Abstract
Aim
The American Heart Association (AHA) recommends monitoring cardiopulmonary resuscitation (CPR) quality using end tidal carbon dioxide (ETCO2) or invasive hemodynamic data. The objective of this study was to evaluate the association between clinician-reported physiologic monitoring of CPR quality and patient outcomes.
Methods
Prospective observational study of index adult in-hospital CPR events using the AHA’s Get With The Guidelines – Resuscitation Registry. Physiologic monitoring was defined using specific database questions regarding use of either ETCO2 or arterial diastolic blood pressure (DBP) to monitor CPR quality. Logistic regression was used to evaluate the association between physiologic monitoring and outcomes in a propensity score matched cohort.
Results
In the matched cohort, (monitored n=3032; not monitored n=6064), physiologic monitoring of CPR quality was associated with a higher rate of return of spontaneous circulation ROSC; OR 1.22, CI95 1.04 – 1.43, p=0.017) compared to no monitoring. Survival to hospital discharge (OR 1.04, CI95 0.91 – 1.18, p=0.57) and survival with favorable neurological outcome OR 0.97, CI95 0.75 – 1.26, p=0.83) were not different between groups. Of index events with only ETCO2 monitoring indicated (n=803), an ETCO2 >10mmHg during CPR was reported in 520 (65%), and associated with improved survival to hospital discharge (OR 2.41, CI95 1.35 – 4.30, p=0.003), and survival with favorable neurological outcome (OR 2.31, CI95 1.31 – 4.09, p=0.004) compared to ETCO2 ≤10mmHg.
Conclusion
Clinician-reported use of either ETCO2 or DBP to monitor CPR quality was associated with improved ROSC. An ETCO2 >10mmHg during CPR was associated with a higher rate of survival compared to events with ETCO2 ≤10mmHg.
Keywords: blood pressure, cardiopulmonary resuscitation, heart arrest
Introduction
In-hospital cardiac arrest affects approximately 200,000 patients each year in the United States.1 Although outcomes have been improving over the last decade, more than half of adults who have a cardiac arrest during their hospitalization will not survive.2–4 Many of these arrests now occur in highly monitored intensive care units (ICUs), where patients often have invasive monitoring in place at the time of arrest.2 A resuscitation strategy that takes advantage of this monitoring and uses it to incorporate a patient’s physiologic response into the ongoing resuscitation can be expected to save more lives.5,6 Experimental literature has established that survival following cardiopulmonary resuscitation (CPR) for cardiac arrest depends on provision of adequate myocardial blood flow.7–9 However, measurements of myocardial blood flow during CPR are not available to the rescuer. Therefore, the American Heart Association (AHA) now recommends using clinical surrogates closely related to myocardial blood flow (end tidal carbon dioxide (ETCO2) or diastolic blood pressure (DBP)) to monitor resuscitation quality.10 Although the conceptual relevance of hemodynamic and ETCO2 monitoring during CPR is well established, clinical studies supporting the titration of these parameters during human CPR are lacking.
The primary objective of this study was to evaluate the association between clinician-reported physiologic monitoring of CPR quality using either ETCO2 or DBP and survival outcomes. We hypothesized that use of physiology to monitor resuscitation quality would be associated with higher rates of return of spontaneous circulation (ROSC). We addressed this hypothesis in a propensity-score-matched cohort study of adult in-hospital CPR events reported to the AHA’s large multicenter Get With The Guidelines®-Resuscitation (GWTG-R) registry database.
Methods
The GWTG-R registry is an AHA sponsored prospective multisite database of patients undergoing in-hospital resuscitation that utilizes Utstein-style data reporting.11–13 Hospitals voluntarily participate in the registry for the primary purpose of quality improvement. The design and reporting of GWTG-R has been described in detail previously (www.heart.org/resuscitation).3,14,15 Quintiles (Cambridge Massachusetts), through their online, interactive case report form and Patient Management Tool (PMT), serves as the data collection coordination center for the AHA/American Stroke Association Get With The Guidelines programs. The University of Pennsylvania acts as the data analytic center and prepares the data for research purposes. Institutional review board (IRB) approval is not required for hospital participation in this quality improvement database; however, the present research investigation was reviewed by the IRB at The Children's Hospital Of Philadelphia and determined to be IRB exempt.
