Abstract
Cellular redox balance plays a significant role in the regulation of hematopoietic stem-progenitor cell (HSC/MPP) self-renewal and differentiation. Unregulated changes in cellular redox homeostasis are associated with the onset of most hematological disorders. However, accurate measurement of the redox state in stem cells is difficult because of the scarcity of HSC/MPPs. Glutathione (GSH) constitutes the most abundant pool of cellular antioxidants. Thus, GSH metabolism may play a critical role in hematological disease onset and progression. A major limitation to studying GSH metabolism in HSC/MPPs has been the inability to measure quantitatively GSH concentrations in small numbers of HSC/MPPs. Current methods used to measure GSH levels not only rely on large numbers of cells, but also rely on the chemical/structural modification or enzymatic recycling of GSH and therefore are likely to measure only total glutathione content accurately. Here, we describe the validation of a sensitive method used for the direct and simultaneous quantitation of both oxidized and reduced GSH via liquid chromatography followed by tandem mass spectrometry (LC-MS/MS) in HSC/MPPs isolated from bone marrow. The lower limit of quantitation (LLOQ) was determined to be 5.0 ng/mL for GSH and 1.0 ng/mL for GSSG with lower limits of detection at 0.5 ng/mL for both glutathione species. Standard addition analysis utilizing mouse bone marrow shows that this method is both sensitive and accurate with reproducible analyte recovery. This method combines a simple extraction with a platform for the high-throughput analysis, allows for efficient determination of GSH/GSSG concentrations within the HSC/MPP populations in mouse, chemotherapeutic treatment conditions within cell culture, and human normal/leukemia patient samples. The data implicate the importance of the modulation of GSH/GSSG redox couple in stem cells related diseases.
Keywords: Glutathione, HSC’s, LC-MS/MS, Method Validation
Graphical abstract

Introduction
Glutathione, γ-L-glutamyl-L-cysteinylglycine, (GSH) is an endogenous tripeptide involved in many cellular processes including apoptosis, cellular detoxification, and redox signaling[1, 2]. Currently, GSH is thought of as a major cellular reducing agent, with high intracellular concentrations reported to range from 0.5–10 mM, that aids in protection from ROS mediated injury [3–5]. GSH/GSSG homeostasis is tightly regulated with depletion or oxidation of the cellular GSH pool leading to the activation of anti-oxidant signaling pathways, gene transcription, and GSH synthesis accomplished via glutamate-cysteine ligase (GCL) activity. Oxidizing cellular conditions lead to the heterodimerization of the GCL subunits, GCLC (GCL catalytic subunit) and GCLM (GCL modifier subunit) [6, 7] which, results in increased GCL activity. GSH activity is subsequently regulated via cycling the cysteinyl thiol (pKa= 9.2) through oxidized and reduced states. GSH-mediated cellular detoxification may be accomplished by the direct conjugation of GSH, to xenobiotics and other endogenously produced small molecules via glutathione-S-transferase (GST) activity or through the action of glutathione peroxidase (GPx), which reduces hydrogen peroxide while GSH is co-oxidized to its disulfide form (GSSG)[1, 2, 7, 8]. Additionally, GSH may reversibly modulate cellular redox signaling via direct glutathionylation of thiol groups within redox sensitive signaling proteins. This post-translational modification may also protect thiol groups within redox sensitive signaling proteins from permanent modification under oxidizing conditions[9, 10]. Similarly, GSH functions to protect mitochondrial 1-Cys peroxiredoxins from damaging oxidation as the resolving thiol group in thioredoxin catalyzed peroxidase activity in saccharomyces cerevisiae [11]. These functions and aspects of GSH homeostasis demonstrate the importance of the GSH/GSSG redox pair in the maintenance of the cellular redox state.
The cellular redox state is commonly characterized by examining the ratio of reduced to oxidized species within cellular redox pairs. High intracellular concentrations and redox buffer capacity makes this especially true of the GSH/GSSG redox couple[1, 12–14]. Biochemically, GSH and GSSG may be thought of as components of an electrochemical half-cell in which the flux of single electron transfers can be quantified by their electrical potential or electromotive force, characterizing the proclivity of the GSH/GSSG pair to donate or accept electrons in varying redox states. As a result, defining the individual absolute cellular concentrations of GSH and GSSG and applying these concentrations, along with measured values for intracellular pH (pHi) and cellular volume to the Nernst equation allows for a more specific analysis of the 2GSH/GSSG redox state, the electrical half-cell reduction potential (Ehc)[12, 13]. Although living biological systems never rest at a state of equilibrium, characterization of the 2GSH/GSSG reduction potential provides a practical snapshot of cellular redox balance[12, 15]. Furthermore, evaluation of the 2GSH/GSSG Ehc is a strong indicator of the existing redox state of thiol-containing signaling proteins regulated by glutathione.
The cellular GSH/GSSG ratio is characterized by the equilibrium half-cell reaction of glutathione species resulting in the synthesis of two moles of GSH from the reduction of one mole of GSSG, thus the glutathione based redox state is dependent on cellular GSH concentrations[12, 13]. Alternatively, the individual concentrations of GSH and GSSG may be considered when characterizing small dynamic changes in the cellular redox state over time. Consequently, an effective evaluation of the glutathione based redox state requires a sensitive and accurate method for the quantitation of absolute concentrations for both GSH and GSSG. This is particularly important for evaluation of the cellular redox state within hematopoietic malignancies manifesting in hematopoietic stem-progenitor cells (HSC/MPPs); a tissue that has inherently limited availability for study in vivo. For example, in our experience, purification of murine bone marrow typically results in the isolation of approximately 20,000 HSC/MPPs from a single animal that demonstrate the Lin−, Sca-1+, c-kit+ (LSK) phenotype.
HSC/MPP location and function require cellular quiescence and protection from oxidative insult[16–18]. Thus, antioxidant defense is vital to stem cell function. This concept is demonstrated by the increase in stem cell function resulting from treatment with the anti-oxidant and GSH precursor N-acetylcystiene (NAC)[19]. This is further demonstrated by the major cellular regulator of transcriptional anti-oxidant signaling Nrf2; wherein, Nrf2−/ − mice are characterized by an increase in HSC/MPP differentiation and a decrease in stem cell function, indicating that a loss of cellular antioxidant machinery is detrimental to the maintenance of HSC/MPP pools in vivo[20]. These observations indicate a potential role for glutathione metabolism and maintenance in the regulation of redox balance and the resulting effect on differentiation and self-renewal, within normal and malignant HSC/MPPs.
It is well recognized that regulation of glutathione metabolism is significantly affected by the expression and activity of the ABC transporter ABCC1/MRP1 (multi-drug resistance protein 1)[21–24]. MRP1 demonstrates general ubiquitous tissue expression with the exception of the liver hepatocyte, functioning to efflux GSH, GSSG, and glutathione adducted metabolites with a km in the low mM range for GSH and nM to μM range for GS-X adducted metabolites[21–26]. While the role of MRP1 in metabolism within peripheral and specialized tissues has been well documented, the function of MRP1 and its effect on glutathione concentrations as well as the HSC/MPP redox state within primitive HSC’s is less understood. This is partially due to the inherently limited availability of lineage primitive hematopoietic tissues, which display low glutathione concentrations in vivo, and is compounded by the lack of sensitive methodologies capable of discerning small dynamic changes in glutathione concentrations within these specialized hematopoietic populations. However, the ability to accurately quantitate cellular GSH pools within these rare tissues is needed because aberrant GSH metabolism may lead to an alteration of the HSC/MPP redox state. These changes in HSC/MPP redox balance are closely associated with genetic instability as well as proliferation, differentiation, and mobility within the HSC/MPP populations in which hematopoietic disorders are thought to initiate and reside[18, 27–29]. As such, examining the GSH/GSSG based HSC/MPP redox state may provide insight to the role of MRP1 and the glutathione redox pair in the onset and progression of hematopoietic neoplasms. Additionally, many cancer therapies, including radiation and chemotherapeutics, such as the anthracyclines, are known to exert a portion of their tumor killing effect through the production of ROS, which may be remediated by adduction of GSH and efflux via MRP1 further altering the malignant cell redox state. Together, these facets indicate that accurate measurement of GSH/GSSG will aid interpretation of MRP1 function in HSC/MPPs and how alterations in the cellular redox state may affect hematopoietic disease onset, progression, and treatment.
