Abstract
The structures of the leucine transporter, drosophila dopamine transporter, and human serotonin transporter show a secondary binding site (designated S2) for drugs and substrate in the extracellular vestibule towards the membrane exterior in relation to the primary substrate recognition site (S1). The present experiments are aimed at disrupting S2 by mutating Asp476 and Ile159 to Ala. Both mutants displayed a profound decrease in [3H]DA uptake compared with wild-type associated with a reduced turnover rate kcat. This was not caused by a conformational bias as the mutants responded to Zn2+ (10 µM) similarly as WT. The dopamine transporters with either the D476A or I159A mutation both displayed a higher Ki for DA for the inhibition of [3H]CFT binding than did the WT transporter, in accordance with an allosteric interaction between the S1 and S2 sites. The results provide evidence in favor of a general applicability of the two-site allosteric model of the Javitch/Weinstein group from LeuT to DAT and possibly other monoamine transporters.
Keywords: dopamine binding; transporter point mutants; cocaine analog binding; CFT; WIN 35,428; Zinc
Introduction
The dopamine, serotonin, and norepinephrine transporters accumulate monoamine substrate against its concentration gradient with the required energy provided by the electrochemical gradient for Na+ (Schmitt & Reith 2010). Structural information for the monoamine transporters comes from the crystals of the leucine transporter (LeuT), drosophila DAT (Yamashita et al. 2005, Zhou et al. 2007, Penmatsa et al. 2013), and recently the human serotonin transporter (Coleman et al. 2016), showing an additional secondary site (S2) in the extracellular vestibule to a primary binding site (S1) for substrates and various types of inhibitors (Yamashita et al. 2005, Zhou et al. 2007, Penmatsa et al. 2013, Coleman et al. 2016, Zhou et al. 2009). It was proposed by the Javitch/Weinstein group that the S2 site in LeuT plays a crucial role in substrate transport: Binding of substrate to the S2 site triggers the release of Na+ and substrate from the S1 site (Shi et al. 2008). On the other hand, studies by the Gouaux group suggested that LeuT has only one binding site for leucine measured by various biophysical methods (Piscitelli et al., 2010); S1-mutation F253A-LeuT still only showed leucine binding to S1 (Wang & Gouaux 2012); and LeuT structures obtained in bicells in the absence of S2-interfering n-octyl-β-D-glucopyranoside did not contain S2-bound substrate (Piscitelli et al. 2010, Wang et al. 2012b, Wang & Gouaux 2012). The Javitch/Weinstein group, however, showed destruction of the S2 site by protein preparation procedures used for crystallography, resulting in a substrate: LeuT stoichiometry of 1 (Quick et al. 2012). In a convincing computational study, the two-site substrate transport model has been successfully applied to human (h) DAT (Shan et al. 2011), and a functional role for the S2 site has been firmly established in the serotonin transporter where it allosterically interacts with the S1 site (Zhu et al. 2016, Plenge et al. 2012). The S2 and S1 sites are separated by 11–13 Å, and can be bridged by bivalent substrate ligands in our computational models (Schmitt et al. 2010). Enhanced binding affinities were observed with ligands bearing two active heads of DA, or heterologous combinations of DA, β-phenethylamine and amphetamine heads, up to two orders of magnitude over the original monovalent substrate (Schmitt et al. 2010).
The present experiments are aimed at studying the role of the S2 site in transport of DA by hDAT. To this end, the S2 site was compromised in D476A and I159A in the computational model (Shan et al. 2011). Ile159 corresponds to Ile111 in LeuT, known to be crucial in interaction with the substrate (Shi et al. 2008) and Asp476 in a dDAT-based computational model of hDAT forms a salt-bridge with Arg85 at the bottom of the S2 site as part of the closed external gate in the occluded form of DAT with DA bound to the S1 site (Cheng et al. 2015). We hypothesized a major impact of S2 disruption on DAT function, pointing to an important role of the S2 site in the substrate translocation process. In order to better understand the two-site model for DA transport, we studied (i) the capability of the two S2 mutants to transport DA, (ii) their conformational bias with micromolar concentrations of Zn2+ known to promote outward-facing DAT (see Discussion), and (iii) their interaction with DA as measured by inhibition of binding of the cocaine analog [3H]CFT.
