3439 (73.1) | 343 (60.1) | 2695 (56.5) | 11.71 | .0045 |
| Discussed safer sex | 182 (26.5) | 2096 (44.7) | 164 (28.9) | 1471 (30.8) | 6.18 | .0273 |
| Discussed using condoms every time patient has sex | 388 (56.2) | 2913 (62.0) | 252 (44.6) | 2136 (44.8) | 4.47 | .0544 |
| Provider gave condoms to patient | 202 (29.2) | 1342 (28.7) | 59 (10.4) | 608 (12.8) | 3.13 | .1001 |
| Disclosure and partner testing | ||||||
| Assessed HIV status disclosure to partner | 194 (29.3) | 1758 (40.1) | 180 (33.8) | 1274 (27.2) | 16.05 | .0015 |
| Asked if partner has been tested for HIV | 24.57 | <.0001 | ||||
| Kenya | 43 (32.6) | 825 (84.3) | 92 (66.7) | 555 (65.9) | 5.28 | .0003 |
| Namibia | 124 (59.3) | 876 (39.4) | 71 (62.8) | 181 (8.4) | 13.37 | <.0001 |
| Tanzania | 63 (58.3) | 651 (71.7) | 37 (46.3) | 676 (53.5) | 1.27 | .2312 |
| Family planning (FP) | ||||||
| Assessed pregnancy status | 83 (12.2) | 1267 (27.2) | 145 (26.1) | 1107 (23.2) | 1.32 | .2715 |
| Offered appropriate FP | 50 (7.3) | 823 (18.1) | 113 (20.1) | 626 (13.2) | 12.30 | .0039 |
| Medication adherence | ||||||
| Assessed ARV adherence | 413 (61.9) | 3054 (70.3) | 395 (71.0) | 3594 (77.9) | 0.01 | .9264 |
| Alcohol use | ||||||
| Assessed alcohol use | 240 (34.7) | 2539 (54.0) | 218 (38.2) | 1616 (33.9) | 11.47 | .0049 |
Note Observations are based on a 3% sampling of clinic visits. Clients observed were not necessarily enrolled in the trial
Significant difference (p < .05) between trial arms at follow-up
Health Care Provider Questionnaires
At baseline and 6- and 12-months post-intervention, HCPs in all clinics were asked to rate the feasibility and importance of offering HIV prevention services to patients during routine visits and to rate how comfortable they were discussing prevention issues with patients. They were also asked if patients were comfortable receiving prevention information from them. All responses were on a 5-point Likert scale with higher scores indicating stronger agreement or greater comfort.
Patient Questionnaires
Sociodemographic variables included age, gender, education, and paid work in the past 6 months. Participants provided the name and dose of their HIV medications and indicated whether they had missed any doses in the last 30 days. Participants were asked to name up to five sex partners in the past 3 months. For each named partner, participants were asked whether the partner was a spouse, main, or non-main partner and whether they had unprotected sex at last vaginal sex and/or within the past 2 weeks with the partner. Participants indicated whether they had disclosed their HIV status to the partner, whether the partner had been tested for HIV and if so, whether the participant knew the partner’s HIV status. To assess pregnancy intention, participants were asked whether they were pregnant or desired a pregnancy in the next 6 months (female) or whether they desired their spouse or main partner to become pregnant in the next 6 months (male). They were also asked whether they (or their partner) were using condoms and/or a highly effective contraceptive method [e.g., pills, injectable, intra-uterine device (IUD), implant, male or female sterilization] to prevent pregnancy. The 10-item Alcohol Use Disorders Identification Test (AUDIT) [15] was used to categorize participants as non-drinkers (AUDIT = 0), non-problem drinkers (AUDIT 1–8), problem drinkers (AUDIT 8–12 for women, 8–14 for men), and likely dependent on alcohol (AUDIT ≥13 for women, ≥15 for men). The AUDIT has been widely used and validated in a variety of settings throughout sub-Saharan Africa [16–19].
