Abstract
Higher prevalence of several pain disorders in women and sexual dimorphism in G-protein coupled receptor-induced analgesia have been reported. We have previously shown that α2-adrenoceptor-induced antinociception is sex-specific and attenuated by estrogen in the female rat. However, this evidence was obtained using reflexive withdrawal-based nociceptive assays conducted on restrained animals that may not involve cerebral processing. Hence, we evaluated whether activation of the trigeminal α2-adrenoceptor produces sex-specific antinociceptive and antihyperalgesic effects in the orofacial region of the rat using a reward conflict-based operant paradigm in which animals must tolerate nociceptive thermal stimulation to be rewarded. Male and ovariectomized (OVX) Sprague-Dawley rats were implanted intracisternally with a PE10 cannula for drug injections. A group of OVX rats (OVX+E) was administered subcutaneously with estradiol 48 hours before the test. Effect of clonidine, an α2-adrenoceptor agonist, was determined on the operant pain assay using a fully automated Orofacial Pain Assessment Device. Number of spout licks, thermode contacts, and amount of reward intake were automatically recorded by the ANY-maze software. Using acute pain modeling, clonidine produced a dose-dependent increase in all three parameters in male and OVX groups, however, it was ineffective in the OVX+E group. Similarly, using inflammatory pain modeling, clonidine significantly increased these parameters in carrageenan-treated male and OVX groups but not in the OVX+E group. Thus, α2-adrenoceptor activation produces sex-specific antinociception and antihyperalgesia and estrogen attenuates these effects in female rats using an operant pain assay. These findings may help the discovery of effective analgesics for each sex.
Keywords: Pain, operant, sex-differences, clonidine, α2-adrenoceptor, estrogen
1. Introduction
Sex-related differences in pain have been reported with women exhibiting a higher prevalence of several pain disorders, e.g. temporomandibular joint disorder, migraines, irritable bowel syndrome and fibromyalgia as well as some women-specific pain conditions viz., endometriosis, vulvodynia and menstrual pain [1–9]. Relatively high levels of gonadal hormones present in women during the reproductive years have been implicated for enhanced pain perception [10–12]. Further, sexual dimorphism in the G-protein coupled receptor (GPCR)-mediated analgesia have been reported in clinical [13–15] and animal studies [5,16–27]. In this regard, we have previously shown that antinociception induced by activation of the α2-adrenoceptor (a GPCR) is sex-specific, attenuated by estrogen in the female and requires testosterone in male rats [20–23]. However, experimental evidence thus far has been predominantly obtained from studies conducted using traditional, reflexive withdrawal-based pain assays in often restrained animals that may not involve cerebral processing. With the advent of the recent orofacial nociceptive assay, based on the reward-conflict, operant paradigm in which animals must tolerate nociceptive thermal stimulation in the orofacial region to obtain access to the reward, it has become feasible to access nociception in a manner that better approximates complex, sensory and emotional human pain state in experimental rodents [28,29] using the Orofacial Pain Assessment Device (OPAD). Hence, we investigated whether intracisternal administration of clonidine (an α2-adrenoceptor agonist) produces antinociception in the orofacial region of the rat using the OPAD. In addition to a test for acute thermal nociception, an inflammation-induced thermal hyperalgesia model using carrageenan injection in the orofacial region was also employed since it mimics an important, persistent pain and hyperalgesia condition commonly observed in patients with pain disorders as well as following injuries and surgical procedures. Moreover, we investigated whether estrogen attenuates α2-adrenoceptor-mediated antinociception and antihyperalgesia using this operant assay of pain.
2. Materials and methods
2.1 Subjects
Sprague–Dawley male and ovariectomized (OVX) female rats (225–249 g; Harlan Sprague-Dawley, Inc., Indianapolis, IN) were housed in the animal care facility at Meharry Medical College certified by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) under a 12-h light/dark cycle (lights on: 7:00 AM) and had free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Meharry Medical College and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain (IASP).
2.2 Intracisternal cannulation
As previously described [21,22,30] animals were anesthetized using ketamine and xylazine anesthesia (80 and 4 mg/kg respectively; i.p.), their heads were shaved and secured in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The skin above the head/neck region was incised, the atlantooccipital membrane was removed and the dura was exposed. A tiny opening was made in the dura using a 25 gauge sterile needle and the tip of a PE-10 cannula (dead volume 7 μl) was inserted approximately 1.5 mm through the opening. The cannula was then secured to the skull with dental cement and the wound closed using 9 mm Autoclips (BD, Franklin, NJ). Animals were kept on a heating pad (36°C) till they regained consciousness and were then returned to their home cages for recovery.
