Abstract
The auditory brainstem response (ABR) is a sound-evoked non-invasively measured electrical potential representing the sum of neuronal activity in the auditory brainstem and midbrain. ABR peak amplitudes and latencies are widely used in human and animal auditory research and for clinical screening. The binaural interaction component (BIC) of the ABR stands for the difference between the sum of the monaural ABRs and the ABR obtained with binaural stimulation. The BIC comprises a series of distinct waves, the largest of which (DN1) has been used for evaluating binaural hearing in both normal hearing and hearing-impaired listeners. Based on data from animal and human studies, we discuss the possible anatomical and physiological bases of the BIC (DN1 in particular). The effects of electrode placement and stimulus characteristics on the binaurally evoked ABR are evaluated. We review how inter-aural time and intensity differences affect the BIC and, analyzing these dependencies, draw conclusion about the mechanism underlying the generation of the BIC. Finally, the utility of the BIC for clinical diagnoses are summarized.
Keywords: Binaural difference potential, auditory brainstem, BIC measurement, BIC generation, clinical use, animal models
Introduction
Objective measures of hearing ability are commonplace in clinical practice to obtain information about auditory function. The auditory brainstem response (ABR) is a commonly used objective clinical measure used for newborn hearing screening and other applications. The ABR represents the synchronized electrical activity of the neurons in the auditory pathway, which can be recorded non-invasively by electrodes placed on the skin. Since the different ABR waves broadly represent the activity of different parts of the auditory pathway (e.g. Boettcher 2002), it is possible to draw conclusions about the functioning of distinct stages of the auditory pathway. This includes the binaural processing stages if the binaural interaction component (BIC) of the ABR is investigated.
Binaural hearing constitutes an important mechanism for localizing sound sources, and it facilitates the perceptual segregation of sounds of interest from noise (Middlebrooks & Green 1991). It enhances the ability of humans to communicate in an environment with many sound sources (e.g. the cocktail-party setting), and, especially for animals, directional hearing is essential for survival (e.g. for detecting prey or predators). Even without any overt peripheral hearing loss due to inner ear deficiencies, elderly persons may have compromised ability to understand speech in a noisy environment (Frisina & Frisina 1997) or localize sound sources (Olsen et al. 1976) which may reflect more central processing deficits. Additionally, children that have experienced persistent hearing loss due to common conditions such as otitis media with effusion (ear infections, of which the resultant conductive hearing loss is the number one cause of hearing loss in children; Dhooge 2003) can show binaural and spatial hearing impairments even years after the cause of the hearing loss has passed or been surgically corrected and peripheral hearing has returned to normal (Tollin 2010; Whitton & Polley 2011). One of the current difficulties is that even though a child or adult may present as audiologically normal in the clinic (e.g. normal audiometric thresholds, etc.), speech and language perception and learning in noisy reverberant environments such as classrooms, restaurants and office spaces may still be compromised due to the persistently impaired binaural hearing capabilities. Clinical detection of these deficits has been elusive, thus precluding intervention and optimization of rehabilitative approaches. The so-called binaural interaction components of the ABR have emerged as a potential objective measure of binaural hearing function. For example, a prominent wave in the BIC was found to be reduced or absent in children who experienced chronic conductive hearing loss during infancy and/or were diagnosed with central auditory processing disorders (CAPD, Gunnarson & Finitzo 1991; Delb et al. 2003). Moreover, studies of the BIC have demonstrated that changes in BIC latency and amplitude not only vary systematically with the binaural cues to sound location in normal-hearing subjects (the interaural time (ITD) and level (ILD) differences) but are also predictive of the perceived stimulus lateralization (e.g. Furst et al. 1990; ).
Here we review the methods by which the BIC is measured, assess the caveats and controversies surrounding the BIC, discuss the anatomical and physiological bases of the BIC, and finally discuss the potential clinical interpretations and diagnostic uses of the BIC. Since there are many open questions regarding the emergence of the BIC and its modification by pathologies, a review comparing animal and human studies is timely that can serve to clarify these points based on physiological evidence and help to provide a causal basis for clinical measures of binaural function.
Measuring the BIC: Computation and Waveform, Nomenclature
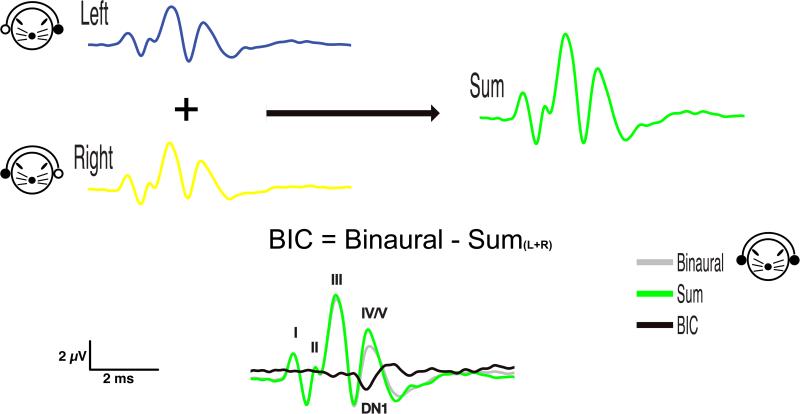
Recording and calculation of the BIC is conceptually straightforward, but requires careful consideration and control of multiple factors and parameters. In theory, in the absence of neural binaural processing, the sum of left and right monaural ABRs should be equal to the binaural ABR. This is, however, not the case given normal binaural processing mechanisms. Dobie and Berlin (1979) described a procedure of computing the BIC in the auditory brainstem response whereby the BIC is derived by subtracting the sum of monaural responses elicited by stimuli to the right and left ear from the response evoked by binaural stimulation (e.g., Dobie & Berlin 1979; Dobie & Norton 1980 , see also Figure 1). Alternatively, the BIC can be calculated with an inversed arithmetic operation by subtracting the binaural response from the sum of left and right ABRs, resulting in a BIC with an opposite sign but the same absolute values and latencies (e.g. Levine 1981; Furst et al. 1985; Gunnarson & Finitzo 1991; Delb et al. 2003). For the purposes of this review, we follow the convention of subtracting the sum of monaural responses elicited by stimuli to the right and left ear from the response evoked by binaural stimulation.
Figure 1.
Exemplary calculation of the binaural interaction component (BIC) of the auditory brainstem response (ABR) from data collected in the Guinea pig (Cavia porcellus) using the convention first described by Dobie and Berlin (1979). Left and right ABR waveforms recorded from monaural stimulus presentation to each ear are summed. The sum (violet) is then subtracted from the binaural waveform (gray), which is recorded from simultaneous presentation of stimuli to both ears. In the absence of binaural interaction, the sum of monaural waveforms would be predictive of the binaural waveform. Instead, there is a discrepancy between the sum and binaural waveforms: the binaural interaction component (BIC, green).
Further considerations must be made in the calculation of the BIC when experiments involve the use of ITDs or ILDs in stimulus presentation. When ITDs other than 0 μs are present in the binaural stimulus condition, the left and right monaural ABR waveforms must be appropriately time-shifted to temporally match their respective presentations as part of the binaural stimulus before they are summed (e.g. Dobie & Berlin 1979; Riedel & Kollmeier 2006). Correspondingly, when an ILD is present, the sum of the monaural ABRs from the left and right ears must be derived from monaural waveforms acquired using stimuli presented at amplitudes that correspond to what was presented to the left and right ears in the binaural waveform.
The BIC has proven to be an evoked response that can be identified in most, but interestingly not all, audiologically normal hearing human subjects (Dobie & Norton 1980; Levine 1981; Kelly-Ballweber & Dobie 1984). Upon initial consideration, a lack of a BIC in normal hearing subjects might seem to limit the clinical utility of the BIC. Procedural factors described below may, however, explain failures to reliably observe the BIC in normal-hearing subjects. As one example, in order to examine test-retest reliability, Dobie and Berlin (1979) and Dobie and Norton (1980) repeated BIC recordings in subjects during the same measurement session. They observed a good repeatability of BIC measurements in some – but again not all – subjects, even when these tests occurred just minutes apart. If the repeatability of recordings across subjects is considered, there is great variation in BIC morphology (amplitude, latency, etc.) observed even in normal hearing subjects (e.g. Stollman et al. 1996). This within- and across-subject variability highlights the importance of considering factors and recording parameters described below to minimize variability that can be attributed to methodological sources.
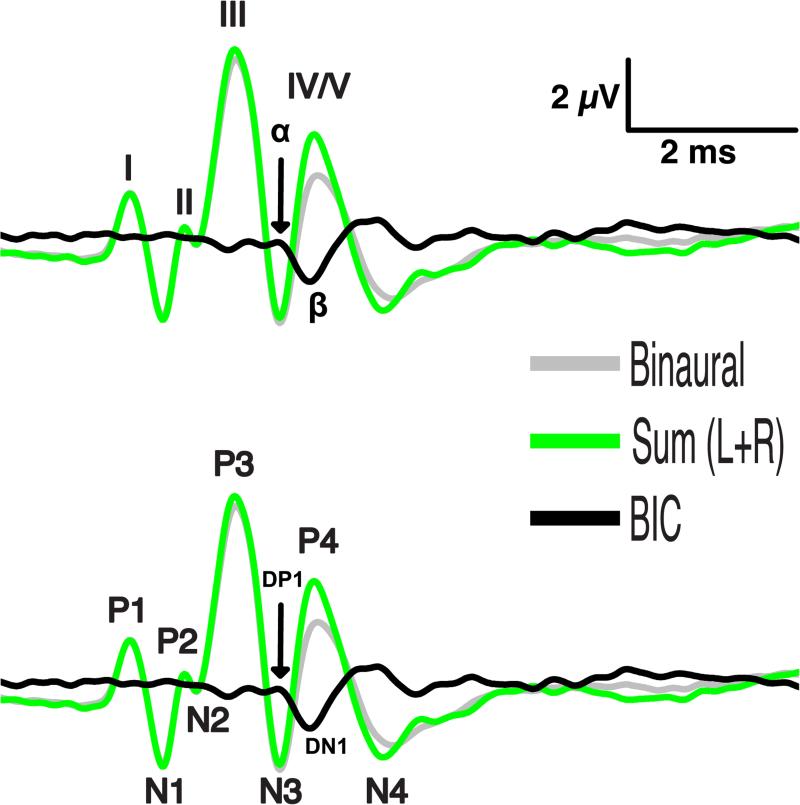
Before discussing analysis of the BIC, it is useful to briefly describe conventions for polarity and peak naming for both the BIC and ABR. Two ABR nomenclatures are most common (Fig. 2). The first is that used by Jewett and Williston (1971), in which vertex-positive wave peaks following stimulus onset are numbered sequentially with Roman numerals, and vertex-negative peaks are not considered. In the second convention (e.g. Goksoy et al. 2005), the first vertex-positive peak following stimulus onset is numbered P1 and successive positive peaks are numbered P2, P3, etc., and vertex-negative peaks are numbered N1, N2, etc., accordingly. Similarly, successive peaks in the BIC waveform are labeled as α, β, γ, δ (as in Levine 1981) following the first convention, which correspond to the peaks named DP1, DN1, DP2, DP3 (Dobie & Berlin 1979), respectively, following the second convention. In this review, we will always use the term DN1 for the major peak in the BIC instead of the synonyms β (e.g. Furst et al. 1985), P1 or N1 (e.g. Dobie & Norton 1980; Jones & Van der Poel 1990), A (e.g., Wrege & Starr 1981), b (e.g., Melcher 1996) and DV (McPherson et al. 1989) referring to the potentials reported in these other studies. Some other studies also included smaller peaks next to the DN1 peak in their discussion of the BIC (e.g., Wada & Starr 1989).
Figure 2.
Nomenclature. Various nomenclatures describing peaks in the ABR waveforms and the BIC have been used in the literature since the 1960's. The schemes depicted here are derived from calculation of the BIC using this formula: BIC = Binaural – Sum(L+R). TOP: Jewett and Williston (1971) used Roman numerals to label vertex-positive peaks only in the ABR waveforms. This convention is often, but not always, associated with use of the Greek alphabet to label consecutive BIC peaks, whether positive or negative. BOTTOM: A more formulaic approach to peak nomenclature applies sequential numbering of vertex-positive (‘P’) and vertex-negative (‘N’) peaks. This convention gave rise to labeling the BIC (i.e. ‘difference’ potential D) using formulaic positive and negative descriptors. For example, ‘DN1’ would indicate the first negative peak of the difference potential (i.e. the BIC). Note that different conventions for identifying ABR peaks can be combined with various conventions for BIC peak nomenclature.