Many current methodologies utilized for GSH and GSSG quantitation are based on free thiol conjugation followed by the observation of a fluorescent product, enzymatic reduction of glutathione disulfide pools, and the chemical derivation of parent glutathione molecules allowing for chromatographic separation[30–34]. These methodologies require complicated chemical reactions, which may not reach completion, and large amounts of sample tissue. Moreover, some methodologies may only accurately measure total glutathione content and are not effective or ideal for accurately quantitating GSH/GSSG in small cell populations in vivo, such as HSC/MPP’s. However, emerging methods which combine high performance liquid chromatography with single or tandem mass spectrometry (LC-MS, LC-MS/MS) with and without post column sample modification have quantitated GSH/GSSG in physiological fluids[35, 36]. While these methods demonstrate the potential power of LC-MS/MS analysis, they do so within an effectively unlimited sample population. Here we describe the development and validation of a simple tissue extraction combined with a robust and sensitive LC-MS/MS method, demonstrating high throughput potential, for the direct and simultaneous quantitation of oxidized and reduced forms of glutathione in small HSC/MPP populations. The application of this methodology is demonstrated in cell culture systems as well as mouse bone marrow, including purified Lin−, c-kit+, Sca-1+ (LSK) populations. Validation procedures performed were based on the recommended guidelines for LC-MS/MS based analysis of small molecules in industry as set forth by the United States Food and Drug Administration[37]. As a method and model control we examine cell populations demonstrating differential expression of MRP1 resulting in the characterization of MRP1 functional effect on glutathione concentrations within primitive HSC/MPP populations in vivo. This control allows us to evaluate the ability of our LC-MS/MS method to detect fine variations in glutathione concentrations with both in vitro and in vivo systems. We have found that the over expression of MRP1 in MCF7 cells results in decreased intracellular GSH/GSSG concentrations, while loss of Mrp1 expression in Mrp1−/− HSC/MPPs resulted in the cellular accumulation of GSH and GSSG. These results indicate that MRP1 expression may have a direct impact on the cellular redox state of the HSC/MPP population. Additional evaluation of the utility for this methodology is completed through the quantitation of glutathione within cultured MDSL cells treated with chemotherapeutics (Doxorubicin and Lenalidomide) that have been previously used for the treatment of hematopoietic disorders such as acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS). Furthermore, we characterize glutathione concentrations in normal human bone marrow as well as mononuclear cells isolated form patients afflicted with acute myeloid leukemia. We found that acute myeloid leukemic cells derived from human bone marrow demonstrate elevated levels of GSH, indicating a potential mechanism by which leukemic stem cells balance elevated levels of oxidative stress produced during proliferation.
LC-MS/MS Materials
γ-L-Glutamyl-L-Cysteinyl-Glycine (GSH), γ-glutamyl-L-cyteinyl-glycine disulfide (GSSG), ethylenediaminetetraacetic acid (EDTA), were purchased from Sigma-Aldrich (St. Louis, MO). Trichloroacetic acid purchased from J. T. Baker (Center Valley, PA). Chromatographic columns were purchased from Phenomenex (Torrance, CA). HPLC grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). Formic acid and ammonium formate were purchased from Acros Organic (Pittsburgh, PA).
Cell Culture
MDSL cells were cultured in IMDM media (ATCC; Manassas, VA) supplemented with 20% FBS (GE Healthcare; Pittsburgh, PA), 1% penicillin/streptomycin (Life Technologies; Grand Island, NY), and 15 ng/mL recombinant human IL-3 (Peprotech; Rocky Hill, NJ). MDSL cells were cultured at both 5% and 21% O2, 5% CO2, at 37° C. MCF7 cells were cultured in DMEM media (Life Technologies; Grand Island, NY) supplemented with 10% FBS and 1% penicillin/streptomycin in 5% CO2 at 37°C. MRP1 overexpressing MCF7 (MRP1-10, a gift from Dr. Charles Morrow, National Institute of Health, Bethesda, Washington DC) cells were cultured in DMEM media supplemented with 10% FBS and 1% penicillin/streptomycin, 1.0 mg/mL G418, in 5% CO2 at 37°C.
LC-MS/MS Methodology
Mass spectrometric analysis was performed on a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham MA) coupled with an ion max electrospray ionization source containing a HESI II probe operated in positive ion mode. The GSH/GSSG MS/MS method development was accomplish by direct infusion of a GSH/GSSG standard (10 μg/mL) into the mass spectrometer at a flow rate of 5 μL/min. Single reaction monitoring (SRM) was used to simultaneously analyze samples for GSH and GSSG. The SRM’s for GSH (m/z 308.022 → m/z 84.056 + 162.002) and GSSG (m/z 613.99 → m/z 231.034 + 354.993) were completed with collision energies and S-lens voltages optimized for each individual transition. Other MS/MS method settings follow: Q1 and Q3 resolution at 0.7 FWHM, scan width at 0.1 amu, scan rate at 0.1 seconds, and collision gas pressure at 1.0 mTorr. Tune parameters were as follows: spray voltage at 2700 V, vaporizer temperature at 200° C, capillary temperature at 250° C, sheath and auxiliary gas pressures at 35 and 10 arbitrary units respectively. Liquid chromatography was performed on a Shimadzu LC system containing a CMB-2A controller, a SIL-2A auto sampler, and two LC-20 AD pumps (Canby, OR). Liquid chromatographic separation of 10 μL sample injections were achieved on a Phenomenex Luna PFP(2) analytical column (100 mm x 2.0 mm, 3 μm) and completed under isocratic conditions, 99% mobile phase A (H2O, 0.75mM ammonium formate, 0.01% formic acid), 1% solvent B (methanol) at 250 μL/minute over an 11 minute total run time.
Calibration Standards and Quality Control’s
Stock solutions of GSH and GSSG were prepared at 1 mg/mL by dissolving 1.0 mg of pure powder stock in 1.0 mL of 2% TCA (1mM EDTA). One hundred μL aliquots were then frozen at −80° C for no more than 7 days. Working stock solutions were created by 1:10 serial dilutions of the 1.0 mg/mL stock solutions in 2% TCA (1mM EDTA). A working GSH/GSSG stock solution was mixed at 10 μg/mL and subsequently used to dilute working standard concentrations of 500.0, 250.0, 100.0, 50.0, 25.0, 10.0, 7.5, 5.0, 2.5, 1.0, 0.5 ng/mL for simultaneous GSH/GSSG standardization of the LC-MS/MS system. QC solutions of 5.0, 25.0, and 250.0 ng/mL (five injections at each concentration) were also diluted from the 10 μg/mL working GSH/GSSG stock solution. Standard curves and QC’s were prepared and run on each day of analysis. Data acquisition and sample peak integration analysis was completed with Xcalibur software, version 2.1 (Thermo Fisher). Standard, QC, and sample concentrations were calculated with sample peak areas and linear equations (form y = mx + b) generated by external standard curves for both GSH and GSSG.
Sample Extraction Methodology
Samples, prepared as described below were pelleted in a 5 mL round bottom tube by centrifugation in a swinging bucket rotor at 1300 rpm for five minutes. Supernatants were carefully discarded by vacuum aspiration. Cells were then re-suspended in 75 μL of extraction buffer (2% TCA; 1.0 mM EDTA) and incubated on ice for 15 minutes after which samples were vortexed for 45 seconds and incubated on ice for a further 15 minutes. Sample pH was adjusted to 2.0 by mixing 50 μL of sample lysate with 50 μL of Mobile phase A (HPLC grade H2O, 0.75 mM ammonium formate, 0.01% formic acid, pH=3.0) and cellular debris was subsequently pelleted by centrifugation at 4,000 x g for 10 minutes. Supernatants were collected for LC-MS/MS analysis.
