Abstract
Objectives
The level-dependent growth of distortion product otoacoustic emissions (DPOAEs) provides an indirect metric of cochlear compressive nonlinearity. Recent evidence suggests that aging reduces nonlinear distortion emissions more than those associated with linear reflection. Therefore, in this study we generate input/output (I/O) functions from the isolated distortion component of the DPOAE to probe the effects of early aging on the compressive nonlinearity of the cochlea.
Design
Thirty adults whose ages ranged from 18–64 years participated in this study, forming a continuum of young to middle-aged subjects. When necessary for analyses, subjects were divided into a young-adult group with a mean age of 21 years, and a middle-aged group with a mean age of 52 years. All young-adult subjects and 11 of the middle-aged subjects had normal hearing; four middle-aged ears had slight audiometric threshold elevation at mid-to-high frequencies. DPOAEs (2f1–f2) were recorded using primary tones swept upward in frequency from 0.5–8 kHz, and varied from 25–80 dB SPL. The nonlinear distortion component of the total DPOAE was separated and used to create I/O functions at one-half octave intervals from 1.3–7.4 kHz. Four features of OAE compression were extracted from a fit to these functions: compression threshold, range of compression, compression slope, and low-level growth. These values were compared between age groups and correlational analyses were conducted between OAE compression threshold and age with audiometric threshold controlled.
Results
Older ears had reduced DPOAE amplitude compared to young-adult ears. The OAE compression threshold was elevated at test frequencies above 2 kHz in the middle-aged subjects by 19 dB (35 versus 54 dB SPL), thereby reducing the compression range. In addition, middle-aged ears showed steeper amplitude growth beyond the compression threshold. Audiometric threshold was initially found to be a confound in establishing the relationship between compression and age; however, statistical analyses allowed us to control its variance. Correlations performed while controlling for age differences in high-frequency audiometric thresholds, showed significant relationships between the DPOAE I/O compression threshold and age: Older subjects tended to have elevated compression thresholds compared to younger subjects and an extended range of monotonic growth.
Conclusions
Cochlear manifestations of nonlinearity, such as the DPOAE, weaken during early aging, and DPOAE I/O functions become linearized. Commensurate changes in high-frequency audiometric thresholds are not sufficient to fully explain these changes. The results suggest that age-related changes in compressive nonlinearity could produce a reduced dynamic range of hearing, and contribute to perceptual difficulties in older listeners.
INTRODUCTION
Otoacoustic emissions (OAE) offer an index of cochlear function and are linked to outer hair cell health (Kemp 2002). It has long been established that OAEs decrease in amplitude with increasing hearing loss (e.g., Lonsbury-Martin & Martin 1990; Gorga et al. 1993) but there is also evidence that aging, independent of hearing loss, reduces OAE amplitude (e.g., Dorn et al. 1998; Uchida et al. 2008). When strict audiometric inclusion criteria are implemented (in particular at high-frequencies where older adults show threshold elevation), age is still negatively correlated with the amplitude of the distortion-product (DP)OAE (Dorn et al. 1998). However, this work is countered by studies reporting that reductions in OAE amplitude attributed to age can be accounted for by variability in audiometric thresholds (Lonsbury-Martin et al. 1991; Prieve & Falter 1995; Oeken et al. 2000). For example, while DPOAE reductions are observed in aging subjects with clinically normal audiograms, they are often restricted to frequencies above 2 kHz, where the audiometric thresholds are worse (although still “normal”) in older- compared to young-adult ears (Lonsbury-Martin et al 1991).
In the present study, we did not target the well-documented impact of aging or even audiometric threshold on OAE amplitude; rather, we sought to describe age-related effects on one specific cochlear property: compressive nonlinearity. Previous work from our lab (Abdala & Dhar 2012) has shown that the distortion component of the DPOAE is more reduced in elderly subjects than the reflection component. The DPOAE is made up of two parts: a distortion component generated around the overlap of the two primary tones by nonlinearities linked to OHC transduction (e.g. Hudspeth & Corey 1977), and a typically smaller reflection component generated by linear reflections from the DP place (2f1–f2). Others have also reported that older subjects show more significant reductions in DPOAE amplitude than amplitude of the transient-evoked OAE, another reflection-type emission (Hoth et al. 2010). Otoacoustic emissions generated by nonlinear distortion in the cochlea may be impacted by aging more than OAEs arising from linear reflection.
DPOAEs Provide an Estimate of Cochlear Compression
The level-dependent growth of the DPOAE as depicted by an input/output (I/O) function provides an indirect metric of cochlear compressive nonlinearity and is similar in gross form to that measured directly from the basilar membrane of laboratory animals, most notably at the peak of the traveling wave (Ruggero et al. 1997; Robles & Ruggero 2001); it is also similar to compression inferred from human perceptual data (Buus et al. 2001; Boege & Janssen 2002; Johannesen & Lopez-Poveda 2008, 2010; Rasetshwane et al. 2013). Basilar membrane and DPOAE I/O functions are both characterized by linear response growth at low stimulus levels followed by compressive growth at moderate-to-high stimulus levels. This compression is also observed in the input/output function of sensory cells and sustained by the firing rates of auditory nerve fibers. It is key to the large dynamic range of hearing (Yates et al. 1990; Robles & Ruggero 2001). In cases where there is cochlear damage, the basilar membrane response to sound becomes linear and loses its compressive nature (Ruggero & Rich 1991; Ruggero et al. 1996).
Earlier studies have measured DPOAE I/O functions to quantify the DPOAE threshold (the lowest stimulus level at which a DPOAE can be distinguished from the noise) during aging (Lonsbury-Martin et al. 1991; Gates et al. 2002). However, the current study examines specifically how early aging affects the compressive features of this I/O function as a metric of cochlear nonlinearity. Compressive DPOAE features have been described in normal and impaired infant and adult ears previously, but not during aging. These existing studies describe human DPOAE I/O functions characterized by steep growth at low stimulus levels and amplitude saturation at moderate-to-high levels (Abdala 2000; Dorn et al. 2001; Neely et al. 2003, 2009; Gorga et al. 2003, 2007). Hearing-impaired ears, in contrast, show truncated I/O functions due to increases in the DPOAE threshold, an extended region of monotonic growth, and slightly steeper slope of DPOAE growth beyond the compression threshold (Dorn et al. 2001; Neely et al. 2003, 2009). Features measured from the DPOAE I/O function have shown to correlate with perceptual measures of compression such as temporal masking curves and categorical loudness scaling (Williams & Bacon, 2005; Johannesen & Lopez-Poveda 2008, 2010; Moore et al. 2014; Neely et al. 2003; Muller & Janssen, 2004; Thorson et al. 2012; Rasetshwane et al. 2013), suggesting they can provide objective measures of perceptual phenomena.
The current study describes a method for generating and analyzing distortion-component OAE I/O functions using swept-tone stimuli, and presents initial results applying this metric of cochlear compression to an early-aging group. In order to isolate the nonlinear distortion component of the total DPOAE and eliminate reflection energy, we have applied offline signal processing and constructed all I/O functions using only the nonlinear distortion component. In most studies, the distortion component has not been isolated from the total DPOAE when generating I/O functions. However, past work suggests that distortion-component I/O functions decrease response variability across frequency and produce functions that correlate more closely with perceptual measures of compression (Mauermann & Kollmeier 2004; Long et al. 2009; Dalhoff et al. 2013; Moore et al. 2014).
MATERIALS AND METHODS
Subjects
Thirty adults whose ages ranged from 18–64 years participated in this study. This range was chosen to represent a continuum from young adulthood to middle age. When required for statistical purposes, we divided the participants into two age groups: 15 young adults (12 females, 3 males) with a mean age of 21 years (range = 18–32 years) and 15 middle-aged adults (13 females, 2 males) with a mean age of 52 years (range = 39–64 years). Twelve right and three left ears were tested in the young-adult group; ten right and five left ears were tested in the middle-aged group. The test ear was chosen randomly unless one ear had markedly higher DPOAEs or better audiometric thresholds in which case the better ear was chosen. Subjects were excluded if they reported recent middle-ear pathology or a history of ear surgery or occupational noise exposure. All participants provided informed consent in accordance with the guidelines of the Institutional Review Board (IRB) of the University of Southern California.
