Abstract
Vascular endothelial growth factor-A (VEGF) is one of the most important growth factors for regulation of vascular development and angiogenesis. Since bone is a highly vascularized organ and angiogenesis plays an important role in osteogenesis, VEGF also influences skeletal development and postnatal bone repair. Compromised bone repair and regeneration in many patients can be attributed to impaired blood supply; thus, modulation of VEGF levels in bones represents a potential strategy for treating compromised bone repair and improving bone regeneration. This review (i) summarizes the roles of VEGF at different stages of bone repair, including the phases of inflammation, endochondral ossification, intramembranous ossification during callus formation and bone remodeling; (ii) discusses different mechanisms underlying the effects of VEGF on osteoblast function, including paracrine, autocrine and intracrine signaling during bone repair; (iii) summarizes the role of VEGF in the bone regenerative procedure, distraction osteogenesis; and (iv) reviews evidence for the effects of VEGF in the context of repair and regeneration techniques involving the use of scaffolds, skeletal stem cells and growth factors.
Keywords: vascular endothelial growth factor; bone repair, bone regeneration; inflammation; endochondral ossification; membranous bone formation; bone remodeling; distraction osteogenesis
1. Introduction
Unlike many other organs, human bones heal in response to injury or surgical treatments. Bone repair is a process that utilizes endogenous regenerative potential to restore original bone structure without increasing bone volume. Although it shares certain similarities with bone repair, bone regeneration is a complicated, well-orchestrated process, usually involving external elements to promote formation of new mineralized tissues, and leads to an increase in bone volume [1]. Generally, bone repair is a rapid and efficient process. However, the process may be compromised in certain pathological situations. For example, about 10% patients with bone fracture have delayed union or nonunion and develop large unhealed bone defects [2, 3]. Certain bone regenerative procedures, such as bone grafting, reconstruction of large bone defects in the craniofacial region and distraction osteogenesis, sometimes fail in patients. Possible causes of such failure include, but are not limited to, impaired blood supply, damage to the periosteum, reduced number of osteoprogenitor cells as a result of age or osteoporosis, inadequate immobilization, and infection at the injury site [4]. The cellular mechanisms underlying such defective repair and impaired regeneration processes are not fully understood, making improvements of current therapies difficult.
Communication between blood vessels and bone cells ensures that the cells maintain physical proximity and functional codependency [5]. VEGF functions not only as one of the most important regulators of vascular development and angiogenesis [6, 7], it also plays critical roles in skeletal development [8-10]. Postnatal bone repair and regeneration recapitulate some steps of skeletal development [1, 11], such as intramembranous and endochondral ossification, but they also include additional features, such as recruitment of inflammatory cells and decreased numbers of mesenchymal stem cells [12, 13]. VEGF has been reported to participate in several stages of bone repair and regeneration; however, most studies have primarily examined the consequences for bone repair when VEGF levels were manipulated at local injury sites or systemically. Evidence related to the detailed mechanisms by which cells generate VEGF and how cells respond to VEGF has been lacking. Here we review VEGF-based strategies for improvement of bone repair and regeneration in the context of recent data on VEGF functions in bone.
2. Overview of VEGF
2.1. VEGF family, receptors and signaling
VEGF belongs to a family of homodimeric proteins consisting of at least 6 members: VEGF-A (VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E and Placental growth factor (PlGF) [14, 15]. VEGF, the most abundant form, plays important roles in proliferation, migration and activation of endothelial cells as well as in promotion of permeability and fenestration of blood vessels [15]. VEGF-C and -D are important for lymphangiogenesis [16, 17]; VEGF-B has a role in embryonic angiogenesis [18] and PlGF is a critical component of pathological angiogenesis [19].
Depending on alternative splicing, VEGF mRNA is translated into four major isoforms in humans, including VEGF121, VEGF165, VEGF189 and VEGF206 [20, 21], and three isoforms in mice, including VEGF120, VEGF164, and VEGF188 [22]. VEGF121/120 can diffuse freely, whereas VEGF189/188 and 206 are almost sequestered within the extracellular matrix (ECM). VEGF165/164, the most abundant VEGF isoform and the isoform that is usually used to explore VEGF functions in animal models of bone healing and in vitro experiments, is mostly bound to extracellular matrix (ECM) [23].
VEGF receptors include VEGFR1, VEGFR2, VEGFR3, Neuropilin1 (Npr1) and Neuropilin 2 (Npr2) [24]. VEGFR2 is the main VEGF signaling receptor and is mostly expressed in endothelial cells to mediate angiogenesis and vasculogenesis, as well as promotion of vessel permeability in response to VEGF [15]. The functions of VEGFR1, the firstly discovered VEGF receptor, are still being debated. In addition to binding to VEGF, VEGFR1 also binds to VEGF-B and PlGF [25, 26]. VEGFR1 is expressed as both soluble and membrane-bound forms, depending on alternative splicing. Decreased VEGFR1 expression in endothelial cells from infantile hemangioma tumors leads to constitutive VEGFR2 activation and abnormal angiogenesis, indicating that membranous-bound and soluble forms of VEGFR1 function as decoy receptors of VEGF [15, 27]. In contrast, other studies showed that VEGFR1 is capable of transducing a mitogenic signal in a similar way as VEGFR2 in certain circumstances [28]. For example, monocyte migration in response to VEGF depends on the tyrosine kinase domain of VEGFR1 [29]. VEGFR3 mainly binds to VEGF-C and VEGF-D in lymphatic endothelial cells, and plays an important role in regulation of lymphangiogenesis [30, 31]. Upon binding to ligands, VEGF receptors undergo dimerization. This results in phosphorylation of certain receptor tyrosine residues, and this mediates downstream mitogenic, chemotactic and pro-survival signals [15, 32].
2.2. Factors regulating VEGF and genes regulated by VEGF
VEGF is regulated by many factors, including growth and transcription factors, hormones and mechanical stimuli. Hypoxia is considered a major driver of VEGF expression, especially in tumor tissues and bones [33, 34]. The transcription factor, hypoxia induced factor-1 (HIF-1α), is greatly up-regulated under low oxygen tension in tumor cells or osteoblasts, and this promotes transcriptions of various angiogenic factors, including VEGF [35, 36]. Under normal aerobic conditions, HIF-1α is hydroxylated and targeted for proteasomal degradation by the von Hippel-Lindau (VHL) tumor suppressor [37]. Deletion of HIF-1α in osteoblasts causes reduction of VEGF expression, leading to interruption of both angiogenesis and osteogenesis, while deletion of VHL in osteoblasts increases both expression of HIF-1α and VEGF, leading to promotion of bone formation and angiogenesis [34]. In addition to HIF-1α, VEGF is also regulated by the transcription factor Osterix, expressed in osteoblastic lineage cells and a regulator of their differentiation [38]. Certain hormones, including estrogen and parathyroid hormone, regulate VEGF levels as well. VEGF plasma levels are decreased in women after menopause [39], and animal experiments demonstrate decreased VEGF levels in ovariectomized mice [40]. Several growth factors that play critical roles in bone development and postnatal bone repair also regulate VEGF expression, particularly in osteoblastic cells. These factors include, but are not limited to, members of the Transforming growth factor beta (TGF-β) superfamily, such as TGF-β1, TGF-β2, Bone morphogenetic protein (BMP) 2 (BMP2), BMP4 and BMP7 [41, 42], insulin-like growth factor (IGF) [43] and Fibroblast growth factor 2 (FGF2) [44]. Inflammatory factors, such as prostaglandin E1 and E2, interleukin-1 (IL-1), IL-6 and IL-8, which are increased during the inflammation phase of bone repair, also induce VEGF expression [45-47]. Mechanical strain is another regulator of VEGF expression. Under mechanical stress, osteoblasts release VEGF and this VEGF stimulates biological responses [48, 49]. All these VEGF regulatory factors play critical roles in bone development and homeostasis, suggesting that modulation of VEGF levels in osteoblasts may provide a basis for strategies aimed at controlling bone repair and regeneration.