Materials and methods
Site-directed mutagenesis and heterologous expression of hDAT mutant constructs
The mutants I195A and D476A were generated (WT-hDAT cDNA is from Dr. Jonathan Javitch at Columbia University) with the Quick-change site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s protocol and confirmed by DNA sequencing (Genewiz Inc., South Plainfield, NJ, USA). The pair of primers for I159A were 5'-cttcttttacaacgtcgccattgcctgggctctcc-3' and 5'-ggagagcccaggcaatggcgaxcgttgtaaaagaag-3' while primers for D476A were 5’-gaatctacgtgttcaccctcctggaccacttcgctgccggcagcac-3’ and 5’-gtgccggcagcgaagtggtccaggagggtgaacacgtagattc-3’.
Culturing and stable transfection of Lewis Lung Carcinoma-Porcine Kidney (LLC-PK1) cells (gift from Dr. Roxanne Vaughan at University of North Dakota) with human dopamine transporter (hDAT) constructs by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was carried out as in our previous work (Chen et al. 2001). Stable cells were maintained in minimum-essential medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 200 µg/mL G418 with 5% CO2 at 37°C in a cell incubation chamber. Cells were grown to about 70% confluence in culture plates prior to experimentation. To enhance the expression of hDAT I159A, sodium butyrate (NaBt) was added into cell culture plates at final concentration of 5 mM and incubated for 24 hrs before each experiment. Constitutional approval applied to the work with cloned human transporters in heterologous cell lines and bacterial amplification (Biosafety Level 2).
[3H]CFT binding assay and [3H]dopamine uptake assay
[3H]CFT (CFT=2β-carbomethoxy-3β-[4-fluorophenyl]-tropane, 85.9 Ci/mmole) and [3H]DA (48 Ci/mmole) are from Perkin Elmer (Boston, MA, USA). Binding of [3H]CFT (Chen et al. 2001) and [3H]DA uptake assays (Ng et al. 2014) to stably transfected LLC-PK1 cells were measured by procedures as described by us previously. Briefly, intact cell suspensions were incubated with [3H]CFT (4 nM final concentration) and varying concentrations of non-labelled CFT or DA spanning their IC50 values for 20 minutes at 21°C. A final concentration of 10 µM CFT was used to define nonspecific binding. [3H]DA (5nM final concentration) and non-labelled DA were added into 24-well cell culture plates and incubation continued for 5 min at 21°C.
Immunoblotting studies
Surface DAT was assessed with sulfo-NHS-SS-biotin as described by us previously (Ng et al. 2014). Total lysates and biotinylated proteins were resolved on 8% Tris-Glycine mini gels and probed with polyclonal anti-DAT antibody against the N-terminal of DAT (Millipore, Billerica, MA, USA), followed by HRP-conjugated goat anti-rabbit antibody (Thermo scientific, Waltham, MA, USA). Polyclonal anti–β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA) was used as an internal control for loading. The transporter signal was visualized using Thermo Scientific SuperSignal West Pico Chemiluminescent Substrate solution (Thermo Scientific, Waltham, MA, USA) and quantified using Image-J (from website of National Institute of Heath).
Data analysis
The Michaelis-Menten constant (Km) and maximal velocity (Vmax) of [3H]dopamine uptake and the binding affinity (Kd) and the maximum binding site (Bmax) of [3H]CFT binding were calculated by non-linear regression with the Radlig software (KELL program). Half maximal inhibitory concentration (IC50) was estimated by logistic fitting of data by the ORIGIN software (Origin Lab Co.) and converted to inhibition constant (Ki) by the Cheng- Prusoff equation. Statistical analyses were done with SigmaPlot software (Systat Software Inc. San Jose, CA, USA). Results are expressed as the Mean±SEM averaged from at least three independent experiments.
