Summary
Apical constriction is a change in cell shape that drives key morphogenetic events including gastrulation and neural tube formation. Apical force-producing actomyosin networks drive apical constriction by contracting while connected to cell-cell junctions. The mechanisms by which developmental patterning regulates these actomyosin networks and associated junctions with spatial precision are not fully understood. Here, we identify a myosin light chain kinase MRCK-1 as a key regulator of C. elegans gastrulation that integrates spatial and developmental patterning information. We show that MRCK-1 is required for activation of contractile actomyosin dynamics and elevated cortical tension in the apical cell cortex of endodermal precursor cells. MRCK-1 is apically localized by active Cdc42 at the external, cell-cell contact-free surfaces of apically constricting cells, downstream of cell fate determination mechanisms. We establish that the junctional components α-catenin, β-catenin, and cadherin become highly enriched at the apical junctions of apically-constricting cells, and that MRCK-1 and myosin activity are required in vivo for this enrichment. Taken together, our results define mechanisms that position a myosin activator to a specific cell surface where it both locally increases cortical tension and locally enriches junctional components to facilitate apical constriction. These results reveal crucial links that can tie spatial information to local force generation to drive morphogenesis.
Introduction
Morphogenesis is driven by forces produced within individual cells [1]. The molecular machines that produce these forces must be localized precisely within cells. Understanding the links between developmental biology and cell biology that can determine exactly where force-producing mechanisms are positioned is fundamental to understanding how complex morphologies form.
Apical constriction, the shrinking of apical cell surfaces, is a cell shape change that drives diverse tissue shape changes including gastrulation in many systems and neural tube formation in vertebrates [2]. Apical constriction is driven by contraction of networks composed of actin filaments and non-muscle myosin II that are localized near apical cell surfaces and that connect to adhesive, apical cell-cell junctions [3]. In cells undergoing apical constriction these networks can be organized in at least two types of structures: junctional belts that are found at cell-cell junctions and that contract via a purse-string mechanism [3, 4], and medio-apical networks that crisscross the entire apical cortex [5]. Recent experiments in diverse animal systems demonstrate that medio-apical networks are under tension and contribute forces that drive cell shape change [6–8]. To understand apical constriction mechanisms, we are investigating how these medio-apical networks, and the junctions that they connect to, are deployed and maintained with spatial and temporal precision by developmental patterning mechanisms.
The gastrulation movements in the early C. elegans embryo are a valuable system to address these questions. The internalization of the endoderm precursor cells occurs through contraction of apical actomyosin networks [9, 10]. There exists a strong understanding of how embryonic cell fates are specified in C. elegans [11], as well as an understanding of how the embryonic cells become polarized along their apicobasal axis [12]. Apicobasal polarization in the early embryo is regulated by a system that distinguishes apical cell surfaces, which are free of contacts with other cells, from basolateral surfaces, which make contact with other embryonic cells. The current model for apicobasal polarization involves classical cadherins recruited basolaterally—to sites of cell-cell contact—through homotypic binding of cadherin ectodomains. Cadherin cytoplasmic tails then sequentially recruit p120-catenin, the coiled-coil protein PICC-1, and finally the Rho family GTPase Activating Protein (RhoGAP) PAC-1, which locally inactivates CDC-42 at sites of cell-cell contact [13]. In a set of elegant experiments, it was shown that generating ectopic cell contacts can change in predictable ways the localization of cadherin, PAC-1 and other polarity proteins in C. elegans embryos, confirming that this system relies on positional information defined by sites of cell-cell contact [13, 14]. Many of these proteins show conserved interactions in mammalian cells [15, 16], but how these apicobasal polarization mechanisms deploy force-producing mechanisms to specific parts of cells is not well understood in any system.
For actomyosin-based contractile forces to drive changes in tissue shape, the forces must be mechanically propagated to neighboring cells. The cadherin-catenin complex has been shown to be a force-bearing link between the actomyosin cortices of adjacent cells [17, 18]. Interestingly, actomyosin dynamics, regulated by Rho-family small GTPases, have been shown to have significant effects on the behavior of cadherin-catenin based adherens junctions. However the nature of these effects can vary from system to system. For example, actomyosin based contractility can enhance junctional stability in some systems [19] and promote junctional turnover in other systems [20]. During Drosophila melanogaster gastrulation there is an apical enrichment of adherens junctions, which is lost if apical constriction is inhibited [19, 21], and during Xenopus gastrulation, myosin activity leads to altered C-cadherin dynamics [22]. These studies raise the interesting possibility that modulation of actomyosin networks might result in enrichment of junctional complexes at network connection sites.
Here, we have identified molecular links between developmental patterning mechanisms and cytoskeletal force-producing mechanisms, as a step toward understanding how developmental patterning can accurately position both force-producing and force-transmitting systems. We report the identity of a myosin activator, MRCK-1, that is required for activation of myosin in the apical cortex of the gastrulating cells and for apical constriction in vivo. We further show that MRCK-1-regulated myosin contractility is a key regulator of cell-cell adhesion components during these cell movements.
Results
MRCK-1 is required for myosin activation, cortical tension, apical constriction, and early gastrulation in C. elegans
In wild type embryos, two endoderm precursor cells (EPCs), called Ea and Ep, move from the embryo's surface to its interior starting at the 26–28 cell stage (Figure 1A). Other cells move in later, but for convenience, we refer here to the internalization of the endoderm precursors as gastrulation. We have previously shown that myosin is activated apically in these endoderm precursors through phosphorylation of myosin regulatory light chain [23]. We therefore searched for molecular links between developmental patterning mechanisms and cytoskeletal force-producing mechanisms by seeking to identify myosin activators required for C. elegans gastrulation. The myosin light chain kinases LET-502 (Rho-associated kinase) and MRCK-1 (myotonic dystrophy kinase-related Cdc42-binding kinase) are known to activate myosin and are essential for normal C. elegans development [24, 25]. LET-502 is required for early cytokinesis, while both LET-502 and MRCK-1 have roles in embryonic elongation. Unlike traditional myosin light chain kinases, MRCK-family kinases and Rho-associated kinases are not regulated by calcium binding. Instead their activities are modulated by interaction with the Rho family small GTPases and other binding partners [26, 27].
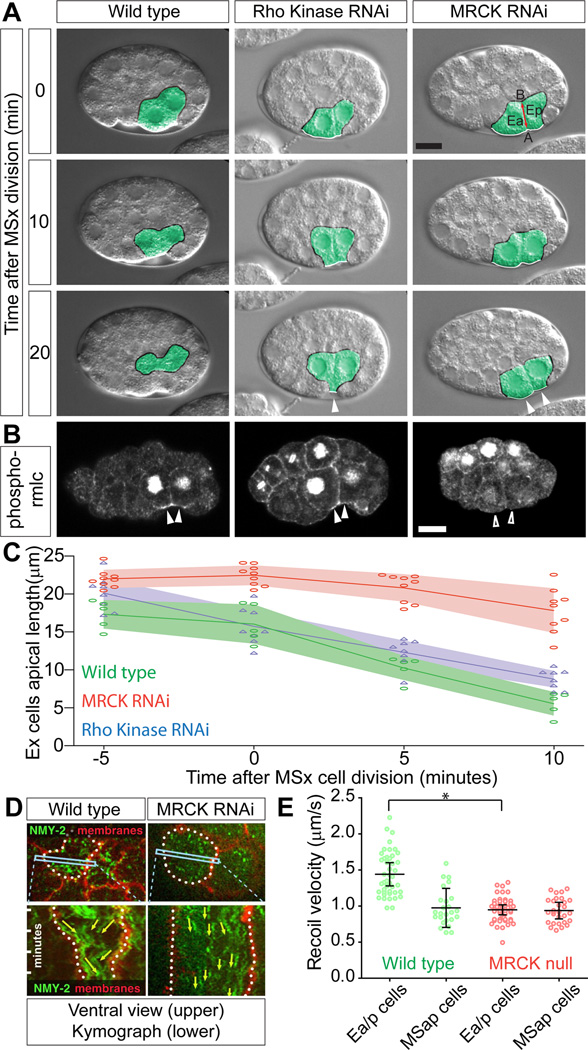
Figure 1. MRCK-1 and Rho Kinase are required for early gastrulation movements in C. elegans, and MRCK-1 regulates apical myosin. See also Figure S1 and Movie S1.