On the day of test, air and bone conduction audiometric thresholds were established using a modified Hughson–Westlake procedure and tympanograms were recorded. All young-adult subjects had audiometric thresholds ≤ 15 dB HL from 0.25 through 8 kHz (including inter-octave frequencies) in both ears. Of the middle-aged subjects, 11 had audiometric thresholds ≤ 20 dB HL at all frequencies, while four had slightly elevated thresholds for frequencies beyond 3 kHz as shown in Fig. 1. The mean difference in audiometric thresholds between young-adult and middle-aged groups was 9.4 dB for the frequency interval from 2–8 kHz. This group threshold difference was statistically significant (F = 34.38, p < 0.001) and its potential influence on the results will be discussed. All prospective subjects were screened with a DP-gram generated using equi-level 65 dB SPL primary tones at f2 frequencies from 0.5 to 8 kHz. A 6 dB signal-to-noise (SNR) across frequency was required for study inclusion.
Figure 1.
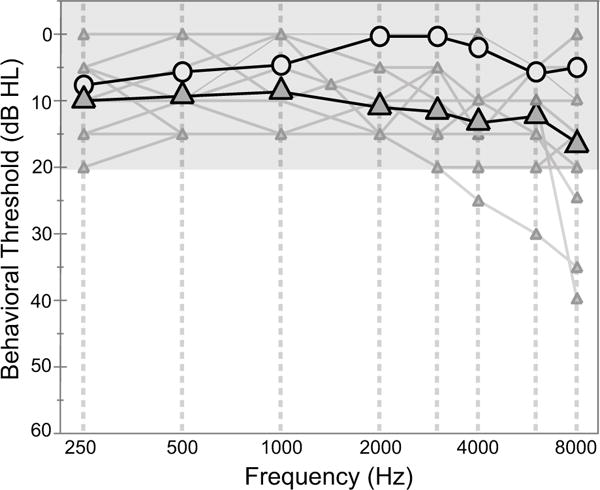
Group mean audiograms for young (circle) and middle-aged adults (triangle). The mean thresholds for both age groups are within the clinical range of normal. The individual thresholds (shown as thin gray lines/small triangles) are also provided. Four middle-aged subjects show thresholds that fall just outside of this normal range for frequencies > 3 kHz.
Instrumentation and Protocol
Otoacoustic emission and audiometric data were collected in a sound-attenuating booth (IAC, Industrial Acoustics Company). For OAE recordings, the participant was semi-reclined in an ergonomic reclining chair for enhanced comfort and stillness during the test sessions. Stimuli were generated using custom-developed software (by Carrick Talmadge; modified by P. Luo, C. Abdala) on a Macintosh laptop. The signal was routed to a MOTU 828 Mk II audio device (44.1 kHz, 24 bit) for digital-to-analog conversion then amplified and delivered to the ear via Etymotic Research ER2 tube phones attached to the ER10B+ probe microphone assembly. The output of the microphone was pre-amplified and passed through an analog high-pass filter with 300-Hz cutoff frequency before being digitized by the MOTU and stored on disk.
DPOAEs were recorded using tones logarithmically swept upwards in frequency at rates of 0.5 octaves/second for f2 = 0.5–4 kHz, and 0.125 octaves/second for f2 = 4–8 kHz at a fixed ratio: f2/f1 = 1.22. The level of the higher-frequency primary tone, L2, was presented from 25–80 dB SPL with 5-dB step resolution and L1 was determined applying the formula: 0.4 L2 + 39 dB SPL (Kummer et al. 1998). This method, commonly referred to as the “Scissors” method, varies the separation of L1 and L2 systematically as a function of stimulus level to maintain the optimal and most consistent overlap of the primaries at the site of DPOAE generation. Between 16 and 24 individual sweeps were presented and averaged for each DPOAE estimate. The middle-aged subjects were presented with more sweeps (n = 24) in order to achieve adequate signal-to-noise ratio given the lower DPOAE levels in this group.
To calibrate stimulus level, half-wave peak frequencies measured in an ear stimulator were coupled with estimates of probe-insertion depth (IEC 60318–4; BK 4157) as described in Lee et al. (2012). The half-wave resonance was then measured in each ear canal prior to the test, the depth-insertion in each ear estimated, and the appropriate frequency-equalization filter selected to produce a flattened frequency response at the tympanic membrane, mitigating the effect of ear canal standing waves. System distortion was measured by running the full DPOAE protocol in the ear simulator. Mean system distortion levels ranged from −30 to −17 dB SPL with a mean of −25 dB SPL across frequency and levels. DPOAEs < 6 dB above system distortion were eliminated.
DPOAE Analysis
DPOAE level and phase were estimated using a least-squares-fit (LSF) procedure (Long et al. 2008; Kalluri & Shera 2013). The LSF technique is a non-FFT–based OAE analysis technique that is routinely applied when swept stimulus presentation is used to evoke OAEs. LSF creates models of the primary tones and OAE and fits them to the averaged OAE measured at the microphone by minimizing the sum of the squared residuals between model and response. The noise floor is estimated as a least squares fit to the average difference between pairs of sweeps.
A sweep rate of 0.5 octaves/second with an LSF analysis window of 125 ms was used for f2 = 1–4 kHz, yielding an analysis bandwidth of 0.06 octaves across frequency which has shown to be an optimal combination of parameters (Abdala et al. 2015). For f2 = 4–8 kHz, the DPOAE was analyzed with an LSF window of 500 ms. The window was four times longer in duration (compared to the low-to-mid frequency segment) because the stimulus sweep rate was four times slower. This yielded a relatively constant analysis window of 0.06 octave across frequency, which optimizes swept-tone DPOAE measures.
Isolating the Distortion Component of the DPOAE
The DPOAE consists of two components: nonlinear distortion generated at the overlap of the two primary tones and a linear reflection component, which arises from the DP place at 2f1–f2. The two components have distinct generation mechanisms (Shera & Guinan 1999; Talmadge et al. 1999; Knight & Kemp 2000, 2001). In this study we were interested in the distortion portion of the total DPOAE because it is a manifestation of the nonlinearities we are interested in studying here. Consequently, the reflection energy in the total DPOAE was eliminated leaving only nonlinear distortion, referred to here as the “distortion OAE”.
We used an inverse fast Fourier transform or IFFT method to isolate the distortion component (Stover et al. 1996). The IFFT exploits the distinct delays of the distortion and reflection components to isolate each and produce independent estimates of amplitude and phase. (Note: We have been applying this DPOAE component-separation method for many years; details can be found in numerous publications e.g., Abdala & Dhar 2010, 2012; Abdala et al. 2015). During the IFFT procedure, the total DPOAE measured in the frequency domain is transferred to the time domain in small segments. A rectangular time-domain filter is applied to each windowed segment and centered on the maximum peak of energy within a search range appropriate to the distortion component (and guided by its latency). The filtered windows of data are then transformed back to the frequency domain by FFT and the level and phase of the isolated distortion component reconstructed (Long et al. 2008).
For comparison, the distortion component of the DPOAE was also isolated using a second technique. If one uses a fast sweeping rate, such as the 0.5 octaves/second combined with an LSF analysis window that is too long to capture the total DPOAE (e.g. 500 ms), reflection energy can be excluded from the DPOAE estimate (Long et al. 2008; Abdala et al. 2015). The distortion component was isolated via IFFT and LSF for comparison; however, all the I/O functions were constructed using the IFFT-derived distortion OAE.