Statistical Analysis of ABR Waveforms for Deriving the BIC
Once the BIC has been calculated, the next step is to identify relevant peaks. The earliest and perhaps simplest such method of peak identification was by visual identification (Dobie & Berlin 1979; Dobie & Norton 1980; Kelly-Ballweber & Dobie 1984). Visual identification is simple because it does not require computer programming or statistical analysis to complete. It is, however, time consuming, requires expertise in ABR/BIC waveforms, lacks objectivity, and may not perform as well at low signal-to-noise ratios (Arnold 1985; Hall 1992; Stollman et al. 1996). Several approaches of automated (and objective) peak identification methods have been described. Don et al. (1984) describes an automated detection method based on variance analysis of the recorded ABR waveform amplitude versus the background noise. Objective detection has also been achieved via template-matching using cross-correlation of responses (Elberling 1979; Kileny 1987; Stollman et al. 1996), or by signal-to-noise ratio measurement (e.g. Furst et al. 1985; Gunnarson & Finitzo 1991; Levine & Davis 1991; Stollman et al. 1996; Brantberg et al. 1999a; Riedel & Kollmeier 2002a,b, 2006). Stollman et al. (1996) compared the effectiveness of template-matching (Elberling 1979; Kileny 1987) versus signal-to-noise ratio evaluation in which significant peaks were identified as those deviating by more than 3 standard deviations from the distribution of values of the pre-stimulus noise (“3SD method”). Stollman et al. (1996) concluded that the 3SD method shows very good specificity, being efficient and sensitive for detecting the BIC. It also was found to be superior to the template-matching method, which is susceptible to inter-subject differences in BIC waveform morphology (including latency). They noted, however, that the 3SD method is vulnerable to noise artifacts such as muscle contraction in the pre-stimulus interval and thus requires effective artifact-rejection techniques. Variants of the “3SD” method have been applied in more recent studies on humans. Brantberg et al. (1999) used a 4SD criterion and required the BIC peak to occur during the downslope of ABR wave V and not being preceded by a larger peak. Others have used less conservative criteria such as sqrt2*2SD (Riedel & Kollmeier 2002a) or sqrt2*3SD (Riedel & Kollmeier 2002b, 2006).
Finally, there is a notable difference between studies regarding measurement of the peak(s) of the BIC. Some studies measure BIC peak amplitudes peak-to-peak (Ito et al. 1988; Riedel & Kollmeier 2002a,b, 2006) while others measure amplitudes baseline-to-peak (Gunnarson & Finitzo 1991 (with baseline correction); Ungan et al. 1997), or use a combination of the two approaches (Levine & Davis 1991). While either method of measurement is acceptable, it is important to note how a measurement is made, since peak-to-peak measurements by definition include information from more than one peak, each of which may have different origins. Perhaps the better method of the two is to measure amplitudes baseline-to-peak, but this requires a proper filtering and a baseline correction in order to be feasible (Gunnarson & Finitzo 1991). It may be suitable or necessary to normalize DN1 amplitudes for within- or between-subjects comparisons, since large amplitude variations can occur due to factors including electrode impedance or differences/changes in animal size (Furst et al. 2004; Ferber et al. 2016).
Anatomical and Physiological Source of the BIC
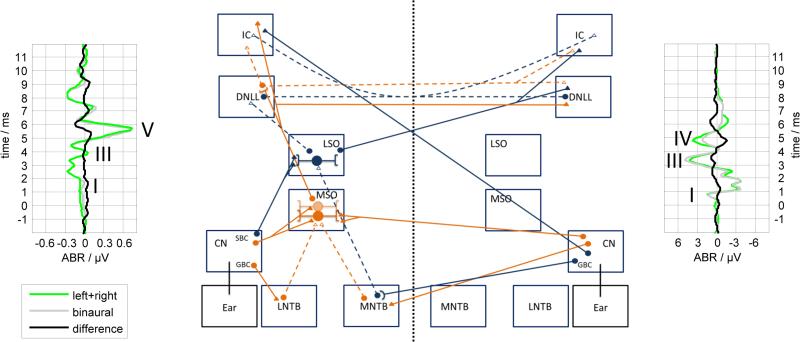
It is generally assumed that the BIC is generated by the activity of binaural neurons in the auditory pathway below the inferior colliculus (IC). The most likely candidate structures providing sites of binaural interaction that produce the DN1 component of the BIC are two nuclei of the superior olivary complex (SOC), i.e., the medial superior olive (MSO) and the lateral superior olive (LSO) and their outputs. Figure 3 provides a schematic of the connections to and from the SOC nuclei that are potential sources of the BIC. The MSO receives excitatory inputs from spherical bushy cell (SBCs) of the anteroventral cochlear nuclei (AVCNs) from both sides and some inhibitory input from the lateral (LNTB) and medial nucleus of the trapezoid body (MNTB) that receive their input from globular bushy cells (GBCs) from the ipsilateral and contralateral side, respectively (see Fig. 3). The LSO gets excitatory input from the ipsilateral site via SBCs in the AVCN and inhibitory input from the contralateral site by the MNTB which gets its input by GBCs in the AVCN (see also reviews by Tollin 2003; Grothe et al. 2010; Malmierca & Hackett 2010). The axons of the excitatory MSO neurons project to the ipsilateral IC and to the ipsi- and contralateral dorsal nucleus of the lateral lemnicus (DNLL). The axons of excitatory LSO neurons project to the contralateral IC and to the contralateral DNLL. The axons of inhibitory LSO neurons project to the ipsilateral DNLL. Commissural inhibitory projections between the ipsi- and contralateral DNLL and between both ICs are known. With these structures and connections in mind, we are provided with a framework to evaluate the arguments regarding the source of the BIC that are outlined below.
Figure 3.
Schematic of the mammalian interaural level (ILD) and interaural time difference (ITD) circuit. The boxes indicate the different brain nuclei (CN: Cochlear Nucleus, GBC: Globular Bushy Cells, SBC: Spherical Bushy cells, LNTB: Lateral Nucleus of the Trapezoid Body, MNTB: Medial Nucleus of the Trapezoid Body, MSO: Medial Superior Olive, LSO: Lateral Superior Olive, DNLL: Dorsal Nucleus of the Lateral Lemniscus, IC: Inferior Colliculus). Blue solid lines represent excitatory input for the ILD circuit, whereas blue dashed lines indicate inhibitory input. Orange solid lines show excitatory input for the ITD circuit and orange dashed lines inhibitory input. An exemplary ABR from a human subject (Riedel & Kollmeier 2002a) and from a gerbil are shown on the left and right side, respectively. The complete set of connections is obtained by mirroring the figure with respect to the dotted line since all connections shown have a counterpart originating from the other side. Cell bodies are indicated by circles. Synapses are indicated by triangles, whereas filled triangles represent excitatory synapses and empty triangles inhibitory ones.
The peaks of the monaural ABR can be roughly assigned based on their latencies to the activity of different nuclei, or at least different general levels within the auditory brainstem (Figure 3). Although just as there are anatomical differences between species, species-related discrepancies between attributed wave origins also arise. An all species, wave I is likely to arise in the distal part of the auditory nerve, whereas wave II in humans is due to the activity of the auditory nerve at the auditory nerve-brainstem junction (Boettcher et al. 1993; Boettcher 2002). Wave III in humans corresponds to the wave complex II-III complex in gerbils, which originates in the cochlear nucleus, although wave III in guinea pigs (Gardi & Bledsoe 1981), cats (Tsuchitani 1994) and mice (Jalabi et al. 2013) has been attributed to the MNTB. Wave IV in humans is generated by the SOC, whereas wave IV in gerbils is comparable to wave V in humans being likely to originate from fibers of the LL (Boettcher 2002).
Conclusions Derived from ABR Characteristics
The most common method to predict the generator of the BIC relies on indirect assumptions correlating the BIC peaks to a specific wave in the ABR. For example, Jewett (1970) reported that the first three waves of the binaural ABR in cats were equal to the sum of the monaural ABR, whereas the amplitude of wave IV in the binaural condition was smaller than in the sum of the amplitudes in the monaural condition suggesting a form of binaural interaction was occurring at the brainstem site(s) that generates wave IV. Based largely on latencies of DN1, authors have suggested that the generators are the IC (Wrege & Starr 1981; Jiang 1996), third order neurons in the SOC (McPherson & Starr 1993) or afferents from the SOC to the LL (e.g., Ito et al. 1988; Jones & Van der Poel 1990; Jiang 1996; Riedel & Kollmeier 2002a; see Fig. 3). Thus, DN1 latency does not provide clear evidence regarding the source of the BIC.
Generally, the DN1 component is negative, which has been interpreted as evidence that the excitation elicited by binaural stimulation is less than the sum of both the left and right monaural excitation. Since the LSO consists mainly of so-called ‘EI’ neurons, which receive excitatory (E) input from the ipsilateral side and inhibitory (I) input from the contralateral side, the negative value of the DN1 amplitude could be interpreted to reflect the reduced output of the LSO when stimulated binaurally that is due to the inhibition (e.g., Riedel & Kollmeier 2002a). The observation that the DN1 amplitude generally is smaller for higher-frequency stimuli (e.g., Ito et al. 1988; Melcher, 1996) does not provide conclusive evidence for the source of the BIC because like is the case for MSO neurons, a part of the population of LSO neurons are also sensitive to low-frequency ITDs (Tollin & Yin 2005). . Some studies have proposed that the MSO, consisting mainly of so-called ‘EE’ coincidence detector neurons that receive excitatory (E) input from both cochlear nuclei, could be the source of DN1 (e.g., Ungan et al. 1997; Riedel & Kollmeier 2002a).
Conclusions Derived from Field Potentials
A more direct method of identifying the source of the BIC involves simultaneous recording of ABRs and auditory field potentials (AFPs) using electrodes placed into the brainstem near the potential sources. Studies in the cat showed that the binaural ABR wave IV, which exhibits binaural interaction, reflects fast ripples in field potentials recorded in or close to the SOC (Caird et al. 1985; Sontheimer et al. 1985; Ungan & Yagcioglu 2002). Caird et al. (1985) and Sontheimer et al. (1985) assign the small ripples to action potentials in efferent MSO fibers in the LL. Sontheimer et al. investigated the effect of ITD/ILD trading (i.e., the change of the binaural ITD necessary to compensate 1 dB of ILD) on the BIC and the AFPs and concluded that the trading ratio of the binaural wave IV (6.4-17.6 μs/dB) and DN1 (9-20 μs/dB) corresponds to the trading ratio for the MSO. In contrast, based on the asymmetry of the ITD effects in ipsilateral-leading versus contralateral-leading stimulation on AFPs being due to the nonlinearity in the response of EI neurons, Ungan and Yagcioglu (2002) concluded that the MSO cannot be involved due to its predominant EE processing. However, some theories regarding MSO processing of ITDs do involve EI interactions (review by Grothe et al. 2010) that could lead to such an asymmetry whereas others do not see relevant exactly timed EI interactions in MSO (e.g. Roberts et al. 2013; van der Heijden et al. 2013). With regard to the effects of LSO, Ungan and Yagcioglu (2002) concluded that this nucleus contributes to the BIC since in the LL only contralateral stimulation produces fast AFP ripples that show a binaural interaction corresponding to the contralateral projection of the LSO output neurons. Thus, the AFP studies assigning the BIC to LL fibers cannot directly distinguish the contributions of the LSO from that of the MSO and the BIC cannot be unequivocally assigned to one of the SOC nuclei.
Conclusions Derived from Lesion Experiments
Surgical or pharmacological lesion experiments provide another method for identifying possible sources of the BIC. Kainate-acid induced lesions of the AVCNa reduced the DN1 component of the BIC in cats (Melcher 1996). The spherical bushy cells in the AVCNa provide bilateral excitatory input to the MSO suggesting that the output activity is reduced due to the lesion resulting in a reduced BIC (Melcher 1996). But since spherical bushy cells also provide the excitatory input to the ipsilateral LSO as well (Tollin, 2003) a similar argument could be made for the LSO as the source of the DN1 component of the BIC because a reduction/elimination of excitatory input to the LSO will reduce the binaural interaction in LSO and thus also reduce the amplitude of DN1. Lesions in nuclei of the SOC severely affected the BIC (Wada & Starr 1983b, 1989; Melcher, 1996; Zaaroor & Starr, 1991). Zaaroor and Starr (1991) noted that the BIC amplitude was reduced in a way correlated with the extent of the lesions of the LSO and MNTB. Lesions to the fibers of the trapezoid body (TB), which cross the midline in the brainstem to innervate the nuclei of the SOC relevant for binaural interaction, may lead to a reduction of the DN1 amplitude because it contains axons of the spherical and globular bushy cells (c.f. Melcher 1996) that ultimately drive binaural interaction in the MSO and LSO, respectively. Midsagittal section of the brain stem reduced the BIC and its remaining amount was linearly related to the size of the remaining TB (Wada & Starr 1983c, 1989). Similarly, pharmacological disruption of the neurons in the TB abolished the BIC (Wada & Starr 1983a). Wada and Starr (1983a) observed that the binaural wave IV of the ABR increased as a result of a pharmacological lesioning of the TB abolishing the BIC, which could be interpreted as resulting from the loss of the inhibition in binaural processing.
