Abstract
Mitochondria-dependent programmed cell death (PCD) in yeast shares many features with the intrinsic apoptotic pathway of mammals. With many stimuli, increased cytosolic [Ca2+] and ROS generation are the triggering signals that lead to mitochondrial permeabilization and release of proapoptotic factors, which initiates yeast PCD. While in mammals the permeability transition pore (PTP), a high-conductance inner membrane channel activated by increased matrix Ca2+ and oxidative stress, is recognized as part of this signaling cascade, whether a similar process occurs in yeast is still debated. The potential role of the PTP in yeast PCD has generally been overlooked because yeast mitochondria lack the Ca2+ uniporter, which in mammals allows rapid equilibration of cytosolic Ca2+ with the matrix. In this short review we discuss the nature of the yeast permeability transition and reevaluate its potential role in the effector phase of yeast PCD triggered by Ca2+ and oxidative stress.
Keywords: Yeast Mitochondria, Programmed cell death, Ca2+, Reactive oxygen species, Permeability transition, F-ATP synthase
Graphical Abstract
1. Mitochondria and yeast programmed cell death
Occurrence of programmed cell death (PCD) in yeast and its role in population dynamics and aging is increasingly understood [1,2]. Many of the signaling events of the mammalian intrinsic (mitochondrial) pathway to apoptosis take place in yeast [3]. It was reported that yeast expresses a BH3 domain-containing protein (Ybh3p, also called Ynl305cp or Bx1p) that upon treatment with lethal stimuli translocates to mitochondria and triggers apoptosis [4]. Ybh3p was initially proposed to be a member of the Bax Inhibitor 1 protein family (Bl-1), due to the presence of a BI-1 transmembrane motif contrasting the death-inducing effect of Bax [5] through a Ca2+-dependent mechanism triggered by ER stress [6]. To date, the physiological function of Ybh3p is still under debate, yet these findings suggest that yeast cells may possess a homolog of the mammalian Bax/Bak pathway for outer mitochondrial membrane (OMM) permeabilization and cell death initiation [7]. Interestingly, S. cerevisiae is also endowed with Yca1p, a metacaspase that may be functionally related to mammalian effector caspases [8,9].
Key events of yeast apoptosis include increase of intracellular [Ca2+] and reactive oxygen species (ROS), which are causally involved in yeast PCD induced by oxidative stress itself [10], acetic acid [11-13], pheromone or amiodarone [14,15], ethanol [16] and osmotic [17] or ER stress [18]. As in mammals, increased intracellular [Ca2+] precedes the surge of ROS levels, cristae remodeling, mitochondrial depolarization, ATP depletion, matrix swelling and outer membrane permeabilization eventually leading to release of cytochrome c and other proapoptotic proteins [1-3]. In yeast, the link between increased intracellular [Ca2+]/ROS and OMM permeabilization remains puzzling because (i) yeast does not possess a mitochondrial Ca2+ uniporter (MCU) [19-21], which in mammals mediates rapid equilibration of Ca2+ across the inner mitochondrial membrane (IMM) [22,23]; and (ii) there is no homolog of mammalian Apaf-1 in yeast, so that cytochrome c release does not trigger caspase activation while it may certainly contribute to decreased respiration, increased ROS production and ATP depletion.
Matrix Ca2+ is essential for opening of the permeability transition pore (PTP), an IMM high-conductance channel that can form under conditions of oxidative stress and mediates a permeability increase (the permeability transition, PT) causing many of the above-mentioned mitochondrial events of PCD in mammals [24]. The defining features of the mammalian pore are high conductance, up to 1.3 nS [25,26], which allows permeation of solutes up to about 1.5 kDa in mass [27]; absolute requirement for matrix Ca2+[27]; inhibition by Mg2+ and adenine nucleotides [28]; inhibition (desensitization to Ca2+) by cyclosporin A (CsA) [29,30], an effect exerted through matrix cyclophilin (CyP) D [31]; and requirement for inducing factors such as oxidants and Pi [32] (Table I). Whether a bona fide PTP exists in yeast, and whether it plays a role in yeast PCD, has been the matter of debate [33].
Table I. Properties of the PTP across species.
The Table summarizes the conductance of F-ATP synthase channels, the effect of the indicated treatments on the PTP, and the presence of a mitochondrial CyP. For further explanation see text.
| Conductance (pS) | Ca2+, oxidants | Mg2+ ADP | Pi | CsA | Matrix CyP | |
|---|---|---|---|---|---|---|
| S. cerevisiae | 300 | Activate | Inhibit | Inhibits | No effect | Yes (Cpr3) |
| D. melanogaster | 53 | Activate | Inhibit | Inhibits | No effect | No |
| B. taurus | 500 | Activate | Inhibit | Activates | Inhibits | Yes (CyPD) |
2. The mitochondrial permeability transition pore in yeast
Before discussing the features of the yeast PTP, we would like to mention that yeast mitochondria appear to possess multiple dissipative pathways, a situation that is further complicated by strain-specific differences. These include an ATP-dependent H+-conductive pathway [34,35] and a variety of both selective [36-39] and unselective channels [40-45].