Method Validation
The linear range of detection for both GSH and GSSG was determined and validated by the analysis of the standard curve and QC samples generated as described above (n=3). A linear regression was used to determine the correlation coefficient (r2 value) after plotting the analyte peak area over the standard concentration. The inter-day slope precision was expressed as the percent of the coefficient of variance ( ). Analyte stability at various temperatures was determined by repeat analysis at the lowest QC concentration (n=5) and is presented as percent of the concentration originally measured. Inter and intra-day precision was determined by repeat standard and QC evaluation at three different concentrations within the determined linear range in replicates of five (n=3). Again, precision is presented as %CV. The lower limit of detection (LLOD) was determined by the lowest peak height that generated a signal to noise ratio (S/N) greater than or equal to three (S/N ≥ 3) for both GSH and GSSG. The lower limit of quantitation (LLOQ) for both analyte species was identified as the minimum analyte concentration required to generate a signal to noise ratio greater than or equal to five (S/N ≥ 5) as determined by standard addition analysis described below.
Because GSH and GSSG are produced endogenously, the cellular lysates produced during sample extraction serve as the biological matrix. Thus, precision, accuracy and percent recovery were evaluated by standard addition of known analyte concentrations to whole cell lysates which were extracted at 30,000 cells per aliquot of lysate. Mouse whole bone marrow was used for cross validation. Here, individual lysates of 30,000 cells/sample were spiked with known amounts of standard GSH/GSSG solution. To accomplish standard addition of MDSL cell lysates, 1.2 million MDSL cells were lysed in 3.0 mL of extraction buffer (30,000 cells/ 75 μL of extraction buffer). Then, 75 μL of lysate was added to 75 μL of blank extraction buffer or extraction buffer containing four times the target analyte (GSH/GSSG) concentration and samples were then incubated on ice for 30 minutes. After incubation, sample pH was adjusted to 2.0 by mixing 50 μL of sample containing the blank lysate, +/− standard with 50 μL of mobile phase A. Samples were then spun down as previously described and analyzed via LC-MS/MS. Standard addition of mouse whole bone marrow was completed by preparing individual aliquots of 30,000 cells to which 75 μL of either blank extraction buffer or extraction buffer containing two times the target analyte concentration of GSH/GSSG was added. Samples were then incubated on ice for 15 minutes after which the samples were vortexed for 45 seconds each, followed by another 15 minute incubation period on ice. Cellular debris was then pelleted by centrifugation at 4,000 x g, and the supernatants were collected for LC-MS/MS analysis. Standard addition for both cell types was completed at 4 concentrations; 0.0, 5.0, 50.0, 250.0 ng/mL for GSH and 0.0, 1.0, 5.0, 50.0 ng/mL for GSSG. Each standard addition concentration was evaluated in replicates of n=5. Target analyte concentrations were evaluated by subtracting the basal GSH/GSSG concentrations obtained by LC-MS/MS analysis of the blank samples from the GSH/GSSG concentrations calculated from the standard addition samples. Precision values at each standard addition concentration are expressed as % CV, described above. Accuracy values are expressed as % bias, which is taken as the percent deviation of the determined experimental concentration from the proposed theoretical concentration ( ). The percent recovery values were determined as the [ ].
GSH/GSSG Analysis of Cultured Cells
MRP1 protein expression in MCF7 WT and MRP1 overexpressing MCF7 (MRP1-10) cells was evaluated by flowcytometry analysis after incubation with a FITC conjugated antibody targeted to the first nucleotide binding domain (amino acids 617-932) of human ABCC1/MRP1. MCF7 and MRP1-overexpressing MCF7 (MRP1-10) cells were plated at 100,000 cells per well in 12 well plates and were subsequently incubated in 5% CO2 at 37° C for 24 hours to allow for cellular attachment. Cells were then mechanically harvested, counted by hemocytometer and 50,000 cells per sample were aliquoted into 500 μL of PBS (pH=7.0 for LC-MS/MS analysis of GSH/GSSG as described above. Similarly, prior to analysis, MDSL cells were plated at 200,000 cells/mL in a 24 well plate and were then incubated in 5% or 21% O2, 5% CO2, at 37° C for 24 hours. Cells were then counted by hemocytometer and samples were diluted in 500 μL of PBS (pH=7.0) prior to LC-MS/MS analysis of GSH/GSSG. Total GSH and GSSG concentrations (ng/mL) were converted to ng/sample by multiplying by total sample volume, sample concentrations were then divided by the total number of cells extracted, normalized to 20,000 cells per sample and reported as [GSH] and [GSSG] in ng/20,000 cells.
LSK Purification by FACS Analysis
Femurs and tibias were harvested from wild type (WT) and Mrp1 knock out (KO) mice of the C57BL/6 background. Subsequently, bone marrow was aspirated with FACS buffer (PBS, 2% HI-FBS pH=7.4) using a 2 mL syringe and a 27.5 gauge needle (BD biosciences, San Jose, CA). Whole bone marrow was then pelleted by centrifugation at 1600 rpm for 3 minutes and the supernatant was discarded. Cells were then washed twice with FACS buffer. Whole bone marrow was then re-suspended in red blood cell lysis buffer (150 mM NH4Cl, 10mM NaHCO3, 1 mM EDTA), filtered through a 5 mL (12 x 75 mm) polystyrene round-bottom tube with a cell strainer cap (BD Falcon, San Diego, CA) and incubated on ice for 5 minutes. Cells were then spun down at 1600 rpm for 3 minutes and the supernatant was discarded. Cells were then washed twice with FACS buffer and re-suspended in 100 μL of FACS buffer. After re-suspension cells were incubated with 2 μL (1:50 ratio) of conjugated antibody corresponding to cell surface markers used for sorting Lin−, Sca-1+, c-kit+ hematopoietic stem cells (LSKs) on ice for 60 minutes. The antibodies used for sorting LSK’s were as follows: Sca-1, Ly-6A/E-PE-Cy7; c-kit, CD-117-PerCP-Cy5.5; Lineage, Cd45R-APC-Cy7, Cd3e-APC-Cy7, Ter199-APC-Cy7, Cd19-APC-Cy7, and Cd11b-APC-Cy7 (all Abs listed are raised in rat, anti-mouse and were purchased at 0.2 mg/mL from BD Pharmigen, San Diego, CA). Subsequent to incubation, cells were washed and then re-suspended in 500 μL of FACS buffer for fluorescence assisted cell sorting of LSKs at the University of Kentucky flow cytometry facility. There, Lin−, Sca-1+, c-kit+ HSC/MPP’s form both WT and Mrp1 KO mice were sorted such that LSKs from two mice were pooled as one animal for GSH/GSSG analysis. Thus, six mice of each genotype were sorted as three separate animals. LSKs were then taken directly to the University of Kentucky proteomics core for analysis of absolute GSH/GSSG concentrations via LC-MS/MS.
Cell Viability
MDSL cells were plated at a density of 200,000 cells/mL in complete MDSL cell medium containing either vehicle or drug (10 μM Lenalidomide, Cayman Chemical, Ann Arbor MI; or 25 nM Doxorubicin, Pfizer, New York, NY) in a 24 well plate and were incubated for 48 hours. Cells were then transferred to 5 mL round bottom tubes and washed twice with warm HBSS (pH=7.4)(Gibco-Thermo Fischer, Waltham, MA). Cells were then re-suspended in MTT solution (0.5 mg/mL 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide, Sigma Aldridge St. Louis, MO; in RPMI media with no phenol red Gibco-Thermo Fischer, Waltham, MA), aliquoted into a 96 well plate, and incubated at 37°C for 4 hours. Equal volumes of lysis buffer (50/50, v/v, isopropanol/DMSO) were added to each well followed by a 30 minute incubation at 37°C. Samples were then evaluated for absorbance at 560 and 690 nm using a Molecular Devices SpectraMax Plus 384 plate reader with SoftMax Pro software.