Cohort Development – Inclusion / Exclusion Criteria
All adult patients (≥ 18 years of age) with a CPR event requiring chest compressions with an invasive airway or arterial catheter in place at the time of arrest were eligible for inclusion. We then subsequently excluded events lasting < 1 minute, any delivery room events, and any events missing either outcome or important confounding variables necessary for propensity matching (Figure 1).
Figure 1.
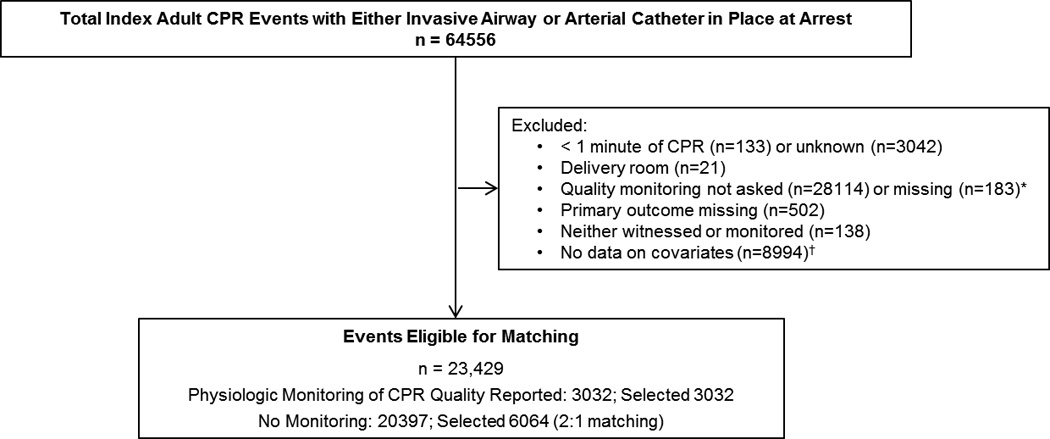
Consort-like diagram. *The primary predictor variables were taken from questions added to the data collection form in 2007. †All data for pre-existing medical conditions and cause of arrest were missing which prohibited adjustment for missing in the propensity model as we did with variables with sporadic missingness.
Study Variables
The primary independent variable was taken from specific questions regarding use of physiologic monitors in the GWTG-R database optional section on resuscitation quality Figure 2). Physiology was deemed to have been used to monitor resuscitation quality if either the following data fields were answered in the affirmative: 1) “Was continuous end tidal CO2 monitoring used to monitor quality of CPR?” or 2) “Was arterial line diastolic pressure used to monitor compression quality?” In the subset of events in which CPR quality monitoring with ETCO2 was indicated, we evaluated the association between the level of ETCO2 achieved during CPR and patient survival. In this analysis, the primary independent variable was ETCO2 >10mmHg versus ETCO2 ≤10mmHg16,17,, the only question specifically asked in the quality section of the GWTG-R registry regarding level of ETCO2 achieved (“was an end tidal CO2 value of >10 mmHg achieved?”). The primary outcome for all analyses was ROSC. This acute resuscitation outcome was chosen a priori because: 1) it is the most proximate outcome to the monitored CPR (the primary predictor variable); and 2) we prospectively determined that we would be underpowered to detect survival differences between monitoring groups. Therefore, secondary outcomes included survival to hospital discharge and survival to discharge with a favorable neurological outcome. Survival with a favorable neurological outcome was defined as a cerebral performance category (CPC) score of 1, 2 or no change from baseline.11,15 In accordance with standard Utstein-style definitions11, potential confounders were: patient factors, such as demographic characteristics (e.g., race18, gender19), preexisting conditions, and illness categories; and arrest characteristics, such as interventions in place at time of arrest, first documented rhythm, immediate cause of arrest, time of arrest, duration of CPR, and pharmacologic interventions (Supplementary Table 1). “First documented rhythm” was defined as the first electrocardiographic rhythm documented during a CPR event. All patients with CPR events regardless of the presence of pulse and rhythm at the onset of CPR were included.