Distortion OAE Input/Output Functions
Input/Output functions were generated from the isolated distortion component of the DPOAE at 6 half-octave intervals: f2 = 1303, 1847, 2607, 3696, 5207 and 7394 Hz. Noise was calculated in third-octave bins centered on each f2 frequency, and only DPOAEs with amplitude ≥ 6 dB above both noise floor and system distortion, and above −20 dB SPL were accepted for further analysis. As noted in Fig. 2C, the actual SNR was much higher than 6 dB for both groups at most levels. To maintain at least 5-dB step resolution for all the fits to the I/O function, only functions with at least three consecutive data points were included in subsequent analyses.
Figure 2.
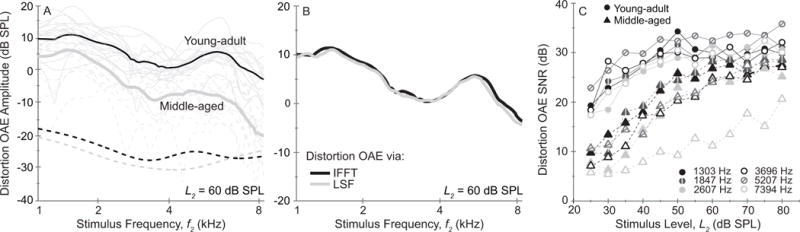
(A) Mean distortion-OAE amplitude as a function of frequency at one primary-tone level, L2 = 60 dB SPL. Thick lines show the mean amplitude for young-adult (black) and middle-aged (gray) groups while thin lines display individual values (dashed gray lines are used for middle-aged subjects). The dashed lines near the bottom of the figure show the mean noise floors. (B) Mean distortion-OAE amplitude as a function of frequency calculated in the young-adult group using the inverse FFT method (black line) and the LSF method (i.e. a fast sweeping rate combined with a long analysis window; gray line). (C) Mean signal-to-noise (SNR) of the distortion component as a function of primary-tone level for each age group with f2 parameterized (circles = young adult; triangles = middle-aged adult.)
Six I/O functions in the middle-aged group (out of a total of 90 collected) could not be included due to low SNR: two I/O functions were eliminated at f2 = 3696 Hz and four at f2 = 7394 Hz. After implementing the acceptance criteria (i.e., 6 dB SNR criteria, the need for three consecutive-points, and OAE values > −20 dB SPL) 76% of the measured DPOAE data points were accepted from the middle-aged dataset and 98% from the young-adult group. To estimate the compression threshold of each I/O function, a fit adapted from Kalluri and Shera (2007) was applied. This fit was initially formulated to characterize the amplitude growth of stimulus-frequency OAEs in normal ears, and it mimics the features of healthy cochlear response growth1. It has more recently been applied to distortion OAE I/O functions for both normal and impaired ears (Ortmann & Abdala 2015).
Four features were analyzed for each I/O function to characterize DPOAE compression: (1) compression threshold (CT) in dB SPL which defines the knee point of the function where growth of distortion OAE amplitude deviates from a linear form and begins to change more slowly in response to increases in stimulus level; (2) the low-level growth slope in dB/dB, which characterizes the segment below the compression threshold where the response is growing approximately linearly; (3) compression range in dB, which is the range of stimulus levels over which compressive growth of the OAE is observed; it is the interval between compression threshold and the highest stimulus level presented; and (4) compression slope in dB/dB, which is the growth slope measured at levels above the compression threshold. It was calculated by fitting a regression line to the data points in the compression range.
Alternative Fits
The fit applied to characterize the distortion OAE I/O function was effective in young normal ears and produced mean residuals of ~0.09 dB across frequency and acceptable confidence intervals (CI) that averaged ~10 dB for estimates of the compression threshold. However, in older ears the fit was not always effective as determined by residuals and CI for each estimate of compression threshold (CT) derived from the fit. Whenever the goodness-of-fit metrics were unacceptable (as defined below) we applied alternative fits and/or assigned CT estimates.
In just over 10% of young-adult I/O functions the fit was not acceptable. In these functions, distortion OAE amplitude compression was immediate at the lowest levels presented (slope averaged 0.06 dB/dB). The fit could not characterize this pattern of growth and produced flawed CT estimates at stimulus levels well below those presented. In these cases, the CT was assigned a value equal to 30 dB SPL (5 dB above the lowest level presented) to reflect immediate compressive growth throughout the function.
In middle-aged ears, 24% of I/O functions triggered alternative strategies for deriving the CT: For ten I/O functions growth was monotonic throughout the level range; however, this occurrence did not reflect immediate compression because OAE amplitude increased at rates well above those associated with typical compressive growth (> 0.3 dB/dB). In these cases, compression threshold was assigned at 75 dB SPL to reflect an elevated CT. This occurred at f2 = 5207 and 7394 Hz for six of the ten I/O functions requiring an alternative fit. Figure 3 provides an example of this type of function in one middle-aged subject at f2 = 7394 Hz (See the lowest right-hand panel).
Figure 3.
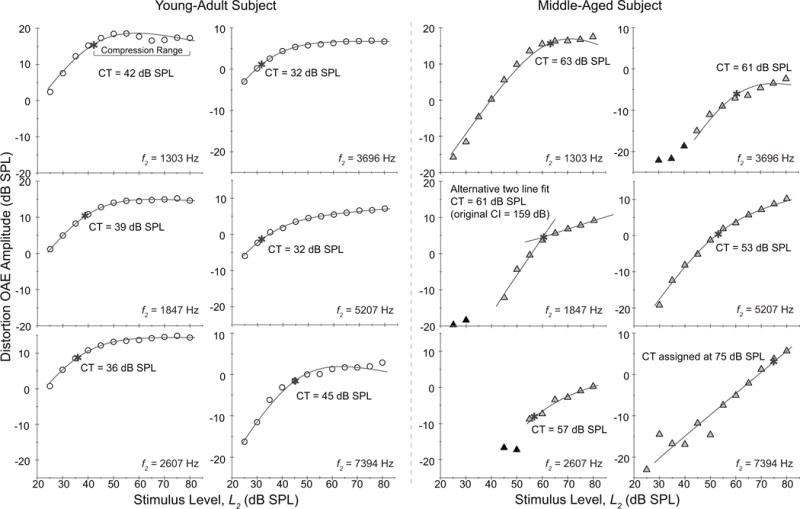
Distortion OAE I/O functions at 6 f2 frequencies for a 20 year-old (circle) and a 49-year-old (triangle) subject with matched audiometric thresholds. Solid black symbols represent values that did not meet 6 dB SNR. A fit (black line) is superimposed on each function and the compression threshold is provided (also shown by an asterisk). The form of distortion-OAE compression differs between these two ears as further elucidated by group results in Fig. 4. The older adult shows more monotonic amplitude growth and elevated compression thresholds. Two alternative fits/values are also shown (f2 = 1847 Hz and 7394Hz, middle-aged subject) as detailed in the Methods.
A second alternative-fitting strategy was employed in middle-aged adults when the confidence interval for an estimated CT value was unequivocally unacceptable as exemplified in Fig. 3 for one middle-aged ear at f2 = 1847 Hz. In this example, the confidence interval associated with the CT estimate was 159 dB indicating an unreliable fit. The unacceptable confidence intervals typically occurred when the low-level linear growth was expansive (> 1) and the compression knee was abrupt. In these cases the I/O was fit with a two-line model to better characterize both the growing and saturated portions of the function and the compression threshold was taken as the stimulus level at the intersection of the two lines. This alternative two-line fit was triggered for four I/O functions.
The third alternative fit occurred in six middle-aged I/O functions when OAE amplitudes were low and the DPOAE threshold exceeded 50 dB SPL. These functions provided a limited number of points for the fit. Figure 4 at f2 = 1303 Hz and f2 = 5207 in the middle-aged group shows two individual functions (thick black lines) with this abbreviated form. Because these low-level emissions are immediately compressed, the compression threshold was assigned as the individual DPOAE threshold plus 5 dB. These three strategies allowed us to extract approximate estimates of the compression threshold in almost all functions with sufficient SNR.
Figure 4.