Statistical analysis
Multiple comparisons of group means were performed by one-way ANOVA followed by Dunnett’s Method. The Student’s t-test was used for comparing two group values, and the one-sample t-test for comparing effects with a theoretical effect of 0%, two-tailed in both cases.
In preparing the present work for publication, the guide for ethical behavior in publishing research was followed as described in the COPE Report 2003 (available from the Committee on Publication Ethics (COPE)).
Results
Effect of mutations on DAT expression and function
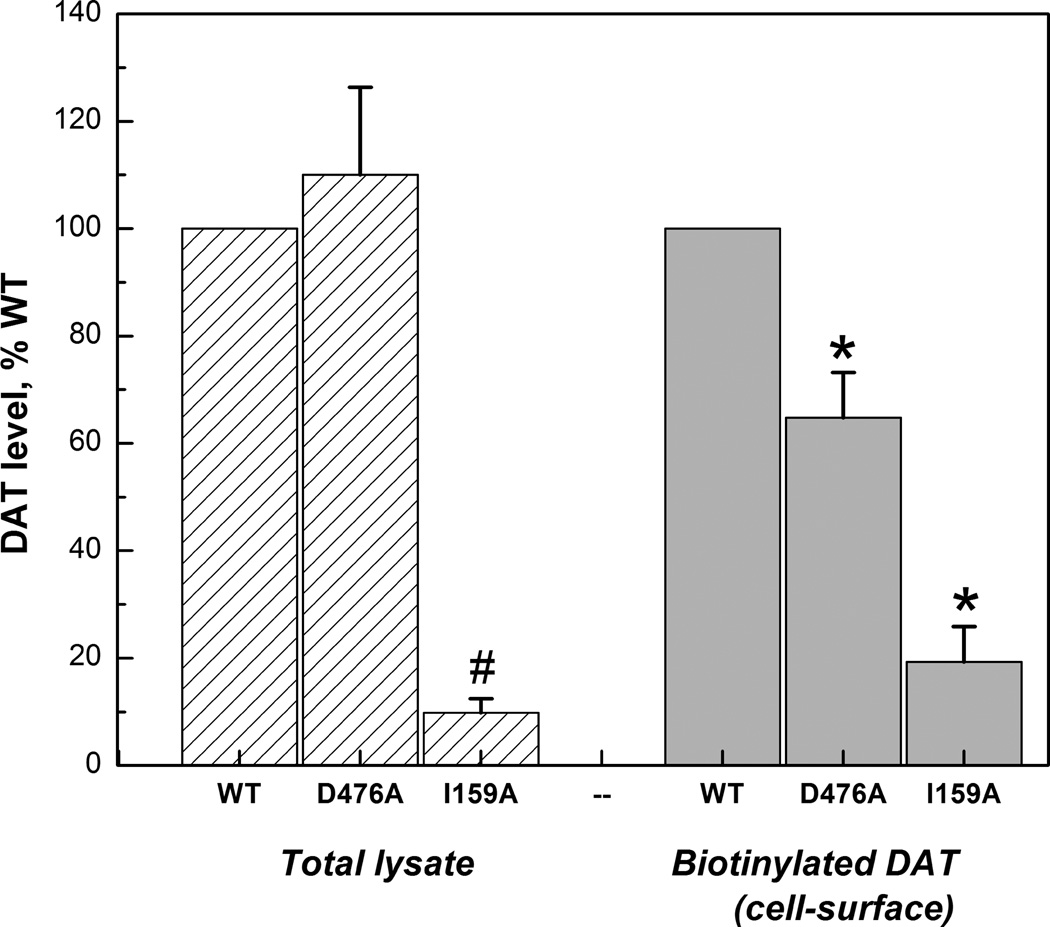
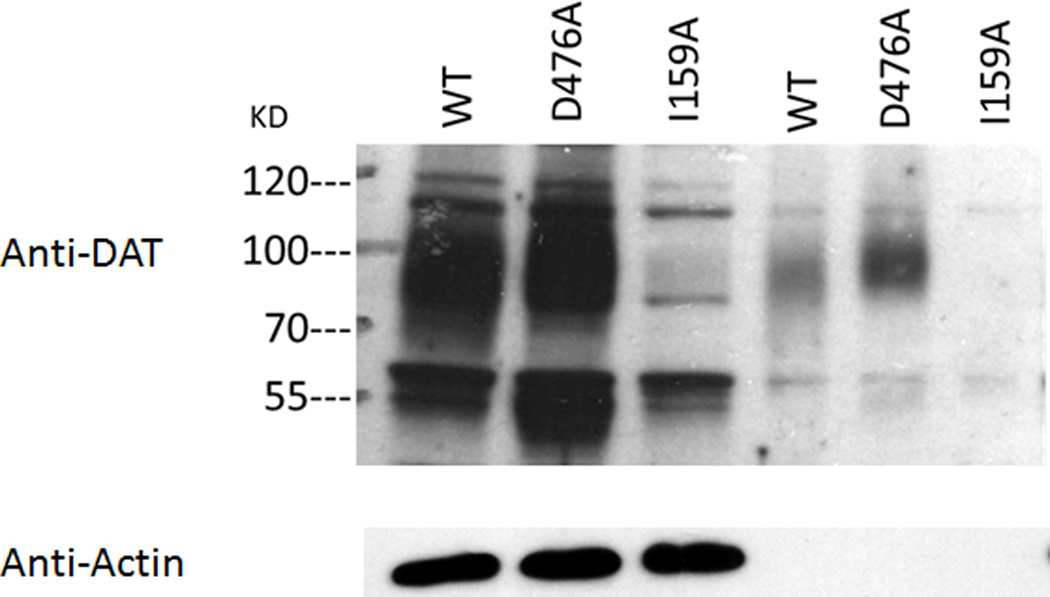
Surface expression of hDAT expressed in LLC-PK1 cells was evaluated by [3H]CFT binding and biotinylation. The Bmax of [3H]CFT binding at intact cells reflects surface DAT (Chen et al. 2004b) and indeed a close correspondence was observed between the Bmax values (WT > D476A > I159A, Table 1) and the biotinylation density values (WT > D476A > I159A, Fig. 1A). D476A and I159A displayed 55.2% and 23.9% of the Bmax vaIue of WT, and 64.8% and 19.3% of the biotinylation density value of WT. It should be noted here that in order to enhance the surface expression (Liang et al. 2013, Chen & Reith 2007) of I159A cells, 5 mM NaBt was applied; without NaBt the expression of I159A was extremely low. No differences between the constructs were observed for the [3H]CFT binding Kd, in contrast to the affinity of DA as measured by competition for [3H]CFT binding. Both mutants displayed much weaker affinities for DA than WT: DA’s Ki was increased sixteen-fold in D476A (from 4.82 to 83.6 µM) and five-fold in I159A (to 26.1 µM) (Table 1, top panel).
Table 1.
[3H]CFT saturation binding parameters (Kd and Bmax) and inhibition constant (Ki) of dopamine in competing for the binding of [3H]CFT to hDAT constructs WT, D476A and I159A with (n=4) and without Zn2+ treatment (n=3).
| Cells | Kd of CFT, nM | Bmax, pmole/mg | DA Ki, µM |
|---|---|---|---|
| WT | 21.5±3.10 | 2.01±0.22 | 4.82±0.79 |
| D476A | 29.0±7.48 | 1.11±0.33* | 83.6±3.75 * |
| I159A | 25.1±4.7 | 0.48±0.08** | 26.1±3.88 * |
| + 10 µM ZnCl2 | |||
| WT | 6.85±1.01 # | 1.28±0.20 | 67.4±5.49 # |
| D476A | 5.65±1.91 # | 1.26±0.02 | 91.8±21.6 |
| I159A | 11.39±1.14 # | 0.80±0.21 | 29.73±5.86 |
: P<0.05 vs WT (One-way ANOVA with post-hoc Dunnett’s test)
: P<0.05 vs without Zn2+ (Student’s t-test)
Figure 1.