Therapeutic Drug Treatments
MDSL cells were plated at a density of 200,000 cells/mL in complete MDSL cell medium containing either vehicle or drug (10 μM Lenalidomide, Cayman Chemical, Ann Arbor MI; or 25 nM doxorubicin, Pfizer, New York, NY) in a 24 well plate and were incubated for 6 hours. Samples were then transferred to 5 mL round bottom centrifuge tubes, extracted, and GSH/GSSG concentrations were analyzed via LC-MS/MS as previously described.
Patient Sample Collection and Analysis
Peripheral blood and bone marrow samples were obtained by Dr. Diana Howard, from the Markey Cancer Center after individuals gave informed consent for tissue donation. White blood cells were isolated by ficol gradient centrifugation as follows; patient tissue (21 mL of blood or bone marrow) was diluted to 50 mL with phosphate buffered saline (sigma Aldridge). Samples were then layered over 13 mL of Histopaque (Sigma Aldridge) and centrifuged at 1400 rpm for 45 minutes. The white blood cell layer was then carefully removed, diluted in freezing media (IMDM, 10% FBS, 1% penicillin/streptomycin, 10% DMSO) and stored in liquid nitrogen (−180° C) until the time of LC-MS/MS analysis as described above.
Results
LC-MS/MS Method Development and Validation
Past liquid chromatographic separation of glutathione has been accomplished with common reverse phase C18 columns. However, we have found that GSH retention on these analytical columns is minimal. Recently, Squellerio et al., described utilization of the Luna PFP-2 reverse phase column (Phenomenex) for the efficient retention of both glutathione species in the LC-MS/MS analysis of GSH and GSSG from human whole blood samples. In agreement with their findings, we found the Luna PFP-2 column to the optimal tool for chromatographic retention and separation of the oxidized and reduced glutathione species. While previous mass spectrometric based methodologies for the analysis of GSH/GSSG in physiological fluids have been characterized, these methods are applied to the analysis of samples for which there effectively is a nearly unlimited supply[35, 36]. To our knowledge, none have examined the limits of sensitivity and potential for analysis of fine changes in GSH/GSSG concentrations within rare and limited tissue populations, such as hematopoietic stem cells in vivo.
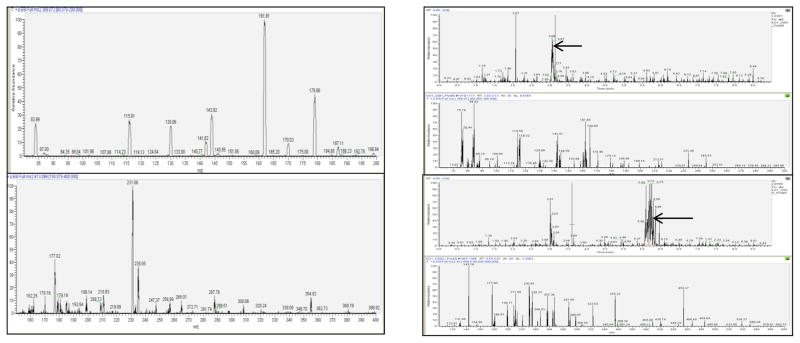
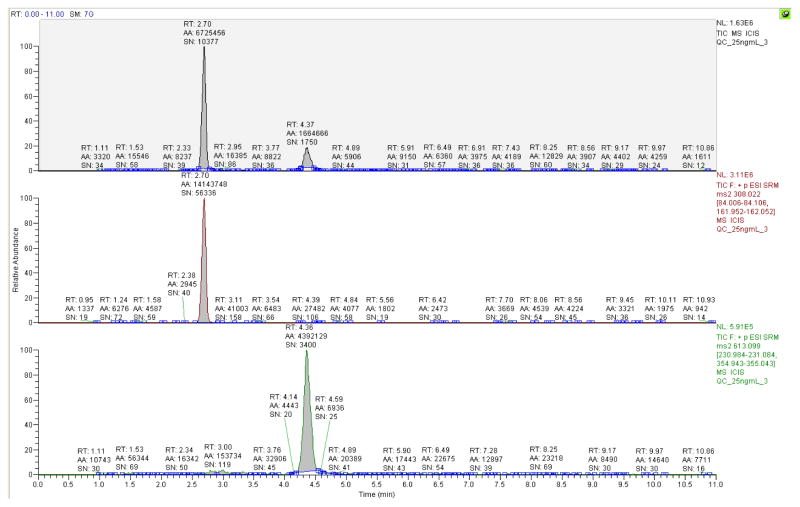
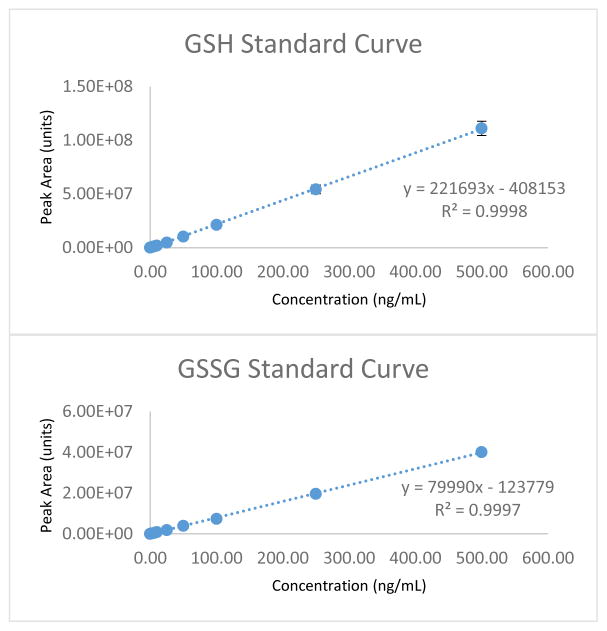
To determine the quantitative limit for tissue sample analysis of GSH/GSSG concentrations in vitro and demonstrate the effectiveness and sensitivity of these analytical parameters in vivo, we developed a modified LC-MS/MS method for direct and simultaneous GSH/GSSG quantitation. The product ion spectra obtained were evaluated by the comparison to product ion spectra acquired from the direct infusion of pure GSH/GSSG standard (figure 1A). The chromatographic resolution of GSH and GSSG was confirmed by the collection and evaluation of full product ion spectra resulting from the analysis of GSH and GSSG from a biological sample (figure 1B). Upon completion of the LC-MS/MS SRM method development, the average retention times for GSH and GSSG elution from the Luna-PFP2 reverse phase column, calculated from QC analysis were 2.69 +/− 0.02 and 4.37 +/− 0.03 minutes respectively (mean +/− SD, n=15). A typical extracted ion chromatogram obtained from LC-MS/MS analysis of a 25 ng/mL standard is shown in Figure 2. Figure 3 demonstrates the typical results obtained from GSH/GSSG standard curve analysis. Although separate external GSH and GSSG standard curves are generated, standard data acquisition occurred simultaneously.
Figure 1.
A.) Both GSH (top) and GSSG (Bottom) were infused in line with a 50% solvent A, 50% solvent B LC flow (200 μL/min) at a rate of 5 μL/min and at concentrations of 10μg/mL. The resulting product ion spectra were obtained from GSH and GSSG parent ions. B.) The chromatograms and spectra above resulted from the injection of a cellular extract isolated from mouse LSK cells. The spectra below each chromatogram display the product ion spectrum for the highlighted chromatographic peak above. Comparison of these spectra with the infusion spectra (A) shown above confirm the presence of GSH and GSSG.