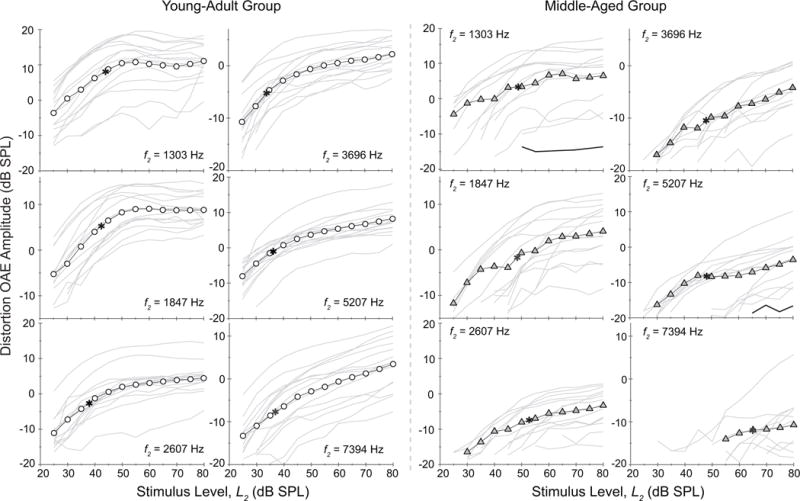
Mean young-adult (circle) and middle-aged (triangle) distortion OAE I/O functions at 6 f2 frequencies. Individual I/O functions are shown around the mean by thin gray lines. Means are provided only when at least 5 values were available. The young-adult group shows typical linear amplitude growth at low-levels and compressive growth at moderate-to-high levels for all frequencies. The middle-aged group shows a comparable pattern at low f2 frequencies but an extended range of monotonic growth and elevated compression thresholds (asterisk) at higher frequencies. Note: two black lines in panels f2 = 1303 and f2 = 5207 for the middle-aged group provide examples of alternative fits/values as explained in the Methods section.
Statistical Analysis
Group Tests
Subjects were divided into two discrete groups for difference tests: young-adult and middle-aged. Age (2) by frequency (5) repeated measures mixed-model analyses of variance (ANOVA) (SPSS ver. 22.0) were applied to assess group and frequency differences for each of the four compression metrics: compression threshold (CT), low-level growth, compression range, and compression slope. Five of the six test frequencies were included in each group ANOVA but too few I/O functions were available at f2 = 7394 Hz in the middle-aged group, and many had assigned CTs. However, data at 7394 Hz are still plotted in all figures. If an interaction between frequency and age was observed, post-hoc pair-wise age comparisons were performed at each frequency, applying a Bonferroni correction factor to hold the experiment-wise error rate constant at alpha = 0.05.
Correlations
Correlational analyses were conducted between age and compression threshold at each f2 frequency separately. Pearson Product moment correlations were conducted: (1) between age and compression threshold and (2) between and an average of audiometric thresholds at 0.5, 1 and 2 kHz and compression threshold. Following these simple correlations, a forced-entry step-wise regression analysis was conducted to separate the effects of age and audiometric threshold on the compression threshold. In this regression analysis, hearing was defined by two PTAs derived from the audiogram: the standard PTA described above and a high-frequency PTA of audiometric thresholds at 2, 4, and 8 kHz. These PTAs were entered into the regression model individually as the first step in determining the association between hearing and compression threshold with age unspecified. Age was subsequently entered into the model as the second variable to determine its relationship to compression threshold independent of hearing.
RESULTS
General Observations
The middle-aged group had reduced-amplitude OAEs at all stimulus levels and frequencies compared to the young-adult group. Fig. 2A displays mean distortion OAE spectra for both age groups at one of these stimulus levels (L2 = 60 dB SPL); data from individual ears are shown in light-gray and dashed lines. At this stimulus level, the older group had distortion OAE amplitudes reduced by 11.5 dB overall relative to the young-adult group. The noise floor, in contrast, was similar between groups as shown by the dashed lines toward the bottom of Fig. 2A (the mean group noise-floor levels were −28 and −26 dB SPL). Although not displayed in Fig. 2, the greatest distortion OAE amplitude reductions for the middle-aged group were noted at the lowest primary-tone levels and highest frequencies.
Figure 2B shows the amplitude of the isolated distortion OAE at L2 = 60 dB SPL calculated in young adults using two methods: (1) inverse FFT and (2) Least squares fitting (see Methods section). The overall mean difference in estimates of distortion OAE amplitude between these two methods was 0.5 dB and the standard deviations of the two means were within 0.6 dB of each other, indicating that either can be used to isolate the distortion component. Figure 2C shows the mean SNR for each group in third-octave bins as a function of stimulus level. One can see that although 6 dB was the minimum criteria for data acceptance, SNRs were generally much higher than this for both groups. Mean SNR across frequency and level was 28 dB for the young-adult group and 22 dB for middle-aged group.
Distortion OAE Input-Output Functions
Figure 3 displays individual input/output (I/O) functions at each test frequency for one representative young-adult and middle-aged subject who had matched audiometric thresholds (±5 dB). The young-adult subject (open circle) shows compression thresholds (CT, denoted by asterisk) ranging from 32 to 45 dB SPL across frequency, linear growth below CT (mean = 1.02 dB/dB), and highly compressed growth (mean = −0.05 dB/dB) at levels beyond the CT for all frequencies. The middle-aged subject (closed triangle) showed elevated compression thresholds (53 to 75 dB SPL), which produced a reduced compression range. At levels above the compression threshold, the mean growth was 0.27 dB/dB, suggesting continued, though slowed, amplitude growth. The low-level slope was approximately linear. The middle-aged ear also provides an example of two I/O functions requiring alternative fits and/or assigned values: 1847 and 7394 Hz (See Methods section, Alternative Fits). While Fig. 3 displays data from only two subjects, their data are representative of group results.
Age-Group Comparisons
Figure 4 shows the group mean distortion OAE I/O functions with individual subject data plotted (gray thin lines) around the mean at each test frequency. Because middle-aged subjects showed elevated DPOAE thresholds (i.e., the lowest stimulus level at which a DPOAE was measured above the noise), some of the group functions are abbreviated at the low-level end. DPOAE threshold was 26 dB SPL overall in the young-adult group versus 40 dB SPL in the middle-aged group. In the older adults, DPOAE threshold was most elevated for the mid-to-high-frequency functions. The data in Fig. 4 also suggest that at most frequencies the marked amplitude plateau associated with compression, if present, was elevated in the middle-aged group. (Group statistics shown in Fig. 5 confirm this anecdotal observation about age differences in the compression threshold). Also, the rate of growth for distortion OAE amplitude is more rapid in the middle-aged subjects. The exception is noted at 7394 Hz where the young adult amplitudes also grow throughout out the function and a compression knee is less evident.
Figure 5.
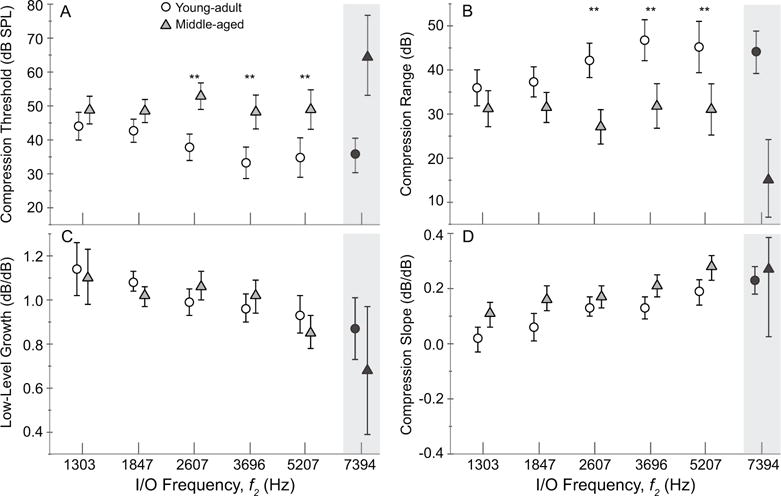
Mean (A) compression threshold, (B) compression range, (C) low-level growth and (D) compression slope are displayed for each age group (young adult = circles; middle-aged = triangles) as a function of test frequency. Error bars represent 95% confidence intervals. The middle-aged group exhibits significantly elevated compression thresholds and shortened compression ranges at all but the two lowest frequencies (top two panels). The compression slope was significantly steeper in the middle-aged group as well. There was no age effect on the approximately linear growth shown at lower-levels. Note: ANOVAs did not include data at 7394 Hz due to reduced observations as detailed in Methods section.