VEGF signaling shares downstream signaling pathways with other growth factors, such as Epidermal growth factor (EGF) and Platelet-derived growth factor (PDGF). Therefore, profiles of genes that are regulated by VEGF signaling overlap with those of genes regulated by other growth factors, especially those of common downstream pathways of receptor tyrosine kinases, like RAS-Raf-ERK1/2 and PI3K-Akt. Currently, the list of genes that are specifically regulated by VEGF signaling is incomplete. Schweighofer et al. characterized the genes in Human umbilical vascular endothelial cells (HUVECs) induced by VEGF, EGF and IL-1, and found that Nuclear receptor related 1 protein (NURR1) and early growth response factor 3 (EGR-3) were selectively regulated by VEGF [50]. Other studies demonstrated that NURR1 and EGR-3 are essential mediators of VEGF-induced endothelial activation and angiogenesis [51, 52]. The profiles of genes that are regulated by VEGF signaling in other cell types, such as mesenchymal progenitor cells or osteoblasts, are not fully characterized.
3. Cellular mechanisms during bone repair and regeneration
Bone repair represents the endogenous healing capability of human bones. Facture healing, the most common form of bone repair, is characterized by several overlapping stages, namely, an inflammation phase, soft callus phase, cartilage turnover (replacement by bony callus) phase and bone remodeling phase [53]. After fracture or many other forms of bone injury, a hematoma is formed at the injury sites due to disruption of blood vessels. In the first couple of days, neutrophils are recruited to the hematoma and phagocytose tissue debris and microorganisms [54]. This is followed by influx of macrophages to remove the dead neutrophils, promote angiogenic responses and initiate the repair cascade [55]. With invasion of blood vessels, mesenchymal cells migrate to the injury site, particularly to the intramedullary region or fracture gap, where they proliferate and differentiate to either osteoblasts or chondrocytes [53]. Depending on the stability of fracture fixation and supply of blood vessels, endochondral or intramembranous ossification occurs at the facture sites. Instability or lack of blood supply may lead to endochondral bone formation during the repair, while a stable facture or fracture rich in vasculature facilitates intramembranous ossification [56]. In addition, a third type of ossification called ‘transchondroid bone formation’ has been proposed in a model of distraction osteogenesis. During transchondroid ossification, chondrocyte-like cells induced by mechanical strains form chondroid bone, which is gradually transformed to bone [57, 58]. In endochondral ossification, the cartilage is gradually resorbed, and replaced by bony callus consisting of woven bone. In both intramembranous and endochondral sites, the newly formed woven bone is gradually remodeled to lamellar bone. The human body can restore small bone defects and repairs bone fractures that are not severe, but it hardly heals large bone defects without intervention. Distraction osteogenesis is a good example of mechanical strain-induced bone regeneration procedures where mechanical forces elicit cellular responses. In addition, exogenous bone, either in the form of autograft, allograft, or xenograft, or bone forming cells can be transplanted into the injury sites when relatively large amounts of bone are needed.
3.1. The role of VEGF in the inflammation phase during bone repair
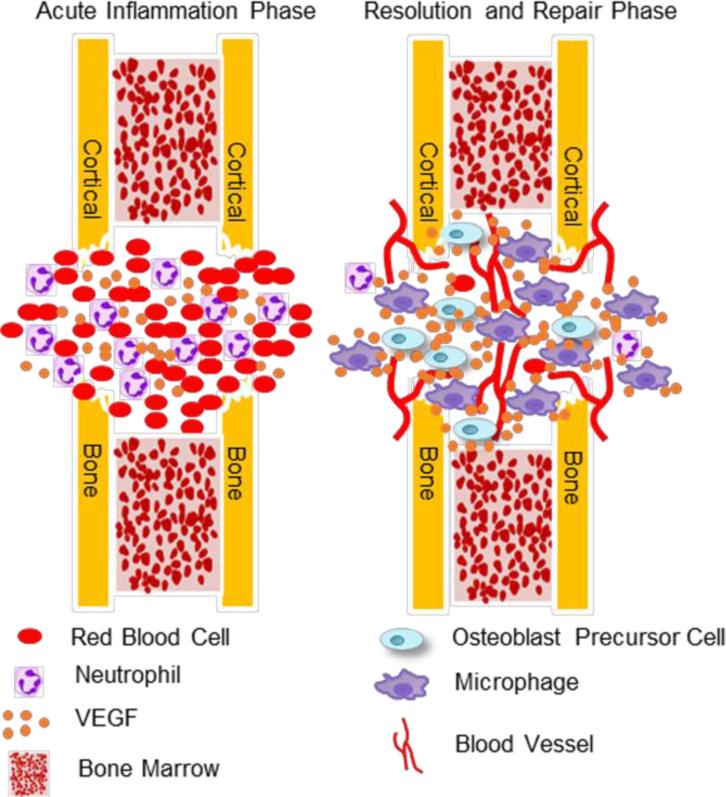
The expression and potential function of VEGF during inflammation phase is illustrated in Figure 1. The hematoma after bone trauma and the subsequent inflammatory responses initiate bone repair. VEGF plays a critical role in this process and is concentrated in the hematoma after bone injury. It has been reported that VEGF concentrations in hematoma can be 15-fold higher than in plasma, although VEGF levels in plasma were already increased significantly compared with healthy controls [59]. The hypoxia in the hematoma induces VEGF expression in surrounding bone cells or recruited inflammatory cells. VEGF released from fragmented bone matrix, platelets or infiltrated innate immune cells may bind to the heparin associated with fibrin(nogen), and be sequestrated in the fibrin matrix, making fibrin matrix in the hematoma a reservoir of VEGF [59-61]. Shortly after injury, the number of neutrophils in the peripheral circulation increases as neutrophil retention in the bone marrow is reduced by inflammatory stimuli [62]. Neutrophils are recruited to the hematoma and help remove bone debris and microbial pathogens. Osteoblastic cells regulates the release of neutrophils to the circulation through the chemokine ligand 12 (CXCL12)-chemokine receptor type 4 (CXCR4) axis [63]. VEGF has been reported to induce neutrophil chemotaxis and increase sinusoid permeability in the bone marrow [64, 65]. During acute inflammation induced by injuries in mice with Vegfa deleted from osteoblastic cells, the reduced release of neutrophils from bone marrow into the circulation suggests that osteoblast-generated VEGF facilitates the entry of neutrophils into the circulation in the acute inflammation stage [66]. Following neutrophil infiltration, macrophages and other inflammatory cells are recruited to the injury sites. This results in a release of cytokines, such as Tumor necrosis factor alpha (TNF-α), IL-1α, and IL-1β [67]. These cytokines activate endothelial cells and promote revascularization at the injury sites. They are also efficient inducers of VEGF expression in inflammatory and osteoblastic cells. VEGF is a chemotactic factor for macrophage/monocytes [29, 68]. Since macrophages release angiogenic factors [55, 69], VEGF may stimulate angiogenesis both indirectly via macrophages as well as directly by targeting endothelial cells. In the resolution phase of inflammation, macrophages phagocytose dead or aging neutrophils. This uptake of apoptotic neutrophils causes a switch of macrophage phenotype from an inflammatory (M1) to a reparative state (M2). This stimulates macrophages to release mediators, such as TGF-β1, which suppress the inflammatory response and initiate the repair process [70, 71].
Figure 1.
Expression and functions of VEGF in the inflammation phase during bone repair. During the acute inflammation phase, a short period of time after bone trauma, hematoma consisting of red blood cells and neutrophils is formed at the injury site. VEGF is concentrated in the hematoma after bone injury as the hypoxia in hematoma induces VEGF expression, and the fibrin matrix in hematoma may serve as a reservoir of VEGF. Shortly after injury, the number of neutrophils in the peripheral circulation increases as neutrophil retention in bone marrow is reduced by inflammatory stimuli, and circulating neutrophils are recruited into the hematoma to remove bone debris and microbial pathogens. VEGF also induces neutrophil chemotaxis and increases sinusoid permeability in bone marrow. Following neutrophil infiltration, macrophages and other inflammatory cells are recruited to the injury site. This results in a release of cytokines, such as TNF-α, IL-1α, and IL-1β, that activate endothelial cells and promote revascularization at the injury sites. In addition, they are efficient inducers of VEGF in inflammatory and osteoblastic cells. VEGF is a chemotactic factor for macrophages/monocytes. Considering that macrophages release angiogenic factors, the pro-angiogenic effect of VEGF may be mediated by macrophages in addition to direct targeting of endothelial cells. In the resolution phase of inflammation, macrophages are recruited to phagocytose dead or aging neutrophils. Subsequent uptake of apoptotic neutrophils causes a switch of macrophage phenotype from M1 to M2. This stimulates macrophages to release mediators, such as TGF-β1, which can suppress the inflammatory response and initiate the repair process.