Figure 2.
The chromatograph above demonstrates the simultaneous separation of reduced (GSH, middle) and oxidized (GSSG, bottom) forms of glutathione. This injection sample consisted of 10 μl of a 25 ng/mL QC sample (0.25 ng on column) routinely run during instrument standardization.
Figure 3.
The linear plots above are examples of typical standard curves for both GSH and GSSG. Although separate curves are generated, GSH and GSSG standardization of the LC-MS/MS system takes place simultaneously. 1 mg/mL standards for GSH and GSSG are prepared in 1% TCA (1.0 mM EDTA, 50% solvent A). These standards are then mixed at 10 μg/mL each and are subsequently diluted down in a stepwise fashion with 1%TCA (1.0 mM EDTA, 50% solvent A) to create the working solutions of standard and QC concentrations displayed in the linear range above. Fresh standards and QC’s are prepared the day sample analysis takes place.
The method validation summary (Table 1) includes all parameters for LC-MS/MS method validation as recommended by the USFDA[37]. The lower limit of detection for both GSH and GSSG was 0.5 ng/mL as demonstrated by chromatographic peaks that have a signal to noise ratio greater than 3. Precision, lower limits of quantitation (LLOQ) as determined by accuracy measurements at various QC concentrations, and analyte recovery were all determined by standard addition analysis. The lower limits of analyte quantitation were 5.0 ng/mL for GSH and 1.0 ng/mL for GSSG.
Table 1.
The table above summarizes the results of the LC-MS/MS method validation for the simultaneous analysis of reduced and oxidized forms of glutathione. The Lower Limit of Detection and Quantitation were taken as the concentrations which exhibit a signal greater than or equal to that of three (LLOD) and five (LLOQ) times the intensity of a blank matrix injection respectively. All standards and QC’s had an injection volume of 10 uL and were analyzed using the LC-MS/MS method previously summarized. Inter-day standard and slope analysis was completed over 3 days with a linear range of 0–500 ng/mL. QC’s levels evaluated were at 5, 25, and 250 ng/mL over 3 days for inter-day precision analysis. Intra-day precision analysis was completed with an n=5 for each concentration on the same day.
| Method Validation Summary | ||
|---|---|---|
|
| ||
| Stability (%) | GSH | GSSG |
| RT (24 hr) | 77 | 91 |
| +4 Deg C (24 hr) | 111 | 94 |
| −80 Deg C (7 Days) | 91 | 95 |
|
| ||
| LLOD (ng/mL) | 0.5 | 0.5 |
| LLOQ (ng/mL) | 5.0 | 1.0 |
|
| ||
| Inter-day St. Curve Slope Precision (%CV) | 6.2 | 3.5 |
|
| ||
| Intra-day QC Precision (% CV) | ||
|
| ||
| GSH 5 ng/mL; GSSG, 5 ng/mL | 4.9 | 6.5 |
| GSH 50 ng/mL; GSSG, 50 ng/mL | 2.0 | 3.3 |
| GSH 250 ng/mL; GSSG, 250 ng/mL | 2.0 | 2.4 |
|
| ||
| Inter-day QC Precision (%CV) | ||
|
| ||
| GSH 5 ng/mL; GSSG, 5 ng/mL | 4.3 | 8.7 |
| GSH 50 ng/mL; GSSG, 50 ng/mL | 3.0 | 3.5 |
| GSH 250 ng/mL; GSSG, 250 ng/mL | 1.4 | 2.3 |
|
| ||
| Accuracy (Mean % Bias Relative to Theoretical Standard Concentration) | ||
|
| ||
| MDSL Cell Line | ||
|
| ||
| GSH 5 ng/mL; GSSG, 1 ng/mL | 17.4 (5.87 ng/mL) | −1.6 (0.98 ng/mL) |
| GSH 50 ng/mL; GSSG, 5 ng/ | 1.1 (50.55 ng/mL) | 1.8 (5.09 ng/mL) |
| GSH 250 ng/mL; GSSG, 50 ng/mL | 1.2 (253.06 ng/mL) | 0.1 (50.94 ng/mL) |
|
| ||
| Mouse Whole Bone Marrow | ||
|
| ||
| GSH 5 ng/mL; GSSG, 5 ng/mL | 2.1 (5.10 ng/mL) | 0.4 (5.07 ng/mL) |
| GSH 50 ng/mL; GSSG, 50 ng/mL | −9.7 (45.16 ng/mL) | 0.2 (50.09 ng/mL) |
| GSH 250 ng/mL; GSSG, 250 ng/mL | −2.4 (243.95 ng/mL) | 0.5 (251.28 ng/mL) |
|
| ||
| Precision (% CV) | ||
|
| ||
| MDSL Cell Line | ||
|
| ||
| GSH 5 ng/mL; GSSG, 1 ng/mL | 11.3 | 14.5 |
| GSH 50 ng/mL; GSSG, 5 ng/mL | 4.3 | 2.4 |
| GSH 250 ng/mL; GSSG, 50 ng/mL | 1.1 | 1.7 |
|
| ||
| Mouse Whole Bone Marrow | ||
|
| ||
| GSH 5 ng/mL; GSSG, 5 ng/mL | 13.2 | 5.1 |
| GSH 50 ng/mL; GSSG, 50 ng/mL | 7.6 | 1.9 |
| GSH 250 ng/mL; GSSG, 250 ng/mL | 5.0 | 2.1 |
|
| ||
| Recovery (% Compared to Measured Blank Concentration) | ||
|
| ||
| MDSL Cell Line | ||
|
| ||
| GSH 5 ng/mL; GSSG, 1 ng/mL | 101.5 | 99.6 |
| GSH 50 ng/mL; GSSG, 5 ng/mL | 100.9 | 102.4 |
| GSH 250 ng/mL; GSSG, 50 ng/mL | 105.1 | 101.2 |
The USFDA recommends that accuracy values, which are defined as the closeness of a concentration value obtained by the analytical method to the actual concentration value, and precision values, defined as the closeness of individual measures of an analyte when the procedure is applied to multiple aliquots of a single homogenous solution of biological matrix, must be evaluated at three different concentrations within the analyte’s linear range and must have five repeat evaluations at each concentration. These analyte evaluations must have mean coefficient of variance values within 15% at medium and high concentrations within the analyte’s linear range, and 20% at the LLOQ[37]. Table 1 summarizes the results of this LC-MS/MS method validation. Analyte stability was evaluated at the lowest QC concentration (5 ng/mL) for storage at 3 different temperatures. Our results agree with those previously reported, demonstrating good GSH/GSSG stability within 15%, at +4° C and −80° C. However, GSH stability, at the LLOQ, was slightly diminished (−23%) at room temperature after a period of 24 hours. Slope and inter/intra-day precision values had coefficient of variance values less than 10% (Table 1). A full method validation was completed in MDSL cell lysates in which the accuracy values were all within 20% at the LLOQ (GSH, 17.4%; GSSG, −1.6%) and medium to high concentrations demonstrated CV values of less than 5% (Table 1). Precision %CV values were less than 15% at all concentrations for both GSH and GSSG. Analyte recovery values, evaluated by standard addition, were all within 10% of full analyte recovery for both GSH and GSSG. Finally, a method cross validation by evaluation of accuracy and precision via standard addition was completed in the mouse whole bone marrow biological matrix. Accuracy and precision coefficient of variance values were determined to be within 15% at all concentrations for both GSH and GSSG.