In contrast to lesions in the AVCNa, lesions of the AVCNp/PVCNa had no effect on DN1, which in Melcher's (1996) view excludes LSO as a generator because it receives contralateral input from globular cells (via the MNTB) in these nuclei. Bilateral sectioning of the LL in dogs, cats, guinea pigs and rats abolished the second wave of the BIC but not the DN1 component (Huang 1980). Therefore, it was concluded that both the LL and the IC, which receives input from the LL, contribute to the BIC. The effect of bilateral lesions of the LL was confirmed by Wada and Starr (1983b, 1989), but an effect of lesions of the IC on the BIC could not be confirmed (Gardi & Berlin 1981; Wada & Starr 1983b, 1989). Unilateral lesions of the LL were less effective (Wada & Starr 1983b, 1989). Studies in human subjects investigated the influence of a small pontine lesion in patients on the BIC (Pratt et al. 1998). The BIC component occurring at peak IV was decreased if lesions of the TB were observed in the patients, whereas lesions of the ventral TB led to a deviant orientation of second BIC component, which is comparable to the DN1 component. In contrast the third BIC component seemed to be relying on the contribution of the rostral LL (Pratt et al. 1998). In summarizing the lesion studies, we can conclude that the binaural interaction in nuclei of the SOC via the TB is a necessary requirement for generating the DN1 peak of the BIC. However, we cannot pinpoint any specific nucleus as the source.
Conclusions Derived from Models
A few studies have involved computational models reflecting the neural circuitry to predict experimental data and draw conclusions on the involvement of different auditory brainstem nuclei (e.g., Ungan et al. 1997; Goksoy et al. 2005; Riedel & Kollmeier 2006). Ungan and colleagues (1997) developed the model prototype to predict latency and amplitude of cat click-evoked BICs in relation to the ITD. They assumed that the MSO contribution to the BIC reflects the response of EE neurons whereas the LSO contribution reflects the response of EI neurons and that the DN1 component depends on the activity of SOC neurons projecting with their axons to the LL. Unfortunately, the model investigated by Ungan et al. (1997) only included the LSO activity. This decision was based on the assumption that EI interactions are needed to generate a reduction of the binaural ABR resulting in a negative DN1, and at that time these were seen to be limited to the LSO.
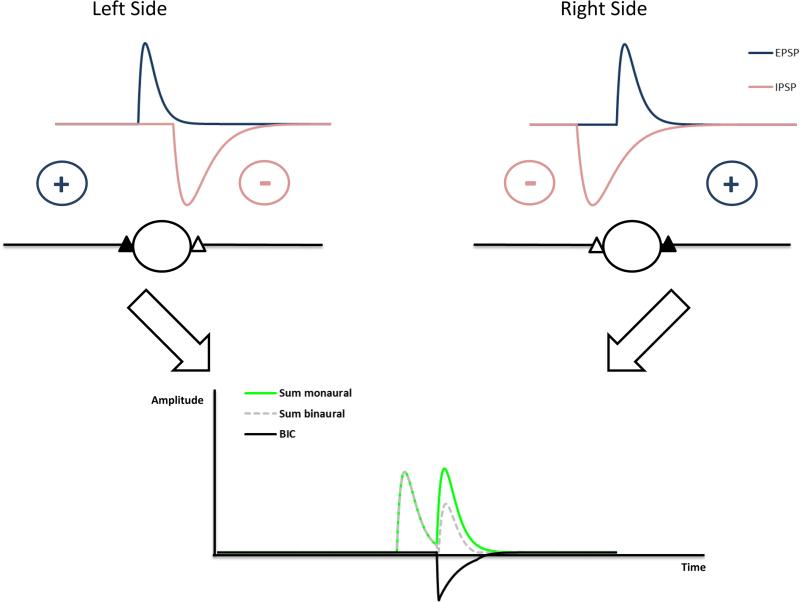
The mechanism underlying the generation of the BIC is exemplified in the schematic shown in Fig. 4. With monaural stimulation, only the summed excitatory postsynaptic potentials (EPSPs) contribute to the ABR. With binaural stimulation, EPSPs and inhibitory postsynaptic potentials (IPSPs) interact. If the ITD is 0μs the interaction is strongest reducing the binaural summed potential the most. This should result in the most negative deflection of the BIC. The more the ITD deviates from 0μs the smaller is the interaction between EPSPs and IPSPs and negative deflection of the BIC is reduced accordingly. This effect is mainly due to the interaction at the side of the brainstem in which the inhibition precedes the excitation. The model parameters adjusted by Ungan et al. (1997) were the relative timing of the ipsi- (excitatory) and contralateral (inhibitory) inputs to LSO units with the inhibition slightly preceding the excitation at ITD of 0 μs, variance of arrival times of excitatory and inhibitory input at the LSO units described by a Gaussian distribution and the duration of inhibition in LSO units. By choosing the appropriate physiological parameters, their model was able to predict the latency of DN1 and the DN1 amplitude at least over a range of ITDs up to 600 μs. With proper choice of model parameters, this model was also successfully applied in the guinea pig (Goksoy et al. 2005) and in human subjects (Riedel & Kollmeier 2006).
Figure 4.
Circuit underlying the generation of the BIC based on neurons integrating excitatory inputs from the ipsilateral side and inhibition from the contralateral side. In the example, the stimulus reaches the left ear earlier than the right ear (ITD). The ITD will shift the relative timing of excitatory and inhibitory inputs. In this circuit, the DN1 component will show a latency shift of ΔITD with an increase of ΔITD. If spike probabilities are taken into account, the latency shift can be smaller (Ungan et al. 1997).
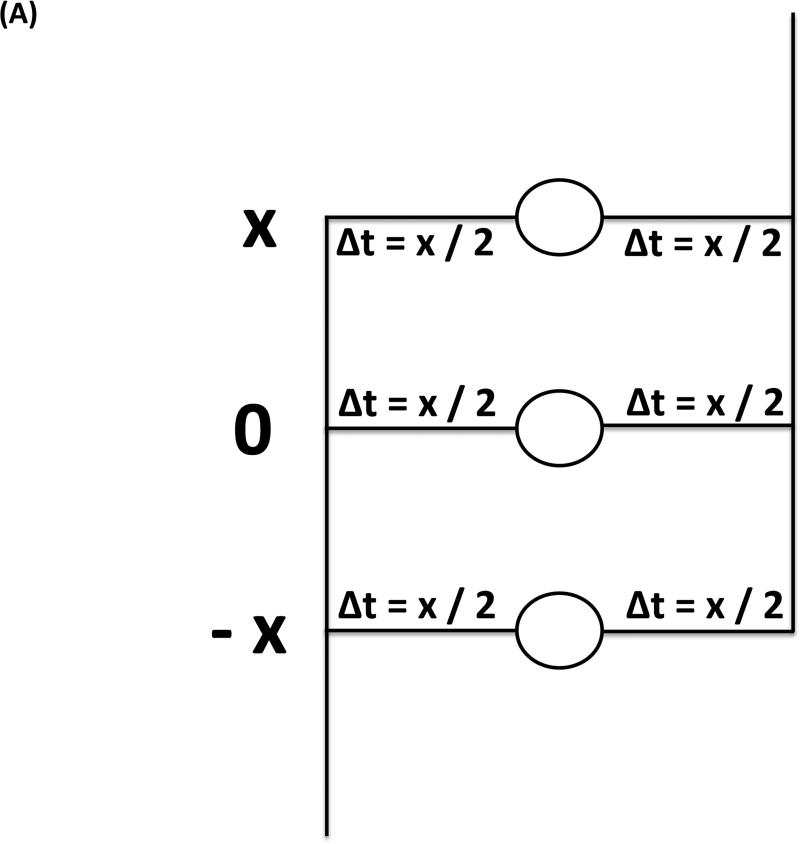
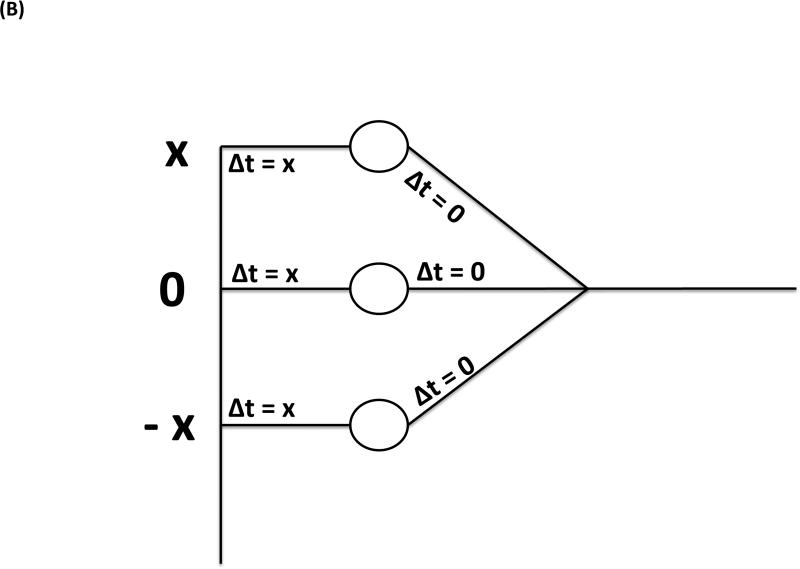
As an alternative, the interaction of the inputs to the MSO has been discussed as the cause of the BIC involving the classical Jeffress model (Jeffress 1948). It assumes bilaterally symmetrical neuronal delay lines predicting that the latency shift of the BIC corresponds to half the size of the ITD since the MSO coincidence detector neurons are excited if the delays from both sides corresponding to ITD/2 match (Fig. 5A). The deviation from this relation was interpreted as evidence against a Jeffress-like mechanism and, thus, against the involvement of the MSO in the generation of the BIC. However, if the excitation is only delayed from one side (Fig. 5B), it is predicted that the latency shift of the BIC corresponds to the size of the ITD (referred to as the avian nucleus laminaris model by Ungan et al. 1997; nucleus laminaris being the avian homologue of the MSO). Another argument against the contribution of MSO to the BIC rests upon the constant ratio between the wave IV amplitude and the DN1 component at different sound intensities that could not be explained by the presumed compressive nonlinearity of EE neurons (Gaumond & Psaltikidou 1991). Thus, the physiological function of the delay lines will determine the ITD dependency of the BIC latency, and this relation per se cannot be used to distinguish an involvement of the MSO from that of the LSO.
Figure 5.
Circuit underlying the generation of the BIC based on neurons integrating excitatory inputs from both sides. A, Circuit representing the classical Jeffress model with two symmetrical delay lines: due to symmetric contributions of the input from both sides, the DN1 component will show a latency shift of ΔITD/2 with an increase of ΔITD. B, Circuit representing a modification of the Jeffress model with unilateral delay lines: due to the contribution of only one side to the delay, the DN1 component will show a latency shift of ΔITD with an increase of ΔITD (For in depth discussion see Ungan et al. 1997).
How Does the BIC Depend on ITD and ILD
The effect of two main cues for sound source localization, ITDs and ILDs, on the BIC has been studied extensively. A preponderance of behavioral and physiological data indicate that ITDs carried by the ongoing, or fine structure, portion of sounds are mainly encoded and used in the low-frequency range (< 2 kHz) and ILDs are mainly encoded and used for higher frequencies (>2 kHz, e.g., see review by Tollin 2003; Grothe et al. 2010). These two cues are predominantly processed by different nuclei of the SOC: ITDs by the medial superior olive (MSO) and ILDs by the lateral superior olive (LSO). LSO neurons are also known to be exquisitely sensitive to the ITDs in transient stimuli, such as clicks, as well as the low-frequency envelopes of amplitude modulated stimuli (Joris & Yin 1995) and the ongoing ITDs of low-frequency stimuli (Tollin 2003; see Tollin & Yin 2005).