Medical Chart and Clinic Record Abstraction
Data abstracted from patient medical charts included date of HIV diagnosis, dates of HIV clinic visits in the past 6 months, most recent CD4 count, prescribed HIV medications and dosages, and pregnancy status. Number of contraceptive pills and injections delivered to women in the study clinics were abstracted from clinic registers.
Data Analysis
Baseline sociodemographic and HIV-related variables of interest were compared using the GLIMMIX procedure in SAS 9.3 to fit mixed model logistic regressions with trial arm as the dependent variable and the variable of interest as the independent variable. All models included a random effect for clinic to adjust for within-clinic correlation. For the outcome analyses, GLIMMIX models were used with fixed effects for country, time (6 or 12 month follow-up), trial arm (intervention or standard of care) and all possible interactions, and random effects for clinic and participant (to control for correlation within clinic and within person over time). The baseline value for the outcome of interest was included as a covariate in these models, both to control for baseline differences across arms and to reduce the intraclass correlation coefficient, where possible. When interaction terms were significant, odds ratios were estimated within country and/or time. When no interaction terms were significant, these were dropped from the model, and if there were no intervention effects, we tested for a significant time effect (modeling baseline as one of the timepoints instead of as a covariate).
Ethical Considerations
The study protocol was approved by ethics review committees at the U.S. Centers for Disease Control and Prevention and at all collaborating organizations including the Columbia University Medical Center, Kenya Medical Research Institute (KEMRI), Namibia Ministry of Health and Social Services (MOHSS), Tanzania National Institute of Medical Research (NIMR), and the Zanzibar Medical Ethical Committee (ZAMEC). All patients, health care providers, and lay counselors signed written informed consent forms prior to the start of data collection procedures.
Results
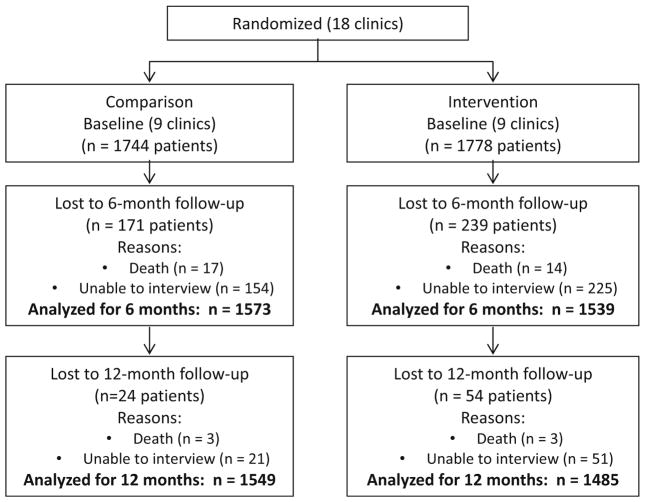
A total of 3538 HIV-positive patients were enrolled between September 2009 and April 2010. Sixteen patients were removed from the analysis because the gender recorded at the baseline and follow-up interviews did not match, leading to uncertainty whether the same person had completed all interviews. This left an analytic sample of 3522 HIV positive patients (intervention: 1778; comparison: 1744) (Fig. 2). The majority of participants (58 %) were female (Table 1). Nearly half were between 30 and 39 years of age with a median age of 36 years. Male participants were significantly older than female participants (median age: 41 vs. 35 years, p <0.0001). Most had completed primary (53 %) or secondary (34 %) school although 10 % reported receiving no schooling. Less than half (44 %) reported having paid work in the last 6 months. Sixty-one percent were married or cohabiting, with nearly all (95 %) reporting only one sex partner in the past 90 days.
Fig. 2.
Flow diagram showing follow-up of HIV-positive patients enrolled in a group randomized control trial. Note Unable to interview includes unable to locate, transferred to another clinic, and declined interview
Table 1.
