Abstract
Increased error monitoring, as measured by the error-related negativity (ERN), has been shown to persist after treatment for obsessive-compulsive disorder in youth and adults; however, no previous studies have examined the ERN following treatment for related anxiety disorders. We used a flanker task to elicit the ERN in 28 youth and young adults (8–26 years old) with primary diagnoses of generalized anxiety disorder (GAD) or social anxiety disorder (SAD) and 35 healthy controls. Patients were assessed before and after treatment with cognitive-behavioral therapy (CBT) or selective serotonin reuptake inhibitors (SSRI), and healthy controls were assessed at a comparable interval. The ERN increased across assessments in the combined sample. Patients with SAD exhibited an enhanced ERN relative to healthy controls prior to and following treatment, even when analyses were limited to SAD patients who responded to treatment. Patients with GAD did not significantly differ from healthy controls at either assessment. Results provide preliminary evidence that enhanced error monitoring persists following treatment for SAD in youth and young adults, and support conceptualizations of increased error monitoring as a trait-like vulnerability that may contribute to risk for recurrence and impaired functioning later in life. Future work is needed to further evaluate the ERN in GAD across development, including whether an enhanced ERN develops in adulthood or is most apparent when worries focus on internal sources of threat.
Keywords: error-related negativity, anxiety disorders, social anxiety disorder, cognitive behavior therapy, pharmacotherapy, endophenotype
1. Introduction
Anxiety disorders often emerge in childhood or adolescence and are characterized by a chronic course, high rates of recurrence and comorbidity, and persistent impairments in functioning (Bruce et al., 2005; Copeland et al., 2015; Woodward and Fergusson, 2001). Thus, identifying underlying factors that predispose to anxiety disorders and to outcomes later in life is of paramount importance. There is evidence that increased error monitoring may be a vulnerability for anxiety disorders that predicts the later development of symptoms (Lahat et al., 2014; Meyer et al., 2015) and persists following treatment for obsessive-compulsive disorder (OCD; Hajcak et al., 2008; Huyser et al., 2011; Riesel et al., 2015), though it has yet to be examined following treatment for other anxiety disorders.
Neural responses to errors are often measured using the error-related negativity (ERN), a negative deflection in the event-related potential (ERP) wave around the time of commission of an error (Falkenstein et al., 1991; Gehring et al., 1993). The ERN has been localized to the anterior cingulate cortex (Brázdil et al., 2005; Miltner et al., 2003), and is thought to reflect conflict between the stimulus and response following an error, which aids in reactivating task goals and adjusting behavior (Yeung et al., 2004). An enhanced ERN has been proposed to indicate increased sensitivity to endogenous sources of threat and perceptions of errors as catastrophic (Weinberg et al., 2016) or compensatory processes to account for limited cognitive resources (e.g., due to worry) that inhibit maintainance of task goals (Moser et al., 2013).
There is consistent evidence that OCD in youth and adults is characterized by an enhanced ERN (e.g., Carrasco et al., 2013b; Endrass and Ullsperger, 2014; Gehring et al., 2000). Importantly, there is also evidence that an enhanced ERN reflects an endophenotype or vulnerability for OCD, as it has been observed among unaffected first-degree relatives of patients with OCD (Carrasco et al., 2013a; Riesel et al., 2011), and has been shown to persist after completion of cognitive behavioral therapy (CBT) for OCD (Hajcak et al., 2008; Riesel et al., 2015). That is, hypersensitivity to errors appears to be relatively trait-like and contribute to risk for OCD, a disorder often characterized by close monitoring of behavior to prevent negative outcomes.
Generalized anxiety disorder (GAD) and social anxiety disorder (SAD) are relatively common disorders that often emerge early in life (Kessler et al., 2005; Mohatt et al., 2014). Like OCD, GAD in adults has been characterized by increased performance monitoring, as measured by the ERN (Weinberg et al., 2012, 2010; Xiao et al., 2011; Zambrano-Vazquez and Allen, 2014). Consistent with conceptualizations of the ERN as reflecting sensitivity to negative outcomes as a result of one’s own behavior (Weinberg et al., 2016), there is also evidence of an enhanced ERN in adults with SAD or elevated symptoms of social anxiety (Endrass et al., 2014; Judah et al., 2016). Relatively little work has examined the ERN in specific anxiety disorders in youth. Though there is some evidence of an enhanced ERN in children and adolescents with GAD, these studies are limited to small samples with mixed anxiety disorders (Carrasco et al., 2013b; Ladouceur et al., 2006). In addition, several studies have found an enhanced ERN among children high in behavioral inhibition (BI; Lahat et al., 2014; McDermott et al., 2009), a temperamant style characterized by fearfulness and increased risk for anxiety disorders, particularly SAD (Hirshfeld-Becker et al., 2007).