Biological Method Evaluation
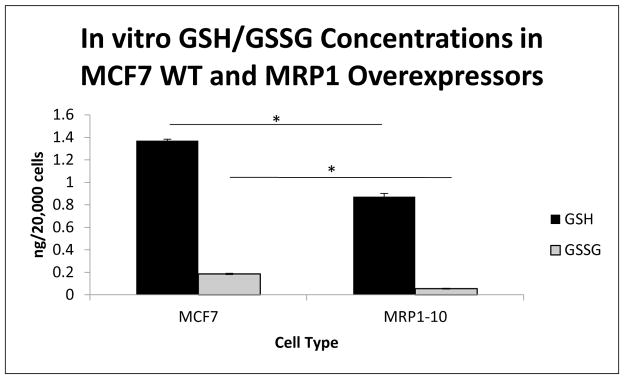
To test the potential for this method to evaluate varying glutathione levels in biological systems, we utilized a system with altered expression of Multidrug Resistance-associated Protein 1 (ABCC1/MRP1). We first measured GSH/GSSG levels in MCF7 wild type cells, known to not express MRP1, and compared these results to the GSH/GSSG measurement within MCF7 cells overexpressing MRP1 (MRP1-10 cells). Differential MRP1 expression levels within the two MCF7 cell lines was confirmed by flow cytometometric analysis of FITC conjugated antibody directed to the first nucleotide binding domain of human MRP1(data not shown). Figure 4 shows that overexpression of MRP1 resulted in a significant decrease in cellular GSH and GSSG concentrations, −0.50 ng/20k cells for GSH, and −0.13 ng/20k cells for GSSG (p=0.001), and that these changes lead to a significant increase in the GSH/GSSG ratio, +8.42 (p=0.01, data not shown).
Figure 4.
Mean GSH/GSSG concentrations were evaluated in MCF7 WT and MCF7 cells overexpressing MRP1 (MRP1-10). Significant differences in GSH concentration between MCF7 (1.37 ng/20k cells) and MRP1-10 cells (0.87 ng/20k cells) were determined (p=0.001). Additionally, significant differences in GSSG concentration between MCF7 (0.19 ng/20k cells) and MRP1-10 cells (0.06 ng/20k cells) were determined (p=0.001). These changes resulted in a significant alteration to the GSH/GSSG ratio between the two cell lines 7.4 (MCF7) vs 15.82 (MRP1-10) (p=0.01).
This method was then used to evaluate GSH/GSSG concentrations in the absence of MRP1 in vivo. Both WT and Mrp1−/ − C57BL/6 mice were obtained and their hematopoietic stem cells (Lin−, sca-1+, c-kit+; LSK’s) were isolated via fluoresce assisted cell sorting (Figure 5). The typical yield for this isolation was approximately 20,000 cells from a single animal. The results demonstrated that the LSK cells from Mrp1−/ − mice had a significant increase in cellular GSH concentrations (+4.6 ng/20k cells, p=0.01). Interestingly, although differences in the GSSG concentration were observed in LSK cells isolated from Mrp1−/ − and WT mice, these changes were not judged to be statistically significant (Figure 6). These results demonstrate the robustness and sensitivity of our LC-MS/MS methodology and its ability to detect fine differences in GSH/GSSG concentrations within very limited tissue populations.
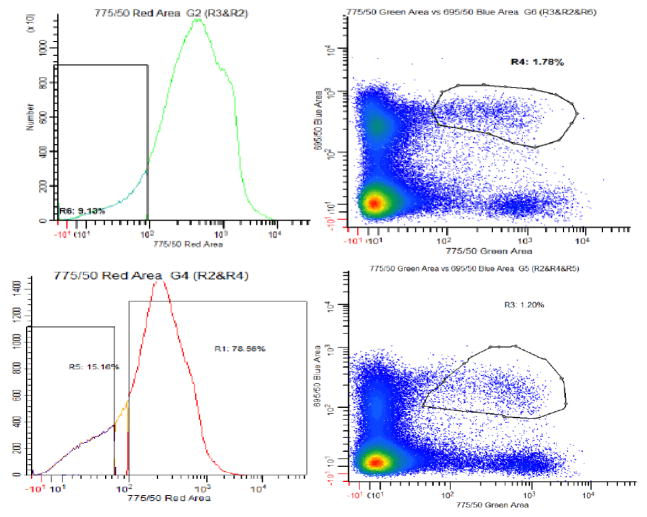
Figure 5.
Fluorescence Assisted Cell Sorting (FACS) purification of Lin−, Sca-1+, c-kit+ (LSK) hematopoietic stem cells (HSC’s). Bone marrow from C57BL6 wild type (WT, Bottom) and Mrp1 knock out (KO, Top) mice used for GSH/GSSG analysis. This analysis routinely resulted in the purification of approximately 20,000 cells per mouse.
Figure 6.
The concentrations of GSH, GSSG and their cellular ratio determined in WT and Mrp1 KO mouse LSK cells purified as described earlier. The graph depicts the differences in analyte concentrations between genotypes. A significant difference in mean GSH concentration is demonstrated between the Mrp1 KO (5.64 ng/20k cells) and WT (1.04 ng/20k cells) animals (p=0.01). A difference in the GSSG concentration between genotypes was found (KO, 1.07 ng/20k cells vs WT, 0.17 ng/20k cells) but this difference was not statistically significant. Similarly no significant difference in GSH/GSSG ratios was determined (KO, 8.56 vs WT, 6.16).
Both the over expression and knock out of Mrp1 expression within various cell types resulted in an increase of the GSH/GSSG ratio. This indicates that the evaluation of the individual cellular concentrations of GSH and GSSG and not only their concentration ratio is useful for an accurate interpretation of the GSH/GSSG based cellular redox state.
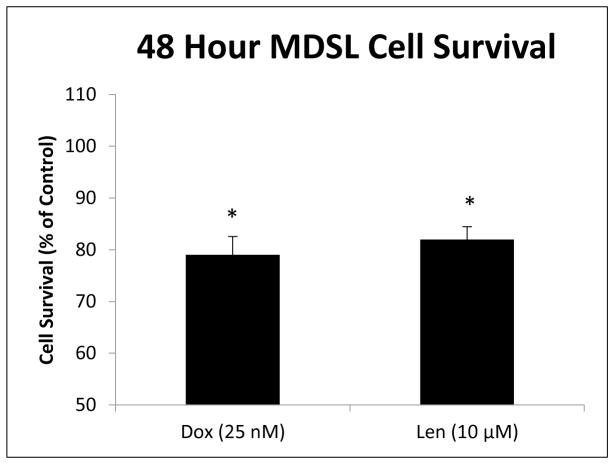
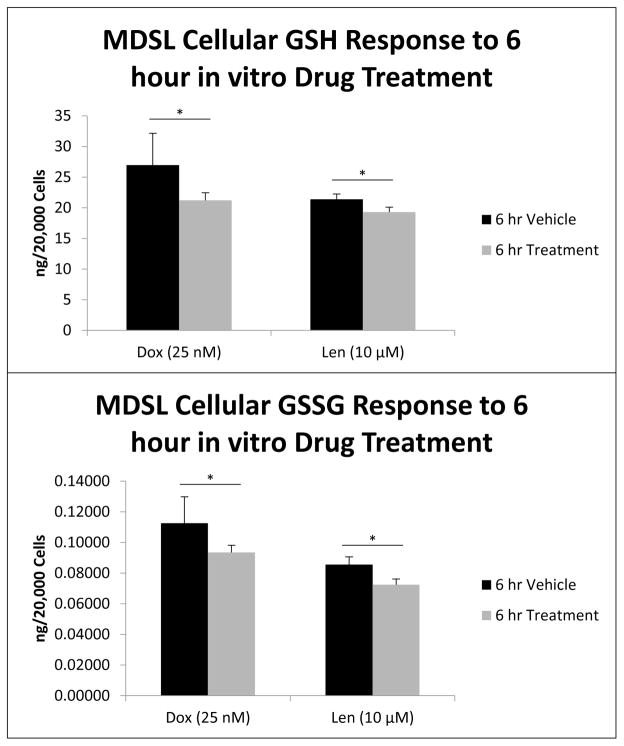
To further validate the utility of the methodology, MDSL cells were evaluated for GSH and GSSG concentrations after treatment in vitro with two clinically relevant chemotherapeutic agents, Doxorubicin (Adriamycin) and Lenalidomide (Revlimid). Traditionally, high risk MDS patients presenting with blast crisis have been treated with high dose Cytarabine plus an additional course of an anthracycline such as Doxorubicin. Because Doxorubicin is a known ROS inducer, due its metabolic formation of an intermediate semi-quinone structure, we compare the treatment effect and generation of ROS via doxorubicin to that of Lenalidomide. Lenalidomide is known to act as an anti-inflammatory agent as well as a stimulant of lymphocytes and erythropoiesis [38–41]. However, there is evidence that suggests Lenalidomide can, itself, induce oxidative stress. This is observed in the use of Lenalidomide for the treatment of a multiple myeloma model in which the combination of Lenalidomide and the spin trap, ROS scavenger, phenyl-N-t-butylnitrone (PBN) effectively modulates the transcriptional activation of AP-1 family transcription factors [42]. Recently, Lenalidomide has been proven to be effective in the treatment of MDS mouse models and has a significant cytotoxic effect in vitro, at 10 μM [43]. Doxorubicin (25 nM) and Lenalidomide (10 μM) treatment of MDSL cells results in 21% (p=0.04) and 18% (p=0.02) decreases in MDSL cell viability respectively (figure 7). Similar treatment effects are also demonstrated on cellular GSH and GSSG concentrations in vitro. A 6 hour treatment of MDSL cells with Doxorubicin resulted in a 19% depletion of GSH (−5.72 ng/20k cells, p=0.03) and a 15% (−0.019 ng/20k cells, p=0.03) depletion of GSSG compared to vehicle control (figure 8). Similarly, 6 hour in vitro treatment of MDSL cells with Lenalidomide resulted in a 10% (−2.09 ng/20k cells, p=0.01) depletion of GSH and a 15% (−0.013 ng/20k cells, p=0.02) compared to vehicle controls (figure 8). Because the decreases in GSH and GSSG upon treatment were very fine, there was no significant difference in the GSH/GSSG ratio’s between treatment and control groups (data not shown). This again points to the importance of individual species concentration determination rather than the examination of the ratio between the reduced and oxidized forms of glutathione alone. Furthermore, the elucidation of such minor, yet significant, changes in analyte concentration demonstrates the sensitivity and robust nature of this methodology.