Age differences in compression threshold (CT) become more apparent at higher frequencies. The mean compression features derived for each group are shown in Fig. 5. Results of repeated measures mixed-model ANOVA confirmed an age effect on CT, indicating that compression was elevated in the older group (F = 62.6, p < 0.001). There was no main effect of frequency on CT but there was an interaction between age and frequency (F = 3.4, p = 0.017). Post hoc paired comparisons showed that compression thresholds were significantly higher in the middle-aged group at all but the lowest two frequencies (p < 0.001 for all significant comparisons). Furthermore, the young-adult group showed decreasing CT with increasing frequency (F = 6.23, p < 0.001), which is consistent with previous descriptions of the typical DPOAE I/O function (Gorga et al. 2007), while the middle-aged group did not show a significant frequency effect on the compression threshold. This result might be influenced by the exclusion of data at f2 = 7394 Hz from the ANOVA.
The compression range shown in Fig. 5B likewise showed an age effect (F = 62.6, p < 0.001), indicating that the stimulus range over which compression occurred was reduced in the older group. As noted in Fig. 5C, the slope of the low-level segment of the function below the CT was approximately linear for both age groups and not significantly different between them; however, there was a significant frequency effect indicating steeper low-level growth at lower f2 frequencies for both groups (F = 8.1, p < 0.001). Once the DPOAE amplitude began to saturate at stimulus levels above CT, mean compressive growth (shown in Fig. 5D) was steeper for the middle-aged group, ranging from 0.11 to 0.28 dB/dB across frequencies (F = 38.1, p < 0.001). For young adults, mean compression slope ranged from 0.02 to 0.19 dB/dB. This trend is also evident in the mean I/O functions shown in Fig. 4.
The Effect of Audiometric Threshold
We conducted Pearson product moment correlations to further understand the associations between age and distortion OAE compression features. A simple linear correlation between age and compression threshold was significant at all frequencies except 1303 Hz, accounting for as much as 69% of the variance at 7394 Hz. Figure 6A shows one such correlation for f2 = 3696 Hz. However, correlations between audiometric hearing (as indicated by a pure tone average [PTA] of thresholds at 0.5, 1 and 2kHz) and CT were also significant; one such correlation is shown in Fig. 6B for f2 = 3696 Hz). This is not surprising since middle-aged adults had audiometric thresholds elevated (re: young adults) by 9.4 dB on average for frequencies > 2 kHz even if they were in the “normal” range. Clearly, hearing status can be a confounding variable in describing the effect of age on distortion OAE compression.
Figure 6.
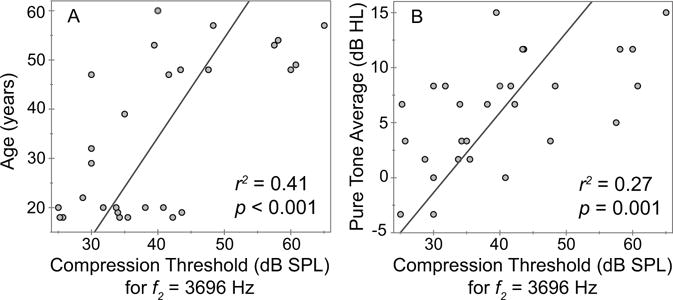
Correlations between the distortion OAE compression threshold at one I/O frequency (3696 Hz) and two variables: (A) age and (B) the average of audiometric thresholds at 0.5, 1 and 2 kHz. Correlations were conducted at all six test frequencies although only one example is displayed here. The increase of both age and audiometric thresholds are strongly related to elevated compression threshold at 3696 Hz and all others (not shown), confirming that these simple correlations cannot define the overlapping influence of these two predictors—age and hearing—on variance of the distortion OAE compression threshold.
A forced-entry stepwise regression allowed us to parse the variance in DPOAE compression threshold accounted for by age and audiometric threshold separately. Using the conventional PTA to represent audiometric status, results confirmed that age was an independent predictor of DPOAE compression threshold at 2607 Hz, 3696 Hz, 5207 Hz and 7394 Hz, accounting for between 14–40% of the total variance. These correlations between age and compression threshold, with the influence of audiometric PTA controlled, are shown in Fig. 7A–D. They indicate that a portion of the variance in CT, unexplainable by the variations in hearing among subjects, can be accounted for by age.
Figure 7.
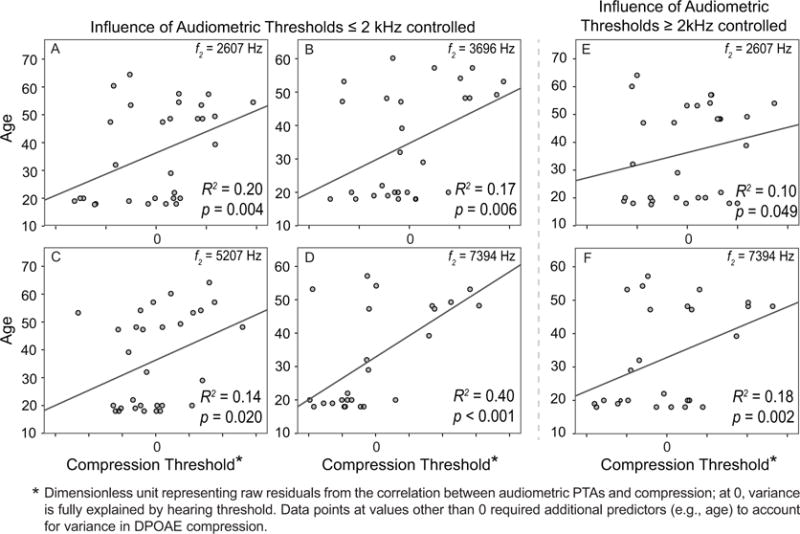
Regression analyses were applied to parse the effects of age and audiometric status on distortion OAE compression threshold. (A-D) Significant correlations between age and compression threshold were present at four f2 frequencies after the variance in audiometric thresholds (defined as a pure tone average, or PTA, of 0.5, 1 and 2 kHz) was controlled; the older the subject, the higher the compression threshold. However, this low-mid-frequency PTA does not capture the audiogram differences between younger and middle-aged ears; hence, a high-frequency audiometric PTA of 2, 4, and 8 kHz was applied and the regression repeated. (E, F) Significant correlations remained at two frequencies (f2 = 2607 and 7394 Hz) after controlling for the variance accounted for by differences in high-frequency audiometric thresholds.
However, the audiometric differences between young and middle-aged groups are confined to frequencies > 2–3 kHz. Therefore, to consider hearing status as a contributor an influence on OAE compression threshold, it is more appropriate to calculate an audiometric threshold average that includes high frequencies. We conducted a second forced-entry stepwise regression using a high-frequency PTA (average of audiometric thresholds at 2, 4 and 8 kHz) as a metric of hearing. Results show that age was still an independent predictor of DPOAE compression threshold at both f2 = 2607 Hz and 7394 Hz, accounting for an additional 10% and 18% of the total variance respectively. Age was no longer a predictor of distortion OAE compression threshold at f2 = 3696 Hz and 5207 Hz (R2 = 0.02, p = 0.28; R2 =0.01, p = 0.97 respectively). The significant correlations at 2607 Hz and 7394 Hz are likely strongly influenced by a cluster of 3–5 middle-aged ears (in the upper right quadrant of the graph) showing elevated compression thresholds, apparently unrelated to hearing their status. Figure 7E–F displays these significant correlations between age and distortion OAE CT with the influence of high-frequency audiometric threshold controlled.