Baseline sociodemographic and health characteristics of study participants
| Overall (n = 3522) n (%) |
Intervention (n = 1778) n (%) |
Comparison (n = 1744) n (%) |
p value | |
|---|---|---|---|---|
| Gender | .4037 | |||
| Male | 1476 (41.9) | 761 (42.8) | 715 (41.0) | |
| Female | 2046 (58.1) | 1017 (57.2) | 1029 (59.0) | |
| Age, years | .7267 | |||
| 18–29 | 615 (17.5) | 317 (17.8) | 298 (17.1) | |
| 30–39 | 1659 (47.1) | 822 (46.2) | 837 (48.0) | |
| 40–49 | 974 (27.7) | 490 (27.6) | 484 (27.8) | |
| ≥50 | 274 (7.8) | 149 (8.4) | 125 (7.2) | |
| Education | .9174 | |||
| No school | 340 (9.7) | 176 (9.9) | 164 (9.4) | |
| Primary school | 1883 (53.4) | 943 (53.1) | 940 (54.0) | |
| Secondary school | 1206 (34.3) | 598 (33.7) | 608 (34.9) | |
| More than secondary school | 88 (2.5) | 58 (3.3) | 30 (1.7) | |
| Any paid work, past 6 months | 1559 (44.3) | 777 (43.8) | 782 (44.9) | .9177 |
| Marital status | .7962 | |||
| Married/living together | 2161 (61.4) | 1123 (63.2) | 1038 (59.5) | |
| Single, never married | 775 (22.0) | 353 (19.9) | 422 (24.2) | |
| Separated / divorced | 359 (10.2) | 206 (11.6) | 153 (8.8) | |
| Widowed | 225 (6.4) | 94 (5.3) | 131 (7.5) | |
| Have children | 3181 (90.3) | 1603 (90.2) | 1578 (90.5) | .8321 |
| On antiretrovirals | 2259 (64.2) | 1163 (65.5) | 1096 (62.8) | .2561 |
| Years since HIV diagnosis | .2423 | |||
| <1 years | 923 (26.2) | 532 (29.9) | 391 (22.4) | |
| 1 to <2 years | 803 (22.8) | 411 (23.1) | 392 (22.5) | |
| 2 to < 3 years | 719 (20.4) | 357 (20.1) | 362 (20.8) | |
| ≥3 years | 1075 (30.5) | 477 (26.8) | 598 (34.3) | |
| Most recent CD4 count (per mm3) | <.0001 | |||
| <200 | 822 (23.6) | 486 (27.7) | 336 (19.4) | |
| 200–349 | 1035 (29.7) | 518 (29.5) | 517 (29.9) | |
| 350–500 | 793 (22.8) | 367 (20.9) | 426 (24.6) | |
| ≥501 | 834 (23.9) | 383 (21.8) | 451 (26.1) |
Note Percentages may not total to 100% due to rounding
Twenty-six percent had been diagnosed with HIV within the past year and 70 % within the past 3 years (Table 1). Nearly a quarter had a CD4 count less than 200 cells/mm3 at their last test and another 30 % had a CD4 count between 200 and 349 cells/mm3. Sixty-four percent of participants were on ART at the time of enrollment. By the 12-month follow-up, 81 % were on ART. There were no significant differences between trial arms on any of these demographic or clinical variables. Overall, 86 % of participants (84 % in the intervention group, 89 % in the comparison group; OR = 0.65, 95 % CI 0.47, 0.91) were retained in the study through the 12-month follow-up assessment.
Implementation of the Intervention
Table 2 summarizes the extent to which the intervention was delivered by HCPs during observations of provider-patient interactions. Providers in the intervention clinics more frequently discussed safer sex (45 % vs. 31 %, p = 0.03), consistent condom use (62 % vs. 45 %, p = 0.05), and patients’ use of alcohol (54 % vs. 34 %, p = 0.005) than providers in comparison clinics. They were also more likely to assess whether participants had disclosed their HIV status to a sex partner (40 % vs. 27 %, p = 0.002). There was a significant country by trial arm interaction on discussion of partner testing with providers in intervention clinics in Kenya (84 % vs. 66 %, p = 0.0003) and Namibia (39 % vs. 8 %, p <0.0001) more likely to discuss partner testing than in comparison clinics. There was a similar but non-significant trend on this variable in Tanzania (72 % vs. 54 %, p = 0.23).