To the best of our knowledge the first evidence that yeast mitochondria may possess a PTP-like channel was obtained in studies of Pi transport in an industrial baker's yeast strain, Yeast Foam [40]. Mitochondria suspended in a K+-based medium showed respiration-dependent large-amplitude swelling insensitive to mersalyl (and thus independent of the Pi carrier) and fully inhibited by antimycin A [40]. Subsequent studies demonstrated that a similar pathway was also present in laboratory yeast strains, where it allowed solute permeation with a cutoff of about 1.5 kDa[42,46-49]. At variance from the mammalian PTP, the yeast PTP (i) was unaffected by matrix Ca2+ even when uptake of the cation was made possible by the ionophore ETH129 [46]; (ii) was insensitive to Mg2+ and ADP [46]; (iii) was insensitive to CsA [46] in spite of the presence of a CsA-sensitive matrix CyP [50,51]; and (iv) was inhibited rather than induced by Pi [46,52]. A PT could be detected in strains lacking VDAC or the adenine nucleotide translocator (ANT), both of which were considered to be essential components of the PTP in mammals (see [33] for a thorough discussion), but the demonstration of a CsA-sensitive PTP in mammalian mitochondria lacking ANT [53] and VDACs [54,55] has refuted the paradigm that these proteins are essential for the PT [24].
Many apparent discrepancies with the mammalian PTP have been resolved in recent years.The Ca2+-dependence of the yeast PTP has now been demonstrated beyond doubt in protocols where Ca2+ uptake was permitted by addition of the ionophore ETH129 and the concentration of Pi was optimized to prevent its inhibitory effect on the pore, which would otherwise mask the inducing effects of Ca2+ itself [56]. Lack of inhibition by Mg2+/ADP may depend on the experimental conditions because of the presence of additional permeability pathways in yeast mitochondria, one of which is activated by ATP [34-36]. Finally, it is now clear that PTP opening can occur in the absence of CyP D also in mammalian mitochondria, as demonstrated both after genetic ablation of CyP D [57-61] and in cells and tissues where CyP D is expressed at low levels [62], conditions under which pore opening is obviously insensitive to CsA. The recent demonstration that after treatment with Ca2+ and oxidants F-ATP synthase forms channels with the features expected of the PTP in B. taurus [63], H. sapiens [64], S. cerevisiae [65] and D. melanogaster [66] now provides a unifying frame to address its role(s) in pathophysiology across species [24].
Table I summarizes the channel properties of F-ATP synthases in S. cerevisiae, D. melanogaster and B. Taurus (as determined by electrophysiology) [63,65,66] and the matching properties of their “PTPs” as determined in isolated mitochondria. It can be appreciated that a unique conductance is a feature of each species. A solute exclusion size of about 1.5 kDa has been defined for the PTP of isolated mitochondria of mammals [67,68] and yeast [46]. The size of the D. melanogaster species has not been defined precisely, but it appears to be considerably smaller because Drosophila mitochondria do not swell in sucrose-based media after opening of the “PTP” [69]. In D. melanogaster swelling was not observed even in KCl-based media, suggesting that the channel is selective for Ca2+ and H+, and may be a fast Ca2+ release channel rather than an unselective permeability pathway like the classical PTP of mammals [66,69](see [24] for a detailed discussion). In spite of this striking difference with the mammalian and yeast PTPs, we feel that they are all mediated by the same structure, i.e. the F-ATP synthase. This hypothesis is also supported by the finding that expression of human CyP D in Drosophila S2R+ cells sensitized the channel to Ca2+ without changing the pore size [66].
Inspection of Table I reveals that conserved general features are (i) the requirement for matrix Ca2+ and facilitation by oxidants, which affect the pore at discrete sites [70,71]; and (ii) inhibition by Mg2+(which competes with Ca2+ for a matrix binding site) and adenine nucleotides [28]. The inducing effect of Pi—a unique feature of the mammalian pore—has always been puzzling because Pi decreases matrix free [Ca2+] [72] and would thus be expected to inhibit rather than promote PTP opening. The finding that Pi inhibits the PTP in S. cerevisiae [46,56,65] and D. melanogaster [69] suggests that stimulation of PTP opening by Pi in mammals may be due to a specific mechanism that evolved only later in evolution [24].
In mammals Pi increases CyP D binding to the OSCP subunit of the F-ATP synthase resulting in increased sensitivity of the PTP to Ca2+ [63,73]. We have proposed that CyP D binding, which is reversed by CsA [63,74], favors access of Ca2+ to the catalytic site of F-ATP synthase (which is usually occupied by Mg2+) resulting in a conformational change that is transmitted to the intramembrane portion of the enzyme through the rigid lateral stalk. The conformational change would eventually cause PTP opening at the interface between two F-ATP synthase monomers in a process involving the dimerizations subunits e and g [24,65]. In keeping with this suggestion, in the absence of CyPD (or in the presence of CsA) low concentrations of Pi inhibit the PTP also in mammalian mitochondria [75].
The PTP of S. cerevisiae is insensitive to CsA despite the presence of a matrix CyP, Cpr3p [76]. We have shown that genetic ablation of CPR3 does not affect the PTP, suggesting that Cpr3p does not interact with F-ATP synthase in yeast [65]. We could not detect a matrix CyP in D. melanogaster, whose genome potentially encodes several isoforms including one with a putative mitochondrial targeting sequence [77], but expression of the human species in mitochondria did sensitize the PTP to Ca2+[66]. In summary, the key PTP-forming features of F-ATP synthases may have appeared early in evolution, before the regulatory interactions of matrix CyP D developed. The reader is referred to a recent extensive review for further details on the mechanistic and species-specific features of the PTP [24].