(A) Internalization of the two EPCs (Ea and Ep, pseudocolored green). The exposed surfaces of these cells (marked with white border, changed to black when covered) become completely covered by the neighboring cells within 20 minutes of MSx division. In Rho Kinase (let-502) and mrck-1 RNAi embryos there were still exposed surfaces at the 20 minute time point (arrowheads). Each panel is a single midsagittal optical section from a DIC time-lapse movie, with time since MSx division noted. In this and all figures, embryos are oriented with anterior to the left and dorsal up. In the top right panel, Ea and Ep are labeled, and the apical (A) – basal (B) axis of the EPCs is denoted by a red line. Scale bar represents 10 µm.
(B) Phosphorylated regulatory myosin light chain (rmlc) localization. Activated myosin was detectable at the apical cortex of EPCs in embryos with a temperature-sensitive mutation in Rho Kinase at the non-permissive temperature (arrowheads), but was absent from mrck-1 RNAi embryos (empty arrowheads). The antibody used also strongly marks nuclei prior to nuclear envelope breakdown and during cell division, which we consider as background staining because it is not eliminated by RNAi targeting regulatory myosin light chain (Lee et al., 2006). Scale bar represents 10 µm. Quantification from multiple embryos is found in Figure S1A.
(C) Quantification of the length of the apical (exposed) surfaces of the endoderm precursor cells in multiple embryos. Each point represents an individual embryo, and the lines represent the mean of several embryos. (mrck-1 RNAi n=9, Rho Kinase n=8, Wild type n=5). Shading represents 95% confidence intervals.
(D) Myosin (NMY-2, green) and membranes (marked using the PH domain from PLCδ, red, marked with dotted white line) were visualized in ventral mounts. A kymograph derived from a line across an EPC (box) showed that as the endoderm cell apical surfaces contracted, myosin foci moved toward the center of the apical cortex in wild type but not in an mrck-1 RNAi embryo.
(E) Recoil velocity of myosin particles in embryos that have been cut by UV laser incision, as a measure of relative cortical tension (Movie S1 [7]). Each point represents a single particle measured. The average recoil velocity was calculated in each embryo, and bars represent the mean of multiple embryos (wild type, Ea/p n = 9 embryos, MSap n = 5; mrck-1 null, Ea/p n=10, MSap n=6, * p=0.029). Error bars in all graphs are 95% confidence intervals (95%CI).
We used dsRNA injection in adult C. elegans to disrupt either let-502 or mrck-1 gene functions in their progeny, and we used live-cell time-lapse microscopy to look for gastrulation defects. In embryos in which we targeted mrck-1 by RNAi the EPCs consistently failed to move inward (Figure 1A) and instead divided on the embryo surface (16/16 embryos). In these embryos, the apical surfaces of the endoderm cells constricted significantly more slowly than in wild type embryos (Figure 1C). Failure of the EPCs to internalize was also seen in embryos from adults homozygous for an mrck-1 null mutant, mrck-1(ok586) (embryos born from heterozygotes are rescued by maternal contributions but their progeny fail to develop to adults). Endoderm precursors left on the embryo surface do move in just prior to the next cell division (the division of four E lineage cells to eight cells), suggesting that a redundant mechanism can rescue the cell internalization defect.
It is impossible to produce embryos completely deficient for LET-502/Rho kinase because LET-502 functions in cytokinesis, and null embryos fail to develop [25]. Therefore, we performed partial LET-502 knockdown by imaging embryos 18hrs post-injection, when there were still embryos developing without early cytokinesis defects. In these embryos, we saw a low penetrance defect (3/13) in which the EPCs moved inward but incompletely, leaving one or both of the endoderm precursor cells exposed to the embryonic exterior after division (Figure 1A). Similar results were seen using a temperature sensitive allele of LET-502 (let-502(sb118ts)) shifted to the non-permissive temperature prior to dissection (4/12 embryos with gastrulation defects compared with 0/8 for wild type embryos similarly shifted). Quantification of the apical membrane length of the dsRNA treated embryos over time showed that the apical membranes contracted at a rate similar to that in wild type embryos (Figure 1C).
We next examined myosin activation in these kinase-deficient embryos. In contrast to wild type embryos, mrck-1(RNAi) embryos contained little or no detectable phosphorylated regulatory myosin light chain in the apical domain of the EPCs, suggesting that MRCK-1 is required for the activation of apical myosin in these cells (Figure 1B, Figure S1A). We found that the pattern of activated regulatory myosin light chain in the let-502(sb118ts) embryos at the non-permissive temperature was similar to that of wild type embryos (Figure 1B, Figure S1A). We conclude that MRCK-1 is required for activation of apical myosin during these cell movements and that LET-502 is likely active at this time and makes at least a minor contribution to gastrulation.
To determine whether MRCK-1 upregulates myosin-based cortical tension in the EPCs, we measured myosin movements and cortical tension in mrck-1(RNAi) or mrck-1 null mutant embryos (see Movie S1 for example). Centripetal movements of myosin—from the edges toward the center of apical cell cortexes, which reflect contractile behavior of the actomyosin cortex [7]—are almost completely absent in the EPCs of mrck-1(RNAi) embryos (Figure 1D).
We used laser cutting experiments to estimate tension in the apical cortex of gastrulating cells. As we had previously shown [7], the EPCs showed significantly higher cortical tension than their neighboring mesoderm precursor cells (Figure 1E). In mrck-1 null mutant embryos, the apical cortex of EPCs had lower tension, indistinguishable from that of neighboring cells (Figure 1E). Therefore, we conclude that MRCK-1 is required during gastrulation of the EPCs to activate myosin and thereby increase actomyosin contractility and cortical tension.
We examined whether MRCK-1 is also required for later cell internalization events by imaging embryos through the stage at which mesoderm precursor cells of the MS lineage internalize. In 4/8 mrck-1(ok586) embryos, internalization of MS-derived cells occurred normally, while in the remaining cases, MS descendants failed to internalize correctly and in some cases divided aberrantly on the surface of the embryo (Figure S1B). Because we cannot determine whether failure of MS cells to internalize is a secondary consequence of mis-positioning of endoderm precursor cells, we conclude that MRCK-1 may play either a direct or indirect role in internalization of mesodermal precursor cells. Therefore we focused on the EPCs in our further experiments to understand MRCK-1 regulation and function.
MRCK-1 localizes to the apical domains of cells undergoing apical constriction
Because we found that MRCK-1 is required to activate myosin and increase cortical tension specifically in the apical cortex of internalizing endoderm precursors, we hypothesized that MRCK-1 might be localized to the apical cell cortex of EPCs. Alternatively, MRCK-1 could be distributed more widely, and other mechanisms could limit where myosin can be activated by MRCK-1. To study MRCK-1 localization at its endogenous levels, we used CRISPR-Cas9 triggered homologous recombination [28] to tag the endogenous mrck-1 locus with the yellow fluorescent protein YPet, chosen because of its high brightness and the lower auto-fluorescence in embryos excited at longer wavelengths (Figure S2). Our approach offers three distinct advantages over other tagging methods that have been used commonly in developmental model systems: 1) since endogenous loci are tagged, most, if not all native transcriptional regulatory elements are preserved, 2) 100% of the protein of interest is fluorescently labeled (i.e. there is no unlabeled population), 3) since the genes tagged here are all essential, the viability of homozygous animals carrying the tagged genes can be used to determine whether the tagged proteins are functional. The outcrossed MRCK-1::YPet line showed wild type levels of embryonic viability (Figure S2) and had no discernable gastrulation phenotype, suggesting that the tagged protein functioned normally.