3.2. The role of VEGF in endochondral bone ossification during bone repair
The mechanism of endochondral ossification during bone repair recapitulates the key process of endochondral ossification (EO) during development, including cartilage formation, vascular invasion, osteoclastogenesis and cartilage removal. During bone development, VEGF is generated by osteoblast precursors in the perichondrium and hypertrophic chondrocytes and this induces osteoblastic cells to migrate into the primary ossification center together with blood vessels, osteoclasts and hematopoietic stem cells [8, 9]. In addition to stimulating skeletal vascularization, VEGF is also a critical factor for epiphyseal chondrocyte survival [72].
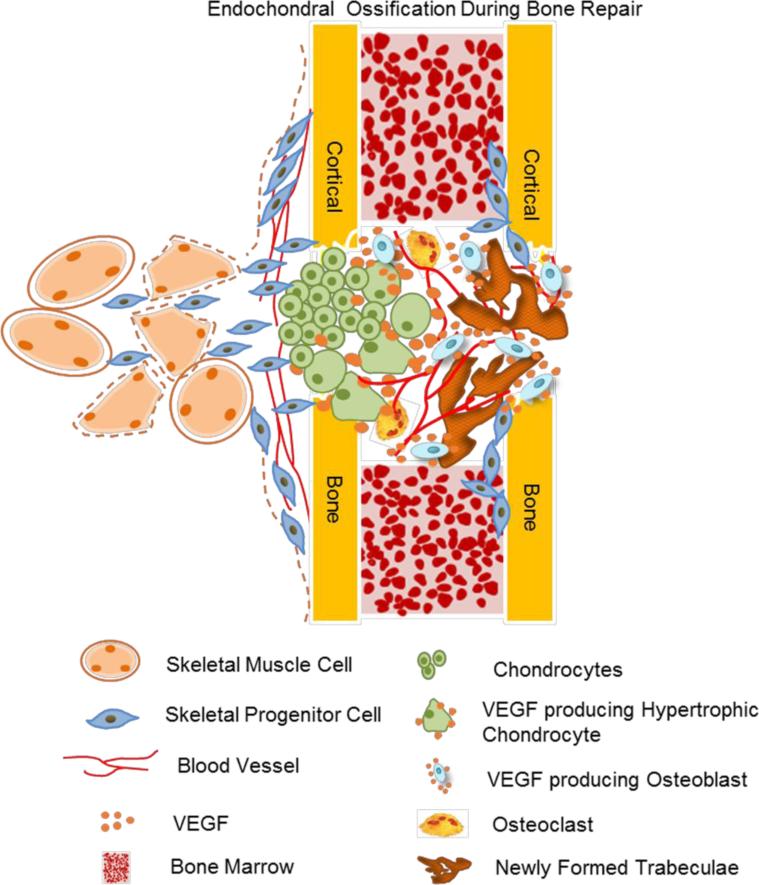
The roles of VEGF in EO during bone healing are illustrated in Figure 2. Cartilage formation is the early step. Large amounts of cartilage are observed in unstable bone fractures, and moderate amounts of cartilage are also observed in the injured periosteum of stable fractures. VEGF may regulate the differentiation of skeletal stem cells from bone marrow, periosteum and surrounding muscles into either chondrocytes or osteoblasts. Systemic VEGF blockade may promote chondrogenesis of explanted grafts containing fetal femur or skeletal stem cells at the expense of osteogenesis [73]. In addition, soluble Flt1 gene therapy improved BMP4-induced chondrogenic differentiation of muscle-derived stem cells (MDSCs) in vitro and promoted articular cartilage repair in vivo [74, 75]. This suggests that VEGF inhibition, in combination with BMP administration, may be a potent therapeutic strategy for regeneration of cartilage.
Figure 2.
The role of VEGF in endochondral ossification-mediated bone repair. VEGF could regulate the differentiation of skeletal progenitor cells from the bone marrow, periosteum and surrounding muscles into either chondrocytes or osteoblasts. Reduced VEGF in early skeletal progenitors facilitates differentiation chondrogenic cells. In endochondral ossification, chondrocytes in the cartilaginous template stop proliferating and matured to hypertrophic chondrocytes. Hypertrophic chondrocytes express Osterix (Osx), a strong inducer of VEGF expression, and produce high levels of VEGF. This VEGF stimulates vessel invasion and recruitment of chondroclasts into the hypertrophic cartilage, and facilitate the replacement of the cartilaginous template by bony callus. In addition, Osx-expressing osteoblast precursors produce high amounts of VEGF, and this VEGF stimulates their differentiation. These cells move into the developing trabecular bone region together with vascular endothelial cells and osteoclasts, where they become stromal cells, pericytes or osteoblasts.
During bone development, VEGF is a critical survival factor for epiphyseal chondrocytes. Maes et al. observed cell death in chondrocytes within epiphyseal regions of long bones in VEGF188/188 mice (only expressing the tightly extracellular matrix-bound VEGF 188 isoform) [76]. The effects of VEGF on chondrocyte survival appear to depend on the chondrocyte differentiation stage. In mice with Vegfa deleted in collagen type II (Col2)-positive chondrocytes, massive chondrocyte death was observed in the central avascular regions of epiphyseal cartilage [72]. In these mice, the detection of the VEGF receptors, Npr1 and Npr2, in epiphyseal chondrocytes and no obvious reduction of blood vessels in the surrounding perichondrium indicated that the cell death might involve a direct effect of VEGF on chondrocytes [72]. Interestingly, a similar cell death phenotype was observed in mice with deficiency of HIF-1α, the upstream transcription factor regulating a set of angiogenic genes including VEGF, in central regions of developing cartilage [77]. In addition, overexpression of VEGF partially rescued the death of chondrocytes in mice lacking HIF-1α, indicating that HIF-1α and VEGF are critical components in the support of chondrocyte survival[78]. In regions of hypertrophic chondrocytes where VEGF levels are greatly elevated, the high levels of VEGF contribute to the cell death in hypertrophic chondrocytes through indirect effects associated with stimulation of vascular invasion [79]. Systemic administration of soluble VEGFR1 (sFlt1) delays the death in hypertrophic chondrocytes. This results in increased numbers of hypertrophic chondrocytes and thus expansion of the growth plate [10]. In addition, inhibition of VEGF leads to reduced apoptosis of articular chondrocytes and improvement of articular cartilage in a mouse model of osteoarthritis [80], consistent with the idea that VEGF may be a detrimental factor in the progression of osteoarthritis.
In the later stages of endochondral ossification during bone development and repair, chondrocytes in the cartilaginous template stop proliferating, enlarge to hypertrophic chondrocytes, and synthesize collagen type X. Hypertrophic chondrocytes express Osterix, a strong inducer of VEGF expression [38], and produce high levels of VEGF [81, 82]. This stimulates vessel invasion and recruitment of chondroclasts into the hypertrophic cartilage [81, 83]. In a model of BMP4-induced ectopic ossification, VEGF enhanced angiogenic responses, cartilage resorption and its replacement by bone [84]. In a murine femoral fracture model, inhibition of VEGF signaling by Flt1, delayed cartilage turnover, disrupted conversion of soft cartilaginous callus to a hard bony callus, and impaired fracture healing [85]. These data are consistent with the delayed endochondral bone formation observed in VEGF120/120 mice (expressing only the VEGF120 isoform) and mice treated with sFlt1 during bone development [9, 10, 86].