2.3 Orofacial thermal pain assay based on operant paradigm
The newly developed, fully automated OPAD (Stoelting, Wood Dale, IL) was used in which animals were presented with a choice of experiencing aversive temperatures to gain access to a liquid reward (1:3 diluted, sweetened condensed milk solution in water [28]. The access to the spout of reward containing bottle was via a thermode-lined opening with which animal's cheeks made contact as it consumed the reward. After a postoperative recovery period of 5-7 days, animals were food deprived overnight and were first trained to consume ~10 g of reward in one, 30 min session. Three to five training sessions (one/day) were administered. Thermode temperature was set at an ambient 24.8°C during this training. On the test day (following the last training day), animal's cheek region on both sides of the head was carefully shaved using hair clippers under 5% isoflurane anesthesia combined with O2 using a vaporizer (VETEQUIP, Pleasanton, CA). Thermode temperature was raised to 48°C for acute pain testing and 45°C to test thermal hyperalgesia following carrageenan injection. The OPAD was under complete control of ANY-maze software (Stoelting) running on a PC that automatically regulated the thermode temperature, recorded number of licks to the spout (licks) and contacts to the thermodes (contacts), and the amount of reward consumed (reward intake), and terminated trial/test at the completion of 30-minute time period. A ratio between number of licks to the spout and thermode contacts was also calculated and analyzed (licks/contact).
2.4 Carrageenan injection
As described [28], vehicle (sterile saline) or lambda carrageenan (4%; 0.15 ml; Sigma Chemical, St. Louis, MO) was injected subcutaneously (s.c.), bilaterally into the mid cheek region using a 27G needle attached to a sterile, disposable 1 ml syringe, under 5% isoflurane anesthesia in trained rats. Cheek region was shaved at the same time. Operant pain testing was conducted at the thermode temperature of 45°C to access thermal hyperalgesia three hours subsequent to the carrageenan injection.
2.5 Estradiol replacement
A group of OVX rats (OVX+E) was administered 17β-estradiol 3-benzoate (100 μg/100 μl; s.c.) 48 hours before the operant pain test. This protocol has been used previously in neuroendocrinology [31,32] and in the pain field by us [20,21,24,26] and others [33,34]. Further, this dose of estradiol has been shown to most reliably induce lordosis behavior in rats [35] and we have previously shown that it yields serum-estradiol levels and vaginal smear cytology comparable to that at proestrous stage of the estrous cycle after 48 h – the time point for behavioral testing in this study [21,24]. OVX group was given the vehicle (sesame oil; s.c.). Normally cycling females at pro- and diestrous stages were not included this in study since estrous cycle is disrupted for prolonged periods following surgery and implantation of cannulae under anesthesia. The unpredictable time period required for the restoration of estrous cycle would further disrupt training/testing protocol due to overtraining and/or uneven time interval between cannula implantation, training and testing.
2.6 Drugs
All drugs were obtained from Sigma–Aldrich (St. Louis, MO) and injected intracisternally. Clonidine (0, 0.875 or 1.75 μg/5 μl) was injected 5 min before the test in separate groups (n=6/group for acute assay and 5/group for hyperalgesia assay). Yohimbine (30 μg/15 μl), an α2-adrenoceptor antagonist was injected 5 min before clonidine. We have used these drug doses successfully in our previous studies employing NMDA-induced scratching behavior and thermal head-withdrawal assays [21]. Previously used higher doses of clonidine (3.5 and 7 μg) produced sedation in our pilot experiments using OPAD and were excluded. All experiments were conducted between 9 AM – 2 PM. Each rat was tested only once and all rats were euthanized by an intraperitoneal injection of Beuthanasia (150 mg/kg; Schering-Plough, Kenilworth, NJ) or CO2 at the end of the experiment. All efforts were made to minimize stress to the animals and the number of animals used.
2.7 Data analysis
Data presented in figures 1 and 2 were analyzed by ANOVA and that in figure 3 by t-test using IBM SPSS (IBM Corp., Armonk, NY, USA) with appropriate between group factor and dependent variables. Fisher's post-hoc test was used for intergroup comparisons where ANOVA yielded a significant main effect. Significance level was set at <0.05. Data are plotted as mean±SEM using SIgmaPlot (Systat Software Inc., San Jose, CA, USA).