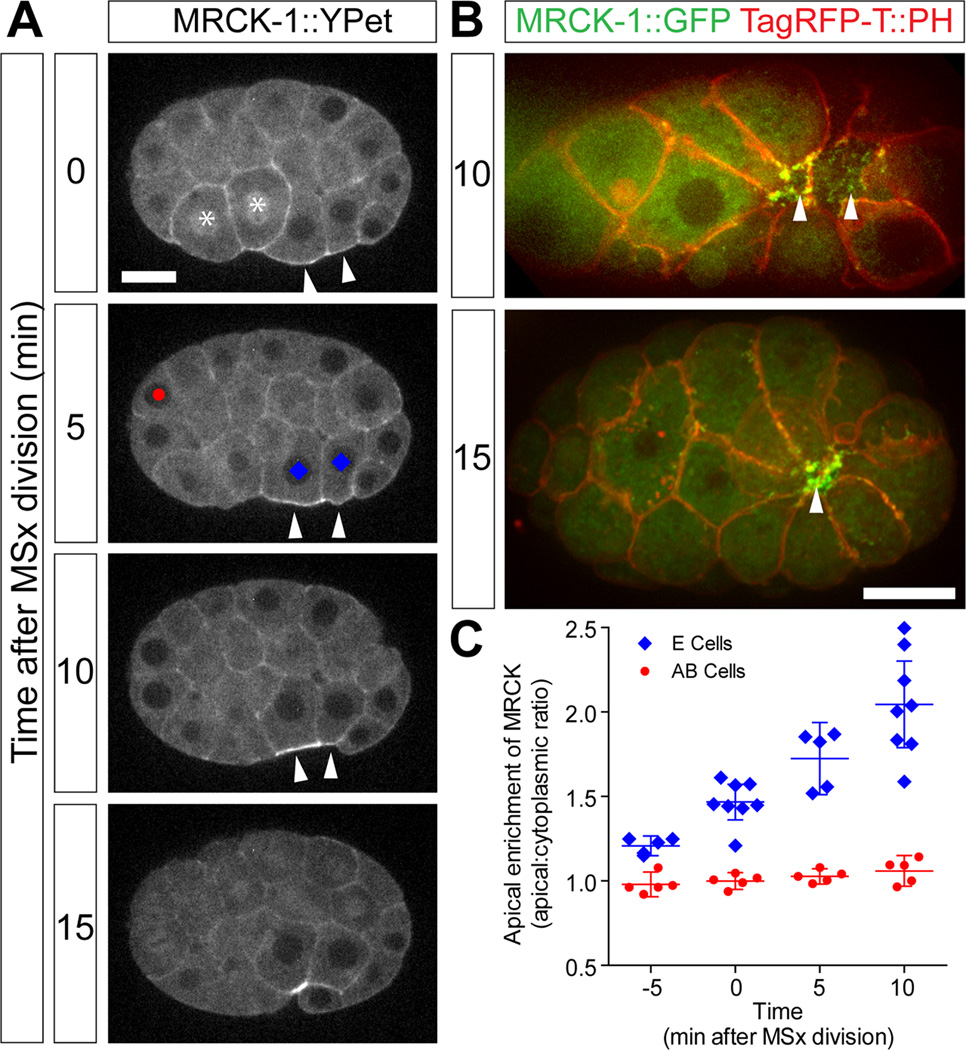
We found that MRCK-1::YPet was enriched in the apical cortex specifically in the gastrulating EPCs (Figure 2A, Movie S2). A GFP tagged strain constructed similarly showed similar localization and allowed us to co-visualize MRCK-1 and an mCherry-tagged PH domain to show membranes (Figure 2B) revealing in ventral views that MRCK-1::GFP localized to puncta across the medio-apical surfaces of EPCs as well as to apical cell-cell borders (Figure 2B). Fluorescently tagged MRCK-1 was not enriched in the apical domain of other early embryonic interphase cells, although some enrichment could be seen in the cortex of dividing cells (Figure 2A). In the gastrulating EPCs, enrichment was detected starting from 5 minutes prior to MSx division (MSx indicating the two daughters of the MS cell), and within 15 minutes had reached two-fold enrichment over cytoplasmic levels (Figure 2C). This was significantly different from non-dividing, non-internalizing AB cells, where little enrichment over cytoplasmic levels was detected in the same time frame. Comparison of the time course of apical constriction and MRCK-1 localization (Figures 1 and 2) shows that apical enrichment of MRCK-1::YPet is concurrent with or even precedes apical constriction and continues throughout the gastrulation movements of the EPCs, consistent with a role for MRCK-1 regulating myosin function during EPC cell movements.
Figure 2. MRCK-1 accumulates apically in endoderm precursor cells during gastrulation. See also Figure S2 and Movie S2.
(A) MRCK-1::YPet accumulated at the apical surface (arrowheads) of the EPCs (labelled with blue diamonds) during gastrulation (Movie S2). Cells marked with asterisk are dividing. Scale bar represents 10 µm.
(B) Ventral views. MRCK-1::GFP (green) decorated the exposed surfaces of the EPCs. Cell membranes were marked with the PH domain of PLCδ tagged with TagRFP-T (red).
(C) MRCK-1::YPet accumulated apically specifically in the EPCs. Each point represents average apical enrichment in denoted cell type in a single embryo (symbols match those drawn at 5 min time point in panel A). Bars represent means of multiple embryos +/− 95%CI.
Endoderm cell fate specification is necessary and sufficient for MRCK-1 localization in the appropriate cells
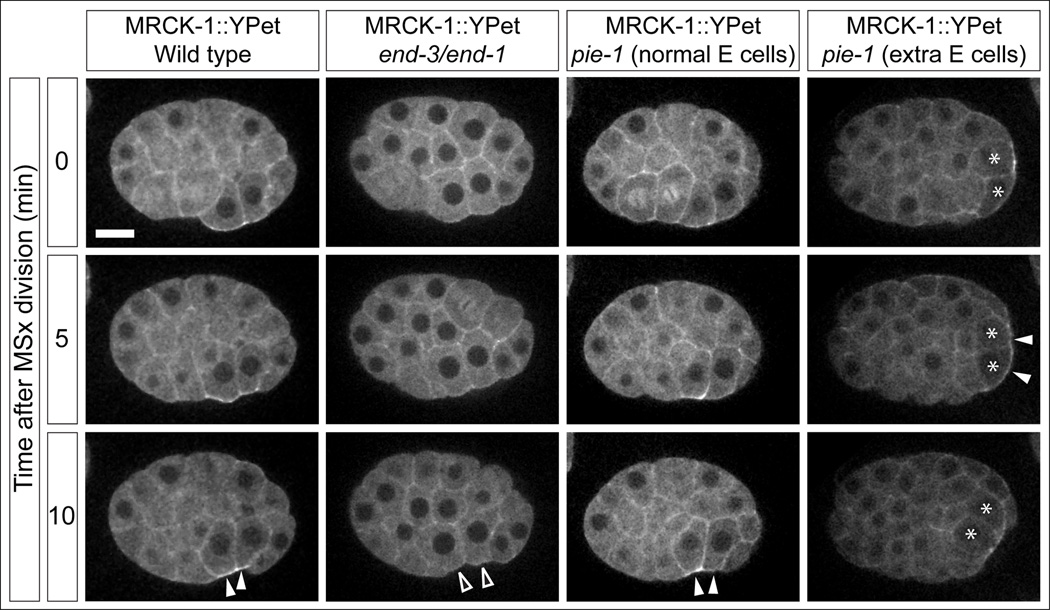
Because apical enrichment of MRCK-1 was only seen in the endoderm precursor cells, we tested whether cell fate specification contributes to the localization of MRCK-1. In C. elegans, endoderm specification is regulated by two partially redundant GATA transcription factors, END-1 and END-3. We crossed MRCK-1::YPet into an end-3 null mutant, end-3(ok1448), and then targeted end-1 by RNAi. We saw complete failure of gastrulation in these embryos, as seen previously for a chromosomal deficiency that deletes both genes [23, 29]. Furthermore, MRCK-1::YPet failed to become apically enriched in endodermal precursor cells (Figure 3; Movie S3, quantified in Figure S3, p<0.001). To determine whether endodermal cell fate is not only necessary but also sufficient for apical MRCK-1 localization in these embryonic cells, we targeted the pie-1 gene by RNAi. pie-1 encodes a CCCH zinc-finger protein that represses RNA-polymerase II-dependent gene expression in germline lineage cells. In its absence, germline lineage cells transform into somatic lineages, producing multiple cell types ectopically, including endodermal precursors in an end-1- and end-3-dependent manner [29, 30]. These additional endoderm precursors have previously been shown to undergo cell internalization shortly after the normal endoderm precursor cells internalize [23]. In pie-1(RNAi) MRCK-1::YPet embryos, MRCK-1 was apically localized in the normal endoderm precursor cells (Figure 3; Movie S3), and later also in the additional, ectopic endoderm precursor cells during their internalization (Figure 3; Movie S3, quantified in Figure S3). We conclude that endoderm cell fate specification is necessary and sufficient for MRCK-1 to localize apically in the appropriate cells, and that endodermal GATA factors play yet-to-be characterized direct or indirect roles in this process.