Quantification of hDAT of total extraction and surface fraction in in LLC-PK1 cells stably expressing hDAT constructs (A) and the representative western blots (B). The integrated-density-value (IDV) for the protein hDAT are expressed as percentage of WT. Note that the bar graphs represent the average and SEM of all obtained results while the gel shown is one typical example.*: P<0.05 vs WT (one-Way ANOVA with post hoc Dunnett’s Method)
Disruption of the S2 site in D476A greatly decreased the [3H]DA uptake activity, mostly due to a decrease in Vmax (an order of magnitude) and to a lesser extent to an increase in Km (4-fold) (Table 2). The Vmax was also decreased (order of magnitude), along with increased Km (2-fold), for I159A. The Vmax effect is necessarily a function of the low membrane expression of hDAT. In order to take that out of the equation, DA turnover kcat (Vmax / Bmax) was computed, and it was appreciably reduced in the mutants (3- to 9-fold) (Table 2). According to the simplified carrier model as used by others (Schomig et al. 1988) and ourselves (Chen et al. 2001), knowledge of the values of the parameters in Table 2 and additionally the DA Ki and [3H]CFT binding Bmax as listed in Table 1, allows the calculation of the forward-rate constant for loaded-transporter k2 and the reorientation-rate constant for empty-transporter k3 (Table 2). As noted in our previous work with multiple mutants of hDAT, kcat largely reflects k3 (Chen et al. 2001), consonant with reorientation of the empty transporter being the rate-limiting step in transport. Noteworthy, k3 shows the same 3- to 9-fold reduction in the mutants compared with WT as kcat does (Table 2). However, there were also notable reductions in k2 (Table 2).
Table 2.
Kinetic properties of [3H]dopamine uptake for WT, D476A and I159A stably expressed in LLC-PK1 cells (n=3).
| Cells | Km, µM | Vmax,pmole/mg/min | kcat, min−1 | k2, min−1 | k3, min−1 |
|---|---|---|---|---|---|
| WT | 0.76±0.06 | 4.54±1.18 | 2.26±0.63 | 14.3 | 2.68 |
| D476A | 3.27±1.27 | 0.29±0.10* | 0.26±0.12* | 6.65 | 0.270 |
| I159A | 1.70±0.34 | 0.33±0.02* | 0.69±0.12* | 10.6 | 0.738 |
Additional parameters, DA Ki and [3H]CFT binding Bmax, needed for these calculations were taken from Table 1.
: P<0.05 vs WT (One-Way Anova with post hoc Dunnett’s Method)
“Equilibrium” binding experiments and the effect of Zn2+
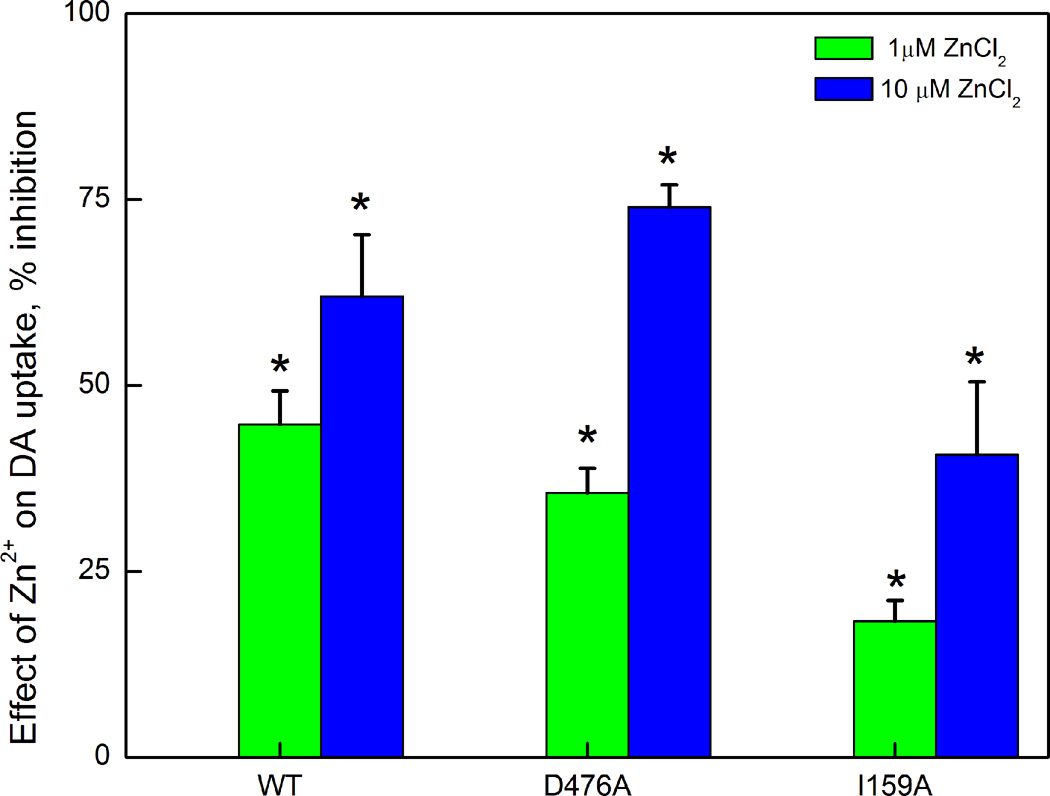
Zn2+ (10 µM) increased the affinity (decreased Kd) of [3H]CFT binding to a similar extent in D476A as in WT; a somewhat smaller decrease in I159A (Table 1 bottom panel). This is in contrast to the enhanced effect seen in inward-facing mutants (Liang et al. 2009, Chen et al. 2004a). Moreover, Zn2+ (1 and 10 µM) inhibited DA uptake equally in WT and D476A; somewhat smaller effects were displayed by I159A (Fig. 2); in contrast, an inward-facing mutant generally responds with an increase in DA uptake (Loland et al. 2002, Loland et al. 2004).