3. Calcium homeostasis in yeast
Ca2+ ions are fundamental regulators of a wide variety of processes in all eukaryotic cells. Ca2+ signaling is triggered by activation of either ion channels or G protein-coupled receptors and affects reactions that range from modulation of enzyme activities to motility, regulation of ion channels and gene transcription. Yeast is no exception, and maintains a tight control of intracellular Ca2+ homeostasis through an integrated array of transport systems [78-80]. In addition to cell growth, Ca2+controls mating between MATa and MATα cells that secrete specific pheromones able to increase its cytosolic concentration, leading to cellular changes required for agglutination [81]. A key role of Ca2+ signaling has also been attributed to the response to an alkaline environment [82] as well as to hypotonic shock through the activation of MAP kinases [83]. Cytosolic free [Ca2+] in yeast is finely regulated and maintained at low levels (50-200 nM) through Ca2+storage in several compartments, i.e. vacuole, endoplasmic reticulum (ER), Golgi apparatus [78-80] and possibly mitochondria. Extracellular Ca2+ influx mainly occurs through the plasma membrane voltage-gated Ca2+ channel, also referred to as Cch1/Mid1 complex, that is activated by several stimuli such as depolarization, hypotonic shock, pheromone stimulation and unfolded protein response [81,84,85]. Following influx Ca2+ binds calmodulin, creating a complex able to activate calcineurin and thus to promote transcription of specific sets of genes required for cell proliferation and for the response to pheromone [86,87]. The signal is terminated by Ca2+ sequestration in the vacuole–the major Ca2+ store–through the Ca2+ ATPase Pmc1 [78] and the high capacity, low-affinity Ca2+/H+ exchanger Vcx1 [88], which may link Ca2+ homeostasis to regulation of intracellular pH. A contribution of ER/Golgi as alternative Ca2+ storage sites has also been recently proposed [89].
4. Mitochondria and Ca2+ homeostasis
In mammals, mitochondria play a crucial role in cytosolic Ca2+ homeostasis through an array of transport systems [90]. In energized mitochondria the inner membrane MCU complex (which is inhibited by lanthanides [91] and ruthenium red [92]) mediates rapid Ca2+uptake down the cation electrochemical gradient [93]. With a membrane potential of 180 mV (negative inside) the equilibrium Ca2+ accumulation is 106, which for a cytosolic [Ca2+] of 100 nM would correspond to a matrix [Ca2+] of 100 mM. Thermodynamic equilibration is never attained, however, due to the existence of the 3Na+/Ca2+ exchanger and of a putative 3H+/Ca2+ exchanger that extrude Ca2+ and thus prevent massive accumulation of Ca2+ and Pi (see [94] for a recent review).
Yeast mitochondria do not possess an MCU complex [93] and therefore their potential role in Ca2+ homeostasis is usually not given much consideration. However, also in yeast the Ca2+ electrochemical gradient favors Ca2+ accumulation with the same predicted equilibrium distribution as that of mammalian mitochondria. To quote the original conclusions of Carafoli and Lehninger “We consider it likely that all mitochondria, whatever the cell type, possess the electrochemical capacity for moving Ca2+ across the membrane. This capacity cannot be expressed, however, unless a pathway for trans-membrane movement of Ca2+ is available, either through the occurrence of a specific Ca2+ carrier system or through simple physical permeability of the mitochondrial membrane to Ca2+” [20]. We contend that the driving force is so large that Ca2+ uptake could be relevant even if it occured through a leak pathway rather than through a specific transport system (which could be present, however, as further discussed in the following paragraph). Consistently (i) S. cerevisiae mitochondria have a Ca2+ content of 8-9 ng atoms/mg protein, which is close to that of rat liver mitochondria [20]; (ii) addition of EGTA or Ca2+ led to relevant changes of mitochondrial matrix free Ca2+ measured with a trapped fluorescent indicator, and mitochondrial Ca2+ release could be elicited by antimycin A or uncouplers [95]; and (iii) electrophoretic Ca2+ uptake coupled to H+ ejection can be easily measured in isolated S. cerevisiae and C. utilis mitochondria when the cation is added at concentrations of 1-10 mM [21]. It is of note that respiration-driven uptake is observed with Ca2+, Sr2+ and Mn2+ but not with Mg2+ [21], suggesting that cation accumulation could be taking place through a low-affinity system whose discrimination for the transported species is strikingly similar to that of the MCU [90]. It is also interesting to recall that yeast mitochondria are endowed with a very effective 2H+/Ca2+ antiporter activated by fatty acids that mediates mitochondrial Ca2+ release [96]. Like in mammals, the antiporter could prevent excessive mitochondrial Ca2+ accumulation but also allow rapid mobilization of the matrix Ca2+pool following activation of phospholipases and perhaps other relevant pathophysiological stimuli.
In contrast to the general assumption that yeast mitochondria lack a specific Ca2+ transport system machinery, a high-capacity Ca2+ uptake system driven by the membrane potential and stimulated by polyamines and ADP has been described in the yeast E. magnusii [97,98]. Rather than acting as an inhibitor, and at variance from the mammalian MCU, ruthenium red affected Ca2+ transport only marginally or even stimulated it under specific conditions[99]. Taken together, these findings suggest that a specific Ca2+ transport system may exist also in yeast mitochondria, and that this putative system could be expressed at varying levels in different yeast strains. Occurrence of Ca2+ uptake in S. cerevisiae mitochondria combined with the sensitivity of the PTP to oxidative stress may provide the missing link between combined increase of intracellular [Ca2+] and ROS, common triggers of yeast PCD [1-3,10-18], and activation of the intrinsic pathwayof apoptosis.