During interviews, participants in intervention clinics were more likely to report receiving prevention messages from their HCPs compared to participants in comparison clinics at the 12-month follow-up (data not shown). This includes messages on disclosure (78 % vs. 62 %, p = 0.04), partner testing (80 % vs. 61 %, p = 0.02), family planning (67 % vs. 47 %, p = 0.002), alcohol reduction (88 % vs. 72 %, p = 0.005), and consistent condom use (97 % vs. 86 %, p <0.0001). Overall 31 % of study participants in intervention clinics reported meeting with a LC at the 6 month assessment, which increased to 52 % at the 12 month assessment. In the 3878 interactions observed, LCs consistently delivered HIV prevention messages to patients including messages on safer sex (77 % of sessions), consistent condom use (85 %), alcohol use (81 %), HIV serostatus disclosure (87 %), and partner testing (81 %).
LCs distributed condoms to patients in 62 % of observed encounters. HCPs distributed condoms to patients in 29 % of observed encounters in intervention clinics compared to 13 % in comparison clinics (p = 0.10). Family planning services were provided more frequently in intervention than comparison clinics (18 % vs. 13 %, p = 0.04). Clinic records indicate that significantly more oral contraceptive pills (mean average: 16.4 pill packs per month vs. 3.0 per month, p = 0.003) and injectables (18.4 vs. 4.3, p = 0.002) were provided in intervention clinics compared to comparison clinics.
Intervention Outcome Analyses
Table 3 compares the outcome variables between the intervention and comparison groups at the baseline, 6-month, and 12-month follow-up periods. Participants in intervention clinics were less likely to report unprotected sex in the past 2 weeks (OR = 0.56, 95 % CI 0.32, 0.99) compared to participants in comparison clinics. A smaller percentage of participants in the intervention clinics in Tanzania reported unprotected sex at last sex compared to participants in comparison clinics (OR = 0.23, 95 % CI 0.14, 0.40, p <0.0001). This effect was not observed in participants from Namibia and Kenya. Similarly, participants in intervention clinics in Tanzania were more likely to report using a highly effective method of contraception compared to participants in comparison clinics (10 %, OR = 2.25, 95 % CI 1.24, 4.10, p = 0.0097). This effect was not observed in Kenya or Namibia.
Table 3.
Study outcome variables at baseline, 6-month and 12-month follow-up by trial arm
| Variable | Intervention
|
Comparison
|
Odds ratio | p value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline N (%) |
6 months N (%) |
12 months N (%) |
Baseline N (%) |
6 months N (%) |
12 months N (%) |
|||
| Sex behavior | ||||||||
| Any unprotected sex (last 2 weeks) | 222 (12.7) | 99 (6.7) | 83 (5.6) | 175 (10.2) | 159 (10.2) | 116 (7.5) | 0.56a (0.32, 0.99) | .0464 |
| Unprotected sex at last vaginal sex (among those who had sex in the past 3 months) | 353 (20.8) | 126 (11.4) | 98 (9.7) | 299 (17.8) | 201 (18.0) | 166 (15.6) | .0018 | |
| Kenya | 105 (17.9) | 50 (11.9) | 35 (8.8) | 114 (20.6) | 56 (14.3) | 33 (8.5) | 0.92b (0.53, 1.59) | .7450 |
| Namibia | 56 (11.0) | 38 (11.5) | 30 (9.7) | 48 (8.9) | 37 (10.7) | 43 (12.5) | 0.81b (0.45, 1.45) | .4674 |