Osteoclasts (chondroclasts) are multi-nucleated terminally differentiated cells derived from monocytes/macrophages. The differentiation and activation of osteoclasts are predominantly regulated by RANK (receptor activator of nuclear factor of NF-λB) ligand (RANKL) and the macrophage colony-stimulating factor (M-CSF) [87-89]. During development, hypertrophic chondrocyte-derived VEGF binds to VEGFR1 in monocytes to promote their migration and differentiation to osteoclasts [90, 91]. VEGF also binds to VEGFR2 in osteoclasts and promotes their survival through stimulation of PI3K/Akt signaling [92]. In vivo and in vitro evidence shows that VEGF can substitute for M-CSF to promote osteoclast differentiation and bone resorption [93, 94]. In addition, VEGF enhances the bone resorbing activity of osteoclasts [95]. Therefore, during endochondral ossification, VEGF from hypertrophic chondrocytes are critical for the invasion of osteoclasts into the cartilaginous template. The enhanced VEGF-dependent osteoclast function during bone development was also observed during postnatal bone repair, and thus the potential influence of osteoclast mediated bone resorption should be considered when using VEGF as a therapeutic strategy to promote bone repair and regeneration.
3.3. Coupling of angiogenesis and osteogenesis by VEGF
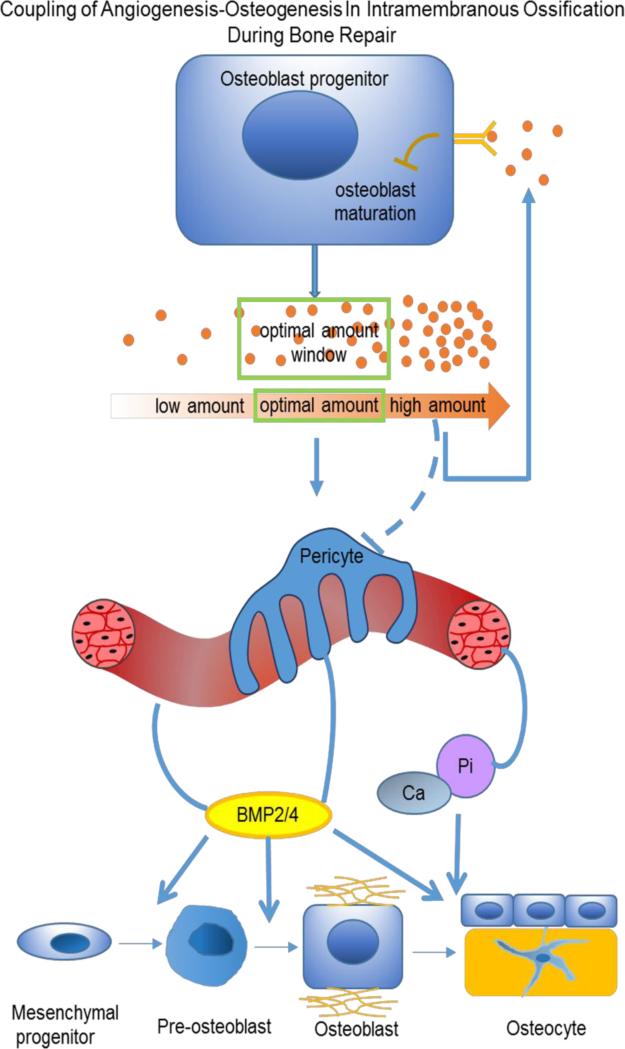
Unlike endochondral ossification, where blood vessels prevent the formation of cartilage, intramembranous bone formation relies on the coupling of angiogenesis and osteogenesis. VEGF has an essential role in this coupling, since both vascular and skeletal morphogenesis is VEGF-dependent (Figure 3). When exposed to hypoxia during inflammation, osteoblasts release factors including VEGF, via the HIF-1α pathway. This activates endothelial cells, and promotes vessel permeability [34]. The increased vasculogenesis and angiogenesis bring bone-forming progenitors as well as nutrition, oxygen and minerals necessary for mineralization. In addition, osteogenic factors, such as BMP2, released from blood vessels, promote osteoblast differentiation and mineralization [96]. In turn, maturing osteoblasts generate angiogenic factors, such as PDGF and VEGF, to further support angiogenesis.
Figure 3.
The role of VEGF in angiogenesis-osteogenesis coupling during intramembranous ossification-mediated bone repair. Intramembranous bone formation relies on coupling of angiogenesis and osteogenesis, and VEGF has an essential role in this process. Stimulated by hypoxia during inflammation, osteoblasts release factors, including VEGF via the HIF-1α pathway. VEGF can act through its receptors on endothelial cells to induce angiogenesis, thereby increasing the supply of oxygen and nutrients required for osteogenesis. Increased vascularization may also lead to influx of skeletal stem cells and/or (pre)osteoblasts and to elevated levels of endothelium-derived osteogenic growth factors, such as BMP2 or BMP4, and anabolic signals to further promote osteoblast differentiation and mineralization. Maturating osteoblasts also generate angiogenic factors, including PDGF and VEGF. During bone repair, physiological levels of VEGF are critical for the outcome. Too little VEGF may interrupt communication between bone and blood vessels, resulting in a compromised bone healing. Too much VEGF has detrimental effects on bone repair, likely due to inhibition of osteoblastic maturation and mineralization via stimulated VEGFR2 signaling, and/or caused by recruitment of osteoclasts that resorb the newly formed bone. (Modified from the supplemental Figure 14 in J Clin Invest. 2016;126(2):509-526.)
3.4. The role of VEGF in bone healing mediated by intramembranous ossification
Intramembranous bone formation during bone repair has been suggested to depend on VEGF signaling (Figure 3). Blocking extracellular VEGF by administering sFlt1 decreased blood vessel formation and bone regeneration in a tibial cortical bone defect model, while administration of exogenous VEGF increased formation of mineralized bone within the bone defects in the same model [85]. However, consequences of exogenous VEGF application in various models of bone repair vary to a certain extent. Exogenous VEGF enhanced bone healing and regeneration in several bone repair models in which endogenous VEGF levels were decreased [97, 98], but failed to improve bone repair in studies of ex vivo gene therapy or delivery of recombinant VEGF [84, 99, 100]. In contrast to repair of large bone defects, it is possible that only small amounts of VEGF are needed for healing of small bone defects. Exogenous VEGF may not help to regenerate bone when endogenous VEGF levels are adequate, and excess VEGF may even have detrimental effects on regeneration [66]. Too much VEGF may recruit excess numbers of osteoclasts, resulting in resorption of newly formed bone, since VEGF regulates the differentiation and migration of osteoclasts [95, 101]. In addition, excess VEGF inhibits the function of pericytes through VEGFR2 mediated inhibition of PDGFR [102], a receptor required for pericyte maturation, and this leads to formation of immature blood vessels and interruption of angiogenesis-osteogenesis coupling. A good example of this was the unexpected increase of mature vessels in tumors of patients treated with anti-VEGF therapy [103, 104], demonstrating the complexity of VEGF functions in therapeutic strategies to treat disorders in humans.
3.5. VEGF signaling in osteoblastic cells
Osteoblast-derived VEGF usually acts in a paracrine manner on adjacent endothelial cells via binding to VEGF receptors to regulate endothelial migration, proliferation and vessel permeability. In addition to endothelial cells, VEGF receptors are also expressed and functional in other cell types, including pericytes and osteoclasts [92, 102]. However, expression of VEGF receptors in osteoblasts is quite variable, particularly in mice, making paracrine/autocrine effects of osteoblast-derived VEGF on osteoblasts difficult to study. In murine osteoblastic MC3T3 cells, VEGF was shown to promote levels of alkaline phosphatase and osteocalcin [105]. However, other studies also showed that primary murine mesenchymal progenitors and osteoblasts failed to respond to exogenous VEGF [106]. Mice with deletion of Vegfr1 or Vegfr2 in osteoblastic cells exhibit reduced bone density two weeks after birth. In addition, reduced number of osteoprogenitor cells was observed in the bone marrow of these mice [106], indicating that both VEGFR1 and VEGFR2 in osteoblastic cells are important for postnatal bone formation. However, in a mouse cortical defect healing model, deletion of VEGFR2 in osteoblast precursors enhanced osteoblastic differentiation and mineralization during bone repair mainly through mechanisms of intramembranous ossification [66].