Previous studies have yet to examine error monitoring after treatment for GAD or SAD. Given that anxiety disorders are characterized by high rates of recurrence and persistent impairments in functioning (Bruce et al., 2005; Copeland et al., 2015; Woodward and Fergusson, 2001), it is likely that some underlying vulnerabilities for the disorder persist after symptom remission, and identifying these processes for more targeted treatment may improve long term outcomes. In addition, existing treatment studies of the ERN have focused on the effects of psychotherapy, and although there is evidence that selective serotonin reuptake inhibitors (SSRI) do not affect the ERN in healthy adults (Bruijn et al., 2006) and that the ERN in OCD patients is unaffected by medication status (Stern et al., 2010), the ERN has yet to be examined in patients before and after SSRI treatment.
The goal of this preliminary study was to examine the ERN before and after treatment in patients with primary diagnoses of GAD or SAD (n = 28) and healthy controls (n = 35) to evaluate whether an enhanced ERN persists following CBT and SSRI treatment for anxiety disorders. Because GAD and SAD often emerge by adolescence or young adulthood (Kessler et al., 2005), we included patients ranging in age from middle childhood through young adulthood. Prior to and following SSRI or CBT treatment, participants completed a flanker task to elicit the ERN in response to errors. Based on prior work in OCD (Hajcak et al., 2008; Riesel et al., 2015), we hypothesized that patients with anxiety disorders would exhibit an enhanced ERN relative to controls both before and after treatment. As studies of the ERN and anxiety in youth have typically included youth with mixed anxiety disorders (Carrasco et al., 2013b; Ladouceur et al., 2006; Meyer et al., 2015), we first examined the effects of both anxiety disorders on the ERN, with secondary exploratory analyses evaluating whether distinct patterns emerge for GAD and SAD examined separately.
2. Methods
2.1 Participants
Participants were youth and young adults recruited through the University of Michigan (UM) and University of Illinois at Chicago (UIC). Patients were recruited through outpatient clinics at each university, and healthy controls were recruited through the surrounding communities. A total of 94 participants (47 patients with SAD and/or GAD and 47 healthy controls) completed the ERN task at each assessment: data from 5 participants were unusable because of too few errors (< 6 trials after artifact rejection), 8 exhibited poor accuracy (< 60% correct of trials with responses), and 18 exhibited excessive artifacts/noise in the EEG at one or both assessments. The final sample included 63 participants (28 patients and 35 healthy controls). The included sample was older than the excluded sample, t(92) = 4.98, p < .001, with a trend for a larger proportion of patients excluded (40.4%) compared to controls (25.5%), χ2(1) = 2.36, p = .09. The exclusion rate for healthy controls was comparable to a previous study examining the ERN in healthy youth across two assessments (29.0%; Meyer et al., 2014). With regard to behavioral performance, on average, excluded participants were lower in accuracy and slower in reaction time (RT) at each assessment (ps < .05). Included patients did not differ from excluded patients with regard to response to treatment (p = .11).
Diagnoses were obtained through semi-structured interviews administered by Master’s or Doctoral level clinicians: the Schedule of Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) for children and adolescents (65.1% of the sample) and Structured Clinical Interview for DSM-IV (SCID; First et al., 2002) for young adults (34.9% of the sample). History of mania, psychotic symptoms, intellectual disability, pervasive development disorders, current substance use disorders, severe depression, or suicidal ideation was exclusionary. Patients with secondary comorbid anxiety, depressive, or externalizing disorders were included (Table 1).
Table 1.