Figure 3. Cell fate specification mechanisms regulate MRCK-1 accumulation. See also Figure S3 and Movie S3.
MRCK-1::YPet localization in wild type embryos and embryos with altered cell fates (Movie S3). end-3/end-1 refers to end-3(ok1448) treated with end-1(RNAi) and causes a loss of endoderm cell fate. pie-1 refers to pie-1(RNAi), which causes additional cells to transform to the endodermal cell fate (extra E cells marked with asterisks). Accumulation is marked with closed arrowheads, failure to accumulate is marked with open arrowheads. Scale bar represents 10 µm. Quantification from multiple embryos is found in Figure S3.
Active Cdc42 recruits MRCK-1 to the apical cell cortex
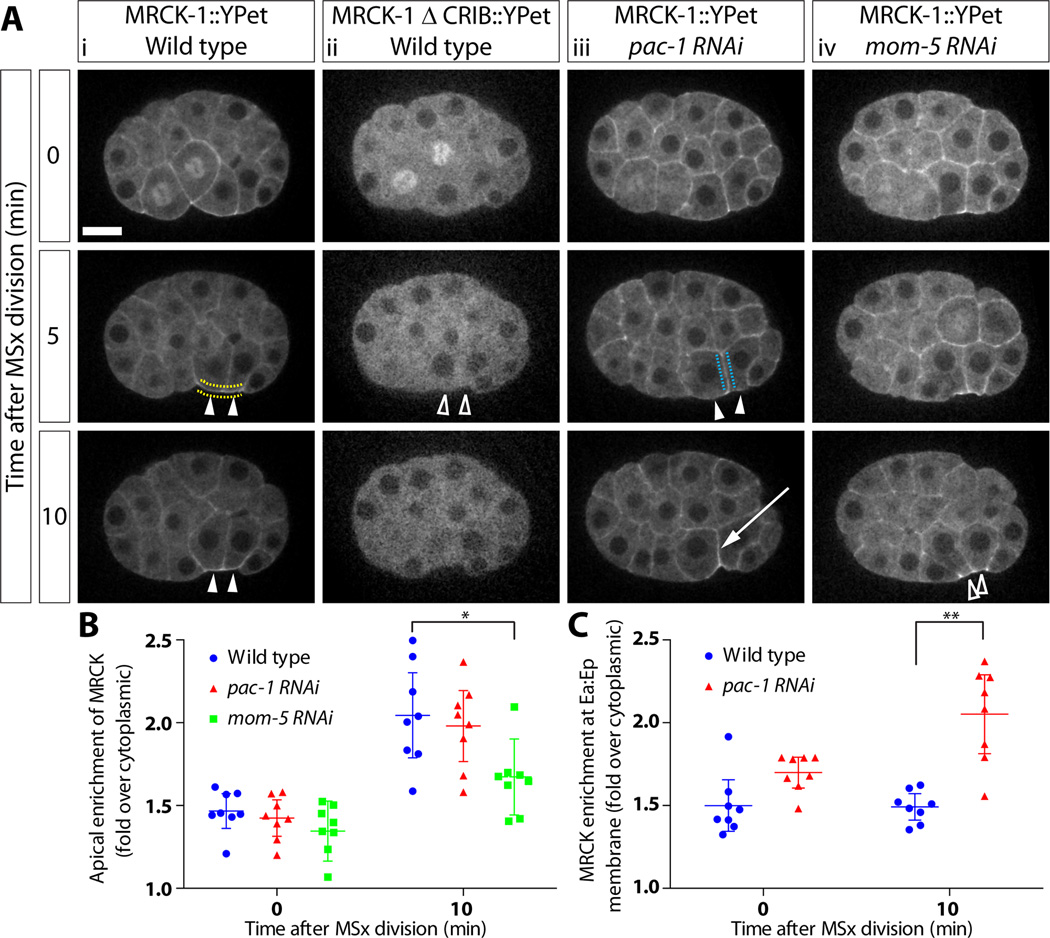
MRCK-1 contains a conserved CRIB (Cdc42- and Rac- interactive binding) motif at its C-terminus that is likely to mediate interactions with active CDC-42 [31]. To determine whether MRCK-1 is localized by active CDC-42, we inserted YPet into MRCK-1 directly upstream of the CRIB motif, adding two stop codons to YPet to produce a version of MRCK-1::YPet lacking a CRIB domain. We were unable to recover a CRISPR line with this construct; therefore we used a fosmid-based transgene to express this fusion protein. A wild type fosmid with an identical YPet insertion to the CRISPR line showed the same expression pattern of MRCK-1 as the CRISPR line (Figure S4A) and was able to rescue an MRCK-1 null strain (mrck-1(ok586)). We found that the MRCK-1 ΔCRIB::YPet, which could not rescue the null strain, was no longer recruited to the cortex and instead appeared to be completely cytoplasmic (Figure 4; Movie S4; Figure S4B).
Figure 4. MRCK-1 localization is regulated by Rho family GTPases and Wnt signaling. See also Figure S4 and Movie S4.
(A)(i) MRCK-1::YPet localization in wild type embryos. (ii) MRCK-1 ΔCRIB::YPet localization in wildtype embryos, accumulation is quantified in Figure S4. (iii) MRCK-1::YPet localization in pac-1(RNAi) embryos. (iv) MRCK-1::YPet localization in mom-5(RNAi) embryos. Apical accumulation is marked with closed arrowheads, failure to accumulate is marked with open arrowheads, and accumulation at basolateral membranes is marked with an arrow (Movie S4) Scale bar represents 10 µm.
(B) Quantification of accumulation at the apical membrane from multiple embryos (dotted yellow line shows quantified membranes). Each point represents average enrichment at denoted membrane in a single embryo. Bars represent mean of multiple embryos +/− 95%CI. Wild type n=8, pac-1(RNAi) n=8, mom-5(RNAi) n=8, *p=0.0209
(C) Quantification of accumulation at the membrane between the two EPCs from multiple embryos (dotted blue line). (Wild type n=8, pac-1(RNAi) n=8), **p=0.006.