Figure 2.
Inhibitory effect of ZnCl2 on the [3H]DA uptake activity of WT, D476A and I159A. Values are expressed as percent inhibition.*: P<0.05 vs theoretical value of 0% (one sample Student’s t-test).
Discussion
This report presents k2 and k3 values for dopamine’s forward translocation by loaded transporter and reorientation by empty transporter, respectively, based on existing kinetic models (Chen et al. 2001, Li et al. 2015). However, it should be considered here that the concentration of DA at the S1 site during the translocation cycle may not be equal to the outside DA in an intact-cell uptake assay with a permeation pathway separating the S2 and S1 sites. Yet, it is this [DA]outside that is used for calculating values for Ki and Km which, in turn, informs k2 and k3. Serious doubt regarding this calculation is raised by the present results for D476A. Thus, when the transporter is fully inward facing (open-to-inside), the external gate, one pair of which is salt-bridge Asp476–Arg85, is normally closed and will open up upon reorientation of the transport to outward facing (Shan et al. 2011). In the D476A mutant, reorientation does not need to open the Asp476–Arg85 bond, and therefore one would expect the reorientation-rate k3 to be, if anything, enhanced, rather than reduced as observed (table 2). Based on this argument, the true magnitude of k3 may well be different from what we calculate; the k2 results, which are based on calculations using the same [DA]i, also need to be viewed with great caution. We do not therefore attach much significance to the relatively small decreases (1.5- to 2-fold) observed for the forward transport rate constant k2 by D476A or I159A mutation (table 2). Decreases of greater magnitude would be likely if the S2 site plays a major role in forward transport of DA.
Micromolar concentrations of Zn2+ are known to promote outward-facing conformations of DAT (Chen et al. 2004a, Liang et al. 2009, Li et al. 2015, Stockner et al. 2013). With Zn2+, DAT spends more time in open-to-out conformations in which D79 is in a salt-bridge with R85 and unavailable for participating in binding to DA (see Cheng et al. 2015). Zn2+ (10 µM) increased DA’s Ki in WT (Table 1 compare top- and bottom-panel). The results, then, can be understood by taking into account that the preferential DAT conformation with DA bound in the S1 site is the occluded form with the external gate closed (Shan et al. 2011, Cheng et al. 2015). In both mutants, the lower affinity for DA (Table 1 top-panel) remained low with Zn2+ (bottom-panel). In a longer-lasting binding experiment, we reasoned that the ambient concentration of DA exposed to S1 can be equated with outside [DA], and therefore the higher Ki truly indicates a lower affinity of the S1 site for DA.