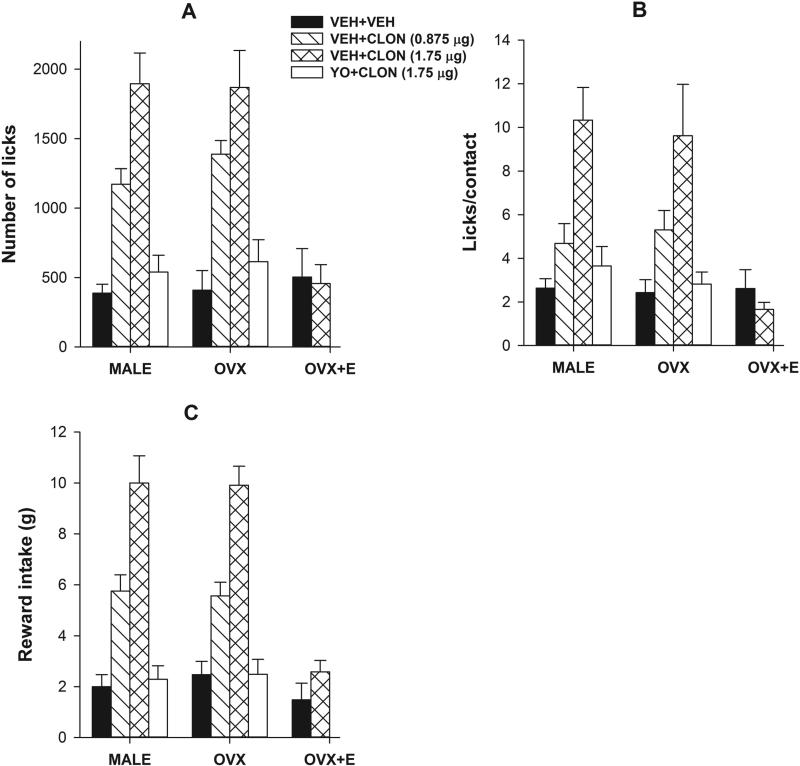
Figure 1.
Selective activation of the trigeminal α2-adrenoceptor produces dose-dependent antinociception on the reward/conflict-based operant assay of acute pain; estradiol attenuates it in females. Data obtained from the acute pain testing with the thermode temperature set at 48°C revealed that intracisternal injection of clonidine significantly and dose-dependently (0 < 0.875 < 1.75 μg) increased the number of licks (A), licks/contact (B), and reward intake (C) in male and OVX groups. However, it failed to produce any effect on any parameter in the OVX+E group (A-C). Pre-treatment with yohimbine abolished the effect of clonidine (1.75 μg) in both male and OVX groups on all three parameters (A-C). Higher doses of clonidine produced sedative effect in pilot experiments and were excluded.
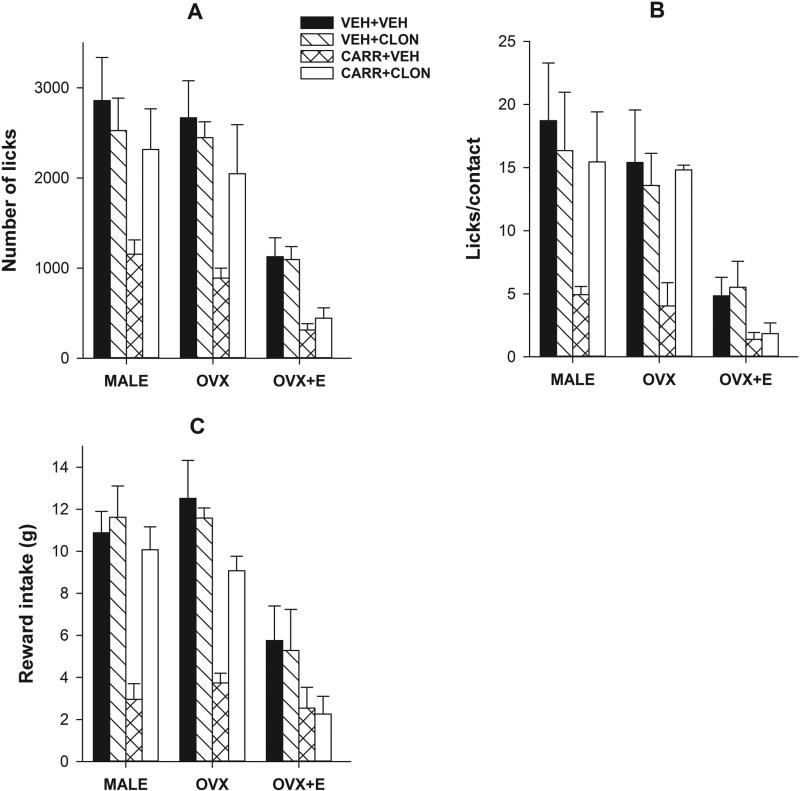
Figure 2.