Figure 2.

Get with the Guidelines® – Resuscitation Patient Management Tool (PMT), Section 7.1 CPR Quality Tab. Arterial line response is optional for the adult database.
Statistical methods
The following analysis plan was prospectively developed, and was approved by both the Adult Research Task Force of the AHA’s GWTG-R registry and the Executive Database Steering Committee of the AHA prior to the authors receiving the data for analysis. In this prospective analysis, propensity score matching20 was used to balance the distribution of potential confounders between patients with versus patients without physiologic monitoring (either ETCO2 or DBP) at the index event. Propensity scores were estimated as a function of the observed covariates (listed in Supplementary Table 1). For any covariate with missing values, ‘missing’ was coded as a separate category so that the propensity score balanced the distribution of missingness between groups.21 Propensity score models only included the main effects for covariates because there were no suspected effect modifiers. Participants were matched 2:1 using nearest neighbor matching, with distance based on the log odds. Matching was performed without consideration of outcome values.
Within the matched cohort, standardized mean differences were used to evaluate balance between groups, with balance achieved if the standardized mean difference was <0.10.22 Logistic regression models were used to compare resuscitation outcomes between matched groups. A robust variance estimator was used to account for the correlation due to clustering of patients within hospitals.23 In sensitivity analyses, we adjusted for hospital characteristics: trauma level designation, teaching versus non-teaching hospital, urban versus rural location, and presence versus absence of an approved residency program. These sensitivity analyses were limited to hospitals with available data.
Secondary analyses
In the subset of patients with only ETCO2 monitoring reported at the index event, we compared patient survival outcomes between events where the reported ETCO2 was >10mmHg during CPR versus events where the reported ETCO2 was lower (≤10mmHg) using a logistic regression model with a robust variance estimator to account for within-hospital correlation and adjusted for age, sex, race, patient illness category, night or weekend event24, and first documented rhythm.2,15
We also evaluated the effect of the release of the 2010 AHA guidelines – which recommended continuous ETCO2 monitoring during CPR25– on the proportion of patients with clinician-reported ETCO2 monitoring at the index event. The independent variable in these models was 2006 – 2010 period vs. 2011 – 2012. A similar analysis was not completed for DBP monitoring because it was not part of the AHA recommendations until the 2013 CPR Quality Consensus Statement10; however, we performed an exploratory analysis to see if the reported use of DBP monitoring changed over time in those patients with an arterial catheter in place at the time of the arrest.
All hypothesis tests were two-sided. All secondary analyses were performed among the unmatched cohort using logistic regression models with a robust variance estimator, as above. All analyses were completed using R 3.1.1 (R Development Core Team, Vienna, Austria), including the MatchIt and geepack extension packages.
Data Integrity
To ensure integrity of the data, the GWTG-R registry uses a detailed periodic reabstraction process, whereby participating hospitals submit randomly selected records that are reabstracted and reviewed for errors by the registry’s oversight committee. Mean (standard deviation) error rates for all data were 2.5% (2.7%). Remediation through a web-based process to support data integrity was continuously available for all sites. Any new hospitals were certified by testing of the accuracy of their data collection prior to data submission centrally.
Results
Between January 1, 2006 and September 7, 2012, there were 64,556 index adult CPR events with either an invasive airway or arterial catheter in place at the time of the arrest, of which 23,429 (36%) met inclusion criteria (Figure 1). Of 21,375 index events with an invasive airway in place at the time of the arrest, clinicians reported using ETCO2 to monitor quality in 803 (4%). Compared to earlier events, clinician-reported use of ETCO2 to monitor resuscitation quality was more common in index events occurring in 2011 and later (5% vs. 3%; OR 1.59, CI95 1.30 – 1.94, p<0.001). Of 7,260 events with an arterial catheter in place at the time of the arrest, clinicians reported using DBP to monitor quality in 2,145 (30%). There was no trend in clinician-reported DBP use over time, which ranged between 25% and 35% per year (p=0.77).