5. Ca2+ and the permeability transition in yeast programmed cell death
As already mentioned, yeast PCD is increasingly recognized as a physiologically relevant event that has intriguing analogies with mammalian apoptosis, which are particularly striking for the mitochondrial pathway [1]. The major mitochondrial changes occurring in yeast apoptosis include cristae remodeling, increased ROS production, matrix swelling and cytochrome c release which are often preceded by increased cytsolic [Ca2+] [1-3]. It is remarkable that the mechanistic basis for these changes has not been fully clarified yet, and it is legitimate to wonder whether the striking analogies with the matching consequences of PTP opening in mammals are just a coincidence, or rather underscore occurrence of a bona fide PT as also suggested in a relevant study of yeast spheroblasts [100].
We have already mentioned that Ybh3p translocation was reported to cause mitochondrial depolarization and intermembrane protein release, which are expected consequences of PTP opening in yeast [4]. The process also involved the Pi carrier, Mir1p, and the core subunit of ubiquinol-cytochrome c oxidoreductase, Cor1p [4]. Whether the PTP could have been involved in this paradigm remains, however, unclear particularly because the site of integration of Ybh3p—whether the IMM or the OMM—was not defined. It is interesting, however, that the stimulus used to trigger apoptosis was acetic acid, which causes increased ROS production [12] and may therefore require a functional respiratory chain and thus Cor1p. The requirement for Mir1p, on the other hand, may depend on the Pi-requirement for mitochondrial Ca2+ uptake which is stimulated up to 8-fold by Pi [21]. In this respect it is very intriguing that cell death induced by acetic acid was greatly reduced in ρ0 cells (where no mitochondrial respiration hence ROS production takes place) and in ATP10 mutants (which cannot assemble the F-ATP synthase) [12]. The latter finding acquires a new meaning in the light of the demonstration that yeast F-ATP synthase forms channels with the features of the yeast PTP when subjected to oxidative stress in the presence of Ca2+ [65].
One of the most relevant studies on the involvement of mitochondria in yeast PCD was performed using the pheromone α factor or amiodarone, which both cause an increase of cytosolic [Ca2+] and cell death. Cell death occurred by apoptosis and required a functional respiratory chain, as ρ0 cells were extremely resistant; and was linked to ROS formation, as it could be protected by antioxidants and by low concentrations of the protonophore FCCP [15]. Amiodarone has complex effects on respiration in intact yeast cells, but consistently increased mitochondrial respiration and ROS production which preceded mitochondral depolarization. The Authors suggest that increased respiration was caused by Ca2+-dependent stimulation of NADH dehydrogenase from the intermembrane space, sequentially causing an increase of membrane potential and of ROS production, followed by IMM permeabilization and cell death [15]. We suspect (i) that ROS-dependent depolarization in this and other paradigms was caused by PTP opening induced by oxidative stress and (ii) that this event could be a final common pathway in a variety of forms of yeast PCD. This hypothesis has so far been hard to test due to the lack of a structure for the PTP and to the insensitivity of the yeast PT to CsA. We have already found that genetic ablation of the e and/or g subunits, whose presence is important for F-ATP synthase dimerization [101], confers at least partial resistance to PTP opening [65]. Identification of the cysteine residues responsible for the sensitizing effects of oxidative stress and the availability of novel, CyP-independent PTP inhibitors [102-104] should soon allow a stringent test of this hypothesis.
6. Summary and conclusions
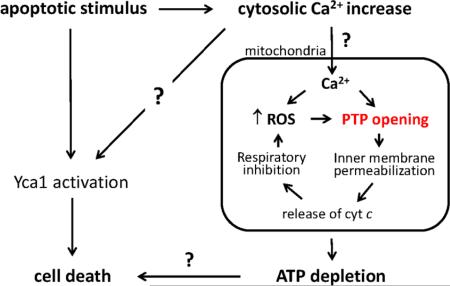
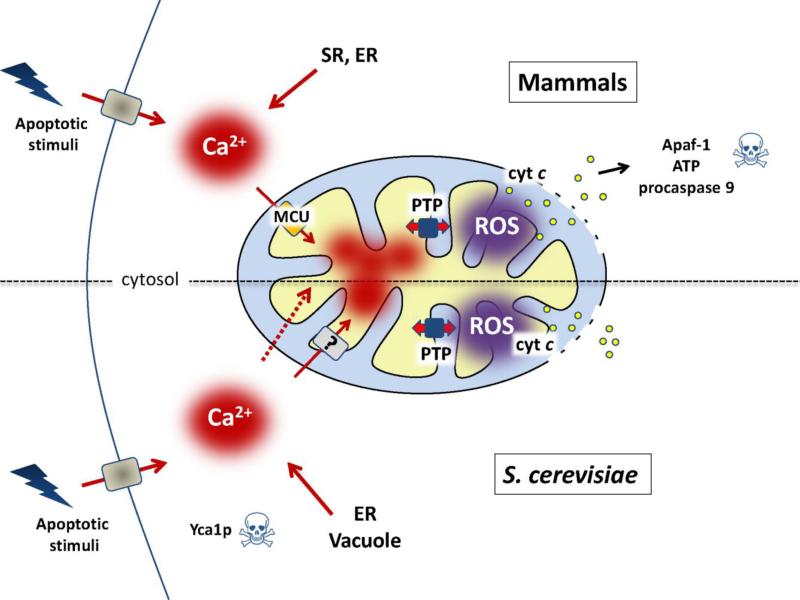
In mammalian cells apoptotic stimuli often lead to increased cytosolic [Ca2+] that is rapidly relayed to mitochondria through MCU-dependent uptake. Under conditions of oxidative stress this Ca2+ signal can cause PTP opening and release of cytochrome c and other proapoptotic factors that eventually may cause activation of caspase 9 and contribute to execution of cell death (upper part of Figure 1). In spite of the absence of the MCU, yeast mitochondria may accumulate enough Ca2+ to undergo the PT when cells are challenged by stimuli that cause yeast PCD, which is accompanied by oxidative stress and increased cytosolic [Ca2+]. Once PTP opening occurs, cytosolic and matrix Ca2+ equilibrate stabilizing the PTP in the open conformation, which is followed by osmotic swelling of the matrix, OMM damage and release of intermembrane proteins causing cell death (lower part of Figure 1). Although in yeast cytochrome c release is not followed by activation of effector caspases, it does cause decreased respiration and increased ROS production, which stabilizes PTP opening and leads to ATP depletion. This set of events could then contribute to cell death together with activation of metacaspases like Yca1p (see also the graphical abstract). The demonstration that PTP forms from F-ATP synthase provides a unifying framework for future studies, and should allow to assess if the transition of the energy-conserving complex into an energy-dissipating device plays a role in yeast PCD.