Participant characteristics by group (healthy controls [HC] or anxiety disorder [AD]) and specific diagnosis (generalized anxiety disorder [GAD] or social anxiety disorder [SAD])
| HC (n = 35) | AD (n = 28) | SAD (n = 18) | GAD (n = 20) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M(SD) | % | M(SD) | % | M(SD) | % | M(SD) | % | |
| Age | 17.40 (3.26) | 17.39(5.08) | 18.78 (3.75) | 17.00 (5.53) | ||||
| Sex (% Female) | 60.0% | 67.9% | 72.2% | 80.0% | ||||
| Site (% University of Michigan) | 17.1% | 25.0% | 22.2% | 25.0% | ||||
| Treatment (% SSRI) | - | 57.1% | 66.7% | 45.0% | ||||
| Comorbid GAD/SAD | 0.0% | 35.7% | 55.6% | 50.0% | ||||
| Other comorbid anxiety | 0.0% | 25.0% | 33.3% | 30.0% | ||||
| Comorbid depression | 0.0% | 14.3% | 22.2% | 10.0% | ||||
| Number of sessions | - | 12.82(2.09) | 12.06(0.87) | 60.58(12.16) | ||||
| Percent change in anxiety severity | - | 64.97(20.70) | 60.58(12.16) | 64.16(22.60) | ||||
| ΔERN at Time 1 | −5.73(4.66) | −6.87(4.93) | −9.09(4.29) | −5.90(5.18) | ||||
| ΔERN at Time 2 | −7.53(4.11) | −8.52(6.32) | −10.79(6.33) | −6.91(5.04) | ||||
The average age of the included sample was 17.40 years (SD = 4.13; range 8 to 26 years). The sample was 63.5% female, 12.7% Hispanic/Latino, 65.1% White, 17.5% Black or African American, 12.7% Asian, 1.6% Native Hawaiian/Pacific Islander, and 3.2% other race.
2.2 Procedure
Procedures were approved by the Institutional Review Boards at both UM and UIC. Written informed consent was obtained from adult participants and parents of minors, with written assent obtained from minor participants.
Patients were treated through outpatient clinics at UM and UIC by a psychiatrist or master’s- or doctoral-level therapist. At UM, participants were offered and self-selected treatment with SSRI or CBT. At UIC, participants were randomly assigned to either SSRI or CBT, but could opt to switch from SSRI to CBT due to intolerable side effects. SSRI treatment consisted of 12 weeks of sertraline for children and adolescents. For young adults, the psychiatrist selected an SSRI based on patient’s prior treatment history and developed a personalized, flexible-dosing schedule. Almost all SSRI patients were treated with sertraline, beginning with a dose of 25 mg/day (12.5 mg/day for children) and increasing at subsequent visits as needed, up to 200 mg/day. Rather than sertraline, one young adult patient was prescribed paroxetine (beginning at 10 mg/day, increasing up to 60 mg/day) and one was prescribed escitalopram (beginning at 5 mg/day, increasing up to 20 mg/day). CBT was delivered through weekly 60-minute sessions by a Master’s or Doctoral level clinician following a manualized intervention for pediatric (Kendall and Hedtke, 2006) or adult anxiety (Craske and Barlow, 2006; Hope et al., 2010). Children and adolescents completed up to 18 CBT sessions, as clinically indicated and to accommodate parent sessions, and young adults completed 12 CBT sessions. CBT manuals included psychoeducation, relaxation, cognitive restructuring, and in vivo or imaginal exposures.
Patients completed the ERN task prior to and after completing treatment. Healthy controls were asked to return for the second assessment approximately 12 weeks after the initial EEG. On average, the second administration of the ERN task was completed 15.77 weeks (SD = 7.30) after the first assessment.
2.3 Measures
2.3.1 Response to Treatment
Clinicians rated participant improvement after the final treatment session using the Clinical Global Impressions scale (CGI; Guy, 1976). Patients with CGI global improvement ratings of 1 or 2 (indicating very much or much improved) were considered treatment responders. To assess changes in symptoms following treatment, patients were administered interviewer-rated measures of anxiety severity at screening and the final treatment session. Young adults were interviewed with the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), and children and adolescents with the Pediatric Anxiety Rating Scales (PARS; The Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002). Percent reduction in anxiety severity was computed to examine individual differences in response to treatment across all participants. One participant was missing a CGI score but was determined to be a treatment responder based on greater than 35% reduction in anxiety severity on PARS (Caporino et al., 2013).