Zn2+ effects on DA transport are more complex, involving membrane potential (Meinild et al. 2004) with changes in both uptake and efflux (Scholze et al. 2002). Most recently, a role for internal [Na+] has been uncovered, such that Zn2+ enhances DA uptake in systems with low [Na+]i but lowers uptake at high [Na+]i (Li et al. 2015). Evidently, our uptake system is in the latter category. Regardless of whether we consider Zn2+ effects on uptake or binding in the present experiments, the mutants responded in a similar way to Zn2+ as WT (Table 1 and Fig. 2), suggesting S2 site disruption did not introduce a conformational bias.
Computational and biochemical experiments in LeuT indicate that binding of a second substrate molecule to the S2 site triggers intracellular release of Na+ and the first substrate molecule from the S1 site thereby affording substrate translocation (Shi et al. 2008). A similar transport mechanism has been proposed for the DAT (Shan et al., 2011). Crucial in the model is the allosteric interaction between the S2 and S1 sites, with ligand occupancy in one affecting the functionality of the other. Such allosterism has been firmly established for the serotonin transporter (Zhu et al. 2016, Plenge et al. 2012). The results of the present study on hDAT provide two lines of evidence in favor of a model with a crucial role of the S2 site in DA transport. First, DA transport is severely impaired in hDAT constructs with mutations of two residues crucial for S2 site functionality: D476 and I159, as evidenced by the considerable reduction in DAT turnover rate (kcat) (table 2). Impairment of substrate transport by disruptions in the S2 site has also been observed for a closely related transporter, hNET (Wang et al. 2012a): mutation of many of its S2 residues to alanine disrupts NE transport, notably mutation of W80, R81, Y152, F317, F388, and D473 (equivalents in hDAT are W84, R85, Y156, F320, F391, and D476, respectively). Second, both D476A and I159A display a higher Ki for DA in inhibiting [3H]CFT binding than WT (Table 1 top panel). As mentioned above, this provides evidence for an allosteric effect of S2 site disruption on the functional state of the S1 site, which is one crucial property of two-site-mediated substrate transport (Shi et al., 2008). Although it could be thought that the sole role of the S2 site is to help along the permeation of DA through its trajectory from out to in by providing low-affinity DA binding along the way, the reduced affinity of DA binding to S2 site-impaired DAT constructs argues in favor of a general applicability of the two-site allosteric model of the Javitch/Weinstein group from bacterial LeuT to DAT and possibly other mammalian monoamine transporters.
Acknowledgments
This study was supported by a grant from the National Institutes of Health National Institute on Drug Abuse (R01 DA019676 to MEAR).
The abbreviations used are
- DA
dopamine
- DAT
dopamine transporter
- CFT
(-)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane
Footnotes
The authors have no conflict of interest to declare.