Physiologic Monitoring of CPR Quality
The propensity score balanced the distribution of potential confounders in the matched cohort of index events (Table 1; monitored n=3,032, not monitored n=6,064), with all standardized mean differences <0.1 (Supplementary Table 1). Clinician-reported physiologic monitoring of CPR quality was associated with higher odds of ROSC (OR 1.22, CI95 1.04 – 1.43, p=0.017) compared to no monitoring with either ETCO2 or DBP. Survival to hospital discharge (OR 1.04, CI95 0.92 – 1.18, p=0.57) and survival with favorable neurological outcome (OR 0.97, CI95 0.75 – 1.26, p=0.83) were not different between groups (Table 2).
Table 1.
Characteristics of the study cohort. Summaries presented as median (25th, 75th percentile) or n (%).
| Physiologic Monitoring of CPR Quality | |||
|---|---|---|---|
| Yes* (N=3032) |
No, Matched* (N=6064) |
No, All (N=20397) |
|
| Demographic characteristics | |||
| Age, y | 62 (50, 73) | 62 (51, 73) | 63 (52, 75) |
| Male sex | 1877 (62) | 3701 (61) | 11824 (58) |
| Race | |||
| White | 1919 (63) | 3846 (63) | 12643 (62) |
| Black | 687 (23) | 1326 (22) | 4485 (22) |
| Hispanic | 199 (7) | 410 (7) | 1427 (7) |
| Other or unknown | 227 (7) | 482 (8) | 1842 (9) |
| Pre-existing medical conditions | |||
| Congestive heart failure | 480 (16) | 957 (16) | 2902 (14) |
| Acute myocardial infarction | 553 (18) | 1167 (19) | 3332 (16) |
| Arrhythmia | 929 (31) | 1910 (31) | 6167 (30) |
| Hypotension or hypoperfusion | 1559 (51) | 2958 (49) | 7711 (38) |
| Respiratory insufficiency | 1732 (57) | 3524 (58) | 11770 (58) |
| Sepsis or pneumonia | 945 (31) | 1839 (30) | 6689 (33) |
| Metabolic or electrolyte abnormality | 599 (20) | 1310 (22) | 4112 (20) |
| Renal insufficiency | 1090 (36) | 2182 (36) | 6947 (34) |
| Hepatic insufficiency | 295 (10) | 682 (11) | 1966 (10) |
| Central nervous system depression | 354 (12) | 711 (12) | 2741 (13) |
| Major trauma | 314 (10) | 557 (9) | 1625 (8) |
| Cancer | 346 (11) | 670 (11) | 2328 (11) |
| Characteristics before cardiac arrest event | |||
| Patient illness category | |||
| Medical cardiac | 843 (28) | 1785 (29) | 5840 (29) |
| Medical non-cardiac | 944 (31) | 1894 (31) | 8836 (43) |
| Surgical cardiac | 440 (15) | 898 (15) | 1790 (9) |
| Surgical non-cardiac | 525 (17) | 1006 (17) | 2576 (13) |
| Trauma | 270 (9) | 463 (8) | 1325 (6) |
| Other or unknown | 10 (<1) | 18 (<1) | 30 (<1) |
| Pulse oximeter placement | 2948 (97) | 5892 (97) | 19014 (93) |
| Electrocardiography monitoring | 3006 (99) | 6012 (99) | 20002 (98) |
| Arterial catheter placement | 2224 (73) | 4465 (74) | 5481 (27) |
| Assisted or mechanical ventilation | 2410 (79) | 4704 (78) | 15070 (74) |
| Vascular access | 2992 (99) | 5973 (99) | 19827 (97) |
| Vasoactive infusion | 1998 (66) | 3948 (65) | 11023 (54) |
| Characteristics of cardiac arrest event | |||
| Location of event | |||
| Intensive care unit | 2279 (75) | 4516 (75) | 13540 (66) |
| Inpatient monitored | 612 (20) | 1240 (20) | 3868 (19) |