2.3.2 Flanker Task
Participants completed a flanker task, which has been shown to reliably elicit an ERN in youth (Meyer et al., 2014). On each trial, horizontally aligned arrowheads were presented for 200 ms, followed by an intertrial interval between 2300 and 2800 ms. The task included 11 blocks of 30 trials (330 trials total), with half of the trials compatible (≫≫> or ≪≪<) and half incompatible (≪>≪ or ≫<≫). Participants were instructed to press the left or right mouse button to indicate the direction of the center arrow. Participants first completed a practice block of 30 trials. During the task, participants received feedback on their performance at the end of each block in order to ensure a sufficient number of error trials. The message “Please try to be more accurate” was displayed if accuracy was less than 75%, and “Please try to respond faster” was displayed if accuracy was above 90%. Otherwise, the message “You’re doing a great job” was displayed.
2.3.3 EEG Data Acquisition and Processing
Data were collected using a BioSemi (Amsterdam, Netherlands) 34-channel cap (32 channels plus FCz and Iz), with electrodes placed on the left and right mastoids, and electrooculogram recorded from four facial electrodes. Data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, and processed offline using Brain Vision Analyzer software (Brain Products, Gilching, Germany). Data were converted to a linked mastoid reference, filtered with high-pass and low-pass filters of 0.1 and 30 Hz, and segmented 500 ms before the response and continuing for 1000 ms after the response. Eyeblinks were corrected (Gratton et al., 1983), and semi-automated artifact rejection procedures removed artifacts with a voltage step of more than 50 μV between sample points, voltage difference of 175 μV within 400 ms intervals, maximum voltage difference of less than 0.5 μV within 100 ms intervals, and additional artifacts removed using visual inspection. ERPs to errors and correct responses were averaged separately and baseline corrected to 500-300 ms prior to responses. The ERN was scored at a pooling of frontocentral sites (Fz, FCz, Cz) 0 to 100 ms after the response. Analyses focused on the error minus correct response difference score (ΔERN) to isolate variation in the ERP wave related to performance monitoring (Luck, 2005).
2.4 Statistical Analyses
Mixed-design ANCOVAs were computed to examine whether group differences in ΔERN were apparent pre- and post-treatment or differed at each assessment. Given developmental increases in the ERN (Davies et al., 2004) and evidence of blunted ERN in depression (Ladouceur et al., 2012; Weinberg et al., 2012), age and diagnosis of comorbid depression were included as covariates. We first examined effects of anxiety group, followed by analyses of GAD and SAD. Effects of GAD and SAD were tested in separate models in order to directly compare those with each diagnosis to healthy controls. Patients with comorbid GAD/SAD were included in each analysis, given the relatively small sample size for each diagnosis.
3. Results
3.1 Participant Characteristics
Among patients, 71.4% (n = 20) had GAD and 64.3% (n = 18) SAD. With regard to comorbidity, 35.7% (n = 10) had both GAD and SAD. With regard to other comorbid disorders, 10.7% (n = 3) had panic disorder, 10.7% (n = 3) specific phobia, 3.6% (n = 1) separation anxiety disorder, 7.1% (n = 2) post-traumatic stress disorder, 3.6% (n = 1) OCD, 14.3% (n = 4) current depression, and 3.6% (n = 1) substance abuse disorder. In addition, 3 child and adolescent patients met criteria for attention-deficit/hyperactivity disorder (ADHD; rates were unavailable for adult patients because ADHD is not assessed by the SCID).
Participant characteristics by group and specific diagnosis are presented in Table 1. Patients and healthy controls did not differ on distribution of sex or study site or mean age (ps > .32). Neither patients with SAD nor patients with GAD significantly differed in age from healthy controls (ps > .17). The proportion of African American and Asian participants was higher among healthy controls, while the anxious group included a greater proportion Latino/Hispanic participants, χ2(5) = 14.35, p = .01. Study sites did not differ on age, treatment response, ΔERN at either assessment, or distribution of race/ethnicity or sex (ps > .11). In addition, patients who completed SSRI vs. CBT did not differ on age, treatment response, ΔERN at either assessment, or distribution of race/ethnicity or sex (ps > .44).
Almost all patients in the final sample responded to treatment (89.3%), with 2 patients (7.1%) exhibiting minimal improvement and 1 patient (3.7%) rated as minimally worse following treatment. Both PARS, t(17) = 12.24, p < .001, and HAM-A, t(9) = 5.28, p = .001, significantly decreased pre- to post-treatment, with a 65.0% reduction in anxiety severity on average (SD = 20.7%).