DISCUSSION
In this study, we examined whether the human cochlea shows reduced compressive nonlinearity with aging. Indicators of nonlinearity can take various forms. The presence of a distortion emission itself is a manifestation of nonlinearity because it represents energy not present in the input stimulus. The subjects tested had measureable DPOAEs for most of the test conditions; therefore, by their inclusion in this study, all subjects showed indications of cochlear nonlinearities. However, these indicators of nonlinearity i.e., DPOAEs, showed reduced amplitude in middle-aged adults, especially at lower stimulus levels and higher f2 frequencies are consistent with past work (Lonsbury-Martin et al. 1991; Dorn et al. 1998; Cilento et al. 2001; Gates et al. 2002; Uchida et al. 2008).
Compressive response growth is another related manifestation of nonlinear cochlear behavior. The biophysical origin of cochlear compression and combination tones (as well as all other peripheral nonlinear behavior such as two-tone suppression) is likely the outer hair cell transduction process (e.g., Hudspeth & Corey 1977). The transduction current through gating channels on sensory cell stereocilia saturates once all of the channels are mechanically forced open at moderate-high stimulus levels. This saturation contributes to the array of nonlinearities we see in the cochlea, including compressive growth (Liberman et al. 2004; Verpy et al., 2008).
The DPOAE I/O as a Metric of Compressive Nonlinearity
Distortion OAE I/O functions do not provide a direct gauge of basilar membrane compressive nonlinearity. However, while an indirect and imperfect metric, they accurately mimic the pattern seen in basilar membrane compressive growth, most notably near the peak of the traveling wave (Sellick et al. 1982; Cooper & Rhode 1992; Ruggero et al. 1997), and of input/output functions of inner and outer hair cells (Russell & Sellick 1978; Cody & Russell 1987). These similarities notwithstanding, DPOAE compression is largely dependent on methodological factors such as primary-tone level separation, which determines the overlap between the traveling waves where the distortion is generated. This overlap influences the magnitude and phase of the DPOAE; therefore, any changes across level will shape the I/O function. By using an optimal L1–L2 separation (L1 = 0.4 L2 +39 dB SPL; Kummer et al., 1998), we attempted to maintain a consistent maximal overlap. Some work has shown that this Scissors method yields the most consistent overlap and generation of distortion across level as evidenced by minimal shifts in DPOAE fine structure (Long et al., 2009). Additionally, we applied an efficient and rapid swept-tone stimulus presentation that allows for extensive averaging to achieve excellent SNR as noted in Fig. 2C.
Another strength of our I/O protocol is that we used the separated distortion component of the DPOAE only. The DPOAE consists of two components: nonlinear distortion and a typically smaller reflection component (Shera & Guinan 1999; Talmadge et al. 1999; Knight & Kemp 2000, 2001). To study the distortion component of the total DPOAE, the composite response must be “unmixed”. Isolating the distortion component decreases the response variability across frequencies and eliminates reflection energy, which has different input/output characteristics than distortion (Mauermann & Kollmeier 2004; Dalhoff et al. 2013; Moore et al. 2014; Ortmann & Abdala 2015; Abdala & Kalluri 2015). Even with these attempts to minimize spurious effects, remotely measured acoustic emissions provide only a partial glimpse of cochlear compressive nonlinearity. This glimpse shows similarity in form to cochlear compression and significant links to perceptual indices of nonlinearity, making it a viable tool for our purposes (Ruggero et al. 1997; Buus et al. 2001; Neely et al. 2003; Johannesen & Lopez-Poveda 2008, 2010; Rasetshwane et al. 2013; Moore et al. 2014).
Aging of Compressive Nonlinearity
The compressive features of the distortion OAE I/O functions recorded from our young-adult group were generally similar to those reported in the literature (e.g., Abdala 2000; Dorn et al. 2001; Neely et al. 2003, 2009). Even using different stimulus parameters, fitting models, analysis strategies, and age ranges, these studies report median DPOAE compression thresholds of ~45 dB SPL in adults and compressive growth ranging between 0.1 and 0.3 dB/dB, which is consistent with our data.
When we compared young-adult OAE I/O functions to those of middle-aged subjects, there were significant differences. Although OAE amplitude grew linearly at low levels for both age groups, it reached its compression knee at stimulus levels that were 19 dB higher on average for frequencies above 2 kHz in the middle-aged group, which suggests an extended range of monotonic growth. Also, once reaching this compression threshold, the continued growth of OAE amplitude, though compressed, was steeper in the middle-aged ear with an average slope of 0.21 dB/dB compared to 0.13 dB/dB in young-adult ears.
The fit used to extract the compression threshold from each OAE I/O function was modeled on the normal response growth of a healthy cochlea which includes two basic segments: linear growth at low-levels and a saturated region with a transition between them. This fit characterized the distortion OAE growth well in young-adult ears, as we would expect given its origin as a metric of typical cochlear growth. However, it was less effective in characterizing the amplitude growth of the distortion OAE in some middle-aged ears: one-quarter of the functions generated by these older individuals (20 of 90 functions involving 13 of the 15 subjects) showed unacceptable estimates of compression threshold, either because the estimate was well below the range of stimulus levels presented, the confidence intervals were unreasonably large, or the function was abbreviated due to elevated DPOAE thresholds. This observation suggests that DPOAE compression in some middle-aged subjects is of a different form than the compression seen in young-adult ears. By association, it suggests that the compressive nonlinearity of the cochlea may be degraded during early aging.
It is noteworthy, however, that the expected compressive response pattern was indeed intact for the majority of functions calculated from this middle-aged group. We tested a generally young and healthy middle-aged cohort including one 39-year-old and subjects mostly in their 40s and early 50s (only one subject was 64 years old). Hence, it is not surprising that the group shows a grossly normal pattern of OAE growth (even though indices of compression derived from these normal-appearing functions differed between age groups). As we begin to test more elderly subjects, the deviation from this normal pattern of response growth will likely become more marked, resulting in a greater percentage of ears with clearly atypical patterns of DPOAE compression.
Targeting Specific Cochlear Properties
In this study, we were not interested in the accretion of one more result elucidating reduced OAEs with age and/or aging-related audiometric threshold variation. Our goal was to target and investigate a specific cochlear property, compressive nonlinearity, to assess whether it becomes dysfunctional in early-aging ears. Hence, we have gone from a general observation of reduced distortion OAE amplitude with aging to a specific cochlear deficit in early aging ears. This more targeted result may help elucidate the mechanisms underlying perceptual difficulties in older listeners. As the deficits that contribute to presbycusis are defined, it may be possible to more effectively guide intervention and hearing aid fittings in this age group rather than rely on a one-size-fits-all approach. If the loss of compressive nonlinearity is one of the deficits among the constellation of aging effects on the auditory system, it might explain a reduced dynamic range, for example. This would impact one’s ability to encode and tolerate a vast range of intensities, possibly impairing perceptions of loudness. The relationship between compressive features of the DPOAE I/O function and loudness scaling has in fact been shown in normal and hearing-impaired ears (Rasetshwane et al. 2013). In general, ears with hearing loss show steeper growth of loudness so that moderately loud sounds (as rated by normal hearers) are considered very loud for those with elevated DPOAE compression and monotonic amplitude growth. In future studies, we will explore the links between loudness scaling and aging-related changes in DPOAE compression.
Audiometric Thresholds
There is a persistent confound in research investigating the effects of aging on various auditory tasks and measures: age-related elevation of audiometric thresholds, even if they are within what is considered a clinically normal range. As reviewed in the Introduction, there are studies in the literature that have landed on both ends of this question, some determining that audiometric status can account for noted OAE changes in amplitude (Lonsbury-Martin et al. 1991; Prieve & Falter 1995; Oeken et al. 2000); and others concluding that some intrinsic feature of aging, apart from threshold elevation, is at play (Dorn et al. 1998; Uchida et al. 2008).
