Abstract
Objective
Cerebral dyschromatopsia is sometimes associated with acquired prosopagnosia. Given the variability in structural lesions that cause acquired prosopagnosia, this study aimed to investigate the structural correlates of prosopagnosia-associated dyschromatopsia, and to determine if such color processing deficits could also accompany developmental prosopagnosia. In addition, we studied whether cerebral dyschromatopsia is typified by a consistent pattern of hue impairments.
Methods
We investigated hue discrimination in a cohort of 12 subjects with acquired prosopagnosia and 9 with developmental prosopagnosia, along with 42 matched controls, using the Farnsworth-Munsell 100-hue test.
Results
We found impaired hue discrimination in six subjects with acquired prosopagnosia, five with bilateral and one with a unilateral occipitotemporal lesion. Structural MRI analysis showed maximum overlap of lesions in the right and left lingual and fusiform gyri. Fourier analysis of their error scores showed tritanopic-like deficits and blue-green impairments, similar to tendencies displayed by the healthy controls. Three subjects also showed a novel fourth Fourier component, indicating additional peak deficits in purple and green-yellow regions. No subject with developmental prosopagnosia had impaired hue discrimination.
Conclusions
In our subjects with prosopagnosia, dyschromatopsia occurred in those with acquired lesions of the fusiform gyri, usually bilateral but sometimes unilateral. The dyschromatopsic deficit shows mainly an accentuation of normal tritatanopic-like tendencies. These are sometimes accompanied by additional deficits, although these could represent artifacts of the testing procedure.
Keywords: hue discrimination, colour perception, face recognition, Fourier analysis, fusiform gyrus
1 INTRODUCTION
Acquired prosopagnosia is the rare disorder of impaired face recognition. It can occur with a variety of lesions, right or bilateral, in occipitotemporal or anterior temporal cortex (Davies-Thompson, Pancaroglu, & Barton, 2014). Prosopagnosic subjects can also have cerebral dyschromatopsia (Meadows, 1974), an impairment of colour perception whose hallmark is a deficit in discriminating hues. This has often been linked to bilateral damage to the lingual and posterior fusiform gyri, as demonstrated in a meta-analysis (Bouvier & Engel, 2006) and consistent with early fMRI studies of colour perception (Sakai, et al., 1995). However, subsequent neuroimaging studies have shown, first, a posterior V4 locus and an anterior V8 locus near the collateral sulcus (Bartels & Zeki, 2000), and more recently, multiple patches with colour-related activity, including more anterior cortex and often in close proximity to face-sensitive regions (Beauchamp, Haxby, Jennings, & DeYoe, 1999; Lafer-Sousa & Conway, 2013). The lesion data has also suggested that colour perception may involve a stream of cortical processing, rather than a single region (Bouvier & Engel, 2006). This raises the possibility that a variety of prosopagnosic lesions may be associated with impaired colour perception. Furthermore, although the association between dyschromatopsia and acquired prosopagnosia has been well documented (Zeki, 1990), there has been no systematic study of colour perception in developmental prosopagnosia a condition whose anatomic correlate remains a subject of debate (Avidan, et al., 2014; Song, et al., 2015).
Unlike congenital dyschromatopsia due to inherited deficits in cone pigments, the colour processing deficits of cerebral dyschromatopsia are often said to be non-specific, affecting all hues. Nevertheless, some describe more pronounced deficits in certain regions of colour space (Adachi-Usami, Tsukamoto, & Shimada, 1995; Fine & Parker, 1996; Rizzo, Smith, Pokorny, & Damasio, 1993; Rondot, Tzavaras, & Garcin, 1967). Almost all of these cases report only a subjective inspection of data; however, when deficits are widespread patterns may be detected more effectively by quantitative mathematical techniques (Victor, 1988). Surprisingly, this has been done in only one case of cerebral dyschromatopsia (Victor, Maiese, Shapley, Sidtis, & Gazzaniga, 1989).
In this study we applied a widely used, sensitive test of hue discrimination, the Farnsworth-Munsell 100-hue test (Farnsworth, 1943), to a large cohort of subjects with acquired or developmental prosopagnosia. Our first goal was to determine whether any type of lesion causing prosopagnosia could be associated with dyschromatopsia or if colour impairments were limited to those with bilateral fusiform lesions. Our second goal was to determine if colour processing deficits occurred in at least some subjects with developmental prosopagnosia. Our third goal was to use Fourier analysis to determine if cerebral dyschromatopsia has relative selectivity for a particular region or axis of hue space.
2 MATERIALS AND METHODS
2.1 Subjects
The Institutional Review Boards of the University of British Columbia and Vancouver Hospital approved the protocol and all subjects gave informed consent in accordance with the principles of the Declaration of Helsinki.