Figure 7.
MDSL cell viability was evaluated via MTT assay after 48 hour treatment with 25 nM Doxorubicin and 10 μM Lenalidomide in vitro. Both treatments resulted in a significant decrease of cell survival, 21% for Doxorubicin (p=0.04) and 18% for Lenalidomide (p=0.02). Results are expressed as percent cell survival normalized to the vehicle control.
Figure 8.
MDSL cell GSH (A) and GSSG (B) concentrations were analyzed after 6 hour drug treatment in vitro. Both Doxorubicin (25 nM) and Lenalidomide (10 μM) caused significant depletion of GSH and GSSG (Dox GSH −5.72 ng/ 20k cells, p=0.03; Dox GSSG −0.019 ng/20k cells, p=0.03; Len GSH −2.09 ng/20k cells, p=0.02; Len GSSG −0.013 ng/20k cells p=0.01).
Patient Sample Evaluation
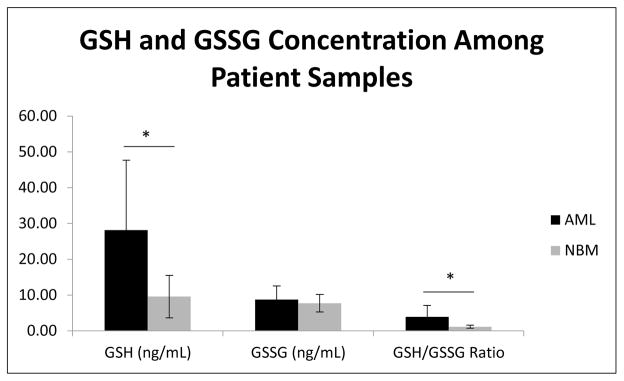
Because cellular GSH/GSSG concentrations could play a significant role in disease onset and progression, changes in cellular GSH/GSSG concentrations and the cellular redox state may potentially serve as a biological marker for disease onset or severity. As such, we set out to evaluate GSH/GSSG concentrations in ficol purified white blood cell populations from patient samples donated by healthy individuals or those who had a confirmed case of a hematopoietic malignancy. We measured GSH/GSSG concentrations in 5 samples collected from healthy bone marrow (NBM), and 6 samples from patients diagnosed with acute myeloid leukemia (AML). Figure 9 shows a significantly higher GSH levels in AML compared to the NBM samples (+ 18.57 ng/mL), resulting in an increased GSH/GSSG ratio (+2.87). This elevated GSH/GSSG redox state may compensate for the elevated oxidative stress levels that malignant cells utilize for proliferation and disease progression.
Figure 9.
Mean GSH, GSSG concentrations and their cellular concentration ratio analyzed within lymphocytes purified from the peripheral blood of AML patients as was the bone marrow obtained from normal patient donors. LC-MS/MS analysis revealed a significant increase in GSH concentrations within the AML (28.15 ng/mL) vs NBM (9.58 ng/mL) sample populations (p=0.03), resulting in a significant increase in the GSH/GSSG ratio, 3.94 in AML vs 1.16 in NBM patients (p=0.04). No significant difference was determined in GSSG concentrations between patient groups (AML, 8.77 ng/mL vs NBM, 7.73 ng/mL).
Discussion
Recently, several mass spectrometric based methodologies have directly evaluated oxidized and reduced glutathione extracted from physiological fluids. These methods have demonstrated the ability to measure GSH/GSSG concentrations without the need for the enzymatic reduction or chemical derivation of glutathione or glutathione disulfide prior to endpoint detection[31, 33, 35, 36]. While effective, previous methods have been applied only to large sample pools in which the limits of sample volume and method sensitivity are not an issue. Following the USFDA Guidance for Industry on Bioanalytical LC-MS/MS Method Validation, we have developed and described the validation of a sensitive and robust LC-MS/MS method for the simultaneous and direct quantitation of the oxidized and reduced forms of glutathione. We apply this methodology to the quantitation of GSH and GSSG at the cellular level, and we use this method to determine the lower limit of tissue volume required for accurate cellular GSH/GSSG quantitation. We have demonstrated the quantitation of cellular GSH/GSSG within the murine stem cell populations and the potential for GSH/GSSG analysis within HSCs derived from an individual animal. Our analyses enable the determination of the physiologically relevant, functional effect of MRP1 expression on the glutathione based redox state within primitive HSC populations. Here, loss of MRP1 expression results in an increase in cellular glutathione concentrations creating a more reducing intracellular environment. This result indicates that MRP1 may play an important role in regulating redox balance within HSC’s and thus influencing the ability or proclivity for HSCs to self-renew or differentiate into downstream functional populations. Additionally, the ability to detect fine clinically relevant treatment induced variations in glutathione concentrations within hematopoietic tissues, as demonstrated by the treatment of MDSL cells with Doxorubicin and Lenalidomide, show that this methodology may be capable of providing valuable insight to treatment toxicity as well as efficacy as it pertains to the modulation of the cellular redox state in vivo.
Because of the critical role of glutathione in cellular redox balance and signaling, we believe that the tissue extraction and LC-MS/MS method described here will become an important tool for examining how the cellular redox state may affect or be affected by the development and treatment of hematopoietic neoplasms. Based on the data obtained from human samples, we speculate that elevated redox stress within malignant hematopoietic cells may be compensated with elevated levels of GSH and that the altered redox state may support disease onset as well as drive disease progression[44]. The results suggest that there is a potential for the future use of cellular GSH/GSSG concentrations as a bio-marker for hematopoietic cancer; however, a much larger patient cohort must be evaluated.