In this study, we tested an early aging group. As such, the majority of these subjects (11 of 15) had audiometric thresholds ≤ 20 dB HL. Four exhibited slight high-frequency threshold elevation, producing a significant difference between the high-frequency threshold averages of young-adult and middle-aged groups. Can this threshold elevation, (averaging 9.4 dB from 2–8 kHz) explain the atypical compression features derived from the DPOAE I/O functions of the middle-aged adults, which can be interpreted as weakened nonlinearity in the early-aging cochlea? After variance attributed to high-frequency hearing was accounted for using statistical methods, correlations between age and I/O compression threshold were significant at two frequencies, 2607 and 7394 Hz, accounting for up to 18% of additional variance. This suggests that age predicts distortion OAE compression threshold (and by association, cochlear compressive nonlinearity) independent of audiometric threshold differences: the older the subject, the more elevated the distortion OAE compression threshold.
Only two of six f2 frequencies showed this predictive effect of age on compression threshold. However, as previously mentioned, we studied a group representative of early aging with a mean of 52 years. The effect of aging on distortion OAE compression in this cohort has likely not peaked. A stronger effect across a broader frequency range might be observed with subjects who are 20 years older than those tested here. Yet even in this early aging group, cochlear nonlinearities such as combination tones (distortion OAEs) and compressive nonlinearities (as gauged by level-dependent growth) are weakening. Changes in the audiogram do not appear sufficient to fully explain these shifts. Changes in outer hair cell health, a diminishing population of viable OHCs, and/or a reduction of the endocochlear potential, which provides the drive for OHC-based amplification, may contribute to this outcome (See Schmiedt, 2010 for review).
Middle-Ear Function and Aging
In considering the potential sources of age effects on distortion OAE compression, one cannot rule out age-related changes in the conductive pathway as a contributor. However, a strong influence of the middle ear on these results is not likely. All subjects had normal tympanograms recorded with a 226 Hz probe tone and normal tympanic membrane by otoscopic examination. Yet studies of conventional tympanometric and immittance measures may not be sufficiently sensitive to detect age effects if present (e.g., Holte 1991; Wiley et al. 1999; Uchida et al. 2000).
Using more sensitive measures of middle-ear function, Feeney and Sanford (2004) applied wideband ear canal reflectance to aging ears and report decreasing stiffness in the middle ear of those over 60 years of age. Studies with inbred strains of mice have also shown changes in middle-ear sensitivity with aging as measured by umbo velocity but the shifts were small (Rosowski et al. 2003). In the present study all but one subject was < 60 years old and the group had a mean age of 52 years; therefore, if aging effects on middle-ear function were present, they are likely slight.
Future Studies and Conclusions
In future work, we will extend the age range studied several decades to view changes in compressive nonlinearity as a continuum of aging. Additionally, we are currently augmenting our number of subjects; a large sample size will allow us to use statistics more effectively for the purpose of disentangling the competing effects of age and hearing deterioration on distortion OAE compression. Although matching each audiometric threshold between young-adult and elderly ears (to effectively eliminate the impact of audiogram variability) is not feasible, we will implement matching of this type for a subset of young and older subjects within our larger sample. Finally, we are conducting studies to link the distortion OAE I/O function as an objective measure of compressive nonlinearity to perceptual measures such as loudness scaling to better understand these relationships during aging and to possibly develop objective tests to guide patient counseling and the fitting of hearing aids.
Acknowledgments
This work was supported by the National Institutes of Health, grants T32 DC009975 and R01 DC003552, and by the University of Southern California. We would like to thank Danielle Fregoni for data collection and analysis, Dr. Laurel Fisher for statistical assistance and Ping Luo for his many technical contributions to this project. We also thank Drs. Christopher Shera and Heather Porter for helpful comments on an earlier version of this manuscript.
Footnotes
Conflicts of Interest: There are no conflicts of interest.
References
- Abdala C. Distortion product otoacoustic emission (2f1–f2) amplitude growth in human adults and neonates. J Acoust Soc Am. 2000;107:446–456. doi: 10.1121/1.428315. [DOI] [PubMed] [Google Scholar]
- Abdala C, Dhar S. Distortion product otoacoustic emission phase and component analysis in human newborns. J Acoust Soc Am. 2010;127:316–325. doi: 10.1121/1.3268611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala C, Dhar S. Maturation and aging of the human cochlea: A view through the DPOAE looking glass. J Assoc Res Otolaryngol. 2012;13:403–421. doi: 10.1007/s10162-012-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala C, Kalluri R. Exploiting dual otoacoustic emission sources. In: Corey DP, Karavitaki KD, editors. Mechanics of Hearing. American Institute of Physics; Melville, NY: 2016. In press. [Google Scholar]
- Abdala C, Luo P, Shera CA. Optimizing Swept-tone Protocols for Recording Distortion Product Otoacoustic Emissions in Adults and Newborns. J Acoust Soc Am. 2015;138:3785–3799. doi: 10.1121/1.4937611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boege P, Janssen T. Pure-tone threshold estimation from extrapolated distortion product otoacoustic emissions I/O-functions in normal and cochlear hearing loss ears. J Acoust Soc Am. 2002;111:1810–1818. doi: 10.1121/1.1460923. [DOI] [PubMed] [Google Scholar]
- Buus S, Obeling L, Florentine M. Can basilar membrane compression characteristics be determined from distortion-product otoacoustic emissions input-output functions in humans. In: Breebart DJ, Houtsma AJM, Kohlrausch A, Prijs VF, Schoonhoven R, editors. Physiological and Psychophysical Bases of Auditory function; Proceedings of the 12th International Symposium on Hearing; Mierlo, Netherlands. 4–9 August 2000; The Netherlands: Shaker; 2001. pp. 373–381. [Google Scholar]
- Cilento BW, Norton SJ, Gates GA. The effects of aging and hearing loss on distortion product otoacoustic emissions. Otolaryngol Head Neck Surg. 2003;129:382–9. doi: 10.1016/S0194-59980300637-5. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol. 1987;383:551–69. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NP, Rhode WS. Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: sharp tuning and nonlinearity in the absence of baseline position shifts. Hear Res. 1992;63:163–190. doi: 10.1016/0378-5955(92)90083-y. [DOI] [PubMed] [Google Scholar]
- Dalhoff E, Turcanu D, Vetešník A, et al. Two-source interference as the major reason for auditory-threshold estimation error based on DPOAE input- output functions in normal-hearing subjects. Hear Res. 2013;296:67–82. doi: 10.1016/j.heares.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Dorn PA, Konrad-Martin D, Neely ST, et al. Distortion product otoacoustic emission input/output functions in normal and impaired ears. J Acoust Soc Am. 2001;110:3119–3131. doi: 10.1121/1.1417524. [DOI] [PubMed] [Google Scholar]
- Dorn PA, Piskorski P, Keefe DH, et al. On the existence of an age/threshold/frequency interaction in distortion product otoacoustic emissions. J Acoust Soc Am. 1998;104:964–971. doi: 10.1121/1.423339. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA. Age effects in the human middle ear: wideband acoustical measures. J Acoust Soc Am. 2004;116:3546–3558. doi: 10.1121/1.1808221. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills DM, Nam B-H, et al. Effects of age on the distortion- product otoacoustic emission growth functions. Hear Res. 2002;163:53–60. doi: 10.1016/s0378-5955(01)00377-x. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman BM, et al. A comparison of transient- evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. J Acoust Soc Am. 1993;94:2639–2648. doi: 10.1121/1.407348. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dierking DM, et al. Low-frequency and high- frequency cochlear nonlinearity in humans. J Acoust Soc Am. 2007;122:1671–1680. doi: 10.1121/1.2751265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dorn PA, et al. Further efforts to predict pure-tone thresholds from distortion product otoacoustic emission input/output functions. J Acoust Soc Am. 2003;113:3275–3284. doi: 10.1121/1.1570433. [DOI] [PubMed] [Google Scholar]