Figure 1. Pathways for Ca2+-dependent cell death in mammals and yeast.
Top, increased cytosolic [Ca2+] often takes place in response to apoptotic stimuli in mammalian cells. Mitochondria quickly accumulate Ca2+ via the MCU complex, leading to increased matrix [Ca2+] that, together with increased ROS levels, can favor PTP opening leading to release of proapoptotic factors including cytochrome c. This eventually results in formation of an active Apaf-1 complex leading to procaspase 9 cleavage and initiation of cell death. Bottom, apoptotic stimuli increase cytosolic [Ca2+] in S. cerevisiae. Ca2+ can enter mitochondria through a membrane leak pathway (dashed red arrow) or via an unidentified transporter (denoted with a question mark) and, under conditions of oxidative stress, trigger PTP opening. The resulting matrix swelling and rupture of the OMM may cause release of cytochrome c, which would further stabilize the open state because of decreased respiratory activity and increased ROS production. The ensuing ATP depletion would add to activation of caspase-like pathways (such as Yca1p) in promoting cell death.
Highlights.
We discuss the mitochondrial permeability transition in Ca2+-dependent yeast programmed cell death
Acknowledgements
This work is in partial fulfillment of the requirements for the PhD of Michela Carraro at the University of Padova. Research in our laboratory is supported by Associazione Italiana Ricerca sul Cancro (AIRC) IG 17067, Italian Ministry for University and Research (MIUR) FIRB RBAP11S8C3 and PRIN 20107Z8XBW, Telethon Italy GGP14037 and GPP14187, and the National Institutes of Health – Public Health Service (USA) R01GM069883.
Abbreviations used are
- ANT
adenine nucleotide translocator
- BI-1
Bax Inhibitor 1
- CsA
cyclosporin A
- CyP D
cyclophilin D
- IMM
inner mitochondrial membrane
- MCU
mitochondrial Ca2+ uniporter
- OMM
outer mitochondrial membrane
- PCD
programmed cell death
- PT
permeability transition
- PTP
permeability transition pore
- ROS
reactive oxygen species
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pereira C, Silva RD, Saraiva L, Johansson B, Sousa MJ, Côrte-Real M. Mitochondria-dependent apoptosis in yeast. Biochim. Biophys. Acta. 2008;1783:1286–1302. doi: 10.1016/j.bbamcr.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Côrte-Real M, Madeo F. Yeast programed cell death and aging. Front. Oncol. 2013;3:283. doi: 10.3389/fonc.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaragnella N, Zdralevic M, Antonacci L, Passarella S, Marra E, Giannattasio S. The role of mitochondria in yeast programmed cell death. Front. Oncol. 2012;2:70. doi: 10.3389/fonc.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Büttner S, Ruli D, Vögtle FN, Galluzzi L, Moitzi B, Eisenberg T, Kepp O, Habernig L, Carmona-Gutierrez D, Rockenfeller P, Laun P, Breitenbach M, Khoury C, Fröhlich KU, Rechberger G, Meisinger C, Kroemer G, Madeo F. A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis. EMBO J. 2011;30:2779–2792. doi: 10.1038/emboj.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae HJ, Ke N, Kim HR, Chen S, Godzik A, Dickman M, Reed JC. Evolutionarily conserved cytoprotection provided by Bax Inhibitor-1 homologs from animals, plants, and yeast. Gene. 2003;323:101–113. doi: 10.1016/j.gene.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Cebulski J, Malouin J, Pinches N, Cascio V, Austriaco N. Yeast Bax inhibitor, Bxi1p, is an ER-localized protein that links the unfolded protein response and programmed cell death in Saccharomyces cerevisiae. PLoS One. 2011;6:e20882. doi: 10.1371/journal.pone.0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aouacheria A, Rech de Laval V, Combet C, Hardwick JM. Evolution of Bcl-2 homology motifs: homology versus homoplasy. Trends Cell Biol. 2013;23:103–111. doi: 10.1016/j.tcb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Fröhlich KU. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 2002;9:911–917. doi: 10.1016/s1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA, Chock PB, Stadtman ER. Knockout of caspase-like gene, YCA1, abrogates apoptosis and elevates oxidized proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17326–17331. doi: 10.1073/pnas.0508120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madeo F, Fröhlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Fröhlich KU. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludovico P, Sousa MJ, Silva MT, Leão C, Côrte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- 12.Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Côrte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:2598–2606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludovico P, Sansonetty F, Silva MT, Côrte-Real M. Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEMS Yeast Res. 2003;3:91–96. doi: 10.1016/s1567-1356(02)00166-6. [DOI] [PubMed] [Google Scholar]
- 14.Severin FF, Hyman AA. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 2002;12:R233–R235. doi: 10.1016/s0960-9822(02)00776-5. [DOI] [PubMed] [Google Scholar]