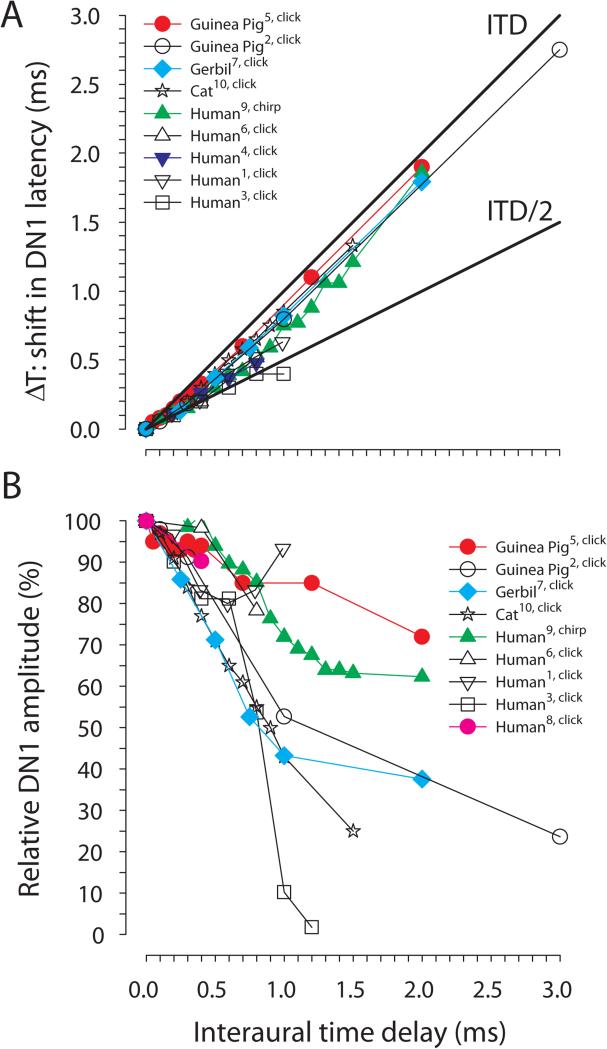
Figure 6 A and B show the effect of ITDs on the latency and relative amplitude of the DN1 component in different species, respectively. In humans, the DN1 amplitude for ITD and ILD of zero is generally very small (0.2-0.6 μV; Brantberg et al. 1999b; Furst et al. 1985; Jones & van der Poel 1990; Riedel & Kollmeier 2002a; Riedel & Kollmeier, 2006). In comparison, a larger DN1 amplitude of 1.8 μV and 2.3 μV was observed for the gerbil and the cat, respectively (Laumen et al. 2016; Ungan et al. 1997). The largest DN1 amplitude of about 5 μV was reported in the guinea pig (Goksoy et al. 2005). This difference between humans and animal models could be due to the smaller distance of the electrodes to the recorded source, electrode configuration, or other methodological issues. In accordance with the size of the anatomical structures, a longer latency for the DN1 component of about 5.6-6.8 ms was found in humans (Brantberg et al. 1999b; Furst et al. 1990; Jones & van der Poel 1990; Riedel & Kollmeier 2002a) compared to 3.7-4.8 ms in the animal models (Dobie & Berlin, 1979; Goksoy et al. 2005; Ungan et al. 1997; Laumen et al. unpublished data) if click stimuli were used. Furthermore, the amplitude of DN1 might scale with the size (e.g., number of neurons) of and or number of brainstem nuclei that produce DN1. With increasing ITD the latency of the DN1 component is increased. The gerbil (Laumen et al. unpublished data), cat (Ungan et al. 1997) and guinea pig (Dobie & Berlin, 1979; Gokosy et al. 2005) animal models show a similar pattern as in humans. It has been proposed (Ungan et al. 1997) that the latency increase reflects on the one hand the anatomical structures and on the other hand the interaction between excitation and inhibition. If the binaural interaction with changing ITDs reflects a Jeffress-like mechanism based on excitation provided by ipsilateral and contralateral inputs and coincidence-detector neurons (e.g., in mammalian MSO or avian Nucleus laminaris (NL), see Carr & Macleod 2010; Ashida & Carr 2011; Karino et al. 2011), then symmetrical delay lines for the input from the ipsi- and contralateral side would predict a latency shift of ITD/2 whereas unilateral delay lines (e.g. as proposed for the chick NL) would predict a latency shift of the DN1 of the size of the ITD (see also Fig. 5B). The observed values, however do not allow a clear distinction, which may reflect anatomical connections that show an intermediate pattern of bilateral and unilateral delay lines (Fig. 6). A study in a species such as the barn owl (Tyto alba), for which the anatomy of the NL is well worked out (Carr & Konishi 1990), will allow for evaluation of the previous hypothesis. An alternative hypothesis builds on the interaction between excitation and inhibition, which is observed, for example, in the LSO. Riedel and Kollmeier (2006), Ungan et al. (1997) and Goksoy et al. (2005) could fit their data with a model reflecting such an interaction (Fig. 4). Although there has been a recent ongoing debate whether not only in the LSO but also in the MSO the output is due to interacting and suitably timed inhibitory and excitatory inputs (see Grothe et al. 2010; Roberts et al. 2013; van der Heijden et al. 2013), the ITD dependence of the latency does not allow for a conclusion regarding the site and type of interaction of the ipsi- and contralateral inputs.
Figure 6.
A, Latency shift of the DN1 component of the BIC in relation to ITD; the reference latency at ITD = 0 is set to 0 ms. B, Relative DN1 amplitude in relation to ITD normalized to the DN1 amplitude for ITD = 0 ms. Data are from Brantberg et al. 1999b (Human1), Dobie and Berlin 1979 (Guinea pig2), Furst et al. 1985 (Human3), Furst et al. 1990 (Human4), Goksoy et al. 2005 (Guinea pig5), Jones and Van der Poel 1990 (Human6), Laumen et al. 2016 (Gerbil7), Riedel and Kollmeier 2002a (Human8), Riedel and Kollmeier 2006 (Human9), Ungan et al. 1997 (Cat10). We excluded data from studies that measured the DN1 in less than three subjects.
The relative change in DN1 amplitude with increasing ITD shows a higher between species variability than the latency (Fig. 6 B). Within the acoustically available range of ITDs in humans (i.e., up to an ITD of 800 μs; Kuhn 1977) there is a decrease of DN1 between ~7-50% in the amplitude. Within the acoustically available range of the gerbil (~130 μs; Maki & Furukawa 2005) the DN1 component is reduced by only 10% being further reduced by 60% for an ITD of 2000 μs. For the guinea pig the reduction is about 5% within its acoustically available range (~250-300 μs; Greene et al. 2014) and up to 28% for an ITD of 2000 μs. For the cat the DN1 amplitude is reduced by 30% in the acoustically available range (~400 μs; Tollin & Koka 2009) and by 75% for an ITD of 1500 μs. The lack of a systematic reduction of the DN1 amplitude at large ITDs in some studies may be due to the DN1 amplitude dropping below the noise floor of the measurements (see Fig. 6). The observation of effects of the ITD on latency and amplitude of the DN1 component outside the acoustically available range in humans (e.g. see Riedel & Kollmeier 2006) and in the animals models (Dobie & Berlin 1979; Ungan et al. 1997; Goksoy et al. 2005; Laumen et al. 2016) raises the question of whether the DN1 component results from just general synaptic delays and integration times that cause the amplitude change and latency shift rather than from direct effects of ITDs in the acoustically available range. Regardless of the mechanism producing DN1, the relative similarity of DN1 amplitude changes with ITD across different species with dramatically different acoustically available ranges of ITD suggests that the acoustically available range of ITD has little bearing on the specific mechanism responsible for DN1. The suggestion by Furst and colleagues (2004) that the BIC does not indicate a recalibration of ITD processing in the brainstem during head growth into adulthood supports the notion that head size per se is unimportant. Thus, more basic physiological and synaptic mechanisms, such as membrane time constants, etc. (which form the basis for the BIC model of Ungan et al., 1997), may explain the similarities across species independent of the physiological range of ITD cues. The integration time window of ~2 ms that was reported by Babkoff & Sutton (1966) for the perceptual fusion of dichotically presented click stimuli differing in time of presentation suggests binaural mechanisms operating with a time constant that matches the duration of interaction between synaptic inhibition and excitation or the time constants of peripheral frequency filters. Physiologically, neurons in the LSO have integration time windows of ~2 ms (Joris & Yin 1995; Koka & Tollin 2014; Wu & Kelly 1992).
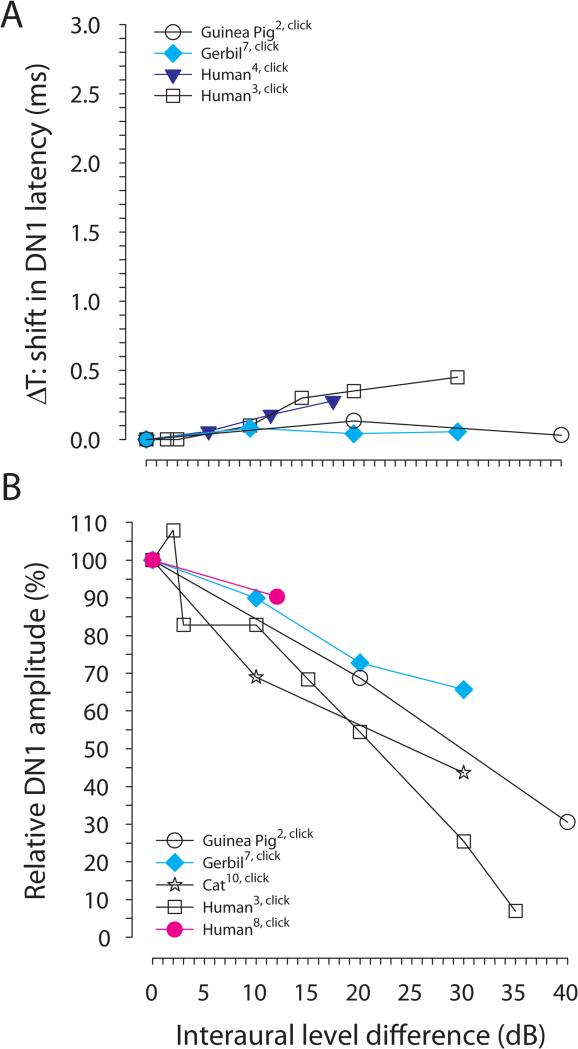
The effect of ILDs on the BIC has been investigated less extensively. Figure 7 shows the effect of ILD on the latency and amplitude of the DN1 component. For the gerbil, varying the ILD has little effect on the DN1 latency (Laumen et al. 2016) whereas for humans an increase of 450 μs was reported for an ILD of 30 dB (Furst et al. 1985). The DN1 amplitude relative to that at 0 dB ILD is typically reduced with increasing ILD. An ILD of 30 dB results in a reduction of the DN1 amplitude that corresponds to an ITD of about 1000 μs (Ungan et al. 1997) and of about 600 μs (Laumen et al. 2016) in the cat and the gerbil, respectively. The amplitude of the DN1 component in humans has been found to decrease as much for an ILD of 35 dB as for an ITD of about 1000 μs (Furst et al. 1985). Similarly, Riedel and Kollmeier (2002a) reported in humans a decrease of the relative DN1 amplitude for an ILD of 12 dB being as large as for an ITD of 400 μs. Non-invasive studies of the effects of ITD and ILD on the amplitudes and latencies of the BIC do not allow to draw conclusions about the origin of the BIC since they represent the sum of activity of the two separate binaural pathways involving both MSO and LSO. In the cat, trading effects between ITD and ILD with a ratio of 11-26 μs/dB and 80-550 μs/dB have been found for MSO and LSO, respectively (Caird & Klinke 1983; Joris & Yin 1995). With a ratio of 20-33 μs/dB for the non-invasive BIC measurements for the different species mentioned above (Figs. 6, 7) the ratio is closer to the one determined for the cat MSO. However, the neural trading ratios were measured with long-duration pure tone stimuli while the BIC trading ratios were measured with transient stimuli. Therefore, conclusions drawn from these comparisons must be done cautiously, if at all. Moreover, Melcher (1996) suggests that the putative MSO neurons reported in Caird & Klinke, (1983) were actually not located within the MSO nucleus, so arguments based on the ITD/ILD trading ratios in production of the BIC cannot definitively implicate the MSO. Moreover, Ungan and colleagues (1997) showed that no complete trading was possible in the cat that could be interpreted as evidence against the MSO as the site of trading.
Figure 7.