3.2 Behavioral Performance
Mixed-design ANCOVAs were computed to examine the effects of time (pre- to post-treatment for patients) and anxiety group (patients vs. healthy controls) on accuracy and reaction time (RT), controlling for age and comorbid depression. Both accuracy and RT increased from the first to the second assessment, F(1, 59) = 14.43, p < .001, η2p = .20, and, F(1, 59) = 5.74, p < .02, η2p = .09, respectively. For RT, the interaction between time and anxiety group was significant, F(1, 59) = 4.00, p = .05, η2p = .06. Patients were slower than healthy controls prior to treatment, F(1, 59) = 8.01, p < .01, η2p = .12, but the groups did not significantly differ after treatment (p = .53). This was primarily driven by an increase in RT from the first to second assessment for healthy controls, t(34) = −2.64, p = .01, while the comparison between assessments was not significant for patients (p = .93). None of the main or interaction effects with anxiety group on accuracy reached significance (ps > .13).
When GAD and SAD were examined separately, patients with GAD exhibited slower RT overall compared to healthy controls, F(1, 51) = 7.63, p = .01, η2p = .13. In addition, the interaction between time and SAD on RT was significant, F(1, 49) = 4.41, p = .04, η2p = .08, with similar patterns as observed with the combined patient group. RT increased between assessments for healthy controls but not for patients with SAD (p = .47). No significant effects of GAD or SAD on accuracy were observed (ps > .13).
With regard to relationships between ΔERN and behavioral performance, in the combined sample, higher accuracy at the first assessment was correlated with an enhanced ΔERN, r(62) = −.40, p < .01. This effect was not significant at the second assessment (p = .34), possibly due to overall improvements in accuracy and a more limited range. In addition, faster RT was related to a larger ΔERN at the second assessment, r(62) = .37, p < .01, with a similar pattern at the first assessment, though the correlation did not reach significance, r(62) = .21, p = .10.
3.3 Anxiety Group Differences in the ERN Pre- and Post-Treatment
A mixed-design ANCOVA was computed to examine the effects of time (pre- and post-treatment) and anxiety group (patients vs. healthy controls) on ΔERN, controlling for age and comorbid depression. The magnitude of ΔERN increased across time in the overall sample, F(1, 59) = 3.97, p = .05, η2p = .06, but the main effect of anxiety group and interaction between time and group were not significant (ps > .42). Though age was correlated with ΔERN at the first assessment r(61) = −.27, p = .04, the main effects of age and depression, as well as interactions with time, were not significant in the ANCOVA (ps > .16).
Next, mixed-design ANCOVAs were computed to examine whether patients with GAD or SAD differed from healthy controls on ΔERN and whether the effect was moderated by time. Neither the main effect of GAD nor the interaction between time and GAD were significant (ps > .40). The main effect of SAD was significant, F(1, 49) = 6.91, p = .01, η2p = .12; the interaction with time was not significant (p = .97).
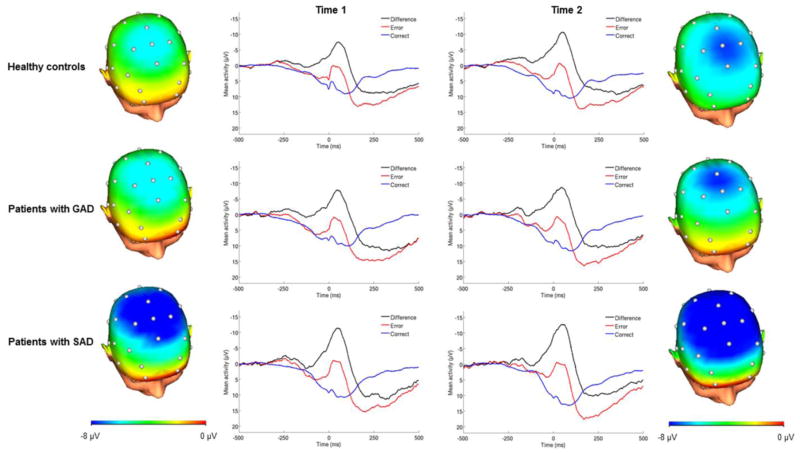
Patients with SAD showed an enhanced ΔERN relative to healthy controls before, F(1, 49) = 5.42, p = .02, η2p = .10, and after treatment, F(1, 49) = 4.53, p = .04, η2p = .09 (Figure 1). To determine whether this was driven by patients who did not respond to treatment, the effect was examined excluding non-responders (n = 3), and the effect of SAD on ΔERN post-treatment remained significant, F(1, 46) = 4.98, p = .03, η2p = .10. Furthermore, change in ΔERN from pre- to post-treatment was unrelated to change in symptom severity (ps > .63). As the majority of the SAD sample (n = 12) was treated with SSRI, we also examined the effect of SAD on ΔERN post-treatment among those treated with pharmacotherapy and the effect remained significant, F(1, 43) = 5.57, p = .02, η2p = .12. The SAD sample completing CBT was too small to examine separately (n = 6).