Activation of the trigeminal α2-adrenoceptor produces antihyperalgesia on the reward/conflict-based operant assay of inflammatory pain; estradiol attenuates it in females. Data obtained from the inflammation-induced hyperalgesia test with the thermode temperature set at 45°C revealed that carrageenan injection (s.c.) into the cheek region produced a significant reductions in the number of licks (A), licks/contact (B), and reward intake (C) in male OVX and OVX+E groups (VEH+VEH vs CARR+VEH). Further, the control OVX+E (VEH+VEH) group had significant decreases on all three parameters as compared to control male or OVX groups (A-C). As expected, intracisternal injection of clonidine (1.75 μg) produced a significant increase on all three parameters in male and OVX groups (CARR+VEH vs CARR+CLON), however, it failed to do so in the OVX+E group (A-C). Clonidine alone did not significantly change any parameter in any group (VEH+VEH vs VEH+CLON) indicating a lack of possible hyperphagic effect when injected intracisternally (A-C).
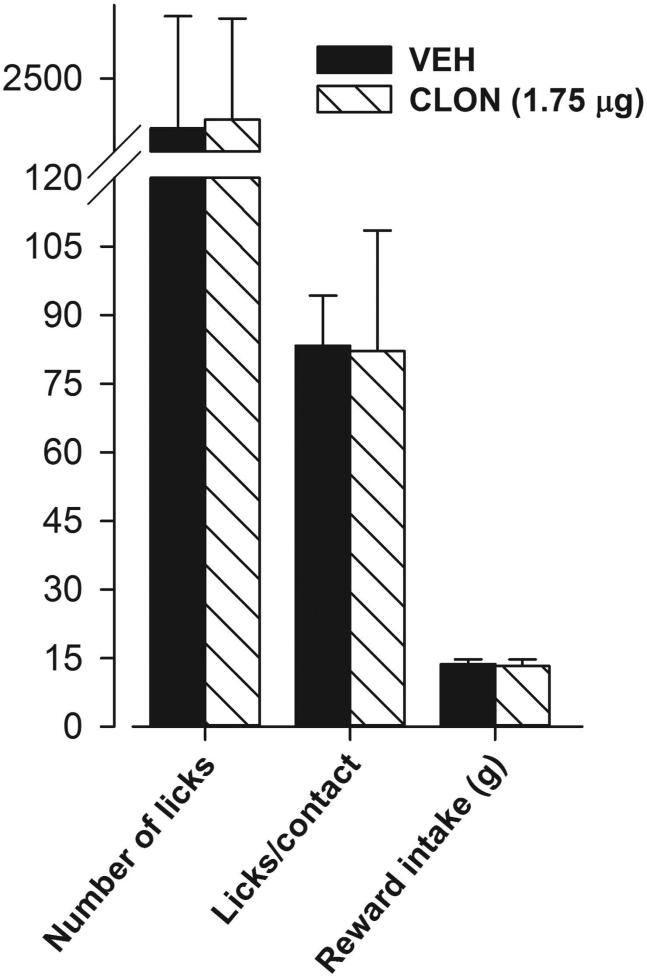
Figure 3.
Lack of a clonidine-induced hyperphagic effect. When testing was conducted at an innocuous thermode temperature of 24.8°C (same as training temperature) in a separate batch of trained male rats, intracisternal injection of clonidine (1.75 μg) did not significantly change the number of licks, licks/contact or reward intake as compared to the vehicle-treated group. A break has been introduced on the Y axis in this figure to accommodate data from all three parameters.