| Inpatient ward | 14 (<1) | 33 (1) | 422 (2) |
| Emergency department | 85 (3) | 183 (3) | 2151 (11) |
| Other or unknown | 42 (1) | 92 (2) | 416 (2) |
| Night or weekend event† | 1434 (47) | 2882 (48) | 10117 (50) |
| Immediate cause of arrest | |||
| Acute myocardial infarction | 248 (8) | 526 (9) | 1684 (8) |
| Hypotension or hypoperfusion | 1931 (64) | 3795 (63) | 10843 (53) |
| Acute respiratory insufficiency | 842 (28) | 1755 (29) | 6572 (32) |
| Inadequate invasive airway | 56 (2) | 120 (2) | 686 (3) |
| Acute pulmonary edema | 54 (2) | 105 (2) | 345 (2) |
| Acute pulmonary embolism | 61 (2) | 100 (2) | 364 (2) |
| Metabolic or electrolyte abnormality | 598 (20) | 1322 (22) | 3980 (20) |
| First documented rhythm | |||
| Asystole or pulseless electrical activity | 2362 (78) | 4615 (76) | 15936 (78) |
| Ventricular fibrillation or tachycardia | 576 (19) | 1239 (20) | 3485 (17) |
| Number of shocks, # | 2 (1, 4) | 2 (1, 4) | 12 (5, 24) |
| Bradycardia | 21 (1) | 43 (1) | 104 (1) |
| Other or unknown | 73 (2) | 167 (3) | 872 (4) |
| Duration of resuscitation, min | 15 (7, 27) | 14 (6, 27) | 13 (6, 25) |
| Pharmacologic interventions during arrest | |||
| Epinephrine bolus | 2719 (90) | 5393 (89) | 18408 (90) |
| Sodium bicarbonate | 1797 (59) | 3566 (59) | 11683 (57) |
| Calcium chloride or gluconate | 1146 (38) | 2227 (37) | 6542 (32) |
| Atropine | 1889 (62) | 3690 (61) | 13282 (65) |
| Fluid bolus | 1104 (36) | 2115 (35) | 6380 (31) |
| Lidocaine | 244 (8) | 502 (8) | 1383 (7) |
| Amiodarone | 675 (22) | 1406 (23) | 3949 (19) |
| Magnesium sulfate | 366 (12) | 702 (12) | 1927 (9) |
| Dextrose bolus | 147 (5) | 334 (6) | 1032 (5) |
Each participant with either physiologic monitoring of CPR quality was matched to two participants without monitoring based on a propensity score that included characteristics listed in Table 1 and calendar year. All covariates were balanced within the matched cohorts (all standardized mean differences >0.1; see Supplementary Table 1).
Night: 11:00pm to 6:59am; Weekend: Friday 11:00pm to Monday 6:59am.
Table 2.
Comparison of resuscitation outcomes among adults with and without monitoring.*
| Outcome | With n (%) |
Without n (%) |
OR (CI95)† | p Value |
|---|---|---|---|---|
| Return of spontaneous circulation |
2117/3032 (70) | 3973/6064 (66) | 1.22 (1.04, 1.43) | 0.017 |
| Survival to hospital discharge |
527/3020 (17) | 1019/6022 (17) | 1.04 (0.91, 1.18) | 0.57 |
| Survival with favorable neurological outcome |
368/2911 (13) | 757/5840 (13) | 0.97 (0.75, 1.26) | 0.83 |
OR, odds ratio; CI, confidence interval
Each participant with monitoring was matched to two participants without monitoring based on a propensity score that included all variables listed in Supplementary Table 1.
Odds ratios compare the odds of an outcome between index events with versus without monitoring, estimated from a logistic regression model with a robust variance estimator to account for within-hospital correlation.