In addition to secreted VEGF, cells also generate intracellular (intracrine) VEGF, which is not secreted but participates in transcriptional regulation and cell survival. This phenomenon was first reported based on studies of VEGF signaling in the survival of hematopoietic and endothelial cells [107, 108]. In early osteoblast precursors, intracellular VEGF also plays a role in the control of osteoblast and adipocyte differentiation [106]. Recombinant VEGF can be used to restore extracellular VEGF levels but do not affect intracrine VEGF. If intracellular VEGF in osteoblasts is also important for osteoblast maturation and mineralization during bone repair, it may explain why exogenous VEGF failed to improve bone repair in some cases. Should this turn out to be the case, modified cell- permeable VEGF, fused to a nuclear localization signal, may enhance intracellular levels of VEGF and promote bone repair and regeneration when application of traditional recombinant VEGF fails.
3.6. The role of VEGF in bone remodeling
In the remodeling phase of bone repair, replacement of woven bone by lamellar bone requires coupling of osteoclast mediated bone resorption and osteoblast mediated bone formation. VEGF influences the function of both types of bone cells, and normal VEGF levels are necessary for maintaining normal bone remodeling. Several osteogenic factors, such as TGF-β1, IGF and PDGF-BB, are released from bone matrix or pre-osteoclasts during the process of bone resorption [109-111]. These factors induce neo-angiogenesis, which is required for angiogenesis and coordination of bone formation. VEGF binds VEGFR1 in osteoclasts and regulates their differentiation and activation. Therefore, reduction of VEGF in bone remodeling may decrease the angiogenic and osteogenic signals indirectly through inhibition of bone resorption. In a mouse femur facture model, inhibition of VEGF by sFlt1 caused reduced callus volume at various stages of repair, including bone remodeling, while treatment with exogenous VEGF increased vascularity and mineral density in the calcified callus [85]. These data indicate that recruitment of osteoclasts by VEGF may be necessary to maintain normal bone remodeling.
4. The role of VEGF in distraction osteogenesis
Unlike bone repair, bone regeneration is a process in which a relatively large amount of new bone is formed in response to external stimulators, such as mechanical force, exogenous bony tissues or bone forming cells [1]. Bone regeneration is widely used in treatment of non-union fractures, craniofacial reconstructions, segmental bone defects after tumor resection, augmentation of jaw bones, lengthening of long bone, promotion of implants, and bone grafting. Distraction osteogenesis involves the use of mechanical forces to induce bone formation and the addition of exogenous bony tissues helps form new bone in bone grafting. As bone grafting is very much like bone repair, we will here only discuss the role of VEGF in distraction osteogenesis.
Distraction osteogenesis (DO) is a surgical procedure used to treat limb length discrepancies, bone deformities and bone loss. It is widely used in orthopedics and oral and maxillofacial surgery. The process of DO involves slow and steady distraction of an osteotomy gap after a period of latency. VEGF expression during active distraction is primarily located to the maturing osteoblasts at the primary mineralization front [58], indicating that the main source of VEGF at distraction sites is the osteoblast lineage cells.
As a key regulator of angiogenesis-osteogenesis coupling, VEGF has multiple roles during DO. First, it is released from osteoblastic cells in response to mechanical forces and regulates osteoblast differentiation and mineralization by an autocrine mechanism [48, 112]. Second, VEGF from osteoblastic cells binds receptors on adjacent endothelial cells, enhancing neo-angiogenesis and promoting the release of osteogenic factors, such as BMP2 and BMP4, from blood vessels [113]. Disruption of VEGFR1 and VEGFR2 using neutralizing antibodies reduced the angiogenic response and bone regeneration in a mouse distraction osteogenesis model [114], highlighting the importance of VEGF signaling mediated angiogenesis. Third, in addition to the autocrine and paracrine effects, intracrine VEGF within osteoblastic cells may play a role in stimulating osteoblast differentiation and mineralization, since exogenous VEGF or antagonists failed to affect the differentiation of early osteoblastic lineage cells [106, 115],
5. Strategies to promote bone repair and regeneration
Skeletal stem cells, cytokines, growth factors, hormones, and bone matrix scaffolds are currently the components of strategies to stimulate tissue regeneration. As a grow factor, VEGF interacts with other factors, such as BMPs and PDGF, regulates stem cell functions, and exhibits various activities in different extracellular matrices or engineered scaffolds. Understanding the biology of VEGF from the perspective of its interactions with stem cells in the context of extracellular matrices, may be of help in developing novel techniques for modulating VEGF activity to promote bone regeneration.
Extracellular matrix or matrix-like engineered scaffolds can serve as reservoirs for VEGF. Different properties of different scaffolds influence the loading capacity, speed of release, and activities of VEGF. VEGF delivered to the distraction gap through a mini-osmotic pump in a rabbit distraction osteogenesis model failed to improve blood flow and bone mineral content [115]. In this case, no scaffold was used to trap VEGF at the injury site, and thus VEGF was possibly disseminated into adjacent tissues or metabolized within a relative short period of time after delivery. Similarly, by using the guided bone regeneration (GBR) technique to repair critical-sized defects in rats, Kaigler et al. found that a bolus of VEGF delivered to the defects without a scaffold failed to improve vasculogenesis and bone formation, while a bolus of VEGF loaded in hydrogels and released at a relatively slow rate, successfully enhanced both angiogenesis and osteogenesis [116]. These studies indicate that an optimal scaffold loaded with VEGF may significantly influence the outcome of bone repair. Loading of VEGF in a heparin cross-linked demineralized bone matrix improved vasculogenesis when implanted subcutaneously [117]. Porous strontium-doped calcium polyphosphate (SCPP) scaffolds have been proposed to promote the secretion of VEGF from human osteoblast-like MG63 cells. These scaffolds, together with mesenchymal stem cells (MSCs), improved bone regeneration in a rabbit segmental bony defect model [118]. The release kinetics of VEGF is also important for the effects of biomaterial on vascularization and bone formation. Several studies showed that sustained release of VEGF increased the efficiency of bone regeneration. Scaffolds with the capability to release VEGF slowly include biomimetic poly (lactide-co-glycolide) (PLGA) scaffolds, cylindrical chitosan sponges, a biomimetic bone matrix modified with heparin, and biphasic calcium phosphate (BCP) ceramics. These scaffolds provide prolonged bioavailability of VEGF.
Skeletal stem cells are heterogeneous cell populations that may be found in cancellous bone, perisinusoidal niche in bone marrow, endosteum, periosteum, and skeletal muscle. Under certain conditions, these cells can further differentiate into multiple cell types, such as osteoblasts, chondrocytes, adipocytes and myoblasts. VEGF from bone marrow mesenchymal progenitors is important for the lineage fate determination between osteoblasts and adipocytes. Reducing VEGF levels in these cells causes adipogenic differentiation at the expense of osteogenic differentiation [106]. Osteochondroprogenitors are present in the periosteum, located in the cambium layer, and can differentiate to osteoblasts or chondrocytes. VEGF levels in the periosteum control the lineage choice during periosteal responses after injury. Inhibition of VEGF by sFlt1 was found to enhance BMP2 induced cartilage formation [119], indicating that reduction of local VEGF levels may facilitate chondrogenesis. This is consistent with findings that inhibition of VEGF signaling by sFlt1 promoted chondrogenesis of transplanted fetal skeletal tissues at the expense of osteogenesis [73].
Many growth factors, in addition to VEGF, such as PDGF and BMPs, have been widely used in animal models of bone repair and regeneration. They share similar downstream signal transduction pathways and a better understanding of their interactions may help to refine strategies based on the use of recombinant proteins to promote bone regeneration.