To further test whether MRCK-1 localization depends on recruitment to active CDC-42, we knocked down PAC-1, a GTPase activating protein (GAP) for CDC-42 that acts to exclude active CDC-42 from sites of cell-cell contact in the early C. elegans embryo [13, 14]. We predicted that if MRCK-1 was recruited to the apical cortex by active CDC-42, then targeting pac-1 with dsRNA would result in ectopic localization of MRCK-1 to sites of cell-cell contact. Consistent with this, pac-1(RNAi) embryos showed normal enrichment of MRCK-1::YPet to the apical cortex of EPCs (Figure 4; Movie S4) and significant ectopic MRCK-1 recruitment to the site of contact between the two EPCs (Figure 4, graph), which was dependent on the CRIB domain of MRCK-1 (Figure S4C). MRCK-1 might activate myosin and cortical tension at this ectopic site, because we sometimes saw an atypical indentation between the Ea and Ep apical surfaces (3/8 pac-1(RNAi) embryos) suggesting that the Ea/Ep border was shortening (Figure 4; Movie S4). Finally, embryos depleted of CDC-42 at gastrulation stage by tagging CDC-42 with a ZF-1 domain, depleted this way to allow it to function earlier, when it is needed for polarization of the zygote [14], show a similar gastrulation defect to the mrck-1 mutant embryos (Figure S4D, 12/13 embryos). Examination of myosin localization in embryos where CDC-42 is partially depleted by RNAi shows that CDC-42 is required to recruit myosin to the apical membranes of the EPCs (Figure S4E). Taken together, the requirement of CDC-42 for gastrulation movements and myosin recruitment, the requirement for MRCK-1’s CRIB domain for cortical localization, and the ectopic localization of tagged MRCK-1 in pac-1 mutant embryos suggest that MRCK-1 is recruited to the apical cortex in the endoderm precursor cells by active CDC-42.
MOM-5/Frizzled contributes to MRCK-1 localization
We previously identified an additional developmental input to myosin regulation during C. elegans gastrulation. We showed that myosin activation was dependent upon Wnt-Frizzled signaling [23]. To determine if Wnt-Frizzled signaling also affects MRCK-1 localization, we targeted the Wnt receptor, Frizzled (MOM-5) by RNAi in the MRCK-1::YPet strain and quantified the MRCK-1 apical recruitment in these embryos (Figure 4; Movie S4). Knockdown of MOM-5 by RNAi results in a partially penetrant gastrulation-defective phenotype and also has effects on cell cycle length in EPCs [23]. To avoid the confounding effects of cell cycle length alteration, we scored mom-5(RNAi) embryos that had gastrulation defects and normal cell cycle timing in the EPCs. In these embryos, MRCK-1 recruitment to the apical cell cortex was still detected but was significantly reduced compared to the wild type levels (Figure 4; Movie S4), suggesting that Frizzled signaling contributes to C. elegans gastrulation and myosin activation at least in part by contributing to apical MRCK-1 recruitment.
Taken together, our results suggest that multiple mechanisms contribute to the spatial specificity of MRCK-1 localization. Cell fate specification mechanisms contribute by determining in which cells MRCK-1 becomes recruited to the cell cortex. MRCK-1 is then recruited to the contact-free cell surfaces via its CRIB domain binding to the active CDC-42-decorated, apical cell cortex. Once at this site, MRCK-1 is required for myosin activation and contractile dynamics of actomyosin in the cell cortex, which increases cortical tension and drives the cell movements.
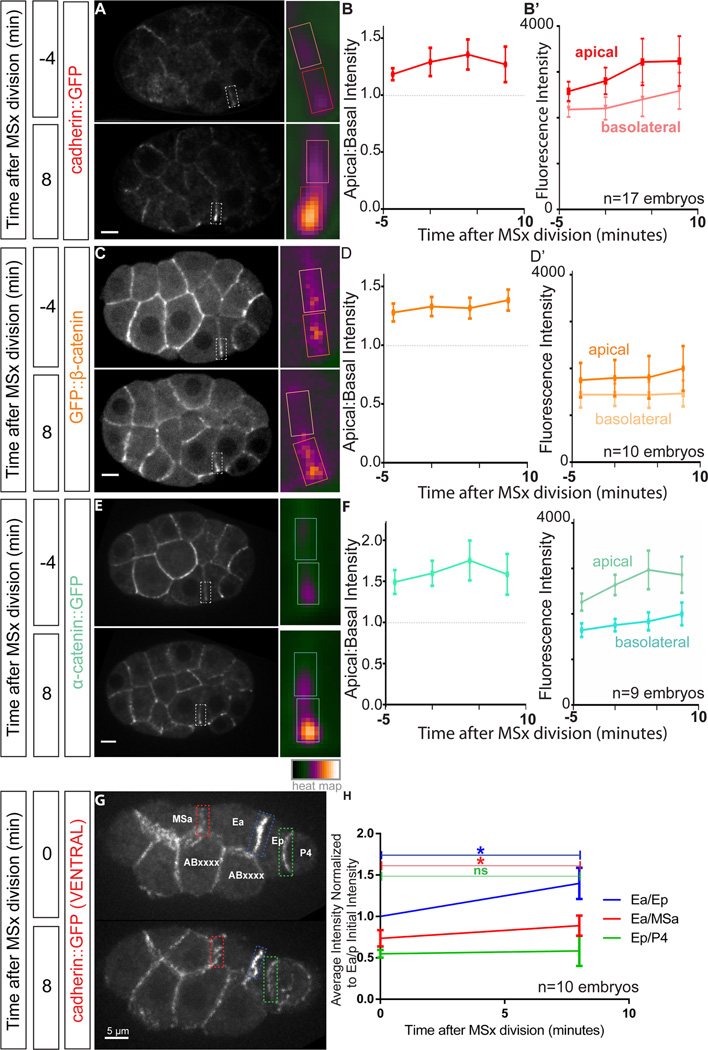
The cadherin-catenin complex enriches at apical junctions in internalizing cells
For MRCK-1 mediated myosin activation to drive apical constriction, actomyosin networks must connect to junctional complexes at the edges of the apical cell cortex. Cortical tension has been proposed to play important roles in adherens junction complex formation and stabilization in purified proteins and in cultured cells [19, 21, 32]. Cadherin complex proteins are known to function in C. elegans gastrulation, albeit redundantly, by promoting the linking of actomyosin networks to membranes at apical cell-cell contact zones [7, 33], but little is known about how these junctional proteins become localized. Therefore we sought to examine the localization of the cadherin complex proteins, and then to determine whether MRCK-1 and myosin activity contribute to junctional complex localization and/or stabilization.
To track the in vivo localization of junctional components, we used CRISPR to make GFP insertions into the endogenous loci encoding the three essential proteins of the cadherin-catenin complex (CCC) (Figure S2A). We were able to produce viable homozygous strains for HMR-1/cadherin-GFP, HMP-1/α-catenin-GFP, and GFP-HMP-2/β-catenin. β-catenin was particularly sensitive to the GFP insertion site as multiple insertion sites abrogated gene function (Figure S2B). Our tagging results suggest that prior transgene studies may have used GFP fusions in which tagged proteins were not fully functional [34]. We used strains in which tagging endogenous loci resulted in 99–100% homozygote viability (Figure S2B) for further experiments.
Consistent with previous immunostaining studies [35, 36], endogenously tagged CCC components localized to sites of cell-cell contact in early embryos (Figure S5A, C, E, Movie S5). The distribution of HMR-1/cadherin-GFP along these contacts was non-uniform, with enrichment often seen at apico-lateral junctions: at sites of cell-cell contact close to the apical cell surface. Previous electron microscopy studies of gastrulation stage embryos have not identified electron-dense adherens junction-like structures [9]. However, for convenience, we refer to these structures as adherens junctions based on the apico-lateral enrichment of CCC components at cell-cell contacts. While several cell-cell contacts displayed some degree of this CCC polarization (Figure S5A–D), this pattern was most striking at the contact between the apically constricting EPCs, Ea and Ep (Figure S5E–F).
To examine whether junctions matured during apical constriction, we quantified the apicobasal polarization of the three CCC proteins along the Ea/Ep border during the period of apical constriction (Figure 5A–F). We found that all three CCC proteins were enriched at the apical-most portion of cell-cell contacts between Ea and Ep throughout this period, with the enrichment being strongest late in apical constriction (Figure 5A–F). These data together suggest that adherens junctions formed and/or were stabilized near the border of Ea and Ep, with specific enrichment proximal to the apical surface.
Figure 5. The cadherin-catenin complex (CCC) polarizes along the apical-basal axis of junctions of apically constricting cells. See also Figure S5 and Movie S5.