References
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. Journal of neurochemistry. 2007;101:377–388. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Rickey J, Berfield JL, Reith ME. Aspartate 345 of the dopamine transporter is critical for conformational changes in substrate translocation and cocaine binding. The Journal of biological chemistry. 2004a;279:5508–5519. doi: 10.1074/jbc.M306294200. [DOI] [PubMed] [Google Scholar]
- Chen N, Vaughan RA, Reith ME. The role of conserved tryptophan and acidic residues in the human dopamine transporter as characterized by site-directed mutagenesis. Journal of neurochemistry. 2001;77:1116–1127. doi: 10.1046/j.1471-4159.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhen J, Reith ME. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. Journal of neurochemistry. 2004b;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- Cheng MH, Block E, Hu F, Cobanoglu MC, Sorkin A, Bahar I. Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding. Frontiers in neurology. 2015;6:134. doi: 10.3389/fneur.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532:334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hasenhuetl PS, Schicker K, Sitte HH, Freissmuth M, Sandtner W. Dual Action of Zn2+ on the Transport Cycle of the Dopamine Transporter. The Journal of biological chemistry. 2015;290:31069–31076. doi: 10.1074/jbc.M115.688275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wang RS, Wang F, Liu S, Guo F, Sun L, Wang YJ, Sun YX, Chen XL. Sodium butyrate protects against severe burn-induced remote acute lung injury in rats. PloS one. 2013;8:e68786. doi: 10.1371/journal.pone.0068786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YJ, Zhen J, Chen N, Reith ME. Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters. Journal of neurochemistry. 2009;109:981–994. doi: 10.1111/j.1471-4159.2009.06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Granas C, Javitch JA, Gether U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. The Journal of biological chemistry. 2004;279:3228–3238. doi: 10.1074/jbc.M304755200. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinild AK, Sitte HH, Gether U. Zinc potentiates an uncoupled anion conductance associated with the dopamine transporter. The Journal of biological chemistry. 2004;279:49671–49679. doi: 10.1074/jbc.M407660200. [DOI] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, et al. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain : a journal of neurology. 2014;137:1107–1119. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 2010;468:1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge P, Shi L, Beuming T, Te J, Newman AH, Weinstein H, Gether U, Loland CJ. Steric hindrance mutagenesis in the conserved extracellular vestibule impedes allosteric binding of antidepressants to the serotonin transporter. The Journal of biological chemistry. 2012;287:39316–39326. doi: 10.1074/jbc.M112.371765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Shi L, Zehnpfennig B, Weinstein H, Javitch JA. Experimental conditions can obscure the second high-affinity site in LeuT. Nature structural & molecular biology. 2012;19:207–211. doi: 10.1038/nsmb.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Mamidyala S, Biswas S, Dutta AK, Reith ME. Bivalent phenethylamines as novel dopamine transporter inhibitors: evidence for multiple substrate-binding sites in a single transporter. Journal of neurochemistry. 2010;112:1605–1618. doi: 10.1111/j.1471-4159.2010.06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Annals of the New York Academy of Sciences. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Scholze P, Norregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH. The role of zinc ions in reverse transport mediated by monoamine transporters. The Journal of biological chemistry. 2002;277:21505–21513. doi: 10.1074/jbc.M112265200. [DOI] [PubMed] [Google Scholar]
- Schomig E, Korber M, Bonisch H. Kinetic evidence for a common binding site for substrates and inhibitors of the neuronal noradrenaline carrier. Naunyn-Schmiedeberg's archives of pharmacology. 1988;337:626–632. doi: 10.1007/BF00175787. [DOI] [PubMed] [Google Scholar]
- Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PloS one. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Molecular cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockner T, Montgomery TR, Kudlacek O, Weissensteiner R, Ecker GF, Freissmuth M, Sitte HH. Mutational analysis of the high-affinity zinc binding site validates a refined human dopamine transporter homology model. PLoS computational biology. 2013;9:e1002909. doi: 10.1371/journal.pcbi.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CI, Shaikh NH, Ramu S, Lewis RJ. A second extracellular site is required for norepinephrine transport by the human norepinephrine transporter. Molecular pharmacology. 2012a;82:898–909. doi: 10.1124/mol.112.080630. [DOI] [PubMed] [Google Scholar]
- Wang H, Elferich J, Gouaux E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nature structural & molecular biology. 2012b;19:212–219. doi: 10.1038/nsmb.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gouaux E. Substrate binds in the S1 site of the F253A mutant of LeuT, a neurotransmitter sodium symporter homologue. EMBO reports. 2012;13:861–866. doi: 10.1038/embor.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith ME, Wang DN. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science (New York, N.Y.) 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nature structural & molecular biology. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Sinwel D, Hasenhuetl PS, et al. Nanopharmacological Force Sensing to Reveal Allosteric Coupling in Transporter Binding Sites. Angewandte Chemie (International ed. in English) 2016;55:1719–1722. doi: 10.1002/anie.201508755. [DOI] [PMC free article] [PubMed] [Google Scholar]