- 15.Pozniakovsky AI, Knorre DA, Markova OV, Hyman AA, Skulachev VP, Severin FF. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J. Cell Biol. 2005;168:257–269. doi: 10.1083/jcb.200408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagaki H, Araki Y, Funato K, Shimoi H. Ethanol-induced death in yeast exhibits features of apoptosis mediated by mitochondrial fission pathway. FEBS Lett. 2007;581:2935–2942. doi: 10.1016/j.febslet.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002;29:649–659. doi: 10.1046/j.0960-7412.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 18.Kajiwara K, Muneoka T, Watanabe Y, Karashima T, Kitagaki H, Funato K. Perturbation of sphingolipid metabolism induces endoplasmic reticulum stress-mediated mitochondrial apoptosis in budding yeast. Mol. Microbiol. 2012;86:1246–1261. doi: 10.1111/mmi.12056. [DOI] [PubMed] [Google Scholar]
- 19.Carafoli E, Balcavage WX, Lehninger AL, Mattoon JR. Ca2+ metabolism in yeast cells and mitochondria. Biochim. Biophys. Acta. 1970;205:18–26. doi: 10.1016/0005-2728(70)90057-5. [DOI] [PubMed] [Google Scholar]
- 20.Carafoli E, Lehninger AL. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem. J. 1971;122:681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balcavage WX, Lloyd JL, Mattoon JR, Ohnishi T, Scarpa A. Cation movements and respiratory response in yeast mitochondria treated with high Ca2+ concentrations. Biochim. Biophys. Acta. 1973;305:41–51. doi: 10.1016/0005-2728(73)90229-6. [DOI] [PubMed] [Google Scholar]
- 22.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Stefani D, Raffaello A, Teardo E, Szabó I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernardi P, Rasola A, Forte M, Lippe G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J. Bioenerg. Biomembr. 1989;21:497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- 26.Petronilli V, Szabó I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- 27.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 29.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 30.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 31.Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 33.Manon S, Roucou X, Guerin M, Rigoulet M, Guerin B. Characterization of the yeast mitochondria unselective channel: a counterpart to the mammalian permeability transition pore? J Bioenerg. Biomembr. 1998;30:419–429. doi: 10.1023/a:1020533928491. [DOI] [PubMed] [Google Scholar]
- 34.Prieto S, Bouillaud F, Ricquier D, Rial E. Activation by ATP of a proton-conducting pathway in yeast mitochondria. Eur. J. Biochem. 1992;208:487–491. doi: 10.1111/j.1432-1033.1992.tb17212.x. [DOI] [PubMed] [Google Scholar]
- 35.Prieto S, Bouillaud F, Rial E. The mechanism for the ATP-induced uncoupling of respiration in mitochondria of the yeast Saccharomyces cerevisiae. Biochem. J. 1995;307(Pt 3):657–661. doi: 10.1042/bj3070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prieto S, Bouillaud F, Rial E. The nature and regulation of the ATP-induced anion permeability in Saccharomyces cerevisiae mitochondria. Arch. Biochem. Biophys. 1996;334:43–49. doi: 10.1006/abbi.1996.0427. [DOI] [PubMed] [Google Scholar]
- 37.Manon S, Guerin M. Evidence for three different electrophoretic pathways in yeast mitochondria: ion specificity and inhibitor sensitivity. J. Bioenerg. Biomembr. 1993;25:671–678. doi: 10.1007/BF00770253. [DOI] [PubMed] [Google Scholar]
- 38.Roucou X, Manon S, Guérin M. ATP opens an electrophoretic potassium transport pathway in respiring yeast mitochondria. FEBS Lett. 1995;364:161–164. doi: 10.1016/0014-5793(95)00380-r. [DOI] [PubMed] [Google Scholar]
- 39.Roucou X, Manon S, Guérin M. Conditions allowing different states of ATP- and GDP-induced permeability in mitochondria from different strains of Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1997;1324:120–132. doi: 10.1016/s0005-2736(96)00215-5. [DOI] [PubMed] [Google Scholar]
- 40.de Chateaubodeau G, Guérin M, Guérin B. Studies on anionic transport in yeast mitochondria and promitochondria. Swelling in ammonium phosphate, glutamate, succinate and fumarate solutions. FEBS Lett. 1974;46:184–187. doi: 10.1016/0014-5793(74)80364-9. [DOI] [PubMed] [Google Scholar]
- 41.Guérin B, Bunoust O, Rouqueys V, Rigoulet M. ATP-induced unspecific channel in yeast mitochondria. J. Biol. Chem. 1994;269:25406–25410. [PubMed] [Google Scholar]
- 42.Perez-Vazquez V, Saavedra-Molina A, Uribe S. In Saccharomyces cerevisiae, cations control the fate of the energy derived from oxidative metabolism through the opening and closing of the yeast mitochondrial unselective channel. J. Bioenerg. Biomembr. 2003;35:231–241. doi: 10.1023/a:1024659615022. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez-Aguilar M, Perez-Vazquez V, Bunoust O, Manon S, Rigoulet M, Uribe S. In yeast, Ca2+ and octylguanidine interact with porin (VDAC) preventing the mitochondrial permeability transition. Biochim. Biophys. Acta. 2007;1767:1245–1251. doi: 10.1016/j.bbabio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez-Aguilar M, Perez-Martinez X, Chavez E, Uribe-Carvajal S. In Saccharomyces cerevisiae, the phosphate carrier is a component of the mitochondrial unselective channel. Arch. Biochem. Biophys. 2010;494:184–191. doi: 10.1016/j.abb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Uribe-Carvajal S, Luévano-Martìnez LA, Guerrero-Castillo S, Cabrera-Orefice A, Corona-de-la-Peña NA, Gutiérrez-Aguilar M. Mitochondrial Unselective Channels throughout the eukaryotic domain. Mitochondrion. 2011;11:382–390. doi: 10.1016/j.mito.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Jung DW, Bradshaw PC, Pfeiffer DR. Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J. Biol. Chem. 1997;272:21104–21112. doi: 10.1074/jbc.272.34.21104. [DOI] [PubMed] [Google Scholar]