A, Latency shift of the DN1 component of the BIC in relation to ILD; the reference latency at ILD = 0 is set to 0 ms. B, Relative DN1 amplitude in relation to ILD normalized to the DN1 amplitude for ILD = 0 dB. Data are from Dobie and Berlin 1979 (Guinea pig2), Furst et al. 1985 (Human3), Furst et al. 1990 (Human4), Laumen et al. 2016 (Gerbil7), Riedel and Kollmeier 2002a (human8), Ungan et al. 1997 (Cat10). We excluded data from studies that measured the DN1 in less than three subjects.
Technical Considerations for Recording the BIC
As BIC is derived from ABR recordings, any parameters that affect the ABR (e.g., stimulus rate and amplitude, asymmetric ABR thresholds, or temperature) will correspondingly affect the derived BIC. For these reasons, in addition to BIC-specific effects, a brief review of some parameters affecting the ABR is also provided if relevant to the BIC.
Rate of Stimulus Presentation
Rate of stimulus presentation should be considered with respect to the effects on the BIC and ABR as well as setting practical constraints including the length of an experimental recording session and recording window length. Practically, presentation rate is limited on the high end to a maximum rate of 100 (click) stimuli/sec by the minimum recording window duration needed for acquiring the ABR (10-13 ms); the only practical low-end rate constraint is excessive time required to complete the minimum number of requisite repetitions per stimulus condition. It is well established that increased stimulus rate results in increased ABR wave latencies as well as decreased ABR peak amplitudes, and this in turn often reduces the BIC amplitude while increasing its latency (Jewett & Williston 1971; van Olphen et al. 1979; Levine 1981; Shipley et al. 1984; Wilson et al. 1985; Fullerton et al. 1987; Jiang 1996; Brantberg et al. 1999a; Parthasarathy & Moushegian 1993). Levine additionally revealed – by applying a form of BIC amplitude normalization in humans – that the ratio of DN1 amplitude to the summed left and right monaural wave V was relatively stable across a wide range of click rates (especially stable when click rate was below the threshold required to activate the middle ear reflex at a given amplitude, see “Mitigating Effects of the Middle Ear Reflex”). That is to say, the absolute amplitudes of DN1 and wave V were not themselves stable, but rather BIC amplitude co-varied with ABR component amplitude. The observation that BIC amplitude scales with the parent ABR waveform (Ferber et al. 2016) likely accounts for any discrepancy as to whether DN1 amplitude decreases or remains stable with increasing presentation rate.
Stimulus Amplitude Effects
Amplitude of the ABR-eliciting stimulus affects the recorded ABR and, thus, also the BIC even if stimulus amplitudes remain beneath the threshold that can result in acoustic crosstalk (see Acoustic Crosstalk section below). Increasing stimulus intensity increases the BIC amplitude and decreases their latency and this effect is most prominent for the DN1 peak amplitude (Dobie & Berlin 1979; Levine 1981; Ito et al 1988; Jiang 1996; Cone-Wesson et al 1997; Riedel & Kollmeier 2002b).
Noise Reduction and SNR
Commonly, a minimum of 500 repetition per stimulus type are averaged to achieve satisfactory signal-to-noise, though it is recommended to assess each individual recording scenario to determine the optimum number of repetitions, where enhanced signal-to-noise meets diminishing returns. As with standard monaural ABR recording, signal averaging is essential for reliable calculation of the BIC. Riedel and Kollmeier (2002a,b; 2006) showed the utility of using an increased number of monaural stimulus-evoked recordings and an iterative weighted averaging method in recording the BIC which they had previously also applied to simple ABR recordings (Granzow et al. 2001; Riedel et al. 2001). Ungan et al. (1997) employed yet another method of increasing the signal-to-noise ratio by including a small but predictable jitter into their high rate of stimulus presentation (50-65 ms inter-stimulus interval (ISI), which serves to mitigate against middle ear reflex effects. Perhaps more importantly, this jitter eliminates the entrainment of the 50 or 60 Hz AC power frequencies. Similarly, using a 37 ms ISI will be advantageous, as this prime number cannot be fit to 50 Hz or 60 Hz frequencies. In animal experiments, the use of skull-based epidural screw electrodes can also improve signal-to-noise ratio (Huang 1980; Goksoy et al. 2005).
Electrode Placement
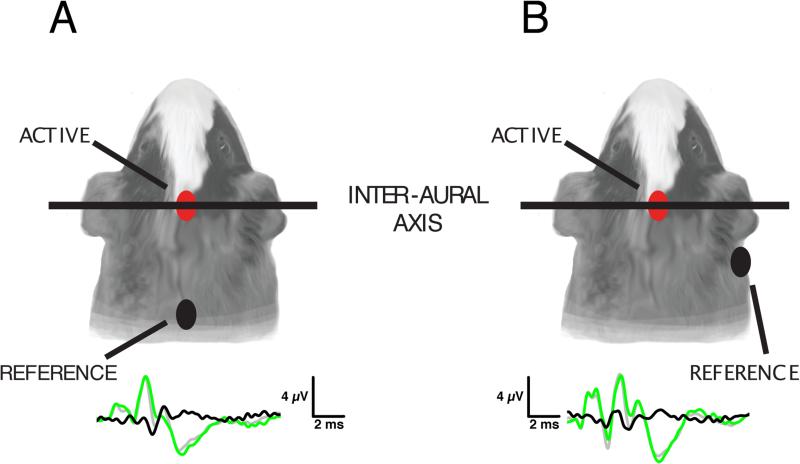
Electrode placement has been used to enhance specific ABR components including the BIC, taking into account the distance from and orientation to various nuclei or fiber tracts that contribute to the component of interest. Electrode placements used to record BIC are generally derived from earlier historical use for recording ABR. By far the most common electrode placements (Fig. 8) are vertex-nape, vertex-mastoid and vertex-earlobe, with a ground electrode either elsewhere on the head (mostly in human) or body (mostly in experimental animals). This is appropriate for differential amplification: van Olphen et al. (1978) determined that in humans the ABR wave V signal amplitude (here referred to as the parent of the BIC DN1 peak) is maximal at the vertex, decreasing to a minimum as one moves the recording electrode systematically toward the nasion, inion, or mastoid. Therefore, in human subjects, electrode placement is often vertex (active; Furst et al. 2004, Levine 1981) or forehead (Brantberg et al. 1999a, b) with forehead ground (Furst et al. 2004, Levine 1981, Ainslie and Boston 1980, Chiappa et al. 1979), and reference electrode(s) located at either mastoid(s) (Ainslie and Boston 1980; Riedel and Kollmeier 2002a), earlobe(s) (Levine 1981, Chiappa et al. 1979) or nape (Furst et al. 2004, Levine 1981, Brantberg et al. 1999a, b). Mastoid electrode placement is better for ABR wave I resolution as well as for signals propagating in the mediolateral (L-R) direction. However, it is biased toward potentials from one side of the head unless yoked bilateral electrodes are used. The vertex-nape montage is preferable as a simple two-electrode strategy that provides left-right balance and favors resolution of ascending/descending potentials. Levine (1981) observed that wave V and BIC were consistently larger using a vertex-nape electrode montage compared to a vertex-earlobe electrode placement. It should be noted that wave I is proportionally small in these recordings compared to the human recordings in Chiappa et al. (1979) using vertex-earlobe montage. Ainslie and Boston (1980) employed a montage for humans that included vertex, bilateral mastoid, and forehead ground electrodes attaining results being similar to the vertex-earlobe montage. Brantberg et al. (1999a,b) applied a forehead and nape electrode montage for recording electrodes for a BIC with a lateral forehead ground, achieving results consistent with other vertex-nape montage studies. Riedel and Kollmeier (2002a) applied a different strategy in humans (vertex-mastoid and vertex-[parieto-occipital junction], bilaterally), producing four sets of simultaneous differential recordings on separate channels. While recordings differed between electrode montages, resulting DP1 and DN1 amplitudes and latencies were ultimately similar, larger muscle-related noise was recorded at the vertex-mastoid electrode placement.
Figure 8.
ABR waveforms in relation to electrode placement. The two most-common electrode placements are depicted here. A: The vertex-nape montage features the active electrode at the vertex and a reference electrode at the nape of the neck, with a third ground electrode placed at a distal site (e.g. hind leg). Both electrodes are midline, resulting in smaller contributions from lateral generators of the ABR waveform. B: The vertex-mastoid montage uses a vertex active electrode and a more lateral reference electrode placed at the mastoid, which enhances the contribution of lateral generators of the ABR waveform.
Animal studies of the BIC tend to use comparable electrode montages to human studies, though needle electrodes are typically used over AgCl electrodes. Two electrode montages dominate in animal studies: vertex-neck (midline at the base of the skull-neck junction) and vertex-mastoid (either unilateral mastoid or yoked bilateral mastoid). Fullerton et al. (1987) described elicitation of large BIC and DN1 in the cat using vertex-nape electrode configuration. Ungan et al. (1997) opted for a bregma and midline neck muscle electrode placement. Similarly, Goksoy et al. (2005) placed epidural screw electrodes near bregma and lambda of the guinea pig with a forehead ground (6mm posterior to bregma). Dum et al. (1981) opted for electrode positions at the vertex, both pinnae and both mastoids for guinea pig experiments.
In the end, electrode montage matters for comparison of specific amplitude and latency values across studies, since peak amplitudes and latencies can vary between electrode montages. However, a BIC of some form can be recorded using any of these montages, and a pattern of peak amplitude or latency change detectable in one montage will show a corresponding similar change in any other. Studies that recorded BIC while altering ITD and ILD cues have used varying electrode montages, yet all arrive at the same conclusion: DN1 amplitude is highest for midline cues, where latencies are also shortest. Furthermore, the DN1 peak is reduced or absent when binaural hearing is compromised (e.g. Gopal & Pierel (1999) vs. Gunnarson and Finitzo (1991)), electrode montage differences. Due to enhancements and advantages from one montage to another (e.g. Riedel & Kollmeier 2002a), results may not be exactly identical per se, but the same trends certainly persist, resulting similar conclusions despite electrode montage differences. Thus, as the data from the studies above confirm, the ultimate results achieved from any of these electrode montages will be analogous. However, in the interest of making relevant comparisons with other studies, use of a “standard” montage is suggested (e.g. apex, nape, [ground at distant site, location dependent upon species]).
Effect of Stimulus Waveform
The ABR is an onset response elicited by brief transient stimuli and ABR amplitude is highly dependent on the stimulus rise time (Suzuki & Horiuchi 1981, Cone-Wesson et al. 1997). Typical stimuli include clicks, tone pips, and specially-designed “chirp” stimuli, which are short-duration stimuli engineered to compensate for temporal dispersion along the basilar membrane by progressively delaying the high-frequency part of the spectrum allowing to stimulate the entire basilar membrane virtually simultaneously (Dau et al. 2000). For the sake of simplicity, most studies have employed broadband click stimuli (e.g., Dobie & Berlin 1979; Dobie & Norton 1980; Furst et al. 1985, 1990; Levine & Davis 1991; McPherson & Starr, 1993, 1995; Pratt et al. 1998). In general clicks yield larger BIC responses than tones, lower-frequency tones yield larger-amplitude BIC than their high-frequency counterparts, and BIC component latency decreases for higher-frequency tones Cone-Wesson et al. 1997; Ito et al. 1988). However, when comparing clicks to the chirp stimulus, Riedel and Kollmeier (2002b), demonstrate that a chirp of equal amplitude elicits significantly larger ABR wave V amplitude as well as a larger DN1 (measured DP1-DN1 peak-to-peak) BIC response than a click. No clear relation was observed between click polarity and the BIC (e.g., Wrege & Starr 1981, Levine & Davis 1991). Furthermore, Wilson et al. (1985) found that there was no advantage of a high or low frequency skewed click on BIC amplitude. They remarked, however, that recordings had a better SNR when using high-frequency clicks to elicit responses. However, when measured by amplitude ratio (versus parent ABR waveform), low-frequency (500 Hz) tones produce the largest BIC (versus click or 4 kHz tone, see Cone-Wesson et al. 1997).
Calibration: Amplitude and Phase
Given the known effects of amplitude and phase spectrum of the click upon the ABR and BIC (e.g., Dobie & Berlin 1979; Riedel & Kollmeier 2002a), it is necessary to control those parameters by calibration and filtering (Beutelmann et al. 2015). A minimum phase filter based on calibration of both amplitude and phase produces a click with a flat energy distribution that has a fast rising onset and a short ringing time eliciting a larger ABR and requiring fewer averages. Beutelmann et al. (2015) also demonstrated that other commonly used filter types, such as the linear phase filter that is often favored for studies of binaural interaction because of the constant group delay (Tsai et al. 2010) may produce stimuli that have a less sharp and concentrated click waveform resulting in a lower ABR amplitude and, consequently, signal-to-noise ratio (SNR).