Of the 334 hospitals that contributed data to this analysis, 262 (78%) had data available on hospital characteristics. In this subset of hospitals, monitoring was associated with increased odds of ROSC (OR 1.23, CI95 1.04 – 1.45, p=0.015). In sensitivity analyses, adjustment for hospital characteristics did not impact the association between monitoring and ROSC (OR 1.22, CI95 1.04 – 1.43, P=0.013).
In the subset of index events with ETCO2 CPR quality monitoring indicated (n=803), an ETCO2 >10mmHg during CPR was reported in 520 (65%) index events, and was associated with improved survival to hospital discharge (24% versus 11%; OR 2.41, CI95 1.35 – 4.30, p=0.003), and improved survival with favorable neurological outcome (18% versus 8%; OR 2.31, CI95 1.31 – 4.09, p=0.004) compared to index events with ETCO2 ≤10mmHg after accounting for within-hospital correlation and adjusting for age, sex, race, patient illness category, night or weekend event, and first pulseless rhythm.
Discussion
In this propensity-matched-cohort study of adults using the AHA’s multicenter GWTG-R registry, clinician-reported use of physiology to monitor CPR quality with either ETCO2 or DBP was associated with improved rates of ROSC. Survival to hospital discharge and survival with favorable neurological outcome were not different between groups. In an adjusted model using the subset of 803 index events during which ETCO2 monitoring was reported as used, an ETCO2 >10mmHg during CPR was associated with a higher rate of patient survival to hospital discharge and survival with favorable neurological outcome compared to events when ETCO2 >10mmHg during CPR was not reported. Of note, in the subset of events with an invasive airway in place at the time of arrest, it was not common (4%) for clinicians to report using ETCO2 to monitor CPR quality.
In a recent consensus statement published in Circulation, the AHA now recommends monitoring the patient’s physiologic response to the resuscitation effort using ETCO2 and invasive hemodynamic data.10 In a hierarchal fashion depending on what is available at the time of the arrest, hemodynamic goals were prioritized over capnography goals. Despite the substantial amount of data that was evaluated in this consensus statement, the authors conceded that clinical studies “supporting the optimal titration of these parameters during human CPR are lacking.” Therefore, this study takes an important first step by providing prospectively collected human data and associating clinician-reported intra-arrest physiologic monitoring of CPR quality with superior clinical outcomes.
Numerous experimental models have demonstrated that ETCO2 correlates well with pulmonary blood flow, cardiac output, and resuscitation success.17,26–28 Clinical data supporting ETCO2 monitoring during CPR dates back to the 1970s when Kalenda described using ETCO2 levels to monitor for rescuer fatigue (declining ETCO2) and for ROSC (sudden rise in ETCO2).29 Similarly, but more recently, Sheak et al. demonstrated that CPR quality is associated with ETCO2 levels during both in- and out-of-hospital resuscitation attempts.30 Other studies have suggested that ETCO2 can be used as a prognostic tool during cardiac arrest, as failure to achieve an ETCO2 of at least 10mmHg is rarely associated with good outcome.16,17 Similar to these previous reports, we now provide data suggesting that when clinicians monitor resuscitation quality with ETCO2, rates of ROSC are higher, and further, than when an ETCO2 >10mmHg was reported during CPR, rates of survival to discharge and survival with good neurological are also higher.
In the AHA consensus statement, hemodynamic goals were prioritized over ETCO2 based upon a large body of experimental literature that has established myocardial blood flow as the primary determinant of successful cardiac arrest resuscitation.7–9 Measures of myocardial blood flow are not easily made available during the resuscitation attempt; therefore, coronary perfusion pressure (CPP; the primary determinant of myocardial blood flow) and arterial DBP (the primary driving force of CPP) were recommended first line as physiologic resuscitation quality monitors because they are most closely related to myocardial blood flow.31 Supporting this recommendation, there is also a growing body of pre-clinical evidence demonstrating that titration of CPR efforts to arterial blood pressure can improve survival.5,6,32 Highlighting a potential limitation of these data, we would have been underpowered to detect an association between individual monitoring modalities and outcome in our study cohorts; therefore, we a priori chose to define physiologic monitoring as use of either ETCO2 or DBP. Consequently, further quantitative human investigations are warranted to not only determine the effectiveness of physiologic monitoring to improve survival outcomes, but also which monitor, and further, which physiologic target is most associated with outcome.