3. Results
3.1 Activation of the trigeminal α2-adrenoceptor produces sex-specific, estrogen-dependent antinociception using acute thermal orofacial operant pain assay
To determine the effect of α2-adrenoceptor activation on thermal, orofacial, operant nociception, we intracisternally injected clonidine in trained male, OVX and OVX+E rats and tested them on the operant paradigm at a noxious thermode temperature of 48°C. First, there was no significant main effect of Sex on number of licks, licks/contact or reward intake (all F2,50 <1; p>0.05) indicating that control male, OVX and OVX+E groups performed similarly on this task (Fig 1). However, ANOVA yielded significant main effect of Drug on the number of licks (F3,50=34.71, 0<0.01; Fig 1A), licks/contact (F3,50=18.88, 0<0.01; Fig 1B), and reward intake (F3,50=63.56, 0<0.01; Fig 1C). Post hoc analysis further revealed that clonidine significantly and dose-dependently increased all three parameters in male and OVX groups as compared to their respective vehicle-treated controls (veh < 0.875 μg < 1.75 μg; all p's<0.05), however, it failed to do so at the highest dose in OVX+E group (Fig 1). The selectivity of α2-adrecoceptor effect as observed in male and OVX groups was determined with a pre-injection of yohimbine (5 minutes before 1.75 μg clonidine) since clonidine has some affinity for imidazoline receptors and yohimbine is a nonimidazoline α2-adrecoceptor antagonist. As expected, yohimbine pre-injection led to abolition of clonidine-induced increase in all three parameters (Fig 1) which did not significantly differ from control levels. Due to a complete lack of an effect of clonidine, yohimbine pretreatment was not required in OVX+E group and was thus excluded.
3.2 Activation of the trigeminal α2-adrenoceptor produces sex-specific, estrogen-dependent thermal antihyperalgesia using orofacial operant pain assay
To determine the effect of α2-adrenoceptor activation on inflammation-induced thermal hypersensitivity, we intracisternally injected clonidine 3 hours post carrageenan injection in trained male, OVX and OVX+E rats and tested them on the operant paradigm at a slightly lower but noxious thermode temperature of 45°C. Overall, ANOVA yielded Significant main effects of i) Sex on number of licks (F2,48=25.82, 0<0.01; Fig 2A), licks/contact (F2,48=16.09, 0<0.01; Fig 2B), and reward intake (F2,48=23.98, 0<0.01; Fig 2C), ii) Carrageenan on number of licks (F1,48=26.24, 0<0.01; Fig 2A), licks/contact (F1,48=11.01, 0<0.01; Fig 2B), and reward intake (F1,48=41.89, 0<0.01; Fig 2C), and iii) Drug on number of licks (F1,48=4.60, 0<0.05; Fig 2A), licks/contact (F1,48=5.39, 0<0.05; Fig 2B), and reward intake (F1,48=7.59, 0<0.01; Fig 2C). Post hoc analysis further revealed that the control OVX+E group (veh+veh) had significant reductions in all three parameters as compared to the control male and OVX groups (all p<0.05) indicating a sex-specific, estrogen-dependent effect in acute thermal nociception (Fig 2). As expected, carrageenan significantly reduced all three parameters in all three groups as compared to their respective vehicle-treated controls (veh+veh vs veh+carr; all p<0.05). Clonidine reversed carrageenan-induced decrease in all three parameters in male and OVX groups (all p<0.05) but not in the OVX+E group. Clonidine had no effect in any vehicle-treated group on any parameter (Fig 2).
3.3 Did clonidine-induced hyperphagia confound its observed antinociceptive/antihyperalgesic effect?
Clonidine has been previously shown to produce hyperphagia [36,37] which could confound our findings of its antinociceptive/antihyperalgesic effect on the reward-conflict paradigm-based operant assay reported above. To determine whether intracisternal injection of clonidine produced hyperphagia, we employed a separate batch of male rats, trained them as before, however, tested them at the innocuous thermode temperature of 24.8°C (same as the training temperature). As shown in figure 3, clonidine (1.75 μg)-treated rats performed similarly to the vehicle-treated rats and no significant differences on any of the parameters recorded were observed between these two groups (p>0.05). Thus, intracisternal injection of clonidine does not produce hyperphagia.
4. Discussion
This is the first study to employ a reward-conflict paradigm-based operant assay of orofacial pain to investigate sex-related differences in the α2-adrenoceptor-mediated antinociception and antihyperalgesia. Clonidine produced robust antinociceptive and antihyperalgesic effects in male and OVX groups, however, estradiol-treatment abolished these effects in the OVX+E group. In addition, OVX+E group displayed significantly higher inflammatory hyperalgesia as compared to male and OVX groups. These results obtained using an operant assay of pain are consistent with those reported in our previous studies using reflexive withdrawal-based orofacial pain assays [21] with some subtle differences. First, due to their sedative effect, higher doses of clonidine (3.5 and 7 μg) that were successfully used in our previous studies using acute thermal stimulus-evoked reflexive head withdrawal or NMDA-induced scratching behavior had to be excluded from the present study. The sedative effect may not have been apparent at these doses in previous studies due to the nature of the nociceptive stimuli applied on restrained rats, i.e. an intense thermal stimulus with a linear, several degrees/second temperature increase in the orofacial region or an intracisternal injection of NMDA that produces intense licking/biting/scratching behavior as compared to a less intense thermal stimulus in the present study (48° or 45°C). Nevertheless, sedation was apparent at clonidine doses above 7 μg in our previous studies and were excluded [21,38]. Second, the maximal effect of clonidine was obtained at 1.75 μg dose in the current study and at 7 μg in our previous studies [21,38]. Therefore, lower doses seem to be more effective using the operant pain assay. Lastly, there were no baseline level differences in the nociception between male and female groups when an acute, operant pain paradigm was used in the present study (Fig 1) or in our previous studies using reflexive withdrawal [21,38].