- 47.Manon S. Dependence of yeast mitochondrial unselective channel activity on the respiratory chain. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1410:85–90. doi: 10.1016/s0005-2728(98)00178-9. [DOI] [PubMed] [Google Scholar]
- 48.Kovaleva MV, Sukhanova EI, Trendeleva TA, Zyl'kova MV, Ural'skaya LA, Popova KM, Saris NE, Zvyagilskaya RA. Induction of a non-specific permeability transition in mitochondria from Yarrowia lipolytica and Dipodascus (Endomyces) magnusii yeasts. J. Bioenerg. Biomembr. 2009;41:239–249. doi: 10.1007/s10863-009-9227-5. [DOI] [PubMed] [Google Scholar]
- 49.Bradshaw P, Pfeiffer DR. Characterization of the respiration-induced yeast mitochondrial permeability transition pore. Yeast. 2013;30:471–483. doi: 10.1002/yea.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tropschug M, Barthelmess IB, Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989;342:953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- 51.Rassow J, Mohrs K, Koidl S, Barthelmess IB, Pfanner N, Tropschug M. Cyclophilin 20 is involved in mitochondrial protein folding in cooperation with molecular chaperones Hsp70 and Hsp60. Mol. Cell Biol. 1995;15:2654–2662. doi: 10.1128/mcb.15.5.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velours J, Rigoulet M, Guerin B. Protection of yeast mitochondrial structure by phosphate and other H+-donating anions. FEBS Lett. 1977;81:18–22. doi: 10.1016/0014-5793(77)80918-6. [DOI] [PubMed] [Google Scholar]
- 53.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1−/− mitochondria. Biochim. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada A, Yamamoto T, Yoshimura Y, Gouda S, Kawashima S, Yamazaki N, Yamashita K, Kataoka M, Nagata T, Terada H, Pfeiffer DR, Shinohara Y. Ca2+-induced permeability transition can be observed even in yeast mitochondria under optimized experimental conditions. Biochim. Biophys. Acta. 2009;1787:1486–1491. doi: 10.1016/j.bbabio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 58.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 60.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Marchi U, Basso E, Szabó I, Zoratti M. Electrophysiological characterization of the Cyclophilin D-deleted mitochondrial permeability transition pore. Mol. Membr. Biol. 2006;23:521–530. doi: 10.1080/09687860600907644. [DOI] [PubMed] [Google Scholar]
- 62.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, Fontaine E. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim. Biophys. Acta. 2012;1817:1628–1634. doi: 10.1016/j.bbabio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr., Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carraro M, Giorgio V, Šileikyte J, Sartori G, Forte M, Lippe G, Zoratti M, Szabó I, Bernardi P. Channel Formation by Yeast F-ATP Synthase and the Role of Dimerization in the Mitochondrial Permeability Transition. J. Biol. Chem. 2014;289:15980–15985. doi: 10.1074/jbc.C114.559633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Stockum S, Giorgio V, Trevisan E, Lippe G, Glick GD, Forte MA, Da-Rè C, Checchetto V, Mazzotta G, Costa R, Szabò I, Bernardi P. F-ATPase of D. melanogaster Forms 53 Picosiemen (53-pS) Channels Responsible for Mitochondrial Ca2+-induced Ca2+ Release. J. Biol. Chem. 2015;290:4537–4544. doi: 10.1074/jbc.C114.629766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massari S, Azzone GF. The equivalent pore radius of intact and damaged mitochondria and the mechanism of active shrinkage. Biochim. Biophys. Acta. 1972;283:23–29. doi: 10.1016/0005-2728(72)90094-1. [DOI] [PubMed] [Google Scholar]
- 68.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 69.von Stockum S, Basso E, Petronilli V, Sabatelli P, Forte MA, Bernardi P. Properties of Ca2+ Transport in Mitochondria of Drosophila melanogaster. J. Biol. Chem. 2011;286:41163–41170. doi: 10.1074/jbc.M111.268375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beatrice MC, Stiers DL, Pfeiffer DR. The role of glutathione in the retention of Ca2+ by liver mitochondria. J. Biol. Chem. 1984;259:1279–1287. [PubMed] [Google Scholar]
- 71.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 72.Zoccarato F, Nicholls DG. The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. Eur. J. Biochem. 1982;127:333–338. doi: 10.1111/j.1432-1033.1982.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 73.Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J. Biol. Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A-sensitive channel. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 75.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226.1–226.6. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pemberton TJ, Kay JE. Identification and Comparative Analysis of the Peptidyl-Prolyl cis/trans Isomerase Repertoires of H. sapiens, D. melanogaster, C. elegans, S. cerevisiae and Sz. pombe. Comp. Funct. Genomics. 2005;6:277–300. doi: 10.1002/cfg.