GSH concentrations in the nucleus account for 10–15% of total cellular GSH, and 1–2% of this total cellular GSH pool residing in the nucleus may be resistant to depletion by chemical agents such as L-buthionine-[S,R]-sulfoximine (BSO)[45–49]. Thus, characterization of nuclear GSH concentration and function can indicate GSH utility in nuclear protection from oxidant insult, resulting in the faithful conservation of DNA replication and improved DNA repair capabilities. Similarly, mitochondrial GSH may account for up to 30% of cellular GSH[1, 8, 50, 51]. This mitochondrial pool of GSH protects other sulfhydryl containing proteins and may partially regulate the function of ATPases, transporters, and dehydrogenases by buffering the cellular redox status in the face of oxidative challenge[8]. Due to its sensitivity, our method may be utilized for the evaluation of GSH/GSSG concentrations within separate subcellular fractions, such as nuclear extracts and purified mitochondrial preparations. Future efforts to characterize subcellular GSH pools within rare tissue populations may be accomplished by adopting this LC-MS/MS methodology into an ultra-high pressure liquid chromatographic (UHPLC) system. The features of UHPLC-MS/MS analysis result in tighter peak widths and greater peak heights resulting in improved overall analyte sensitivity and higher throughput potential. Utilization and further development of the methods we describe here can have an important impact on redox biology research as it pertains to the development of hematopoietic malignancies in limited and rare tissue populations as well as the impact of redox balance on subcellular function.
Highlights.
LC-MS/MS method for the simultaneous analysis of oxidized and reduced glutathione.
The method is validated based on FDA guidelines in vitro and in vivo systems.
Detection of glutathione in rare HSPCs and drug induced variations of GSH levels.
ABCC1 expression alters intracellular glutathione concentrations within HSPCs.
Evaluation of glutathione as a potential biomarker for hematopoietic malignancy.
Acknowledgments
This work is supported by NIH training grant T32 ES007266, the Edward P. Evans Foundation, and the the NCI Cancer Center Support Grant P30 CA177558.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, et al. Emerging regulatory paradigms in glutathione metabolism. Adv Cancer Res. 2014;122:69–101. doi: 10.1016/B978-0-12-420117-0.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–44. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 5.Akerboom TP, Bilzer M, Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982;257(8):4248–52. [PubMed] [Google Scholar]
- 6.Yang H, et al. Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1. Biochem J. 2005;391(Pt 2):399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballatori N, et al. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CV, et al. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140(1):1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 9.Ghezzi P. Regulation of protein function by glutathionylation. Free Radic Res. 2005;39(6):573–80. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne I, et al. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Pedrajas JR, et al. Glutathione Is the Resolving Thiol for Thioredoxin Peroxidase Activity of 1-Cys Peroxiredoxin Without Being Consumed During the Catalytic Cycle. Antioxid Redox Signal. 2016;24(3):115–28. doi: 10.1089/ars.2015.6366. [DOI] [PubMed] [Google Scholar]
- 12.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 13.Kirlin WG, et al. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27(11–12):1208–18. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 14.Asensi M, et al. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–76. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- 15.Flohe L. The fairytale of the GSSG/GSH redox potential. Biochim Biophys Acta. 2013;1830(5):3139–42. doi: 10.1016/j.bbagen.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26(8):426–33. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Parmar K, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104(13):5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu L, et al. Antioxidant N-acetyl-L-cysteine increases engraftment of human hematopoietic stem cells in immune-deficient mice. Blood. 2014;124(20):e45–8. doi: 10.1182/blood-2014-03-559369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai JJ, et al. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol. 2013;15(3):309–16. doi: 10.1038/ncb2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167(1):3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 23.Keppler D. Export pumps for glutathione S-conjugates. Free Radic Biol Med. 1999;27(9–10):985–91. doi: 10.1016/s0891-5849(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 24.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 25.Mueller CF, et al. Role of the multidrug resistance protein-1 (MRP1) for endothelial progenitor cell function and survival. J Mol Cell Cardiol. 2010;49(3):482–9. doi: 10.1016/j.yjmcc.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Ellison I, Richie JP., Jr Mechanisms of glutathione disulfide efflux from erythrocytes. Biochem Pharmacol. 2012;83(1):164–9. doi: 10.1016/j.bcp.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431(7011):997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 30.Robin S, et al. LC-MS determination of oxidized and reduced glutathione in human dermis: a microdialysis study. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(30):3599–606. doi: 10.1016/j.jchromb.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 31.Norris RL, et al. A sensitive and specific assay for glutathione with potential application to glutathione disulphide, using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;762(1):17–23. doi: 10.1016/s0378-4347(01)00304-8. [DOI] [PubMed] [Google Scholar]
- 32.Camera E, Picardo M. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781(1–2):181–206. doi: 10.1016/s1570-0232(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 33.Bouligand J, et al. Liquid chromatography-tandem mass spectrometry assay of reduced and oxidized glutathione and main precursors in mice liver. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832(1):67–74. doi: 10.1016/j.jchromb.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Reed DJ, et al. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 35.Squellerio I, et al. Direct glutathione quantification in human blood by LC-MS/MS: comparison with HPLC with electrochemical detection. J Pharm Biomed Anal. 2012;71:111–8. doi: 10.1016/j.jpba.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Cereser C, et al. Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with fluorescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B Biomed Sci Appl. 2001;752(1):123–32. doi: 10.1016/s0378-4347(00)00534-x. [DOI] [PubMed] [Google Scholar]
- 37.Administration, U.S.F.a.D. Guidance for Industry Bioanalytical Method Validation. 2013. [Google Scholar]
- 38.Corral LG, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 39.Matsuoka A, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24(4):748–55. doi: 10.1038/leu.2009.296. [DOI] [PubMed] [Google Scholar]
- 40.Muller GW, et al. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9(11):1625–30. doi: 10.1016/s0960-894x(99)00250-4. [DOI] [PubMed] [Google Scholar]
- 41.Schafer PH, et al. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther. 2003;305(3):1222–32. doi: 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- 42.Colla S, et al. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109(10):4470–7. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhyasen GW, et al. An MDS xenograft model utilizing a patient-derived cell line. Leukemia. 2014;28(5):1142–5. doi: 10.1038/leu.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorgenson TC, Zhong W, Oberley TD. Redox imbalance and biochemical changes in cancer. Cancer Res. 2013;73(20):6118–23. doi: 10.1158/0008-5472.CAN-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tirmenstein MA, Reed DJ. The glutathione status of rat kidney nuclei following administration of buthionine sulfoximine. Biochem Biophys Res Commun. 1988;155(2):956–61. doi: 10.1016/s0006-291x(88)80589-8. [DOI] [PubMed] [Google Scholar]
- 46.Jevtovic-Todorovic V, Guenthner TM. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol. 1992;44(7):1383–93. doi: 10.1016/0006-2952(92)90540-y. [DOI] [PubMed] [Google Scholar]
- 47.Edgren MR. Nuclear glutathione and oxygen enhancement of radiosensitivity. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51(1):3–6. doi: 10.1080/09553008714550431. [DOI] [PubMed] [Google Scholar]
- 48.Edgren M, Revesz L. Compartmentalised depletion of glutathione in cells treated with buthionine sulphoximine. Br J Radiol. 1987;60(715):723–4. doi: 10.1259/0007-1285-60-715-723. [DOI] [PubMed] [Google Scholar]
- 49.Britten RA, et al. The relationship between nuclear glutathione levels and resistance to melphalan in human ovarian tumour cells. Biochem Pharmacol. 1991;41(4):647–9. doi: 10.1016/0006-2952(91)90642-i. [DOI] [PubMed] [Google Scholar]
- 50.Meredith MJ, Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982;257(7):3747–53. [PubMed] [Google Scholar]
- 51.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13(10):1169–83. [PubMed] [Google Scholar]