Figure 1.
ERPs (negative up) at frontocentral sites following errors, correct responses, and the error minus correct difference wave for healthy controls (n = 35), patients with social anxiety disorder (SAD; n = 18), and patients with generalized anxiety disorder (GAD; n = 20) at Time 1 (pre-treatment) and Time 2 (post-treatment). Scalp distributions depict the error minus correct difference 0–100 ms after the response.
Next, given the wide age range of the sample and to determine whether age-related differences may be driving effects, we examined the effects of SAD separately in children and adolescents 17 and younger (n = 20) and young adults 18 and over (n = 33). Though these analyses were limited by the small sample in each group, similar trends for the effect of SAD across time were observed in each group, F(1, 16) = 3.78, p = .07, η2p = .19 for children/adolescents, and F(1, 29) = 3.29, p = .08, η2p = .10 for young adults.
The effect of SAD on ΔERN across time remained significant when controlling for RT and accuracy at each assessment, F(1, 45) = 4.41, p = .04, η2p = .09. Finally, we tested the effect of SAD on ERN and CRN, separately, and none of the main effects or interactions with time were significant (ps > .25), suggesting that effects are specific to the relative response to errors compared to correct responses.
4. Discussion
We examined error monitoring (i.e., ERN) in a sample of youth and young adults with GAD and/or SAD before and after treatment. Our primary finding provides preliminary evidence that patients with SAD exhibit an increased ERN that persists after remission of symptoms following psychotherapy or pharmacotherapy (i.e., CBT or SSRI). In contrast, we did not find evidence of an enhanced ERN in patients with GAD in this sample before or after treatment.
Our finding of an increased ERN in SAD following treatment is consistent with previous studies indicating that an enhanced ERN in OCD continues to persist after CBT (Hajcak et al., 2008; Riesel et al., 2015), but extends this finding to SAD. In the current study, an enhanced ERN correlated with better behavioral performance, including increased accuracy and faster reaction time, suggesting that an a larger ERN may correspond with closer monitoring of one’s own behavior and greater sensitivity to errors. Consistent with evidence that SSRIs do not affect the ERN in healthy adults (Bruijn et al., 2006) or OCD patients (Stern et al., 2010), our findings also indicate that SSRI treatment does not normalize the ERN in SAD. Taken together, these findings are consistent with conceptualizations of enhanced error monitoring as an endophenotype or vulnerability that predisposes some individuals to develop anxiety, is observable among those with a family history, and remains relatively stable after treatment (Carrasco et al., 2013a; Hajcak et al., 2008; Meyer et al., 2015; Olvet and Hajcak, 2008; Riesel et al., 2015, 2011). Further, given that anxiety disorders are characterized by high rates of recurrence, with heightened risk of developing other forms of psychopathology and impaired functioning later in life (Bruce et al., 2005; Copeland et al., 2015; Woodward and Fergusson, 2001), the persistance of overactive performance monitoring following treatment in SAD may be a factor contributing to difficulties later in life.
Importantly, an enhanced ERN after treatment was observable among patients with SAD even when limiting the analyses to those who responded to treatment. Thus, despite changes in symptomatology, the ERN remained enhanced, suggesting that treatment may have resulted in the development of compensatory processes to adjust for hyperactive performance monitoring, rather than normalization of this system. Interestingly, though standard treatments do not appear to reduce the magnitude of the ERN, recent studies indicated that manipulating the allocation of attentional resources reduced the ERN in OCD (Klawohn et al., 2016), and that attention bias modification reduced the ERN in healthy adults (Nelson et al., 2015). In addition, there is evidence that transcranial direct-current stimulation (tDCS) can modulate the ERN in patients with schizophrenia (Reinhart et al., 2015). Thus, one intriguing possibility for examination in future research is that supplementing established anxiety treatments with attention training or tDCS may impact the ERN and improve long-term outcomes.