The relatively low reported use of physiologic monitoring to guide resuscitation quality deserves comment. Of index events with an invasive airway in place at the time of the arrest, clinicians reported using ETCO2 to monitor quality in only 803 (4%). Because our dataset was comprised of many events that occurred prior to release of the 2010 AHA guidelines25, which recommended the use of ETCO2 to monitor quality, low rates of clinician reported ETCO2 monitoring are not surprising. Similarly, among events with an arterial catheter in place, only about 1/3 (30%) of clinicians reported using DBP to monitor CPR quality. DBP monitoring has also not changed significantly over time, likely because all of the index events in the dataset occurred before the release of the 2013 AHA consensus statement which prioritized hemodynamic monitoring.10 Interestingly, there was an approximately 50% relative increase in the reported use of ETCO2 to monitor quality at index events that occurred after release of the 2010 guidelines. However, the overall percentage for both still remains low, and suggests that educational efforts to increase physiologic monitoring during resuscitation are warranted.
This study has limitations. First, as with all studies of large multicenter databases, analysis of the data may be limited by data integrity and validation issues due to multiple sites submitting data to the registry. However, the aforementioned rigorous training and certification process, reabstraction data validation processes, and large sample size, which are unique attributes of the AHA GWTG-R registry, minimize these sources of bias. Second, because important confounding post-resuscitation variables33–37 were not available for the analysis, the effect of physiologic monitoring during CPR on long term survival was not evaluated. Third, actual values of ETCO2 or DBP, which could have been used to verify the presence of an end tidal or arterial line monitor, and also the level of ETCO2 achieved during CPR, are not routinely recorded in the GWTG-R database. Next, as the section on CPR quality in the GWTG-R database is optional, generalizability outside of hospitals that report this data may be limited. Fifth, it is worth noting that ventilation rate is an important unmeasured confounder. It is both associated with ETCO2 levels during human CPR30 and cardiac arrest survival in animal models38, with overventilation associated with mortality. Therefore, the association between higher reported ETCO2 levels and improved survival may be driven partly by the avoidance of excessive ventilation during CPR. Finally, because only about 15% of hospitals in the US are represented, the generalizability of our findings outside of GWTG-R hospitals remains an unanswered question.
Conclusions
In this propensity-matched-cohort study of adult in-hospital cardiac arrest using the AHA’s GWTG-R registry, clinician-reported use of physiologic monitoring of CPR quality with either continuous ETCO2 or diastolic blood pressure was associated with an improved rate of ROSC compared to no reported physiologic monitoring. However, survival to hospital discharge and survival with favorable neurological outcome were not different between groups. In the subset of index events where CPR quality was monitored with only ETCO2, there were improved rates of patient survival to hospital discharge and survival with favorable neurological outcome when ETCO2was reported to be >10mmHg during CPR monitoring. It was not common for clinicians to report the use of physiology to guide resuscitation quality.
Supplementary Material
Table 3.
Comparison of resuscitation outcomes among index events with ETCO2 monitoring (n=803 index events)
| Outcome | ETCO2>10mmHg n (%) |
ETCO2≤10mmHg n (%) |
OR (CI95)* | p Value |
|---|---|---|---|---|
| Survival to hospital discharge |
125/520 (24) | 31/283 (11) | 2.41 (1.35, 4.30) | 0.003 |
| Survival with favorable neurological outcome |
94/520 (18) | 23/283 (8) | 2.31 (1.31, 4.09) | 0.004 |
OR, odds ratio; CI, confidence interval
In the subset of index events with ETCO2 monitoring indicated, odds ratios compare the odds of an outcome between index events with a reported ETCO2 >10mmHg versus events with a reported ETCO2 ≤10mmHg, estimated from a logistic regression model with a robust variance estimator to account for within-hospital correlation and adjusted for age, sex, race, patient illness category, night or weekend event, and first pulseless rhythm.