Effects of Body Temperature
Especially in the case of animal research where the animal is likely anesthetized and cannot thermo-regulate normally, the animal must be maintained at a physiologically normal temperature for porper measurement of the BIC. A decreased body temperature results in increased ABR peak latencies and decreasing peak amplitudes (Achor & Starr 1980; Rossi & Britt 1984; Hall et al. 1988; Ungan et al. 1997), and can thus reduce BIC amplitude.
Mitigating Effects of Acoustic Crosstalk under Symmetric and Asymmetric Bilateral Hearing Thresholds
Van Olphen et al. (1978) and Ainslie and Boston (1980) suggested that acoustic crosstalk could be responsible for the BIC. Acoustic crosstalk (sometimes referred to as interaural attenuation) refers to the fact that sounds being presented with a high level at one ear can travel around and/or through the head and stimulate the cochlea of the opposite ear. Acoustic crosstalk can be determined in monaurally deaf humans (Levine 1981; Fowler & Swanson 1988; Stollman et al. 1996) or animals with unilateral cochlear ablation (Dobie & Berlin 1979; Goksoy et al. 2005) by comparing the stimulus level at the deafened ear with that at the intact ear eliciting an ABR of the same size. Based on these measurements, a threshold is ascertained below which stimuli can be presented without the possibility of erroneously stimulating the contralateral ear and eliciting a contralateral ABR that would contaminate the BIC waveform (Goksoy et al. 2005). Acoustic crosstalk can indeed produce a contralateral ABR at very high stimulus intensities that may affect the BIC (Chiappa et al. 1979; Levine 1981; Goksoy et al. 2005) and typical relative levels of stimulation due to the crosstalk range from −45 to −65 dB (Dobie & Berlin 1979; Levine 1981; Arnold & Burkard 2000; Mair et al. 1979). However, investigations applying masking dispelled the acoustic crosstalk hypothesis as a contributor to the BIC. For example, Dobie and Wilson (1985) directly investigated the effect of contralateral masking on the BIC and ABR waveforms and found that it had no significant effect upon BIC or ABR waveforms at stimulus levels that would not be audible to the contralateral ear. The studies by Chiappa et al. (1979) and Ozdamar et al. (1986) support these findings. Additionally, Stollman et al. (1996) and Fowler and Swanson (1988) were unable to detect BIC in human subjects with profound unilateral hearing loss or unilateral deafness (respectively), but Stollman et al. (1996) detected BIC under the same conditions in normal-hearing subjects, providing further evidence against the acoustic crosstalk hypothesis.
Acoustic crosstalk should always be considered if asymmetric hearing loss is compensated by increased stimulus amplitude at the impaired ear equalizing input from the two ears as evidenced by symmetric monaural ABR amplitudes to enhance the BIC (e.g., Laska et al. 1992). In that case, it must be ensured that unilateral amplification of the stimulus at the impaired ear does not exceed the threshold for acoustic crosstalk to avoid problems with unwarranted stimulation of the more sensitive ear.
Mitigating Effects of the Middle Ear Reflex
Levine (1981) showed that an increased stimulus rate decreases the amplitude threshold required to trigger the middle ear reflex. This presents a problem for ABR measurements if stimuli are to be presented in a blocked fashion (e.g. Dobie & Norton 1980), that is to say, all monaural left stimuli, followed by all monaural right stimuli, followed by binaural stimuli. One solution to this problem was presented by Ito et al. (1988), who interleaved monaural and binaural stimuli presented at a fast rate to produce a constant loudness and hold the muscle contractions of the middle ear reflex tonic and even in both ears, thus reducing confounding variability. This approach was later expanded by Ungan et al. (1997), Goksoy et al. (2005) and Riedel and Kollmeier (2002a,b, 2006) to include the randomized presentation of stimuli differing in ITD and ILD. In addition to being interleaved into a randomized stimulus train and presented at a relatively high rate, binaural stimuli should all be presented at approximately equal loudness, which can be accomplished by centering bilaterally-symmetric intensity offsets around a common average binaural level (Ungan et al. 1997).
Potential for the BIC of the ABR as a Diagnostic Tool
Psychoacoustic measures of binaural and spatial hearing such as localization/lateralization, binaural fusion, or binaural masking level difference (BMLD) can be clinically useful for evaluating pathology. Conducting behavioral assays in a clinical setting, however, requires a cooperative subject, a subject-appropriate task (which can be difficult for infants, children and the elderly) as well as complex hardware and software, thus limiting usefulness in large-scale applications in the clinic. Hence, an objective evoked potential that correlates with these binaural capabilities in health and disease would be useful.
BIC is Present in Normal-Hearing Humans Throughout Life
The BIC of the ABR is a promising candidate to fill this diagnostic void. The BIC is usually present in human neonates at or soon after birth when measured using binaural stimuli with zero ITD or ILD (Hosford-Dunn et al. 1981; Gunnarson & Finitzo 1991; Furst et al. 2004). As noted by Hosford-Dunn et al. (1981) and Jiang and Tierney (1996), similarities between the neonatal and adult normal hearing BIC provide an opportunity for use in detection of developmental and functional brainstem integrity by way of comparing population BIC amplitudes, latencies and morphology against a standardized clinical measurement. Furst et al. (2004) found that significant BIC is present at birth in 76% of tested newborns (neonates age 1-49 days), whereas Stollman et al. (1996) detected significant BIC in 97% of normal-hearing adults. Testing conditions as well as contamination by muscle artifacts, which are common for neonates (Jiang & Tierney 1996), likely account for discrepancies in the detection of the BIC. Laska et al. (1992) documented that BIC latency matures with age in developing guinea pigs. A similar maturation process presumably occurs during development in humans, as demonstrated by the relative immaturity of neonatal BIC (i.e., prolonged latencies and discrete morphological differences) versus adult humans (Cone-Wesson et al. 1997; McPherson et al. 1989). While the neonatal ABR is immature, it is nonetheless comparable to the adult human ABR and BIC (Hosford-Dunn et al. 1981; Jiang & Tierney 1996). For example, Jiang and Tierney found similar, although not identical, identifiable BIC components in neonates and adults. However, neonatal wave latencies and interpeak intervals were longer and amplitudes smaller than those seen in adults, with neonatal BIC components showing more sensitivity to stimulus intensity and rate changes suggesting immaturity in the corresponding neonatal pathways (Jiang & Tierney 1996). Furthermore, both neonatal (Furst et al. 2004) and adult (Riedel & Kollmeier 2006; Junius et al. 2007) BIC amplitude and latency is modulated by interaural time differences. Whereas Riedel and Kollmeier detected BIC modulation by ITDs of up to 2000 μs in nearly every adult, Furst et al. reported a narrower ITD range of BIC sensitivity (of up to 1000 μs) in about 50% of the neonates that were tested, similarly suggesting maturation of the BIC during development. In summary, ABRs and BIC are detectable in normal-hearing humans throughout the human lifespan and are sensitive to binaural location cues, which provide the basis for potential utility of the BIC as a clinical diagnostic tool for identifying dysfunctional binaural processing.
BIC is a Marker for Binaural Perception, Behavioral Ability and Central Auditory Function
The correlation between the amplitude of the BIC DN1 peak and behavioral binaural phenomena implies that both are mediated by the same neural substrate, and thus dysfunctional spatial hearing may be reflected in the BIC. Diminished or absent BIC in the presence of appropriate binaural stimuli may be indicative of centrally-mediated binaural dysfunction. There is a significant correlation between the amplitude of the BIC DN1 peak and the ability to lateralize (Furst et al. 1985; Furst et al. 1990; McPherson & Starr 1995) a sound image as a function of ITD and ILD. In addition to the dependence of BIC amplitude on stimulus lateralization, BIC DN1 peak presence is also an indicator of binaural stimulus fusion (Furst et al. 1985; McPherson & Starr 1995). Together, this suggests that the BIC reflects the spatial processing of sound in the horizontal plane in the brainstem.
The BIC may also serve as an objective index used for diagnosis of some diseases, further supporting the link between elements of the BIC and spatial processing. Furst et al. (1990) showed that multiple sclerosis patients who were unable to match the perceived locations of two click trains differed from normal performers by the lack of dependence of BIC DN1 peak latency on ILD. Moreover, children with central auditory processing disorder (CAPD, which often stem from conductive hearing loss such as chronic or recurrent otitis media during development) differed from normal-hearing counterparts by either absence (Gunnarson & Finitzo 1991; Delb et al. 2003) or reduced amplitude (Gopal & Pierel 1999) of the BIC DN1 peak.
BIC and Peripheral Dysfunction: BIC is Altered or Disrupted by Peripheral Hearing Loss
Beyond its usefulness as an indicator of central auditory function described above, the BIC also could also be useful for assessing peripheral auditory function. It is clear that peripheral hearing loss affects the BIC via amplitude reduction or peak latency delay. Laska et al. (1992) noted that in the adult animals that experienced a 4-week monaural or binaural temporary conductive hearing loss, the conduction delay caused by the earplug (see also Lupo et al., 2011) resulted in an immediate temporary latency increase that resolved upon occlusion removal. In one subject showing a combined low-frequency and high-frequency hearing loss, Wrege and Starr (1981) similarly found a longer BIC latency compared to no latency effect for high-frequency hearing loss alone. Furthermore, Kelly-Ballweber and Dobie (1984) measured BIC in normal-hearing young versus older subjects with some degree of sensorineural hearing loss, finding an increased DN1 peak latency for the older cohort.
Stollman et al. (1996) yields perhaps the most interesting investigation into the relationship between BIC and peripheral hearing loss. Comparing normal-hearing adults to patients with profound unilateral hearing loss, Stollman et al. found 97% detection of BIC in normal-hearing subjects versus 20% in patients with hearing loss, effectively discriminating between the normal and hearing-impaired groups. (Note: The 20% detection in patients with hearing loss was likely a single false positive due to an artifact, as noted by the authors at the time.) Studies of BIC in bilateral cochlear implantees (using a clinical interface and controlled timing) also contribute to our understanding of peripheral effects on BIC. Large place mismatches between points of cochlear electrical stimulation in bilateral cochlear implant users resulted in elimination of the BIC, whereas smaller place mismatches had no effect on BIC latency or amplitude; BIC amplitude also correlated to perceptual lateralization probability of the bilateral cochlear implant users (Gordon et al. 2012). Similarly, Hu and Dietz (2015) found good correspondence between the cochlear implant electrode pair producing the largest BIC and that producing the maximum ITD sensitivity supporting the diagnostic value of the BIC for evaluating the quality of binaural brainstem processing. Clearly, peripheral dysfunction can affect the BIC yielding opportunities for clinical application.
Clinical Utility of the BIC beyond evaluation of basic auditory brainstem function
As noted in an early ABR study by Hausler and Levine (1980), binaural hearing dysfunction related to multiple sclerosis manifests in detectable changes to the ABR, including the BIC (Furst et al. 1990). Inclusion of BIC testing in multiple sclerosis cases could improve detection of related hearing dysfunction, which is not always apparent from other audiometric testing, and allow to better quantify, document and track the degree of hearing impairment resulting from the disease (Fowler & Swanson 1988).
Other applications for detection of binaural hearing dysfunction include the diagnosis of CAPD, for which reduced sensitivity to binaural cues to location such as ITD and ILD in the presence of audiometrically normal peripheral hearing is observed (American Speech-Language-Hearing Association 2005). BIC amplitude and latency correlate with ITD and ILD differences in binaural signals in normal-hearing individuals (Furst et al. 1985, 1990; McPherson & Starr, 1995; Riedel & Kollmeier 2002a). However, in children who have or are at risk for CAPD, BIC amplitudes are diminished or absent (Gunnarson & Finitzo 1991; Gopal & Pierel 1999; Delb et al. 2003). Application of the BIC toward CAPD assessment could speed the process of diagnosis and subsequent treatment and avoid potential misdiagnoses.
Acknowledgements
Helmut Riedel contributed data to a figure. Kelsey L. Anbuhl contributed an illustration to Figure 8. This work was supported by NIH Grants R01-DC011555 (D.J.T.) and F30-DC013932 (A.T.F.) and by grants (EXC 1077, TRR31) from the Deutsche Forschungsgemeinschaft (G.M.K., G.L.). Support was also provided by a fellowship from the Hanse Institute for Advanced Studies (D.J.T.).