Given previous evidence of an enhanced ERN in GAD in adults (Weinberg et al., 2015, 2012, 2010; Xiao et al., 2011; Zambrano-Vazquez and Allen, 2014), it is surprising that we did not find significant effects of GAD on the ERN before and after treatment in this sample. There are a few possible interpretations for the lack of effect. First, previous studies of the ERN in youth with GAD are limited to small samples with mixed anxiety disorders (Carrasco et al., 2013b; Ladouceur et al., 2006), and the average age of the current sample was younger than adult studies of GAD, raising the possibility that an enhanced ERN may develop later in adulthood for GAD. In addition, the ERN has been argued to reflect sensitivity to internal as opposed to external sources of threat (Weinberg et al., 2015); thus, it is possible that sensitivity to internal threat (e.g., making a mistake and humiliating oneself) is best reflected by a diagnosis of SAD in this sample, while patients with GAD and not SAD may exhibit increased reactivity to external sources of threat (e.g., disasters, loss of a loved one). Additional research with larger samples of youth and young adults with GAD with and without comorbid SAD is needed to evaluate this possibility.
In the combined sample, the ERN increased across assessments, consistent with an adult treatment study that found a similar increase for both patients with OCD and healthy controls (Riesel et al., 2015) and evidence of an increase in the magnitude of the ERN in a study of test-retest reliability (Olvet and Hajcak, 2009). Errors may become more salient with repeated administrations of the task, which is also consistent with an overall increase in accuracy from the first to second assessment. This finding emphasizes the importance of identifying and accounting for normative changes with time when examining neural measures before and after treatment, and the current study is limited in that we were unable to examine typical changes with time in a patient group not receiving treatment. Future studies are needed that include treatment control patients in order to examine trajectories of neural processes in youth and young adults with anxiety disorders to those receiving treatment.
Several additional limitations of the study and directions for future research should be noted. First, the sample size was small and heterogeneous with regard to age, primary diagnosis, and treatment. Though this study is among the first to examine distinct effects of GAD and SAD on the ERN, our relatively small sample size precluded the examination of effects of comorbidity as compared to pure presentations of each disorder. In addition, data for a large proportion of the sample were lost due to poor behavioral performance or noise in the EEG data. This was particularly problematic among patients and younger children, indicating the need for additional research to refine methods of eliciting the ERN in children. Larger samples of patients treated with SSRIs and CBT are also needed in order to directly compare the effects of each type of intervention, as our sample did not have sufficient power to test differences between treatments or the effect of SAD within the CBT group alone. It should also be noted that our effects were apparent only for the relative response to errors compared to correct responses (i.e., ΔERN), which differs from previous work that has identified group differences in the magnitude of the ERN specifically (e.g., Hajcak et al., 2008). Future research is needed to evaluate the functional significance of ΔERN compared to ERN and whether these distinct patterns of effects can further inform understanding of psychopathology. Lastly, though we did not observe any effects of study site on the results, we cannot rule out the possibility that differences in design, providers, or other factors may have contributed to the results. Despite the need for replication of the current findings and extension to larger samples, the current study is the first to examine the ERN following treatment for anxiety disorders other than OCD, providing useful insights for future treatment studies.
5. Conclusions
This study is the first to provide preliminary evidence that, like OCD (Hajcak et al., 2008; Riesel et al., 2015), SAD in youth and young adults is characterized by an enhanced ERN that persists following treatment. Increased error monitoring following treatment for anxiety disorders may be a factor contributing to high likelihood of recurrence and impaired functioning later in life, and additional prospective research is needed to evaluate this possibility.
Highlights.
Enhanced error-related negativity (ERN) in social anxiety disorder (SAD)
SAD patients differed from controls before and after pharmaco- and psychotherapy
No significant effects of generalized anxiety disorder
Support enhanced ERN as trait-like vulnerability or endophenotype for SAD
Acknowledgments
Funding: This work was supported by National Institute of Mental Health (NIMH) Grants R01-MH086517 to C.S.M. and K.L.P and R01-MH101497 to K.L.P. A.K. was supported by NIMH Grant T32-MH067631 to Mark Rasenick. The funding source had no involvement in study design, data collection, or manuscript preparation.