| Tanzania | 192 (32.2) | 38 (10.8) | 33 (10.8) | 137 (23.2) | 108 (28.6) | 90 (27.0) | 0.23b (0.14, 0.40) | <.0001 |
| Any unprotected sex with any negative or unknown status partner (last 2 weeks) | 133 (7.6) | 58 (3.9) | 40 (2.7) | 101 (5.9) | 79 (5.1) | 47 (3.0) | 0.72a (0.44, 1.16) | .1691 |
| Given condoms by health care provider (last 6 months) | 812 (45.8) | 878 (59.8) | 760 (52.4) | 893 (51.3) | 744 (47.6) | 736 (48.1) | 1.57a (0.63, 3.94) | .3238 |
| Disclosure and partner testing | ||||||||
| Disclosure to most recent spouse or main partner (or non-main partner if no spouse or main) | 1318 (77.5)* | 948 (85.8) | 887 (87.3) | 1454 (85.8)* | 1016 (91.0) | 976 (91.4) | 0.80a (0.57, 1.13) | .1924 |
| Most recent spouse or main partner tested for HIV (or non-main partner if no spouse or main) | 1123 (74.4) | 842 (83.5) | 813 (88.7) | 1250 (82.6) | 914 (87.7) | 901 (90.1) | 0.83a (0.56, 1.24) | .3565 |
| Know most recent spouse or main partner’s HIV status (or non-main partner if no spouse or main) | 1080 (63.3) | 821 (74.2) | 802 (79.0) | 1192 (70.3) | 886 (79.3) | 889 (83.3) | 0.81a (0.60, 1.11) | .1837 |
| Family planning | ||||||||
| Dual method use | 232 (24.6) | 210 (28.5) | 205 (28.5) | 288 (30.5) | 236 (29.5) | 232 (29.4) | 1.16a (0.64, 2.11) | .6183 |
| Highly effective method use | 345 (36.6) | 304 (41.3) | 294 (40.8) | 387 (41.0) | 323 (40.4) | 328 (41.6) | .0222 | |
| Kenya | 75 (24.2) | 69 (27.1) | 61 (24.6) | 99 (32.7) | 77 (29.6) | 59 (23.4) | 1.02b (0.57, 1.83) | .9357 |
| Namibia | 131 (43.4) | 95 (42.6) | 103 (44.4) | 133 (42.0) | 126 (47.4) | 146 (55.5) | 0.68b (0.38, 1.23) | .1947 |
| Tanzania | 26 (7.8) | 46 (17.8) | 41 (17.1) | 56 (17.3) | 33 (12.1) | 27 (9.9) | 2.25b (1.24, 4.10) | .0097 |
| Medication adherence | ||||||||
| 100% adherent to HIV medications (among those on ARVs) | 911 (80.4) | 965 (82.3) | 1076 (87.3) | 912 (84.2) | 996 (87.8) | 1064 (88.0) | 0.82a (0.44, 1.53) | .5210 |
| Alcohol use | ||||||||
| Any drinking | 378 (21.3) | 235 (15.7) | 195 (13.2) | 319 (18.4) | 270 (17.2) | 251 (16.2) | 0.73a (0.49, 1.07) | .1037 |
| Harmful or likely dependent drinking | 114 (6.4) | 65 (4.3) | 43 (2.9) | 68 (3.9) | 63 (4.0) | 45 (2.9) | 0.83a (0.45, 1.51) | .5236 |
Note Overall, study retention at the 12-month follow-up was 84% in the intervention group, 89% in the comparison group
Significant difference (p < .05) between trial arms at baseline
No significant country or time differences; odds ratio represents the effect of the intervention on 6 and 12 month outcomes (pooled together, controlling for the correlation between 6 and 12 months)
No significant time differences, but significant country x trial arm effect; odds ratio represents the Tanzania-specific effect of the intervention on 6 and 12 month outcomes (pooled together, controlling for the correlation between 6 and 12 months)
There was no significant difference by trial arm on any of the remaining variables including alcohol use, medication adherence, HIV serostatus disclosure, and partner testing (Table 3). The rate of new pregnancies also did not differ significantly between intervention and comparison clinics (55 and 58 pregnancies, respectively).