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cunningham KW, Fink GR. Ca2+ transport in Saccharomyces cerevisiae. J. Exp. Biol. 1994;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- 79.Cui J, Kaandorp JA, Ositelu OO, Beaudry V, Knight A, Nanfack YF, Cunningham KW. Simulating calcium influx and free calcium concentrations in yeast. Cell Calcium. 2009;45:123–132. doi: 10.1016/j.ceca.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cyert MS, Philpott CC. Regulation of cation balance in Saccharomyces cerevisiae. Genetics. 2013;193:677–713. doi: 10.1534/genetics.112.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell Biol. 1994;14:8259–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serrano R, Ruiz A, Bernal D, Chambers JR, Arino J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 2002;46:1319–1333. doi: 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- 83.Batiza AF, Schulz T, Masson PH. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996;271:23357–23362. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- 84.Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997;419:259–262. doi: 10.1016/s0014-5793(97)01466-x. [DOI] [PubMed] [Google Scholar]
- 85.Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Withee JL, Mulholland J, Jeng R, Cyert MS. An essential role of the yeast pheromone-induced Ca2+ signal is to activate calcineurin. Mol. Biol. Cell. 1997;8:263–277. doi: 10.1091/mbc.8.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 89.D'hooge P, Coun C, Van Eyck V, Faes L, Ghillebert R, Mariaën L, Winderickx J, Callewaert G. Ca2+ homeostasis in the budding yeast Saccharomyces cerevisiae: Impact of ER/Golgi Ca2+ storage. Cell Calcium. 2015;58:226–235. doi: 10.1016/j.ceca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 91.Mela L. Inhibition and activation of calcium transport in mitochondria. Effect of lanthanides and local anesthetic drugs. Biochemistry. 1969;8:2481–2486. doi: 10.1021/bi00834a034. [DOI] [PubMed] [Google Scholar]
- 92.Vasington FD, Gazzotti P, Tiozzo R, Carafoli E. The effect of ruthenium red on Ca2+ transport and respiration in rat liver mitochondria. Biochim. Biophys. Acta. 1972;256:43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- 93.De Stefani D, Patron M, Rizzuto R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta. 2015;1853:2006–2011. doi: 10.1016/j.bbamcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bernardi P, von Stockum S. The permeability transition pore as a Ca2+ release channel: New answers to an old question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uribe S, Rangel P, Pardo JP. Interactions of calcium with yeast mitochondria. Cell Calcium. 1992;13:211–217. doi: 10.1016/0143-4160(92)90009-h. [DOI] [PubMed] [Google Scholar]
- 96.Bradshaw PC, Jung DW, Pfeiffer DR. Free fatty acids activate a vigorous Ca2+:2H+ antiport activity in yeast mitochondria. J. Biol. Chem. 2001;276:40502–40509. doi: 10.1074/jbc.M105062200. [DOI] [PubMed] [Google Scholar]
- 97.Votyakova TV, Bazhenova EN, Zvjagilskaya RA. Yeast mitochondrial calcium uptake: regulation by polyamines and magnesium ions. J. Bioenerg. Biomembr. 1993;25:569–574. doi: 10.1007/BF01108413. [DOI] [PubMed] [Google Scholar]
- 98.Bazhenova EN, Deryabina YI, Eriksson O, Zvyagilskaya RA, Saris NE. Characterization of a high capacity calcium transport system in mitochondria of the yeast Endomyces magnusii. J Biol Chem. 1998;273:4372–4377. doi: 10.1074/jbc.273.8.4372. [DOI] [PubMed] [Google Scholar]
- 99.Bazhenova EN, Saris NE, Zvyagilskaya RA. Stimulation of the yeast mitochondrial calcium uniporter by hypotonicity and by ruthenium red. Biochim. Biophys. Acta. 1998;1371:96–100. doi: 10.1016/s0005-2736(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 100.Kowaltowski AJ, Vercesi AE, Rhee SG, Netto LE. Catalases and thioredoxin peroxidase protect Saccharomyces cerevisiae against Ca2+-induced mitochondrial membrane permeabilization and cell death. FEBS Lett. 2000;473:177–182. doi: 10.1016/s0014-5793(00)01526-x. [DOI] [PubMed] [Google Scholar]
- 101.Arnold I, Pfeiffer K, Neupert W, Stuart RA, Schägger H. Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J. 1998;17:7170–7178. doi: 10.1093/emboj/17.24.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fancelli D, Abate A, Amici R, Bernardi P, Ballarini M, Cappa A, Carenzi G, Colombo A, Contursi C, Di Lisa F, Dondio G, Gagliardi S, Milanesi E, Minucci S, Pain G, Pelicci PG, Saccani A, Storto M, Thaler F, Varasi M, Villa M, Plyte S. Cinnamic Anilides as New Mitochondrial Permeability Transition Pore Inhibitors Endowed with Ischemia-Reperfusion Injury Protective Effect in Vivo. J. Med. Chem. 2014;57:5333–5347. doi: 10.1021/jm500547c. [DOI] [PubMed] [Google Scholar]
- 103.Roy S, Šileikyte J, Schiavone M, Neuenswander B, Argenton F, Aubé J, Hedrick MP, Chung TDY, Forte MA, Bernardi P, Schoenen FJ. Discovery, Synthesis, and Optimization of Diarylisoxazole-3-carboxamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem. 2015;10:1655–1671. doi: 10.1002/cmdc.201500284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy S, Šileikyte J, Neuenswander B, Hedrick MP, Chung TD, Aubé J, Schoenen FJ, Forte MA, Bernardi P. N-Phenylbenzamides as Potent Inhibitors of the Mitochondrial Permeability Transition Pore. ChemMedChem. 2016;11:283–288. doi: 10.1002/cmdc.201500545. [DOI] [PMC free article] [PubMed] [Google Scholar]