(A, C, E) Spinning disk confocal fluorescence images of CCC components tagged with GFP. The border between the apically constricting E cells is highlighted with a dotted white box which is enlarged and pseudocolored in the inset (right). Areas used for quantification of apical and basolateral intensities in A, C, and E are circumscribed with colored boxes.
(B, D, F) Plots depicting apical to basal intensity ratio of fluorescence of CCC components. Apical (dark colors) and basolateral (light colors) fluorescence intensity values over time for HMR-1/cadherin-GFP, GFP-HMP-2/β-catenin, and HMP-1/α-catenin-GFP are plotted in B’, D’, and F’, respectively.
(G, H) Spinning disk confocal fluorescence images of HMR-1/cadherin-GFP-expressing, ventrally-mounted embryos at zero and eight minutes following MSa/p cell division initiation. Cell identities are labelled, and the borders quantified in G are highlighted with colored dotted boxes corresponding to the symbols, lines, and error bars depicted in H. Fluorescence intensity normalized to the brightness of Ea/Ep at the time of MSa/p cell division initiation. Significant increases in HMR-1/cadherin accumulation are observed over time at Ea/Ep and Ea/MSa borders, but not at Ep/P4 (n=10 embryos). Error bars represent 95% confidence interval. *p<0.05.
Although the enrichment of CCC proteins was the most obvious at the Ea/Ep apical contact, we also observed CCC enrichment at apical contacts between the EPCs and their neighbors. We sought to quantify in detail which junctions bordering the E cells accumulated cadherin over time. We measured the levels of cadherin-GFP at the cell-cell contact between the two EPCs and between the EPCs two neighboring cells, MSap and P4 (Figure 5G–H). The intensity of HMR-1/cadherin-GFP displayed the largest fold increase at the apical contact between Ea and Ep, but also displayed a significant increase between Ea and MSap. The level of HMR-1/cadherin-GFP did not display a significant increase at the contact between Ep and P4. Taken together, these results reveal detailed dynamics of CCC components imaged in vivo using fluorescent tags inserted into native loci. The enrichment of CCC components to the edges of apical cortexes under high tension [7] suggested to us that tension might upregulate CCC enrichment, leading us to test whether connections to force-producing components are required for CCC enrichment in vivo in this system.
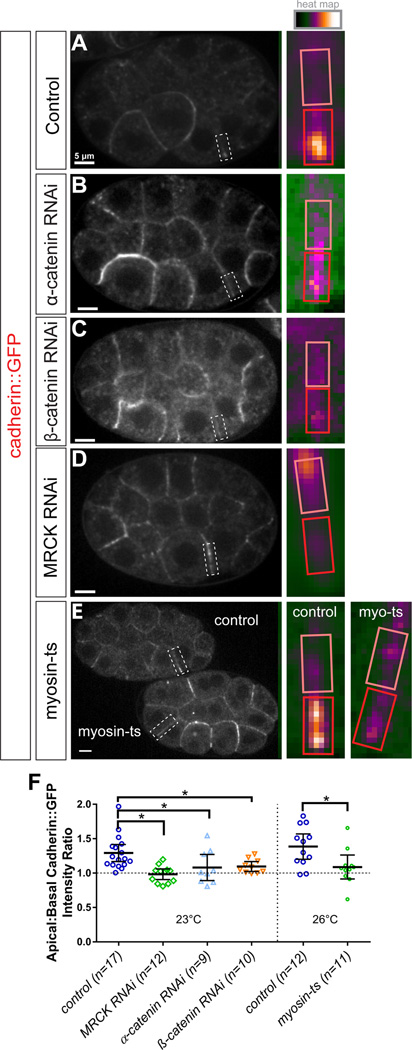
Cadherin requires α- and β-catenin for apical junction enrichment
To test if tension and connections are necessary, we next sought to determine whether HMR-1/cadherin-GFP required β-catenin and α-catenin (Figure S2) to become enriched apically. We used RNAi to knock down the transcripts encoding these proteins and observed the localization of HMR-1/cadherin-GFP. In an independent set of experiments, we assessed quantitatively the extent of HMP-1 and HMP-2 protein knockdown by RNAi. We did this by first mounting three sets of embryos side by side: embryos expressing the fluorescent tags (GFP-HMP-2/β-catenin or HMP-1-GFP/α-catenin), wild type unlabeled embryos, and embryos expressing the fluorescent tags but with the tagged components targeted using dsRNA. This method makes it possible to quantitatively assess knockdown in individual embryos and at localization sites of interest, for example at cell-cell junctions. In all cases, the level of fluorescence in the tagged, knockdown embryos was indistinguishable from that in wild type unlabeled embryos, indicating that RNAi was highly effective (Figure S6). We found that both β-catenin and α-catenin knockdown resulted in a reduction or complete loss of polarization of HMR-1/cadherin-GFP along the apical-basal axis (Figure 6B–C, F), suggesting that cadherin requires β-catenin and α-catenin in order to localize properly in this system.
Figure 6. Myosin II activity is required for apical enrichment of HMR-1/cadherin-GFP in apically constricting cells. See also Figures S6 and S7.
(A–E) Spinning disk confocal fluorescence images of laterally mounted, gastrulation-stage HMR-1/cadherin-GFP-expressing embryos. Genotypes are listed in the boxes to the left. The border between the apically constricting E cells is highlighted with a dotted white box which is enlarged and pseudocolored in the inset (right).
(F) Plot depicting HMR-1/cadherin-GFP apical to basal fluorescence intensity ratio for all above genotypes. Error bars represent 95% confidence interval, n values describe the number of embryos, *p<0.05.
Actomyosin contractility regulates CCC distribution in apically constricting cells
Actomyosin-generated tension is predicted to be highest at the cell-cell contact between Ea and Ep as both cells are actively generating high tension [7]. Given that this cell-cell contact was the site of the greatest CCC polarization along the apico-basal axis, and that cadherin localization depended on β-catenin and α-catenin, which can link cadherin directly to actomyosin networks [17, 18], we hypothesized that apical actomyosin activity might be required to enrich the CCC specifically at apical cell-cell contacts. To test this, we disrupted actomyosin contraction in two ways: first by preventing myosin activation by knocking down MRCK-1, and second using a temperature-sensitive allele of myosin. In mrck-1(RNAi) embryos, the apicobasal polarization of HMR-1/cadherin-GFP was severely disrupted compared to wild type (Figure 6D, F). The accumulation of HMR-1/cadherin-GFP at the apical junction between the Ea and Ep cells was significantly weakened, and instead cadherin-GFP accumulated along the lateral contact between Ea and Ep. Thus, MRCK-1 activity is required for proper apicobasal polarization of cadherin-GFP localization. Consistent with expectations, pac-1(RNAi), which disrupts MRCK-1 localization, resulted in reduced apical HMR-1/cadherin-GFP accumulation (Figure S7).
To directly test a role for myosin, we used a temperature-sensitive allele, ne3409, of the essential C. elegans non-muscle myosin II homolog nmy-2 to disrupt its function specifically during EPC internalization. Previous studies revealed that this allele results in myosin loss of function within 20 seconds of shifting from the permissive temperature (15°C) to the restrictive temperature (26°C) [37]. We mounted four-cell stage HMR-1/cadherin-GFP; nmy-2(ne3409) and HMR-1/cadherin-GFP control embryos side by side at 15–17°C and returned them to 15°C for ~2 hours, until they approached gastrulation stage. Then, we shifted the embryos to the restrictive temperature (26°C), and imaged HMR-1/cadherin-GFP localization (Figure 6E–F). At the restrictive temperature in nmy-2(ne3409) embryos, HMR-1/cadherin-GFP was no longer enriched at the apical junction between the Ea and Ep cells, phenocopying the pattern observed in mrck-1(RNAi) embryos (Figure 6D). HMR-1/cadherin-GFP control embryos displayed normal cadherin-GFP enrichment under these conditions. We conclude that MRCK-1 can polarize HMR-1/cadherin-GFP distribution along the apical-basal axis through its myosin-activating activity.