Discussion
This paper presents findings from a longitudinal, multi-country study examining the impact of integrating HIV prevention interventions into routine clinical care for PLHIV in sub-Saharan Africa. Study findings demonstrate that integrated prevention services are feasible to implement as part of routine care. During observations of patient-provider interactions, providers in intervention clinics were more likely to discuss HIV prevention messages with their patients compared to providers in comparison clinics. In addition, during follow-up interviews, participants in intervention clinics were more likely to report receiving prevention services from their providers.
Despite this high level of integration, effects on behavior change were minimal. Participants in intervention clinics from all three countries were less likely to report unprotected sex in the past 2 weeks. In addition, Tanzanian participants in intervention clinics were less likely to report unprotected sex at last sex and more likely to report using a highly effective method of contraception. These effects were not observed in participants from Namibia and Kenya. Several factors may account for these country-level differences. Among the three countries, Tanzanian participants reported the lowest rates of condom use and hormonal contraception at baseline. In addition, at the time this study was implemented, both Kenya and Namibia had national policies supportive of delivering prevention interventions within HIV clinical settings. Factors such as national policies, laws, and cultural norms have been shown to have a strong influence on health behaviors [20] and may help explain some of the observed differences in this study.
Other factors may also have contributed to the limited impact on behavior change observed in this study. First, high baseline rates were observed on many outcome variables including condom use, disclosure, and knowledge of partner status. This study relied largely on patient self-report and patients may have over-reported their behavior on these variables. This over-reporting should have affected both study arms equally due to the randomization process. However, the high baseline rates may have created a ceiling effect and limited the ability of the study to show improvement over time on key variables. In addition, staff turnover was frequent. While we attempted to continually train new providers in the intervention clinics, participants may have been seen by untrained providers. Moreover, this was a clinic-level intervention that encouraged providers to refer patients to LCs for additional counseling and support. While the percent of participants who reported meeting with a LC did increase over the course of the study, not all participants met with a LC and, thus, were not exposed to additional individualized HIV prevention counseling.
This intervention included multiple components targeting a number of different behaviors. Offering all of these services at every clinic visit may not be feasible given the wide range of operational challenges experienced in these settings including large patient volumes, inadequate staffing, and frequent staff turnover [11]. Further research is needed to determine the optimal service delivery model for delivering prevention interventions to PLHIV within facility settings. In addition, recent research indicates that community-based organizations can increase the awareness, availability, and utilization of facility-based HIV services as well as effectively deliver many prevention, care, and support activities [21, 22]. Delivery of community-based HIV services can reduce patient burden within facility settings, leading to shorter wait times and allowing clinicians to focus on patients with complex medical needs. Operational research to explore delivery of prevention interventions for PLHIV within community settings is therefore urgently needed.
In conclusion, this study found that HIV prevention services are feasible to implement as part of routine care and result in more provider-delivered HIV prevention messages. The intervention led to a self-reported decrease in unprotected sex in the past 2 weeks. However, no change was observed in more objective measures of sexual activity. Therefore, it remains uncertain whether sexual behavior truly changed or not. On other measures, the data were insufficient to determine whether the intervention had its desired impact. Strategies to address operational challenges including high staff turnover and large patient volumes will need to be identified. Further operational research, ideally with biomarkers and costing information, is also needed to determine optimal service delivery models for delivering prevention interventions to PLHIV in diverse resource-limited settings.