Since many of the pain disorders that have higher prevalence in women involve persistent/chronic pain and hyperalgesia [1–9], we also employed an inflammation-induced thermal hyperalgesia model using carrageenan injection in the orofacial region. Indeed, estradiol-treated OVX+E females displayed significantly higher inflammation-induced hyperalgesia as compared to the male and OVX groups (Fig 2). Thus, high estrogen levels may predispose females to significantly higher persistent pain and hyperalgesia. This finding is consistent with evidence presented in other studies reporting significantly higher heat hyperalgesia in proestrous females using paw withdrawal [39] and in females using tail-withdrawal assays [40] as compared to males. Therefore, the nature of pain stimulus may limit the observed differences in pain perception between sexes, e.g., an intense heat stimulus (used in acute pain assay) is likely to produce a “floor effect” where subtle between-group differences will not be evident. In contrast, a less intense nociceptive stimulus (used in inflammation-induced hyperalgesia) is more likely to reveal those differences.
The use of operant pain testing in animals has been suggested recently since traditionally used pain assays are often based on reflexive-withdrawal and hence may not reliably incorporate and evaluate the affective aspect of pain [29]. Procedural stress may further confound the interpretation of results due to commonly employed restrain. These factors may limit translational success of analgesic compounds in clinical trials [29].
Clonidine-induced hyperphagia has been shown before [36,37] which could confound our finding of its antinociceptive effect using the reward/conflict paradigm-based operant assay of pain. Although we did not observe significant differences between vehicle vs clonidine-treated groups (Fig 2), to further evaluate clonidine-induced hyperphagia, we also conducted the operant testing in vehicle or clonidine-treated male rats using an innocuous (24.8°C) thermode temperature. We chose to use only male rats for this aim since clonidine was equieffective in male as well as in the OVX group in acute pain and inflammation-induced hyperalgesia testing (Figs 1, 2). On the other hand, it had no effect in the OVX+E group (Figs 1, 2). Results confirmed that Intracisternal injection of clonidine failed to induce a significant increase in any recorded parameter (Fig 3). Hence, clonidine-induced hyperphagia was not observed in the present study. In fact, α2-adrenoceptors located in median raphe nucleus [36] and/or hypothalamic paraventricular nucleus [37,41] mediate the hyperphagic action of locally injected clonidine and an intracisternal injection with very limited rostro-caudal spread [21] is unlikely to yield similar results.
The α2-adrenoceptor mRNA and estrogen receptors (ER, β and GPR30) have been shown to be present in the medullary dorsal horn and trigeminal ganglia neurons that house orofacial pain circuitry [42–45]. Although we were not able to determine a genomic basis of estrogen-induced attenuation of α2-adrenoceptor-mediated spinal antinociception [38], estradiol has been previously shown to downregulate cortical α2A-adrenoceptor by 50% in OVX+E rats [46]. Further, we did recently uncover a non-genomic, ERK-activation-dependent, Gq-coupled membrane estrogen receptor (Gq-mER)-mediated pathway in the female rat that disrupted clonidine-induced spinal antinociception presumably by inhibiting G-protein-coupled inwardly rectifying potassium (GIRK) channels and/or α2-adrenoceptor - Gi/Go coupling [23]. In the absence of Gq-mER localization studies in the trigeminal region, we can only speculate that similar non-genomic, as well as genomic mechanisms may underlie estradiol-induced attenuation of the α2-adrenoceptor-mediated antinociception in this region.
Finally, spinally targeted clonidine treatment (e.g. intrathecal or epidural) does produce potent analgesia in postoperative patients and those suffering from neuropathic or cancer pain with unsatisfactory opiate analgesia or tolerance to systemic opiates. In addition, adverse side effects such as addiction and tolerance that are commonly associated with systemic opiate treatment are not observed [47–52].