Discussion
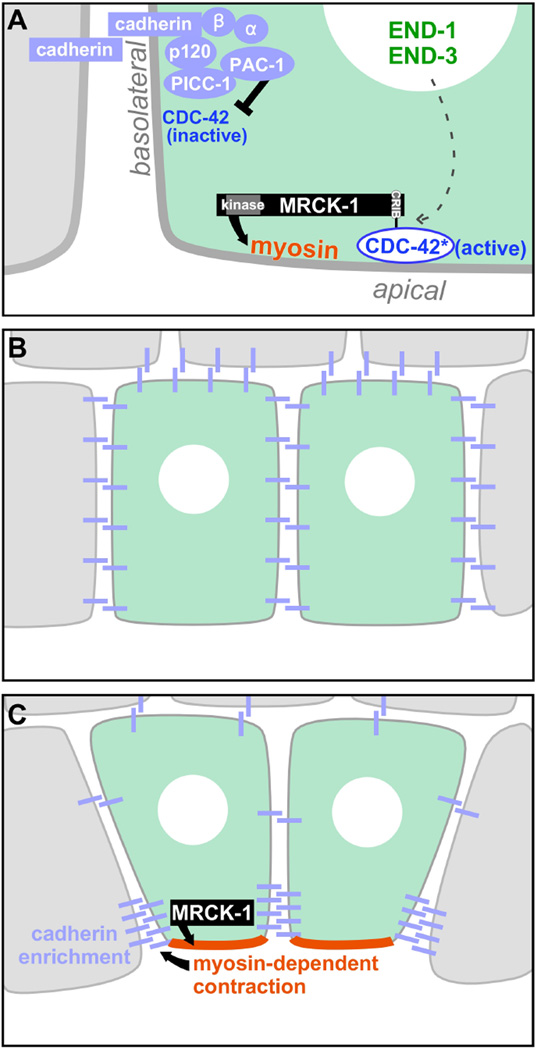
Here we have described how C. elegans early gastrulation movements are regulated by the myosin kinase MRCK-1. We have shown how this kinase is controlled both within the animal, by transcription factors that specify endodermal fate, and subcellularly, through apicobasal patterning of Cdc42 activation. Together these signals specify in which cells and also at which subcellular site constriction will occur. We further show that myosin activation is required to stabilize the force-transmitting cadherin-catenin adherens junctions at the sites of cell-cell contacts between the internalizing cells and their neighbors, presumably leading to a strengthening of adhesion at sites where adhesion is required. In conclusion, this work describes both molecular and mechanistic insights into how developmental patterning mechanisms can result in apical constriction and morphogenesis, summarized in the model in Figure 8.
The mechanisms we identify here show both significant similarities and differences with two other examples of apical constriction that are known to drive morphogenetic movements. In all these systems, including C. elegans, polarized activation of myosin is a key component driving the cell movements [38, 39]. During Drosophila gastrulation and vertebrate neural tube formation, polarized activation of RhoA and/or ROCK leads to myosin activation [40, 41]. In C. elegans, polarized inactivation of Cdc42 leads to polarized activation of myosin at contact-free external surfaces [14]. Although we see a weak effect on gastrulation in LET-502 (ROCK) depleted embryos, suggesting a possible role, and we have previously seen inhibition of gastrulation using MLCK inhibitors (albeit at high enough concentrations to cause non-specific effects [10, 42]) our evidence would suggest that MRCK-1 is the primary activator of myosin during these movements. This is the first example of MRCK-1 being utilized to drive apical constriction and demonstrates that diverse myosin regulators can drive apical constriction in different animal model systems.
Upstream of RhoA and Cdc42, there are more similarities and differences. In Drosophila as in C. elegans, correct patterning is required. In Drosophila, a pair of transcription factors, Snail and Twist, cause autocrine activation of G-protein coupled receptors on the apical surface of the invaginating cells, leading to RhoA activation [43, 44]. During neural tube closure, the protein Shroom is recruited to apical junctions where it recruits Rho associated kinase (ROCK) [45, 46]. RhoA activation downstream of planar cell polarity components [47] is thought to act with Shroom to coordinate ROCK and myosin activation [46, 48]. It remains to be determined whether events analogous to the GPCR activation or Shroom localization of other systems are required for specific localization or activation of MRCK-1 in the internalizing EPCs in C. elegans. Vertebrate MRCK can be activated by regulators binding to its C1 domain [49] suggesting there could be additional regulation in addition to Cdc42 binding. The fact that END-1/3 and MOM-5 regulate MRCK-1 localization during this process suggests further molecular mechanisms to identify.
Previous studies from epithelial cell culture have revealed myosin-dependent enrichment of the CCC at cell-cell junctions [50], and in vivo studies from Drosophila have shown that signaling upstream of myosin activation (i.e. Fog pathway and Rho pathway) is required for proper localization of CCC components during apical constriction in ventral furrow cells [19, 21, 38]. We show that perturbing myosin activity, either indirectly by depleting a kinase required for its activity (MRCK-1) or more directly by a temperature-sensitive mutation in the gene encoding an essential myosin II heavy chain, causes a dramatic rearrangement of cell-cell adhesion components, suggesting that tension-dependent regulation of cadherin enrichment may be evolutionarily widespread. Unexpectedly, we also observed ectopic enrichment of cadherin-GFP to the basolateral cell-cell contact upon myosin disruption, suggesting that the relationship between cadherin recruitment and cortical tension is context-dependent. Recent in vitro results identifying a force-dependent catch bond between α–catenin and F-actin suggest that myosin-based contractile force improves binding of the CCC to the F-actin cytoskeleton [32]. This improved binding could, in principle, promote adherens junction recruitment and/or stabilization. Our results showing myosin-dependent recruitment of adherens junctions during apical constriction provide in vivo data that are consistent with this model.
We previously reported that apical actomyosin contractions precede apical constriction movements, suggesting that apical constriction is triggered by temporally regulated attachments between the actomyosin cortex and apical junctions [7]. We observed a subtle increase in the concentration of CCC components between the early (2–6 minutes after MSx cell division) and late (8–11 minutes after MSx cell division) phases of apical constriction, which might feasibly contribute coupling between cytoskeletal and junctional movements. However, the apical enrichment of CCC components largely precedes the early, uncoupled phase of apical constriction. Thus, while it remains possible that the CCC accumulation we describe here contributes to an improvement in coupling between cytoskeletal and junctional movements, it is likely not sufficient to account for the behavior described previously.
The HMR-1/cadherin complex has been shown in previous studies to play an instructive role in the establishment of apicobasal polarization in early embryonic cells by recruiting the Cdc42 GAP PAC-1 to sites of cell-cell contact through the linker protein PICC-1 [13]. This leads to a decrease in Cdc42 activity along cell-cell contacts relative to the exposed surfaces. We show that the myosin kinase MRCK-1 is downstream of Cdc42 and that its myosin-activating activity positions HMR-1/cadherin to sites of apical cell-cell contact in apically constricting cells. Therefore, HMR-1/Cadherin contributes to its own relocalization: basolateral HMR-1/Cadherin leads indirectly to apical myosin activation, which results in HMR-1/Cadherin relocalization to rings encircling the apical surface, forming the adherens junctions on which actomyosin contractions pull [3]. It will be interesting to see the extent to which this kind of feedback loop between adhesions and myosin contractility might occur in other systems.
Supplementary Material
Figure 7. Model: An outline of how the force-producing mechanisms that drive apical constriction in vivo are spatially regulated.
(A) Active CDC-42, restricted to apical membranes by basolateral inhibition (black inhibitory arrow), recruits MRCK-1 apically via MRCK-1's CRIB domain. MRCK-1 activates myosin (black arrow), as evidenced by regulatory light chain phosphorylation, and increases tension in the apical cortex. MRCK-1 apical enrichment occurs specifically in the two EPCs, dependent (through unknown mechanisms) on the EPC-specific END-1/END-3 transcription factors (dotted arrow). MRCK-1-dependent myosin activity and the actomyosin-cadherin linkers alpha-catenin and beta-catenin contribute through as-yet unexplored mechanisms to junctional cadherin enrichment (B, C).