Footnotes
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Achor LJ, Starr A. Auditory brain stem responses in the cat. I. Intracranial and extracranial recordings. Electroencephalogr Clin Neurophysiol. 1980;48:154–173. doi: 10.1016/0013-4694(80)90301-6. [DOI] [PubMed] [Google Scholar]
- Ainslie PJ, Boston JR. Comparison of brain stem auditory evoked potentials for monaural and binaural stimuli. Electroencephalogr Clin Neurophysiol. 1980;49:291–302. doi: 10.1016/0013-4694(80)90223-0. [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association [December 17, 2015];Causes of Hearing Loss in Children. Available at: http://www.asha.org/public/hearing/Causes-of-Hearing-Loss-in-Children/
- Arnold S, Burkard R. Studies of interaural attenuation to investigate the validity of a dichotic difference tone response recorded from the inferior colliculus in the chinchilla. J. Acoust. Soc. Am. 2000;107:1541–1547. doi: 10.1121/1.428439. [DOI] [PubMed] [Google Scholar]
- Arnold SA. Objective versus visual detection of the auditory brain stem response. Ear Hear. 1985;6:144–150. doi: 10.1097/00003446-198505000-00004. [DOI] [PubMed] [Google Scholar]
- Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr Opin Neurobiol. 2011;21:745–751. doi: 10.1016/j.conb.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babkoff H, Sutton S. End Point of Lateralization for Dichotic Clicks. J. Acoust. Soc. Am. 1966;39:87–102. doi: 10.1121/1.1909878. [DOI] [PubMed] [Google Scholar]
- Beutelmann R, Laumen G, Tollin D, et al. Amplitude and phase equalization of stimuli for click evoked auditory brainstem responses. J. Acoust. Soc. Am. 2015;137:EL71–EL77. doi: 10.1121/1.4903921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA. Presbyacusis and the Auditory Brainstem Response. J Speech Lang Hear Res. 2002;45:1249–1261. doi: 10.1044/1092-4388(2002/100). [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hear. Res. 1993;71:137–145. doi: 10.1016/0378-5955(93)90029-z. [DOI] [PubMed] [Google Scholar]
- Brantberg K, Fransson PA, Hansson H, et al. Measures of the binaural interaction component in human auditory brainstem response using objective detection criteria. Scand Audiol. 1999a;28:15–26. doi: 10.1080/010503999424879. [DOI] [PubMed] [Google Scholar]
- Brantberg K, Hansson H, Fransson P, et al. The binaural interaction component in human ABR is stable within the 0- to 1-ms range of interaural time differences. Audiol Neurootol. 1999b;4:88–94. doi: 10.1159/000013825. [DOI] [PubMed] [Google Scholar]
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res. 1983;52:385–399. doi: 10.1007/BF00238032. [DOI] [PubMed] [Google Scholar]
- Caird D, Sontheimer D, Klinke R. Intra- and extracranially recorded auditory evoked potentials in the cat. I. Source location and binaural interaction. Electroencephalogr Clin Neurophysiol. 1985;61:50–60. doi: 10.1016/0013-4694(85)91072-7. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J. Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Macleod KM. Microseconds matter. PLoS Biol. 2010;8:e1000405–e1000405. doi: 10.1371/journal.pbio.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappa KH, Gladstone KJ, Young RR. Brain stem auditory evoked responses: studies of waveform variations in 50 normal human subjects. Arch Neurol. 1979;36:81–87. doi: 10.1001/archneur.1979.00500380051005. [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B, Ma E, Fowler CG. Effect of stimulus level and frequency on ABR and MLR binaural interaction in human neonates. Hear. Res. 1997;106:163–178. doi: 10.1016/s0378-5955(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Dau T, Wegner O, Mellert V, et al. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J. Acoust. Soc. Am. 2000;107:1530–1540. doi: 10.1121/1.428438. [DOI] [PubMed] [Google Scholar]
- Delb W, Strauss DJ, Hohenberg G, et al. The binaural interaction component (BIC) in children with central auditory processing disorders (CAPD). Int J Audiol. 2003;42:401–412. doi: 10.3109/14992020309080049. [DOI] [PubMed] [Google Scholar]
- Dhooge IJM. Risk factors for the development of otitis media. Curr Allergy Asthma Rep. 2003;3:321–325. doi: 10.1007/s11882-003-0092-8. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Berlin CI. Binaural interaction in brainstem-evoked responses. Arch Otolaryngol. 1979;105:391–398. doi: 10.1001/archotol.1979.00790190017004. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Norton SJ. Binaural interaction in human auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1980;49:303–313. doi: 10.1016/0013-4694(80)90224-2. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Wilson MJ. Binaural interaction in auditory brain-stem responses: effects of masking. Electroencephalog Clin Neurophysiol. 1985;62:56–64. doi: 10.1016/0168-5597(85)90035-8. [DOI] [PubMed] [Google Scholar]
- Don M, Elberling C, Waring M. Objective detection of averaged auditory brainstem responses. Scand Audiol. 1984;13:219–228. doi: 10.3109/01050398409042130. [DOI] [PubMed] [Google Scholar]
- Dum N, Schmidt U, Wedel, Von H. Scalp distribution of the auditory evoked brainstem potentials in the guinea pig during monaural and binaural stimulation. Hear. Res. 1981;5:271–284. doi: 10.1016/0378-5955(81)90051-4. [DOI] [PubMed] [Google Scholar]
- Elberling C. Auditory Electrophysiology: The Use of Templates and Cross Correlation Functions in the Analysis of Brain Stem Potentials. Scand Audiol. 1979;8:187–190. doi: 10.3109/01050397909076320. [DOI] [PubMed] [Google Scholar]
- Ferber AT, Benichoux V, Tollin DJ. Test-retest reliability of the binaural interaction component of the auditory brainstem response. Ear Hear. 2016 doi: 10.1097/AUD.0000000000000315. DOI: 10.1097/AUD.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CG, Swanson MR. Validation of addition and subtraction of ABR waveforms. Scand Audiol. 1988;17:195–199. doi: 10.3109/01050398809070704. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear. Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Fullerton BC, Levine RA, Hosford-Dunn HL, et al. Comparison of cat and human brain-stem auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1987;66:547–570. doi: 10.1016/0013-4694(87)90102-7. [DOI] [PubMed] [Google Scholar]
- Furst M, Bresloff I, Levine RA, et al. Interaural time coincidence detectors are present at birth: evidence from binaural interaction. Hear. Res. 2004;187:63–72. doi: 10.1016/s0378-5955(03)00331-9. [DOI] [PubMed] [Google Scholar]
- Furst M, Eyal S, Korczyn AD. Prediction of binaural click lateralization by brainstem auditory evoked potentials. Hear. Res. 1990;49:347–359. doi: 10.1016/0378-5955(90)90113-4. [DOI] [PubMed] [Google Scholar]
- Furst M, Levine RA, McGaffigan PM. Click lateralization is related to the beta component of the dichotic brainstem auditory evoked potentials of human subjects. J. Acoust. Soc. Am. 1985;78:1644–1651. doi: 10.1121/1.392802. [DOI] [PubMed] [Google Scholar]
- Gardi JN, Berlin CI. Binaural interaction components. Their possible origins in guinea pig auditory brainstem response. Arch Otolaryngol. 1981;107:164–168. doi: 10.1001/archotol.1981.00790390030009. [DOI] [PubMed] [Google Scholar]
- Gardi JN, Bledsoe SC., Jr. The use of kainic acid for studying the origins of scalp-recorded auditory brainstem responses in the guinea pig. Neurosci. Lett. 1981;26:143–149. doi: 10.1016/0304-3940(81)90340-2. [DOI] [PubMed] [Google Scholar]
- Gaumond RP, Psaltikidou M. Models for the generation of the binaural difference response. J. Acoust. Soc. Am. 1991;89:454–456. doi: 10.1121/1.400482. [DOI] [PubMed] [Google Scholar]
- Goksoy CC, Demirtas SS, Yagcioglu SS, et al. Interaural delay-dependent changes in the binaural interaction component of the guinea pig brainstem responses. Brain Res. 2005;1054:183–191. doi: 10.1016/j.brainres.2005.06.083. [DOI] [PubMed] [Google Scholar]
- Gopal KVK, Pierel KK. Binaural interaction component in children at risk for central auditory processing disorders. Scand Audiol. 1999;28:77–84. doi: 10.1080/010503999424798. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Salloum C, Toor GS, et al. Binaural Interactions Develop in the Auditory Brainstem of Children Who Are Deaf: Effects of Place and Level of Bilateral Electrical Stimulation. Journal of Neuroscience. 2012;32:4212–4223. doi: 10.1523/JNEUROSCI.5741-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow M, Riedel H, Kollmeier B. Single-sweep-based methods to improve the quality of auditory brain stem responses Part I: Optimized linear filtering. Z Audiol. 2001;40:32–44. [Google Scholar]
- Greene NT, Anbuhl KL, Williams W, et al. The acoustical cues to sound location in the guinea pig (Cavia porcellus). Hear. Res. 2014;316:1–15. doi: 10.1016/j.heares.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiological Reviews. 2010 doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Gunnarson AD, Finitzo T. Conductive hearing loss during infancy: effects on later auditory brain stem electrophysiology. J Speech Hear Res. 1991;34:1207–1215. doi: 10.1044/jshr.3405.1207. [DOI] [PubMed] [Google Scholar]
- Hall JW. Handbook of Auditory Evoked Responses. Allyn & Bacon; 1992. [Google Scholar]
- Hall JW, Bull JM, Cronau LH. Hypo- and hyperthermia in clinical auditory brain stem response measurement: two case reports. Ear Hear. 1988;9:137–143. doi: 10.1097/00003446-198806000-00006. [DOI] [PubMed] [Google Scholar]
- Hausler R, Levine RA. Brain stem auditory evoked potentials are related to interaural time discrimination in patients with multiple sclerosis. Brain Res. 1980;191:589–594. doi: 10.1016/0006-8993(80)91312-8. [DOI] [PubMed] [Google Scholar]
- Hosford-Dunn H, Mendelson T, Salamy A. Binaural Interactions in the Short-Latency Evoked Potentials of Neonates. Int J Audiol. 1981;20:394–408. doi: 10.3109/00206098109072711. [DOI] [PubMed] [Google Scholar]
- Hu H, Dietz M. Comparison of Interaural Electrode Pairing Methods for Bilateral Cochlear Implants. Trends Hear. 2015;19:2331216515617143. doi: 10.1177/2331216515617143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CM. A comparative study of the brain stem auditory response in mammals. Brain Res. 1980;184:215–219. doi: 10.1016/0006-8993(80)90601-0. [DOI] [PubMed] [Google Scholar]
- Ito S, Hoke M, Pantev C, et al. Binaural interaction in brainstem auditory evoked potentials elicited by frequency-specific stimuli. Hear. Res. 1988;35:9–19. doi: 10.1016/0378-5955(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Jalabi W, Kopp-Scheinpflug C, Allen PD, et al. Sound localization ability and glycinergic innervation of the superior olivary complex persist after genetic deletion of the medial nucleus of the trapezoid body. Journal of Neuroscience. 2013;33:15044–15049. doi: 10.1523/JNEUROSCI.2604-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Hannley M, Rivera V. Auditory brainstem response and the masking level difference. Ann. N. Y. Acad. Sci. 1982;388:466–470. doi: 10.1111/j.1749-6632.1982.tb50809.x. [DOI] [PubMed] [Google Scholar]
- Jewett DL. Volume-conducted potentials in response to auditory stimuli as detected by averaging in the cat. Electroencephalogr Clin Neurophysiol. 1970;28:609–618. doi: 10.1016/0013-4694(70)90203-8. [DOI] [PubMed] [Google Scholar]
- Jewett DL, Williston JS. Auditory-evoked far fields averaged from the scalp of humans. Brain. 1971;94:681–696. doi: 10.1093/brain/94.4.681. [DOI] [PubMed] [Google Scholar]
- Jiang ZD. Binaural interaction and the effects of stimulus intensity and repetition rate in human auditory brain-stem. Electroencephalog Clin Neurophysiol. 1996;100:505–516. doi: 10.1016/s0168-5597(96)96519-3. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Tierney TS. Binaural Interaction in Human Neonatal Auditory Brainstem. Pediatr Res. 1996;39:708–714. doi: 10.1203/00006450-199604000-00024. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Van der Poel JC. Binaural interaction in the brain-stem auditory evoked potential: evidence for a delay line coincidence detection mechanism. Electroencephalog Clin Neurophysiol. 1990;77:214–224. doi: 10.1016/0168-5597(90)90040-k. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J. Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Junius D, Riedel H, Kollmeier B. The influence of externalization and spatial cues on the generation of auditory brainstem responses and middle latency responses. Hear. Res. 2007;225:91–104. doi: 10.1016/j.heares.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Karino S, Smith PH, Yin TCT, et al. Axonal branching patterns as sources of delay in the mammalian auditory brainstem: a re-examination. Journal of Neuroscience. 2011;31:3016–3031. doi: 10.1523/JNEUROSCI.5175-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Ballweber D, Dobie RA. Binaural interaction measured behaviorally and electrophysiologically in young and old adults. Audiology. 1984;23:181–194. doi: 10.3109/00206098409072833. [DOI] [PubMed] [Google Scholar]