- Holte L. Aging effects in multifrequency tympanometry. Ear Hear. 1996;17:12–18. doi: 10.1097/00003446-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Hoth S, Gudmundsdottir K, Plinkert P. Age dependence of otoacoustic emissions: the loss of amplitude is primarily caused by age-related hearing loss and not by aging alone. Eur Arch Otorhinolaryngol. 2010;267:679–690. doi: 10.1007/s00405-009-1106-5. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen PT, Lopez-Poveda EA. Cochlear nonlinearity in normal- hearing subjects as inferred psychophysically and from distortion product otoacoustic emissions. J Acoust Soc Am. 2008;124:2149–2163. doi: 10.1121/1.2968692. [DOI] [PubMed] [Google Scholar]
- Johannesen PT, Lopez-Poveda EA. Correspondence between behavioral and individually “optimized” otoacoustic emission estimates of human cochlear input/output curves. J Acoust Soc Am. 2010;124:2149–2163. doi: 10.1121/1.3377087. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am. 2007;121:2097–2110. doi: 10.1121/1.2435981. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Measuring stimulus-frequency otoacoustic emissions using swept tones. J Acoust Soc Am. 2013;134:356–368. doi: 10.1121/1.4807505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- Knight RD, Kemp DT. Indications of different distortion product otoacoustic emission mechanisms from a detailed f1,f2 area study. J Acoust Soc Am. 2000;107:457–473. doi: 10.1121/1.428351. [DOI] [PubMed] [Google Scholar]
- Knight RD, Kemp DT. Wave and place fixed DPOAE maps of the human ear. J Acoust Soc Am. 2001;109:1513–1525. doi: 10.1121/1.1354197. [DOI] [PubMed] [Google Scholar]
- Kummer P, Janssen T, Hulin P, et al. The level and growth behavior of the 2f1 – f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss. J Acoust Soc Am. 1998;103:3431–3444. doi: 10.1121/1.423054. [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, et al. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 2012;33:315–329. doi: 10.1097/AUD.0b013e31823d7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Zuo J, Guinan JJ., Jr Otoacoustic emissions without somatic motility: Can stererocilia mechanics drive the mammalian cochlea? J Acoust Soc Am. 2004;116:1649–1655. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G, Jeung C, Talmadge C. Dependence of distortion-product otoacoustic emission components on primary-level ratio. In: Cooper NP, Kemp DT, editors. Concepts and Challenges in the Biophysics of Hearing; Proceedings of the 10th International Workshop on the Mechanics of Hearing; Keele University, Staffordshire, UK. 27–31 July 2008; Singapore: World Scientific Press; 2009. pp. 203–208. [Google Scholar]
- Long GR, Talmadge CL, Lee J. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am. 2008;124:1613–1626. doi: 10.1121/1.2949505. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Cutler WM, Martin GK. Evidence for the influence of aging on distortion-product otoacoustic emissions in humans. J Acoust Soc Am. 1991;89:1749–1759. doi: 10.1121/1.401009. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Martin GK. The clinical utility of distortion-product otoacoustic emissions. Ear Hear. 1990;11:144–154. doi: 10.1097/00003446-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Mauermann M, Kollmeier B. Distortion product otoacoustic emission (DPOAE) input/output functions and the influence of the second DPOAE source. J Acoust Soc Am. 2004;116:2199–2212. doi: 10.1121/1.1791719. [DOI] [PubMed] [Google Scholar]
- Moore TM, Hood LJ, Hornsby BWY. Estimates of cochlear compression using distortion product otoacoustic emissions and growth of forward masking. Ear Hear. 2014;35:711–714. doi: 10.1097/AUD.0000000000000083. [DOI] [PubMed] [Google Scholar]
- Muller J, Janssen T. Similarity in loudness and distortion product otoacoustic emissions input/output functions: Implications for an objective hearing aid adjustment. J Acoust Soc Am. 2004;115:3081–3091. doi: 10.1121/1.1736292. [DOI] [PubMed] [Google Scholar]
- Neely ST, Gorga MP, Dorn PA. Cochlear compression estimates from measurements of distortion-product otoacoustic emissions. J Acoust Soc Am. 2003;114:1499–507. doi: 10.1121/1.1604122. [DOI] [PubMed] [Google Scholar]
- Neely ST, Johnson TA, Kopun JG, et al. Distortion-product otoacoustic emission input/output characteristics in normal-hearing and hearing-impaired human ears. J Acoust Soc Am. 2009;126:728–738. doi: 10.1121/1.3158859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeken J, Lenk A, Bootz F. Influence of age and presbycusis on DPOAE. Acta Otolaryngol. 2000;120:396–403. doi: 10.1080/000164800750000630. [DOI] [PubMed] [Google Scholar]
- Ortmann AJ, Abdala C. Cochlear nonlinearity and aging: preliminary results. Assoc Res Otolaryngol Abs. 2015:38. [Google Scholar]
- Prieve BA, Falter SR. COAEs and SSOAEs in adults with increased age. Ear Hear. 1995;16:521–8. doi: 10.1097/00003446-199510000-00009. [DOI] [PubMed] [Google Scholar]
- Rasetshwane DM, Neely ST, Kopun JG, et al. Relation of distortion- product otoacoustic emission input-out functions to loudness. J Acoust Soc Am. 2013;134:369–383. doi: 10.1121/1.4807560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Brinsko KM, Tempel BI, et al. The aging of the middle ear in 129S6/SvEvTac and CBA/CaJ mice: measurements of umbo velocity, hearing function, and the incidence of pathology. J Assoc Res Otolaryngol. 2003;4:371–383. doi: 10.1007/s10162-002-3047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of Corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11:1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC, Recio A. The effect of intense acoustic stimulation on basilar-membrane vibrations. Auditory Neurosci. 1996;2:329–345. [Google Scholar]
- Ruggero MA, Rich NC, Recio A, et al. Basilar membrane responses to tones at the base of the chinchilla cochlea. J Acoust Soc Am. 1997;101:2151–2163. doi: 10.1121/1.418265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA. The physiology of cochlear presbycusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research. The Aging Auditory System. Vol. 34. New York, NY: Springer; 2010. pp. 9–38. [Google Scholar]
- Sellick PM, Patuzzi R, Johnstone BM. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982;72:131–41. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Stover LJ, Neely ST, Gorga MP. Latency and multiple sources of distortion product otoacoustic emissions. J Acoust Soc Am. 1996;99:1016–1024. doi: 10.1121/1.414630. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Tubis A, et al. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J Acoust Soc Am. 1999;105:275–92. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Thorson MJ, Kopun JG, Neely ST, et al. Reliability of distortion-product otoacoustic emissions and their relation to loudness. J Acoust Soc Am. 2012;131:1282–1295. doi: 10.1121/1.3672654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ando F, Shimokata H, et al. The effects of aging on distortion- product otoacoustic emissions in adults with normal hearing. Ear Hear. 2008;29:176–184. doi: 10.1097/aud.0b013e3181634eb8. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Nomura H, Itoh A, et al. The effects of age on hearing and middle ear function. J Epidemiol. 2000;10:S26–32. doi: 10.2188/jea.10.1sup_26. [DOI] [PubMed] [Google Scholar]
- Verpy E, Weil D, Leibovici M, et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;456:255–258. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley TL, Cruickshanks KJ, Nondahl DM, et al. Aging and middle ear resonance. J Am Acad Audiol. 1999;10:173–179. [PubMed] [Google Scholar]
- Williams EJ, Bacon SP. Compression estimates using behavioral and otoacoustic emission measures. Hear Res. 2005;201:44–54. doi: 10.1016/j.heares.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Yates GK, Winter IM, Robertson D. Basilar membrane nonlinearity determines auditory nerve rate-intensity functions and cochlear dynamic range. Hear Res. 1990;45:203–220. doi: 10.1016/0378-5955(90)90121-5. [DOI] [PubMed] [Google Scholar]