BMPs and VEGF have frequently been used together in many studies of bone regeneration. However, the results have been quite variable. VEGF promotes BMP2 induced ectopic bone formation, and accelerates resorption of cartilage and its replacement by bone, while the VEGF blocker sFlt1 inhibits BMP2 induced ectopic bone formation, but promotes cartilage formation and delays resorption of cartilage [119]. In a critical-sized defect model, muscle-derived stem cells in combination with overexpression of VEGF failed to heal the defect [84]. When cells were co-transfected with vectors encoding VEGF and BMP2, a lower ratio of VEGF to BMP2 (1:5) induced greater bone regeneration than BMP2 alone [119] while a higher ratio (5:1) failed to promote bone healing. Similarly, only a low ratio of VEGF to BMP4 (1:5) promoted bone regeneration in a critical-sized defect, while high ratios (5:1) inhibited bone regeneration [84]. This is consistent with the finding that co-expression of VEGF and BMP4 in muscle derived stem cells reduces their bone forming potential [120].
Except for indirect effects on osteoclast recruitment, the inhibitory effects of VEGF on bone regeneration may also be attributed to direct effects of VEGF on osteoblasts. VEGF has been shown to retard the terminal differentiation of osteoblasts by up-regulation of Inhibitor of DNA Binding 1 (Id1) [121]. In endothelial cells, VEGF strongly inhibits TGF-β1 stimulation of Plasminogen activator inhibitor-1 (PAI-1) production and activation of Smad2/3[122]. VEGF also inhibits mesenchymal transition of endothelial cells induced by BMP4 through the Smad2/3 pathway [123]. Although evidence that the inhibitory effects of VEGF on downstream signaling of TGF-β family members, including BMPs, is lacking in the case of osteoblasts, such an effect, if it occurs, may explain, at least in part, why too much VEGF has antagonistic effects on osteoblast activity, particularly in the presence of BMPs.
PDGF is another important factor for bone regeneration [124]. Platelet rich plasma containing PDGF has been widely studied in various bone regenerative procedures [125]. VEGF prevents maturation of pericytes through a VEGFR2 mediated inhibition of PDGFR. This leads to formation of immature blood vessels [102]. Since osteoblasts and pericytes are derived from the same mesenchymal progenitors, the inhibitory effects of VEGF on PDGF-receptor activation may occur in osteoblasts as well. Recombinant VEGF competitively blocks PDGF-dependent activation of PDGFR and its signaling in several cell types [126]. Structural similarities between PDGF-B and VEGF [127], also suggest that VEGF may bind to PDGFRs on mesenchymal stem cells [128]. However, whether such binding would enhance or inhibit PDGFR-mediated downstream signaling pathways and cellular responses in osteoblast lineage cells is unclear.
6. Summary and future directions
Osteoblastic cells represent a major source of VEGF in the bone environment. Mice with reduced levels of VEGF in osteoblastic cells display an osteoporotic postnatal phenotype [106]. It was reported that VEGF levels in osteolineage cells, including mesenchymal progenitor cells, are decreased with age or in some anabolic disorders [129, 130], and this reduction may be related to increased risks of bone fracture or failure to respond to bone regenerative procedures. Thus, procedures to enhance VEGF levels specifically in osteoblasts may have positive effects in age-related osteoporosis or in cases of defective bone healing. Since osteoblasts are metabolically highly active, drugs that are known to up-regulate VEGF levels in osteoblasts, such as statins [131, 132], may be of value in developing such procedures.
The relationship between growth factors, bone forming progenitor cells and scaffolds are important components of procedures to stimulate bone regeneration, and current studies of VEGF functions may provide the basis for novel strategies aimed at improving bone regeneration. Important factors to consider in this context are: 1) Scaffolds with slow release of physiological levels of VEGF are needed and application of exogenous VEGF should be carefully considered, particularly when evidence for reduced levels of VEGF is lacking; 2) The paracrine, autocrine and intracrine effects of VEGF on bone forming stem cells or progenitors add to the complexity of VEGF applications. VEGFR2 modulation and the use of cell-permeable VEGF may be considered in certain scenarios; 3) The combination of VEGF with other growth factors, such BMPs, PDGF, and TGF-β, should be carefully considered, based on the knowledge that VEGF may potentially inhibit downstream signaling of BMPs/TGF-β, and compete with PDGF-BB for binding to PDFGRs. These interactions may vary depending on the repair region, such as bone marrow vs. periosteum, or the type of repair mechanism involved, such as intramembranous ossification vs. endochondral ossification.
Highlights.
VEGF initiates macrophage-related angiogenic response in the inflammation stage.
Hypertrophic chondrocyte-or osteoblast-generated VEGF is required for the recruitment of blood vessels and osteoclasts in endochondral ossification mediated bone repair.
An optimal amount of paracrine VEGF is required for angiogenesis-osteogenesis coupling in intramembranous ossification mediated bone repair.
VEGF is important for regulating osteoclasts in the remodeling stage.
Local administration of VEGF may be useful in treatment of impaired bone healing/regeneration as a consequence of age or osteoporosis.
Acknowledgements
This work was supported by NIH grant AR36819 (to B.R. Olsen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- 1.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Panteli M, Pountos I, Jones E, Giannoudis PV. Biological and molecular profile of fracture non-union tissue: current insights. J Cell Mol Med. 2015;19:685–713. doi: 10.1111/jcmm.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of Compromised Fracture Healing. Journal of the American Academy of Orthopaedic Surgeons. 2012;20:273–282. doi: 10.5435/JAAOS-20-05-273. [DOI] [PubMed] [Google Scholar]
- 5.Clarkin CE, Gerstenfeld LC. VEGF and bone cell signalling: an essential vessel for communication? Cell Biochemistry and Function. 2013;31:1–11. doi: 10.1002/cbf.2911. [DOI] [PubMed] [Google Scholar]
- 6.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 7.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacological Reviews. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 8.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast Precursors, but Not Mature Osteoblasts, Move into Developing and Fractured Bones along with Invading Blood Vessels. Developmental Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelzer E, McLean W, Ng YS, Fulkai N, Reginato AM, Lovejoy S, D'Amore PA, Olsen BR. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 12.Bruder SP, Fink DJ, Caplan AI. Mesenchymal Stem-Cells in in Bone-Development, Bone Repair, and Skeletal Regeneration Therapy. Journal of Cellular Biochemistry. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. Journal of Bone and Mineral Research. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 14.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Sleeman JP. The relationship between tumors and the lymphatics: What more is there to know? Lymphology. 2006;39:62–68. [PubMed] [Google Scholar]
- 17.Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, Sasaki T, Miura M. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005;10:318–327. doi: 10.1007/s10147-005-0508-7. [DOI] [PubMed] [Google Scholar]
- 18.Claesson-Welsh L. VEGF-B taken to our hearts - Specific effect of VEGF-B in myocardial ischemia. Arteriosclerosis Thrombosis and Vascular Biology. 2008;28:1575–1576. doi: 10.1161/ATVBAHA.108.170878. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bon F, Devy L, Beck H, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nature Medicine. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 20.Conn G, Bayne ML, Soderman DD, Kwok PW, Sullivan KA, Palisi TM, Hope DA, Thomas KA. Amino-Acid and Cdna Sequences of a Vascular Endothelial-Cell Mitogen That Is Homologous to Platelet-Derived Growth-Factor. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2628–2632. doi: 10.1073/pnas.87.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepper MS, Wasi S, Ferrara N, Orci L, Montesano R. In-Vitro Angiogenic and Proteolytic Properties of Bovine Lymphatic Endothelial-Cells. Experimental Cell Research. 1994;210:298–305. doi: 10.1006/excr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, DavisSmyth T. The biology of vascular endothelial growth factor. Endocrine Reviews. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 23.Phillips GD, Stone AM, Jones BD, Schultz JC, Whitehead RA, Knighton DR. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994;8:961–965. [PubMed] [Google Scholar]
- 24.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb Journal. 1999;13:9–22. [PubMed] [Google Scholar]