- Kileny PR. Seminars in Hearing. 02. Vol. 8. Thieme Medical Publishers, Inc.; New York: 1987. ALGO-1 automated infant hearing screener: preliminary results. pp. 125–131. [Google Scholar]
- Koka K, Tollin DJ. Linear coding of complex sound spectra by discharge rate in neurons of the medial nucleus of the trapezoid body (MNTB) and its inputs. Front Neural Circuits. 2014;8:144. doi: 10.3389/fncir.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn GF. Model for the interaural time differences in the azimuthal plane. J. Acoust. Soc. Am. 1977;62:157. [Google Scholar]
- Laska M, Walger M, Schneider I, et al. Maturation of binaural interaction components in auditory brainstem responses of young guinea pigs with monaural or binaural conductive hearing loss. Eur Arch Otorhinolaryngol. 1992;249:325–328. doi: 10.1007/BF00179382. [DOI] [PubMed] [Google Scholar]
- Laumen G, Tollin DJ, Beutelmann R, Klump GM. Aging effects on the binaural interaction component of the auditory brainstem response in the mongolian gerbil: Effects of interaural time and level differences. Hear. Res. 2016;337:46–58. doi: 10.1016/j.heares.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA. Binaural interaction in brainstem potentials of human subjects. Ann Neurol. 1981;9:384–393. doi: 10.1002/ana.410090412. [DOI] [PubMed] [Google Scholar]
- Levine RA, Davis PJ. Origin of the click-evoked binaural interaction potential, β, of humans. Hear. Res. 1991;57:121–128. doi: 10.1016/0378-5955(91)90081-j. [DOI] [PubMed] [Google Scholar]
- Lupo EJ, Koka K, Thornton JL, et al. The effects of experimentally induced conductive hearing loss on spectral and temporal aspects of sound transmission through the ear. Hear. Res. 2011;272:30–41. doi: 10.1016/j.heares.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair IW, Elverland HH, Laukli E. Brain-stem responses of the cat and interaural attenuation. Arch Otorhinolaryngol. 1979;222:113–118. doi: 10.1007/BF00469750. [DOI] [PubMed] [Google Scholar]
- Maki K, Furukawa S. Reducing individual differences in the external-ear transfer functions of the Mongolian gerbil. J. Acoust. Soc. Am. 2005;118:2392–13. doi: 10.1121/1.2033571. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Hackett A. Structural organization of the ascending auditory pathway. In: Rees A, Palmer AR, editors. The Oxford Handbook of Auditory Science: The Auditory Brain. Oxford University Press; Oxford, UK: 2010. pp. 9–41. [Google Scholar]
- McPherson DL, Starr A. Auditory time-intensity cues in the binaural interaction component of the auditory evoked potentials. Hear. Res. 1995;89:162–171. doi: 10.1016/0378-5955(95)00134-1. [DOI] [PubMed] [Google Scholar]
- McPherson DL, Starr A. Binaural interaction in auditory evoked potentials: brainstem, middle- and long-latency components. Hear. Res. 1993;66:91–98. doi: 10.1016/0378-5955(93)90263-z. [DOI] [PubMed] [Google Scholar]
- McPherson DL, Tures C, Starr A. Binaural interaction of the auditory brain-stem potentials and middle latency auditory evoked potentials in infants and adults. Electroencephalogr Clin Neurophysiol. 1989;74:124–130. doi: 10.1016/0168-5597(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Melcher JR. Cellular generators of the binaural difference potential in cat. Hear. Res. 1996;95:144–160. doi: 10.1016/0378-5955(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Noffsinger D, Martinez CD, Schaefer AB. Auditory brainstem responses and masking level differences from persons with brainstem lesion. Scand Audiol Suppl. 1982;15:81–93. [PubMed] [Google Scholar]
- Olsen WO, Noffsinger D, Carhart R. Masking Level Differences Encountered in Clinical Populations. Int J Audiol. 1976;15:287–301. doi: 10.3109/00206097609071789. [DOI] [PubMed] [Google Scholar]
- Ozdamar O, Kraus N, Grossmann J. Binaural interaction in the auditory middle latency response of the guinea pig. Electroencephalogr Clin Neurophysiol. 1986;63:476–483. doi: 10.1016/0013-4694(86)90129-x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy TK, Moushegian G. Rate, frequency, and intensity effects on early auditory evoked potentials and binaural interaction component in humans. J Am Acad Audiol. 1993;4:229–237. [PubMed] [Google Scholar]
- Pratt H, Polyakov A, Aharonson V, et al. Effects of localized pontine lesions on auditory brain-stem evoked potentials and binaural processing in humans. Electroencephalog Clin Neurophysiol. 1998;108:511–520. doi: 10.1016/s0168-5597(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Purdy SC, Gardner-Berry K, Sharma M, et al. Electrophysiological measures of binaural interaction in cochlear implantees. International Congress Series. 2004;1273:40–43. [Google Scholar]
- Riedel H, Kollmeier B. Auditory brain stem responses evoked by lateralized clicks: is lateralization extracted in the human brain stem? Hear. Res. 2002a;163:12–26. doi: 10.1016/s0378-5955(01)00362-8. [DOI] [PubMed] [Google Scholar]
- Riedel H, Kollmeier B. Comparison of binaural auditory brainstem responses and the binaural difference potential evoked by chirps and clicks. Hear. Res. 2002b;169:85–96. doi: 10.1016/s0378-5955(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Riedel H, Kollmeier B. Interaural delay-dependent changes in the binaural difference potential of the human auditory brain stem response. Hear. Res. 2006;218:5–19. doi: 10.1016/j.heares.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Riedel H, Granzow M, Kollmeier B. Single-sweep-based methods to improve the quality of auditory brain stem responses Part II: Averaging methods. Z Audiol. 2001;40:62–85. [Google Scholar]
- Roberts MT, Seeman SC, Golding NL. A Mechanistic Understanding of the Role of Feedforward Inhibition in the Mammalian Sound Localization Circuitry. Neuron. 2013;78:923–935. doi: 10.1016/j.neuron.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GT, Britt RH. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr Clin Neurophysiol. 1984;57:143–155. doi: 10.1016/0013-4694(84)90173-1. [DOI] [PubMed] [Google Scholar]
- Shipley C, Strecker G, Buchwald JS. Binaural interaction effects on the auditory brainstem response of the cat and kitten. Brain Res. 1984;321:299–309. doi: 10.1016/0006-8993(84)90182-3. [DOI] [PubMed] [Google Scholar]
- Sontheimer D, Caird D, Klinke R. Intra- and extracranially recorded auditory evoked potentials in the cat. II. Effects of interaural time and intensity differences. Electroencephalogr Clin Neurophysiol. 1985;61:539–547. doi: 10.1016/0013-4694(85)90973-3. [DOI] [PubMed] [Google Scholar]
- Stollman MHP, Snik AFM, Hombergen GCJH, et al. Detection of the binaural interaction component in the auditory brainstem response. Br J Audiol. 1996;30:227–232. doi: 10.3109/03005369609079043. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Horiuchi K. Rise time of pure-tone stimuli in brain stem response audiometry. Audiology. 1981;20:101–112. doi: 10.3109/00206098109072688. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The Lateral Superior Olive: A Functional Role in Sound Source Localization. j biol rhythms. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The development of sound localization mechanisms. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; Oxford, UK: 2010. pp. 262–282. [Google Scholar]
- Tollin DJ, Koka K. Postnatal development of sound pressure transformations by the head and pinnae of the cat: Binaural characteristics. J. Acoust. Soc. Am. 2009;126:3125–3136. doi: 10.1121/1.3257234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Yin TCT. Interaural phase and level difference sensitivity in low-frequency neurons in the lateral superior olive. J. Neurosci. 2005;25:10648–10657. doi: 10.1523/JNEUROSCI.1609-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JJ, Koka K, Tollin DJ. Varying overall sound intensity to the two ears impacts interaural level difference discrimination thresholds by single neurons in the lateral superior olive. J. Neurophysiol. 2010;103:875–886. doi: 10.1152/jn.00911.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchitani C. The brain stem evoked response and medial nucleus of the trapezoid body. Otolaryngology - Head and Neck Surgery. 1994;110:84–92. doi: 10.1177/019459989411000110. [DOI] [PubMed] [Google Scholar]
- Ungan P, Yagcioglu S. Origin of the binaural interaction component in wave P4 of the short-latency auditory evoked potentials in the cat: evaluation of serial depth recordings from the brainstem. Hear. Res. 2002;167:81–101. doi: 10.1016/s0378-5955(02)00351-9. [DOI] [PubMed] [Google Scholar]
- Ungan P, Yağcioğlu S, Ozmen B. Interaural delay-dependent changes in the binaural difference potential in cat auditory brainstem response: implications about the origin of the binaural interaction component. Hear. Res. 1997;106:66–82. doi: 10.1016/s0378-5955(97)00003-8. [DOI] [PubMed] [Google Scholar]
- van der Heijden M, Lorteije JAM, Plauška A, et al. Directional Hearing by Linear Summation of Binaural Inputs at the Medial Superior Olive. Neuron. 2013;78:936–948. doi: 10.1016/j.neuron.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olphen AF, Rodenburg M, Verwey C. Distribution of brain stem responses to acoustic stimuli over the human scalp. Audiology. 1978;17:511–518. doi: 10.3109/00206097809072611. [DOI] [PubMed] [Google Scholar]
- van Olphen AF, Rodenburg M, Verwey C. Influence of the stimulus repetition rate on brain-stem-evoked responses in man. Audiology. 1979;18:388–394. doi: 10.3109/00206097909070064. [DOI] [PubMed] [Google Scholar]
- Wada S, Starr A. Anatomical bases of binaural interaction in auditory brain-stem responses from guinea pig. Electroencephalogr Clin Neurophysiol. 1989;72:535–544. doi: 10.1016/0013-4694(89)90231-9. [DOI] [PubMed] [Google Scholar]
- Wada S-I, Starr A. Generation of auditory brain stem responses (ABRs). I. Effects of injection of a local anesthetic (procaine HCl) into the trapezoid body of guinea pigs and cat. Electroencephalogr Clin Neurophysiol. 1983a;56:326–339. doi: 10.1016/0013-4694(83)90259-6. [DOI] [PubMed] [Google Scholar]
- Wada SI, Starr A. Generation of auditory brain stem responses (ABRs). II. Effects of surgical section of the trapezoid body on the ABR in guinea pigs and cat. Electroencephalogr Clin Neurophysiol. 1983b;56:340–351. doi: 10.1016/0013-4694(83)90260-2. [DOI] [PubMed] [Google Scholar]
- Wada SI, Starr A. Generation of auditory brain stem responses (ABRs). III. Effects of lesions of the superior olive, lateral lemniscus and inferior colliculus on the ABR in guinea pig. Electroencephalogr Clin Neurophysiol. 1983c;56:352–366. doi: 10.1016/0013-4694(83)90261-4. [DOI] [PubMed] [Google Scholar]
- Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J. Assoc. Res. Otolaryngol. 2011;12:535–547. doi: 10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MJ, Kelly-Ballweber D, Dobie RA. Binaural interaction in auditory brain stem responses: parametric studies. Ear Hear. 1985;6:80–88. doi: 10.1097/00003446-198503000-00004. [DOI] [PubMed] [Google Scholar]
- Wrege KS, Starr A. Binaural interaction in human auditory brainstem evoked potentials. Arch Neurol. 1981;38:572–580. doi: 10.1001/archneur.1981.00510090066008. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Binaural interaction in the lateral superior olive: time difference sensitivity studied in mouse brain slice. J. Neurophysiol. 1992;68:1151–1159. doi: 10.1152/jn.1992.68.4.1151. [DOI] [PubMed] [Google Scholar]
- Zaaroor M, Starr A. Auditory brain-stem evoked potentials in cat after kainic acid induced neuronal loss. I. Superior olivary complex. Electroencephalog Clin Neurophysiol. 1991;80:422–435. doi: 10.1016/0168-5597(91)90091-b. [DOI] [PubMed] [Google Scholar]
- Zerlin S, Mowry HJ. Click lateralization and the auditory brain stem response. Audiology. 1980;19:346–354. doi: 10.3109/00206098009072675. [DOI] [PubMed] [Google Scholar]