References
- 1.UNAIDS. The gap report. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. p. 123. [Google Scholar]
- 2.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/ AIDS; 2014. [Accessed October 26, 2015]. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 3.UNAIDS. Fast-tracking combination prevention. Towards reducing new HIV infections to fewer than 500,000 by 2020. Geneva: Joint United Nations Programme on HIV/AIDS; 2015. [Google Scholar]
- 4.UNAIDS and GNP ? Positive health, dignity, and prevention: operational guidelines. Geneva and Amsterdam: Joint United Nations Programme on HIV/AIDS and Global Network of People Living with HIV; 2013. [Accessed November 10, 2015]. Available at: http://www.gnpplus.net/assets/positive_health_dignity_and_prevention_operational_guidelines_-_unaids_gnp_2013.pdf. [Google Scholar]
- 5.Medley A, Bachanas P, Grillo M, Hasen N, Amanyeiwe U. Integrating prevention interventions for people living with HIV into care and treatment programs: a systematic review of the evidence. JAIDS. 2015;68(suppl 3):S286–96. doi: 10.1097/QAI.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20:143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy CE, Medley AM, Sweat MD, O’Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010;88:615–23. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleh-Onoya D, Reddy P, Ruiter R, Sifunda S, Wingood G, van den Borne B. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. AIDS Care. 2009;21(7):817–25. doi: 10.1080/09540120802537823. [DOI] [PubMed] [Google Scholar]
- 9.Wingood GM, DiClemente RJ, Mikhail I, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: the WiLLOW program. JAIDS. 2004;37:S58–67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 10.Grossman D, Onono M, Newmann SJ, et al. Integration of family planning services into HIV care and treatment in Kenya: a cluster-randomized trial. AIDS. 2013;27(suppl 1):S77–85. doi: 10.1097/QAD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 11.Kirigia JM, Barry SP. Health challenges in Africa and the way forward. Int Arch Med. 2008;1:27. doi: 10.1186/1755-7682-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) [Accessed on March 20, 2013.];Hormonal contraception and HIV: technical statement. Available at: http://www.who.int/reproductivehealth/publications/family_planning/rhr_12_8/en/index.html. [PubMed]
- 13.Murray DM. Design and analysis of group-randomized trials. New York: Oxford University Press; 1998. [Google Scholar]
- 14.Kidder D, Bachanas P, Medley A, et al. HIV prevention in care and treatment settings: baseline risk behaviors among HIV patients in Kenya, Namibia, and Tanzania. PLoS ONE. 2013;8(2):e57215. doi: 10.1371/journal.pone.0057215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babor T, Higgins-Biddle JC, Saunders JB, et al. The alcohol use disorders identification test: guidelines for use in primary care. 2. WHO; 2001. [Accessed on January 20, 2011]. Available at: http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. [Google Scholar]
- 16.Kalichman SC, Simbayi LC, Jooste S, Cain D, Cherry C. Sensation seeking, alcohol use, and sexual behaviors among sexually transmitted infection clinic patients in Cape Town, South Africa. Psychol Addit Behav. 2006;20:298–304. doi: 10.1037/0893-164X.20.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Luchters S, Geibel S, Syengo M, Lango D, King’ola N, Tem-merman M, Chersich MF. Use of AUDIT and measures of drinking frequency and patterns to detect associations between alcohol and sexual behavior in male sex workers in Kenya. BMC Public Health. 2011;11:384–92. doi: 10.1186/1471-2458-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use, and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16:2101–18. doi: 10.1007/s10461-011-0087-8. [DOI] [PubMed] [Google Scholar]
- 19.Zetola NM, Modongo C, Kip EC, Gross R, Bisson GP, Collman RG. Alcohol use and abuse among patients with multi-drug resistant tuberculosis in Botswana. Int J Tuberc Lung Dis. 2012;16:1529–34. doi: 10.5588/ijtld.12.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church K, Kiweewa F, Dagupta A, Mwangome M, Mpandaguta E, Gomez-Olive FX, et al. A comparative analysis of national HIV policies in six African countries with generalized epidemics. Bull World Health Organ. 2015;93:457–67. doi: 10.2471/BLT.14.147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakietek J, Geberselassie T, Manteuffel B, Ogungbemi K, Krivelyova A, Bausch S, et al. It takes a village: community-based organizations and the availability and utilization of HIV/AIDS-related services in Nigeria. AIDS Care. 25(suppl 1):78–87. doi: 10.1080/09540121.2012.740158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey MD, Salmen CR, Omollo D, Mattah B, Fiorella KJ, Geng EH, et al. Implementation and operational research: pulling the network together. Quasi-experimental trial of a patient-defined support network intervention for promoting engagement in HIV care and medication adherence on Mfangano Island, Kenya. JAIDS. 2015;69(4):e127–34. doi: 10.1097/QAI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]