5. Conclusion
These results led us to conclude that activation of the trigeminal α2-adrenoceptor produces robust antinociceptive and antihyperalgesic effects in male and ovariectomized female rats using reward conflict-based operant assay of orofacial pain, whereas estradiol replacement abolishes these effects in females. These results support our previous, similar findings obtained from reflexive nociceptive testing and extend them to a more relevant, operant paradigm-based pain assay. These findings may be helpful in the development of better analgesics for each sex.
Highlights.
➢ Trigeminal α2-adrenoceptor activation produces analgesia using an operant pain test
➢ Estrogen abolishes α2-adrenoceptor analgesia and antihyperalgesia in females
➢ Intracisternal administration of clonidine does not produce hyperphagia
Acknowledgements
Research reported here was supported by NIGMS of the National Institutes of Health under award number SC1NS078778 to S.S.M. S.N was supported by research endowment funds to Meharry Medical College. We thank Mr. Jeremy Sprouse for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Authors have no actual or potential conflict of interest pertaining to the reported findings.
References
- 1.Berkley KJ. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380-513. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 2.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 3.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 4.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP, Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. doi:10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain Off. J. Am. Pain Soc. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. doi:10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. doi:10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 8.Melchior M, Poisbeau P, Gaumond I, Marchand S. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.05.007. doi:10.1016/j.neuroscience.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J. Pain Off. J. Am. Pain Soc. 2012;13:228–234. doi: 10.1016/j.jpain.2011.11.002. doi:10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin VT. Ovarian hormones and pain response: a review of clinical and basic science studies. Gend. Med. 2009;6(Suppl 2):168–192. doi: 10.1016/j.genm.2009.03.006. doi:10.1016/j.genm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R245–256. doi: 10.1152/ajpregu.00920.2005. doi:10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- 12.Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146:47–55. doi: 10.1016/j.pain.2009.06.018. doi:10.1016/j.pain.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat. Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 14.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 15.Kshirsagar S, Gear R, Levine J, Verotta D. A mechanistic model for the sex-specific response to nalbuphine and naloxone in postoperative pain. J. Pharmacokinet. Pharmacodyn. 2008;35:69–83. doi: 10.1007/s10928-007-9076-y. doi:10.1007/s10928-007-9076-y. [DOI] [PubMed] [Google Scholar]
- 16.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc. Natl. Acad. Sci. U. S. A. 2003;100:271–276. doi: 10.1073/pnas.0136822100. doi:10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craft RM, Kandasamy R, Davis SM. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain. 2013;154:1709–1717. doi: 10.1016/j.pain.2013.05.017. doi:10.1016/j.pain.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. doi:10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci. Biobehav. Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 20.Nag S, Mokha SS. Estrogen attenuates antinociception produced by stimulation of Kölliker- Fuse nucleus in the rat. Eur. J. Neurosci. 2004;20:3203–3207. doi: 10.1111/j.1460-9568.2004.03775.x. doi:10.1111/j.1460-9568.2004.03775.x. [DOI] [PubMed] [Google Scholar]
- 21.Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex- specific modulation of nociception in the rat. Neuroscience. 2006;142:1255–1262. doi: 10.1016/j.neuroscience.2006.07.012. doi:10.1016/j.neuroscience.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Nag S, Mokha SS. Testosterone is essential for alpha(2)-adrenoceptor-induced antinociception in the trigeminal region of the male rat. Neurosci. Lett. 2009;467:48–52. doi: 10.1016/j.neulet.2009.10.016. doi:10.1016/j.neulet.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nag S, Mokha SS. Activation of a Gq-coupled membrane estrogen receptor rapidly attenuates α2-adrenoceptor-induced antinociception via an ERK I/II-dependent, non-genomic mechanism in the female rat. Neuroscience. 2014;267:122–134. doi: 10.1016/j.neuroscience.2014.02.040. doi:10.1016/j.neuroscience.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. doi:10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claiborne JA, Nag S, Mokha SS. Estrogen-dependent, sex-specific modulation of mustard oil- induced secondary thermal hyperalgesia by orphanin FQ in the rat. Neurosci. Lett. 2009;456:59–63. doi: 10.1016/j.neulet.2009.03.106. doi:10.1016/j.neulet.2009.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson KP, Nag S, Thompson AD, Mokha SS. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151:806–815. doi: 10.1016/j.pain.2010.09.018. doi:10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small KM, Nag S, Mokha SS. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an ERK-dependent non-genomic mechanism. Neuroscience. 2013;255:177–190. doi: 10.1016/j.neuroscience.2013.10.034. doi:10.1016/j.neuroscience.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. doi:10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. doi:10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Choi HS, Jung CY, Ju JS, Kim SK, Bae YC, Ahn DK. Intracisternal NMDA produces analgesia in the orofacial formalin test of freely moving rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:497–503. doi: 10.1016/j.pnpbp.2004.01.001. doi:10.1016/j.pnpbp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Berglund LA, Derendorf H, Simpkins JW. Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology. 1988;122:2718–2726. doi: 10.1210/endo-122-6-2718. doi:10.1210/endo-122-6-2718. [DOI] [PubMed] [Google Scholar]
- 32.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res. Mol. Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 33.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. doi:10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Clark JT. 4 - Analysis of Female Sexual Behavior: Proceptivity, Receptivity, and Rejection. In: Conn PM, editor. Methods Neurosci. Academic Press; 1993. [June 2, 2016]. pp. 54–75. http://www.sciencedirect.com/science/article/pii/B9780121852771500095. [Google Scholar]
- 36.Mansur SS, Terenzi MG, Marino Neto J, Faria MS, Paschoalini MA. Alpha1 receptor antagonist in the median raphe nucleus evoked hyperphagia in free-feeding rats. Appetite. 2011;57:498–503. doi: 10.1016/j.appet.2011.06.017. doi:10.1016/j.appet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Shor-Posner G, Azar AP, Volpe M, Grinker JA, Leibowitz SF. Clonidine hyperphagia: neuroanatomic substrates and specific function. Pharmacol. Biochem. Behav. 1988;30:925–932. doi: 10.1016/0091-3057(88)90121-9. [DOI] [PubMed] [Google Scholar]
- 38.Thompson AD, Angelotti T, Nag S, Mokha SS. Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience. 2008;153:1268–1277. doi: 10.1016/j.neuroscience.2008.03.008. doi:10.1016/j.neuroscience.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–99. doi: 10.1016/s0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 40.Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer 344 rats. J. Pharmacol. Exp. Ther. 2003;307:237–245. doi: 10.1124/jpet.103.054478. doi:10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- 41.McCabe JT, DeBellis M, Leibowitz SF. Clonidine-induced feeding: analysis of central sites of action and fiber projections mediating this response. Brain Res. 1984;309:85–104. doi: 10.1016/0006-8993(84)91013-8. [DOI] [PubMed] [Google Scholar]
- 42.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch. Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. doi:10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118:769–778. doi: 10.1016/s0306-4522(02)01000-x. [DOI] [PubMed] [Google Scholar]
- 44.Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O'Carroll A-M, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. doi:10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. Mol. Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 46.Karkanias GB, Li CS, Etgen AM. Estradiol reduction of alpha 2-adrenoceptor binding in female rat cortex is correlated with decreases in alpha 2A/D-adrenoceptor messenger RNA. Neuroscience. 1997;81:593–597. doi: 10.1016/s0306-4522(97)00359-x. [DOI] [PubMed] [Google Scholar]
- 47.Hassenbusch SJ, Garber J, Buchser E, Du Pen S, Nitescu P. Alternative intrathecal agents for the treatment of pain. Neuromodulation J. Int. Neuromodulation Soc. 1999;2:85–91. doi: 10.1046/j.1525-1403.1999.00085.x. doi:10.1046/j.1525-1403.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- 48.Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- 49.Dobrydnjov I, Axelsson K, Gupta A, Lundin A, Holmström B, Granath B. Improved analgesia with clonidine when added to local anesthetic during combined spinal-epidural anesthesia for hip arthroplasty: a double-blind, randomized and placebo-controlled study. Acta Anaesthesiol. Scand. 2005;49:538–545. doi: 10.1111/j.1399-6576.2005.00638.x. doi:10.1111/j.1399-6576.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 50.Rauck RL, Eisenach JC, Jackson K, Young LD, Southern J. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology. 1993;79:1163–1169. discussion 27A. [PubMed] [Google Scholar]
- 51.Sahni N, Panda NB, Jain K, Batra YK, Dhillon MS, Jagannath P. Comparison of different routes of administration of clonidine for analgesia following anterior cruciate ligament repair. J. Anaesthesiol. Clin. Pharmacol. 2015;31:491–495. doi: 10.4103/0970-9185.169070. doi:10.4103/0970-9185.169070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strebel S, Gurzeler JA, Schneider MC, Aeschbach A, Kindler CH. Small-dose intrathecal clonidine and isobaric bupivacaine for orthopedic surgery: a dose-response study. Anesth. Analg. 2004;99:1231–1238. doi: 10.1213/01.ANE.0000133580.54026.65. table of contents. doi:10.1213/01.ANE.0000133580.54026.65. [DOI] [PubMed] [Google Scholar]