Acknowledgments
We thank Paul Mains (University of Calgary) and Jeremy Nance (NYU School of Medicine) for sharing strains; and Mark Peifer, Steve Rogers, Richard Cheney and members of the Goldstein lab for helpful suggestions and comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This work was supported by NIH T32 CA009156 and a Howard Hughes postdoctoral fellowship from the Helen Hay Whitney Foundation (D.J.D.); NIH F32 GM115151 (A.M.P.), NIH-funded Duke University Cell and Molecular Biology training grant, T32-GM007184 (A.C.H. and R.P.M.); NIH R01 GM033830 (D.P.K.); and NIH R01 GM083071 (B.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, D.J.M., C.D.H., and B.G.; Investigation, D.J.M., C.D.H., K.A.P., T.D.C., D.J.D., A.M.P., R.P.M., and A.H.C.; Writing - Original Draft, D.J.M., C.D.H., and B.G.; Writing - Review & Editing, D.J.M., C.D.H., K.A.P., T.D.C., D.J.D., D.P.K., and B.G.; Funding Acquisition, D.P.K. and B.G.; Resources, D.P.K. and B.G.; Supervision, D.J.M., D.P.K., and B.G.
References
- 1.Lecuit T, Lenne P-F, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annual Review of Cell and Developmental Biology. 2011;27:157–184. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: A cell shape change that can drive morphogenesis. Developmental Biology. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Diaz A, Toyama Y, Abravanel DL, Wiemann JM, Wells AR, Tulu US, Edwards GS, Kiehart DP. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP J. 2008;2:220–237. doi: 10.2976/1.2955565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnside B. MICROTUBULES AND MICROFILAMENTS IN AMPHIBIAN NEURULATION. American Zoologist. 1973;13:989–1006. [Google Scholar]
- 6.Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 7.Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, Gao L, Betzig E, Kiehart DP, Goldstein B. Triggering a Cell Shape Change by Exploiting Pre-Existing Actomyosin Contractions. Science (New York, Ny) 2012;335:1232–1235. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samarage CR, White MD, Alvarez YD, Fierro-Gonzalez JC, Henon Y, Jesudason EC, Bissiere S, Fouras A, Plachta N. Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. Dev Cell. 2015;34:435–447. doi: 10.1016/j.devcel.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Nance J, Priess JR. Cell polarity and gastrulation in C. elegans. Development (Cambridge, England) 2002;129:387–397. doi: 10.1242/dev.129.2.387. [DOI] [PubMed] [Google Scholar]
- 10.Lee J-Y, Goldstein B. Mechanisms of cell positioning during C. elegans gastrulation. Development. 2003;130:307–320. doi: 10.1242/dev.00211. [DOI] [PubMed] [Google Scholar]
- 11.Maduro MF. Cell fate specification in the C. elegans embryo. Dev Dyn. 2010;239:1315–1329. doi: 10.1002/dvdy.22233. [DOI] [PubMed] [Google Scholar]
- 12.Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- 13.Klompstra D, Anderson DC, Yeh JY, Zilberman Y, Nance J. An instructive role for C. elegans E-cadherin in translating cell contact cues into cortical polarity. Nat Cell Biol. 2015;17:726–735. doi: 10.1038/ncb3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science. 2008;320:1771–1774. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markham NO, Cooper T, Goff M, Gribben EM, Carnahan RH, Reynolds AB. Monoclonal antibodies to DIPA: a novel binding partner of p120-catenin isoform 1. Hybridoma (Larchmt) 2012;31:246–254. doi: 10.1089/hyb.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori N, Kuwamura M, Tanaka N, Hirano R, Nabe M, Ibuki M, Yamate J. Ccdc85c encoding a protein at apical junctions of radial glia is disrupted in hemorrhagic hydrocephalus (hhy) mice. Am J Pathol. 2012;180:314–327. doi: 10.1016/j.ajpath.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. Journal of Cell Biology. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng M, Wieschaus E. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J Cell Biol. 2016 doi: 10.1083/jcb.201508056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daneshjou N, Sieracki N, Amerongen GPvN, Schwartz MA, Komarova YA, Malik AB. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. The Journal of Cell Biology. 2015;208:23–32. doi: 10.1083/jcb.201409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development (Cambridge, England) 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 22.Pfister K, Shook DR, Chang C, Keller R, Skoglund P. Molecular model for force production and transmission during vertebrate gastrulation. Development. 2016;143:715–727. doi: 10.1242/dev.128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J-Y, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled Signaling Controls C. elegans Gastrulation by Activating Actomyosin Contractility. Current biology : CB. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gally C, Wissler F, Zahreddine H, Quintin S, Landmann F, Labouesse M. Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development. 2009;136:3109–3119. doi: 10.1242/dev.039412. [DOI] [PubMed] [Google Scholar]
- 25.Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J Cell Sci. 2002;115:2271–2282. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- 26.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Z, Manser E. Myotonic dystrophy kinase-related Cdc42-binding kinases (MRCK), the ROCK-like effectors of Cdc42 and Rac1. Small GTPases. 2015;6:81–88. doi: 10.1080/21541248.2014.1000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nature Methods. 2013;10:1028–1034. doi: 10.1038/nmeth.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maduro MF, Hill RJ, Heid PJ, Newman-Smith ED, Zhu J, Priess JR, Rothman JH. Genetic redundancy in endoderm specification within the genus Caenorhabditis. Dev Biol. 2005;284:509–522. doi: 10.1016/j.ydbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Mello CC, Draper BW, Krause M, Weintraub H, Priess JR. The pie-1 and mex-1 genes and maternal control of blastomere identity in early C. elegans embryos. Cell. 1992;70:163–176. doi: 10.1016/0092-8674(92)90542-k. [DOI] [PubMed] [Google Scholar]
- 31.Pirone DM, Carter DE, Burbelo PD. Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet. 2001;17:370–373. doi: 10.1016/s0168-9525(01)02311-3. [DOI] [PubMed] [Google Scholar]
- 32.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science (New York, NY) 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grana TM, Cox EA, Lynch AM, Hardin J. SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Developmental Biology. 2010;344:731–744. doi: 10.1016/j.ydbio.2010.05.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stetak A, Hajnal A. The C. elegans MAGI-1 protein is a novel component of cell junctions that is required for junctional compartmentalization. Developmental Biology. 2011;350:24–31. doi: 10.1016/j.ydbio.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A Putative Catenin–Cadherin System Mediates Morphogenesis of the Caenorhabditis elegans Embryo. The Journal of Cell Biology. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chihara D, Nance J. An E-cadherin-mediated hitchhiking mechanism for C. elegans germ cell internalization during gastrulation. Development. 2012;139:2547–2556. doi: 10.1242/dev.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies T, Jordan SN, Chand V, Sees JA, Laband K, Carvalho AX, Shirasu-Hiza M, Kovar DR, Dumont J, Canman JC. High-resolution temporal analysis reveals a functional timeline for the molecular regulation of cytokinesis. Developmental Cell. 2014;30:209–223. doi: 10.1016/j.devcel.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin–myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Copp AJ, Greene NDE. Genetics and development of neural tube defects. The Journal of pathology. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 41.Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 44.Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64:447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- 45.Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Plageman TF, Jr, Chauhan BK, Yang C, Jaudon F, Shang X, Zheng Y, Lou M, Debant A, Hildebrand JD, Lang RA. A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination. Development. 2011;138:5177–5188. doi: 10.1242/dev.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huo L, Wen W, Wang R, Kam C, Xia J, Feng W, Zhang M. Cdc42-dependent formation of the ZO-1/MRCKbeta complex at the leading edge controls cell migration. EMBO J. 2011;30:665–678. doi: 10.1038/emboj.2010.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 Is a Key Rho Kinase Target Necessary for the Local Concentration of E-Cadherin at Cell–Cell Contacts. Molecular Biology of the Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.