Footnotes
Author contributions: All authors contributed to study design and revised and approved the final manuscript. A.K. processed/analyzed data and drafted the manuscript. A.W. and N.B. contributed to data analysis and manuscript preparation. K.L.P. designed study and contributed to data analysis/approach and manuscript preparation.
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple Go/NoGo task. J Psychophysiol. 2005;19:244–255. doi: 10.1027/0269-8803.19.4.244. [DOI] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am J Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn E, de Sabbe B, Hulstijn W. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006 doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, Sakolsky D, Birmaher B, Compton SN, Ginsburg G, Rynn M, McCracken J, Gosch E, Keeton C, March J, Walkup JT. Defining treatment response and remission in child anxiety: Signal detection analysis using the pediatric anxiety rating scale. J Am Acad Child Adolesc Psychiatry. 2013;52:57–67. doi: 10.1016/j.jaac.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depress Anxiety. 2013a;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neurosci Lett. 2013b;541:214–218. doi: 10.1016/j.neulet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Shanahan L, Costello EJ. Adult functional outcomes of common childhood psychiatric problems: A prospective, longitudinal study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Barlow DH. Mastery of Your Anxiety and Worry. Oxford University Press; USA, New York: 2006. [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Ann N Y Acad Sci. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, Buhlmann U. Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. J Abnorm Psychol. 2014;123:705–714. doi: 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- Endrass T, Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehav Rev. 2014;46:124–138. doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. I Simple and choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:438–446. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. New York: 2002. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology. US Dep Heal Educ Welfare, Public Heal Serv Alcohol, Drug Abus Ment Heal Adm Natl Inst Ment Heal Psychopharmacol Res Branch, Div Extramur Res Programs 1976 [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assesment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, Rosenbaum JF. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG, Turk CL. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach. Oxford University Press; New York: 2010. [Google Scholar]
- Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F. Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: A fMRI study with a Flanker task before and after CBT. J Child Psychol Psychiatry. 2011;52:1251–60. doi: 10.1111/j.1469-7610.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Frosio KE, White EJ, Taylor DL, Mills AC. Electrocortical evidence of enhanced performance monitoring in social anxiety. Behav Ther. 2016;47:274–285. doi: 10.1016/j.beth.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Hedtke K. Coping Cat Workbook. 2. Workbook Publishing; Ardmore, PA: 2006. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klawohn J, Endrass T, Preuss J, Riesel A, Kathmann N. Modulation of hyperactive error signals in obsessive-compulsive disorder by dual-task demands. J Abnorm Psychol. 2016;125:292–298. doi: 10.1037/abn0000134. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson Da, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson Da, Ryan ND. Altered error-related brain activity in youth with major depression. Dev Cogn Neurosci. 2012;2:351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson Ha, Fox Na. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J Am Acad Child Adolesc Psychiatry. 2014;53:447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. The MIT Press; Cambridge, MA: 2005. [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51:602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol. 2015;124:266–274. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/S0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry. 2014;171:741–748. doi: 10.1176/appi.ajp.2014.13101337. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Jackson F, Amir N, Hajcak G. Single-session attention bias modification and error-related brain activity. Cogn Affect Behav Neurosci. 2015:776–786. doi: 10.3758/s13415-015-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Res. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clin Psychol Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Zhu J, Park S, Woodman GF. Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction error signaling. J Neurosci. 2015;35:12232–12240. doi: 10.1523/JNEUROSCI.1717-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: Evidence from a treatment study. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2014.14070886. appi.ajp.2014.1. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: Evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, Fitzgerald KD, Himle JA, Abelson JL, Taylor SF. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47:913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: A review of the literature. Int J Psychophysiol. 2015;98:276–299. doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J Abnorm Psychol. 2012;121:885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, Hajcak G. Error-related negativity and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53:372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biol Psychol. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1086–93. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, Fan Q, Fromson Ja. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295X.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zambrano-Vazquez L, Allen JJB. Differential contributions of worry, anxiety, and obsessive compulsive symptoms to ERN amplitudes in response monitoring and reinforcement learning tasks. Neuropsychologia. 2014;61:197–209. doi: 10.1016/j.neuropsychologia.2014.06.023. [DOI] [PubMed] [Google Scholar]