Acknowledgments
Robert Sutton and Alexis Topjian are supported by a career development awards (RMS: National Institute of Child Health and Human Development; AAT National Institute of Neurological Disorders and Stroke.
Conflicts of Interest:Robert Sutton has received a speaker’s honoraria from the Zoll Medical Corporation. Raina Merchant has received grant / research support from the NIH (K23 10714038; R01 HL122457), and pilot funding from Physio-Control (Seattle, WA), Zoll Medical (Boston MA), Cardiac Science (Bothell, WA), and Philips Medical (Seattle, WA). Benjamin Abella has received research funding from the NIH, AHA and Medtronic Foundation, speaking honoraria from Philips Healthcare (Seattle, WA) and CR Bard (Murray Hill, NJ), and has advisory board relationships with HeartSine (Newtown, PA) and CardioReady (Conshohocken, PA). Dana Edelson has received research funding from Philips Healthcare (Seattle, WA) and speaking fees from Physio-Control (Seattle, WA). Benjamin French, Peter Meaney, Christopher Parshurum, Stephen Schexnayder, Robert Berg, Melania Bembea, and Vinay Nadkarni have no disclosures.
Abbreviations
- AHA
American Heart Association
- CPR
cardiopulmonary resuscitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38(1):101–108. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: An observational study. Lancet. 2012;380(9852):1473–1481. doi: 10.1016/S0140-6736(12)60862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton RM, Friess SH, Naim MY, et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190(11):1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friess SH, Sutton RM, French B, et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation. 2014;85(9):1298–1303. doi: 10.1016/j.resuscitation.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: A predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16(4):241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: Improved regional blood flow and resuscitation in dogs. Ann Emerg Med. 1984;13(2):79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 9.Halperin HR, Lee K, Zviman M, et al. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med. 2010;28(2):195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: Improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa) Resuscitation. 2004;63(3):233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: The in-hospital 'Utstein style'.A statement for healthcare professionals from the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Australian Resuscitation Council, and the Resuscitation Councils of Southern Africa. Resuscitation. 1997;34(2):151–183. doi: 10.1016/s0300-9572(97)01112-x. [DOI] [PubMed] [Google Scholar]
- 13.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: A report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 14.Chan PS, Krumholz HM, Nichol G, Nallamothu BK American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 15.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA. 1989;262(10):1347–1351. [PubMed] [Google Scholar]
- 17.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 18.Razi RR, Churpek MM, Yuen TC, et al. Racial disparities in outcomes following PEA and asystole in-hospital cardiac arrests. Resuscitation. 2015;87:69–74. doi: 10.1016/j.resuscitation.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topjian AA, Localio AR, Berg RA, et al. Women of child-bearing age have better inhospital cardiac arrest survival outcomes than do equal-aged men. Crit Care Med. 2010;38(5):1254–1260. doi: 10.1097/CCM.0b013e3181d8ca43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Agostino RB, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95(451):749–759. [Google Scholar]
- 22.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234–249. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 24.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 25.Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 26.Sanders AB, Atlas M, Ewy GA, Kern KB, Bragg S. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3(2):147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 27.Sanders AB, Ewy GA, Bragg S, Atlas M, Kern KB. Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med. 1985;14(10):948–952. doi: 10.1016/s0196-0644(85)80235-3. [DOI] [PubMed] [Google Scholar]
- 28.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13(11):907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Kalenda Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation. 1978;6(4):259–263. doi: 10.1016/0300-9572(78)90006-0. [DOI] [PubMed] [Google Scholar]
- 30.Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–154. doi: 10.1016/j.resuscitation.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12(10):871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84(5):696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 35.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 36.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–2113. doi: 10.1161/CIRCULATIONAHA.112.000168. [DOI] [PubMed] [Google Scholar]
- 37.Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. [DOI] [PubMed] [Google Scholar]
- 38.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