- 25.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 26.Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maru Y, Yamaguchi S, Shibuya M. Flt-1, a receptor for vascular endothelial growth factor, has transforming and morphogenic potentials. Oncogene. 1998;16:2585–2595. doi: 10.1038/sj.onc.1201786. [DOI] [PubMed] [Google Scholar]
- 29.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 30.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 31.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. Embo Journal. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wan C, Deng LF, Liu XM, Cao XM, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, et al. The hypoxia-inducible factor a pathway couples angiogenesis to osteogenesis during skeletal development. Journal of Clinical Investigation. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, Longaker MT. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. American Journal of Physiology-Cell Physiology. 2000;278:C853–C860. doi: 10.1152/ajpcell.2000.278.4.C853. [DOI] [PubMed] [Google Scholar]
- 36.Spector JA, Mehrara BJ, Greenwald JA, Saadeh PB, Steinbrech DS, Bouletreau PJ, Smith LP, Longaker MT. Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. American Journal of Physiology-Cell Physiology. 2001;280:C72–C80. doi: 10.1152/ajpcell.2001.280.1.C72. [DOI] [PubMed] [Google Scholar]
- 37.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 38.Tang WJ, Yang F, Li Y, de Crombrugghe B, Jiao HL, Xiao GZ, Zhang C. Transcriptional Regulation of Vascular Endothelial Growth Factor (VEGF) by Osteoblast-specific Transcription Factor Osterix (Osx) in Osteoblasts. Journal of Biological Chemistry. 2012;287:1671–1678. doi: 10.1074/jbc.M111.288472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senel K, Baykal T, Okur SC, Kiziltunc A, Ugur M. Circulating Vascular Endothelial Growth Factor (Vegf) Concentrations in Patients with Postmenopausal Osteoporosis: Correlation between Vegf Levels, and Bone Mineral Density. Osteoporosis International. 2010;21:299–299. [Google Scholar]
- 40.Abdallah D, Abdelzaher E, Khedr G, Elleithy H. The Prognostic Significance of Vascular Endothelial Growth Factor (Vegf) Expression in Wilms' Tumor and Its Relevance to Wt1 Expression. Pediatric Blood & Cancer. 2014;61:S374–S374. [Google Scholar]
- 41.Deckers MML, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CWGM. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 42.Yeh LCC, Lee JC. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Molecular and Cellular Endocrinology. 1999;153:113–124. doi: 10.1016/s0303-7207(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 43.Goad DL, Rubin J, Wang H, Tashjian AH, Patterson C. Enhanced expression of vascular endothelial growth factor in human SaOS-2 osteoblast-like cells and murine osteoblasts induced by insulin-like growth factor I. Endocrinology. 1996;137:2262–2268. doi: 10.1210/endo.137.6.8641174. [DOI] [PubMed] [Google Scholar]
- 44.Saadeh PB, Mehrara BJ, Steinbrech DS, Spector JA, Greenwald JA, Chim GS, Ueno H, Gittes GK, Longaker MT. Mechanisms of fibroblast growth factor-2 modulation of vascular endothelial growth factor expression by osteoblastic cells. Endocrinology. 2000;141:2075–2083. doi: 10.1210/endo.141.6.7502. [DOI] [PubMed] [Google Scholar]
- 45.Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of Vascular Endothelial Growth-Factor Expression by Prostaglandin E(2) and E(1) in Osteoblasts. Journal of Clinical Investigation. 1994;93:2490–2496. doi: 10.1172/JCI117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochemical Pharmacology. 2013;85:531–540. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Jung YD, Liu W, Reinmuth N, Ahmad SA, Fan F, Gallick GE, Ellis LM. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 48.Thi MM, Suadicani SO, Spray DC. Fluid Flow-induced Soluble Vascular Endothelial Growth Factor Isoforms Regulate Actin Adaptation in Osteoblasts. Journal of Biological Chemistry. 2010;285:30931–30941. doi: 10.1074/jbc.M110.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakai T, Yoshimura Y, Deyama Y, Suzuki K, Iida J. Mechanical stress up-regulates RANKL expression via the VEGF autocrine pathway in osteoblastic MC3T3-E1 cells. Molecular Medicine Reports. 2009;2:229–234. doi: 10.3892/mmr_00000088. [DOI] [PubMed] [Google Scholar]
- 50.Schweighofer B, Testori J, Sturtzel C, Sattler S, Mayer H, Wagner O, Bilban M, Hofer E. The VEGF-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thrombosis and Haemostasis. 2009;102:544–554. doi: 10.1160/TH08-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D, Jia HY, Holmes DIR, Stannard A, Zachary I. Vascular endothelial growth factor-regulated gene expression in endothelial cells - KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arteriosclerosis Thrombosis and Vascular Biology. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 52.Liu D, Evans I, Britton G, Zachary I. The zinc-finger transcription factor, early growth response 3, mediates VEGF-induced angiogenesis. Oncogene. 2008;27:2989–2998. doi: 10.1038/sj.onc.1210959. [DOI] [PubMed] [Google Scholar]
- 53.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 55.Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. Bonekey Rep. 2013;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury-International Journal of the Care of the Injured. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, Nomura S. Three modes of ossification during distraction osteogenesis in the rat. Journal of Bone and Joint Surgery-British Volume. 1997;79B:824–830. doi: 10.1302/0301-620x.79b5.7423. [DOI] [PubMed] [Google Scholar]
- 58.Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. Journal of Korean Medical Science. 2002;17:435–447. doi: 10.3346/jkms.2002.17.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clinical Orthopaedics and Related Research. 2000:224–237. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- 60.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 62.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. Journal of Clinical Investigation. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ancelin M, Chollet-Martin S, Herve MA, Legrand C, El Benna J, Perrot-Applanat M. Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Laboratory Investigation. 2004;84:502–512. doi: 10.1038/labinvest.3700053. [DOI] [PubMed] [Google Scholar]
- 65.Lim SR, Zhang Y, Zhang DF, Chen F, Hosaka K, Feng NH, Seki T, Andersson P, Li JR, Zang JW, et al. VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization. Cell Reports. 2014;9:569–580. doi: 10.1016/j.celrep.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ukai T, Shiraishi C, Moriyama H, Yoshimoto M, Hara Y. Expression of receptor activator of NF-kappaB ligand, osteoprotegerin and interleukin-1 beta in mechanical force-induced bone resorption. Bone. 2003;32:S155–S155. [Google Scholar]
- 68.Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. Journal of Pathology. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 69.Sunderkotter C, Goebeler M, Schulzeosthoff K, Bhardwaj R, Sorg C. Macrophage-Derived Angiogenesis Factors. Pharmacology & Therapeutics. 1991;51:195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 70.Brancato SK, Albina JE. Wound Macrophages as Key Regulators of Repair Origin, Phenotype, and Function. American Journal of Pathology. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2015 doi: 10.1016/j.bone.2015.10.019. In press. [DOI] [PubMed] [Google Scholar]
- 72.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 73.Chan CKF, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, Li GH, Fu FH, Huard J. Blocking Vascular Endothelial Growth Factor With Soluble Flt-1 Improves the Chondrogenic Potential of Mouse Skeletal Muscle-Derived Stem Cells. Arthritis and Rheumatism. 2009;60:155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li GG, Usas A, Fu FH, Huard J. Cartilage Repair in a Rat Model of Osteoarthritis Through Intraarticular Transplantation of Muscle-Derived Stem Cells Expressing Bone Morphogenetic Protein 4 and Soluble Flt-1. Arthritis and Rheumatism. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maes C, Stockmans I, Moermans K, Van Looveren R, Smets N, Carmeliet P, Bouillon R, Carmeliet G. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maes C, Araldi E, Haigh K, Khatri R, Van Looveren R, Giaccia AJ, Haigh JJ, Carmeliet G, Schipani E. VEGF-independent cell-autonomous functions of HIF-1alpha regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res. 2012;27:596–609. doi: 10.1002/jbmr.1487. [DOI] [PubMed] [Google Scholar]
- 79.Pfander D, Swoboda B, Cramer T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res Ther. 2006;8:104. doi: 10.1186/ar1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Blocking VEGF as a potential approach to improve cartilage healing after osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:316–317. [PubMed] [Google Scholar]
- 81.Carlevaro MF, Cermelli S, Cancedda R, Cancedda FD. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. Journal of Cell Science. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 82.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, Olsen BR. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mechanisms of Development. 2001;106:97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 83.Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Current Topics in Developmental Biology. 2005;65:169–187. doi: 10.1016/S0070-2153(04)65006-X. 65. [DOI] [PubMed] [Google Scholar]
- 84.Peng HR, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. Journal of Clinical Investigation. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leibbrandt A, Penninger JM. RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv Exp Med Biol. 2009;649:100–113. doi: 10.1007/978-1-4419-0298-6_7. [DOI] [PubMed] [Google Scholar]
- 89.Sells Galvin RJ, Gatlin CL, Horn JW, Fuson TR. TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem Biophys Res Commun. 1999;265:233–239. doi: 10.1006/bbrc.1999.1632. [DOI] [PubMed] [Google Scholar]
- 90.Barleon B, Siemeister G, MartinyBaron G, Weindel K, Herzog C, Marme D. Vascular endothelial growth factor up-regulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer Research. 1997;57:5421–5425. [PubMed] [Google Scholar]
- 91.Aldridge SE, Lennard TWJ, Williams JR, Birch MA. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochemical and Biophysical Research Communications. 2005;335:793–798. doi: 10.1016/j.bbrc.2005.07.145. [DOI] [PubMed] [Google Scholar]
- 92.Yang QL, McHugh KP, Patntirapong S, Gu XS, Wunderlich L, Hauschka PV. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and beta(3)-integrin. Matrix Biology. 2008;27:589–599. doi: 10.1016/j.matbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 93.Kodama H, Yamasaki A, Nose M, Niida S, Ohgame Y, Abe M, Kumegawa M, Suda T. Congenital Osteoclast Deficiency in Osteopetrotic (Op/Op) Mice Is Cured by Injections of Macrophage Colony-Stimulating Factor. Journal of Experimental Medicine. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Nishikawa SI, Kodama H. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. Journal of Experimental Medicine. 1999;190:293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, Hanada K, Kumegawa M, Hakeda Y. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. Febs Letters. 2000;473:161–164. doi: 10.1016/s0014-5793(00)01520-9. [DOI] [PubMed] [Google Scholar]
- 96.Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawao N, Tamura Y, Okumoto K, Yano M, Okada K, Matsuo O, Kaji H. Plasminogen plays a crucial role in bone repair. J Bone Miner Res. 2013;28:1561–1574. doi: 10.1002/jbmr.1921. [DOI] [PubMed] [Google Scholar]
- 98.Behr B, Leucht P, Longaker MT, Quarto N. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11853–11858. doi: 10.1073/pnas.1003317107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaipel M, Schutzenberger S, Schultz A, Ferguson J, Slezak P, Morton TJ, Van Griensven M, Redl H. BMP-2 but not VEGF or PDGF in fibrin matrix supports bone healing in a delayed-union rat model. Journal of Orthopaedic Research. 2012;30:1563–1569. doi: 10.1002/jor.22132. [DOI] [PubMed] [Google Scholar]
- 100.Schonmeyr BH, Soares M, Avraham T, Clavin NW, Gewalli F, Mehrara BJ. Vascular Endothelial Growth Factor Inhibits Bone Morphogenetic Protein 2 Expression in Rat Mesenchymal Stem Cells. Tissue Engineering Part A. 2010;16:653–662. doi: 10.1089/ten.tea.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henriksen K, Karsdal M, Delaisse JM, Engsig MT. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. Journal of Biological Chemistry. 2003;278:48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- 102.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang JH, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–U101. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 105.Jaasma MJ, Jackson WM, Tang RY, Keaveny TM. Adaptation of cellular mechanical behavior to mechanical loading for osteoblastic cells. J Biomech. 2007;40:1938–1945. doi: 10.1016/j.jbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 106.Liu YQ, Berendsen AD, Jia SD, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. Journal of Clinical Investigation. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 108.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crane JL, Cao X. Function of matrix IGF-1 in coupling bone resorption and formation. Journal of Molecular Medicine-Jmm. 2014;92:107–115. doi: 10.1007/s00109-013-1084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie H, Cui Z, Wang L, Xia ZY, Hu Y, Xian LL, Li CJ, Xie L, Crane J, Wan M, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nature Medicine. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thi MM, Iacobas DA, Iacobas S, Spray DC. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Skeletal Biology and Medicine, Pt B. 2007;1117:73–81. doi: 10.1196/annals.1402.020. [DOI] [PubMed] [Google Scholar]
- 113.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. Journal of Bone and Mineral Research. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. Journal of Bone and Mineral Research. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eckardt H, Bundgaard KG, Christensen KS, Lind M, Hansen ES, Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. Journal of Orthopaedic Research. 2003;21:335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 116.Kaigler D, Silva EA, Mooney DJ. Guided Bone Regeneration Using Injectable Vascular Endothelial Growth Factor Delivery Gel. Journal of Periodontology. 2013;84:230–238. doi: 10.1902/jop.2012.110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen L, He ZQ, Chen B, Yang MJ, Zhao YN, Sun WJ, Xiao ZF, Zhang J, Dai JW. Loading of VEGF to the heparin cross-linked demineralized bone matrix improves vascularization of the scaffold. Journal of Materials Science-Materials in Medicine. 2010;21:309–317. doi: 10.1007/s10856-009-3827-9. [DOI] [PubMed] [Google Scholar]
- 118.Cu ZP, Zhang X, Li L, Wang QG, Yu XX, Feng T. Acceleration of segmental bone regeneration in a rabbit model by strontium-doped calcium polyphosphate scaffold through stimulating VEGF and bFGF secretion from osteoblasts. Materials Science & Engineering C-Materials for Biological Applications. 2013;33:274–281. doi: 10.1016/j.msec.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 119.Peng HR, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. Journal of Bone and Mineral Research. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 120.Li GH, Corsi-Payne K, Zheng B, Usas A, Peng HR, Huard J. The Dose of Growth Factors Influences the Synergistic Effect of Vascular Endothelial Growth Factor on Bone Morphogenetic Protein 4-Induced Ectopic Bone Formation. Tissue Engineering Part A. 2009;15:2123–2133. doi: 10.1089/ten.tea.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song XB, Liu SH, Qu X, Hu YW, Zhang XY, Wang T, Wei FC. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochimica Et Biophysica Sinica. 2011;43:796–804. doi: 10.1093/abbs/gmr074. [DOI] [PubMed] [Google Scholar]
- 122.Yamauchi K, Nishimura Y, Shigematsu S, Takeuchi Y, Nakamura J, Aizawa T, Hashizume K. Vascular endothelial cell growth factor attenuates actions of transforming growth factor-beta in human endothelial cells. Journal of Biological Chemistry. 2004;279:55104–55108. doi: 10.1074/jbc.M407423200. [DOI] [PubMed] [Google Scholar]
- 123.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nature Medicine. 2010;16:1400–U1480. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caplan AI, Correa D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. Journal of Orthopaedic Research. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 125.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immunity & Ageing. 2013;10 doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pennock S, Kazlauskas A. Vascular Endothelial Growth Factor A Competitively Inhibits Platelet-Derived Growth Factor (PDGF)-Dependent Activation of PDGF Receptor and Subsequent Signaling Events and Cellular Responses. Molecular and Cellular Biology. 2012;32:1955–1966. doi: 10.1128/MCB.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, DeVos AM. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7192–7197. doi: 10.1073/pnas.94.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. Journal of Cell Biology. 2007;177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costa N, Paramanathan S, Mac Donald D, Wierzbicki AS, Hampson G. Factors regulating circulating vascular endothelial growth factor (VEGF): Association with bone mineral density (BMD) in post-menopausal osteoporosis. Cytokine. 2009;46:376–381. doi: 10.1016/j.cyto.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 130.Wilson A, Shehadeh LA, Yu H, Webster KA. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. Bmc Genomics. 2010;11 doi: 10.1186/1471-2164-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells vial inhibition of protein prenylation. Endocrinology. 2003;144:681–692. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 132.Tsartsalis AN, Dokos C, Kaiafa GD, Tsartsalis DN, Kattamis A, Hatzitolios AI, Savopoulos CG. Statins, bone formation and osteoporosis: hope or hype? Hormones-International Journal of Endocrinology and Metabolism. 2012;11:126–139. doi: 10.14310/horm.2002.1339. [DOI] [PubMed] [Google Scholar]