2.1.1 Acquired Prosopagnosia
These were 12 subjects (6 female, mean age 42 years, range 22 to 60). All had a neuro-ophthalmologic history and examination, with best-corrected acuity of 20/60 or better, and Goldmann perimetry. All complained of impaired face recognition in daily life and were impaired on both a famous faces test and at least one of either the Cambridge Face Memory test or the faces component of the Warrington Recognition Memory test, while performing normally on the word component of the latter (Table 1). A short history of the six who proved to have impaired hue discrimination follows. All six also had noted altered colour perception in their daily life, while none of the other subjects with either acquired or developmental prosopagnosia voiced any complaints about colour perception. Further details on the neuroimaging and neuropsychological testing of this cohort have been published elsewhere (Hills, Pancaroglu, Duchaine, & Barton, 2015; Liu, Pancaroglu, Hills, Duchaine, & Barton, 2014).
Table 1.
Subject data.
| group | subject | etiology | age | gender | visual | CFMT | WRMT | FM-100 | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| yrs | field | face/50 | word/50 | sqrt(TES) | |||||
| controls | mean range | 55.4 (39–70) | 6.8 (2.8–12.8) | ||||||
| 95% prediction limit | <11.8 | ||||||||
| acquired prosopagnosia | |||||||||
| unilateral right occipitotemporal | |||||||||
| R-IOT1 | vascular malformation | 49 | m | LUQ | 44 | 33 | 41 | 10.0 | |
| R-IOT4 | stroke | 57 | m | LUQ | 27 | 39 | 50 | 15.1* | |
| bilateral occipitotemporal | |||||||||
| L-IOT2 | seizure surgery | 56 | m | full | 21 | 27 | 42 | 17.8* | |
| B-IOT1 | strokes | 41 | m | LUQ,RUQ | 45 | 28 | 47 | 16.0* | |
| B-IOT2 | trauma/infarct | 60 | m | RHH,LUQ | 24 | 21 | 42 | 20.3* | |
| bilateral combined occipito and anterior temporal | |||||||||
| B-ATOT1 | HSV encephalitis | 39 | f | LUQ | 30 | 27 | 50 | 19.2* | |
| B-ATOT2 | HSV encephalitis | 22 | f | full | 24 | 19 | 39 | 33.0* | |
| unilateral right anterior temporal | |||||||||
| R-AT1 | lobectomy | 24 | f | full | 38 | 17 | 41 | 4.7 | |
| R-AT2 | HSV encephalitis | 30 | f | full | 40 | 27 | 47 | 7.5 | |
| R-AT3 | HSV encephalitis | 37 | m | full | 31 | 29 | 45 | 8.5 | |
| bilateral anterior temporal | |||||||||
| B-AT2 | trauma, lobectomy | 47 | f | full | 31 | 31 | 46 | 10.0 | |
| developmental prosopagnosia | |||||||||
| DP008 | 61 | f | full | 36 | 36 | 49 | 5.3 | ||
| DP014 | 42 | m | full | 32 | 30 | 48 | 7.2 | ||
| DP016 | 52 | f | full | 41 | 37 | 49 | 8.5 | ||
| DP019 | 44 | f | full | 32 | 32 | 49 | 9.2 | ||
| DP021 | 31 | f | full | 37 | 33 | 50 | 4.0 | ||
| DP024 | 35 | f | full | 41 | 38 | 50 | 2.8 | ||
| DP033 | 49 | f | full | 29 | 39 | 50 | 4.9 | ||
| DP035 | 40 | m | full | 36 | 35 | 49 | 10.0 | ||
| DP044 | 36 | f | full | 40 | 34 | 49 | 7.7 | ||
CFMT = Cambridge Face Memory Test, WRMT = Warrington Recognition Memory Test FM-100 = Farnsworth-Munsell 100-hue test, sqrtTES = square root of Total Error Score HSV = herpes simplex virus LUQ = left upper quadrantanopia, RUQ = right upper quadrantanopia, RHH = right hemianopia
abnormal FM-100 score
L-IOT2 has right fusiform atrophy and had a left fusiform resection for epilepsy at age 39. Afterwards he had aphasia that resolved into a residual word-finding problem. He thought that his car had been repainted a lighter blue when his wife picked him up from hospital. He confuses the jerseys of basketball teams with a similar hue, and has trouble judging skin tone.
B-ATOT2 had herpes encephalitis at age 10, following which she had difficulty reading and spelling, which improved. She has topographagnosic symptoms. Her vision was initially achromatic but partially recovered, although she still cannot identify colours and has labels on her clothes to indicate their colour.
B-IOT2 had a traumatic subdural hematoma that was evacuated at age 27. He has topographagnosic symptoms. He became aware of altered colour vision early on, with hues looking ‘flat’ or ‘turned down’ in vividness.
B-ATOT1 had herpes encephalitis at age 14, leaving a small homonymous paracentral scotoma in the left upper field. She notes difficulty distinguishing blue from green.
B-IOT1 had bilateral sequential occipital infarcts from a vertebral dissection at age 40. He cannot recognize buildings. The world was reduced to black and white initially. After 2 months he began seeing hues again, though he still notes trouble distinguishing similar colours, such as blue versus green.
R-IOT4 had a right occipital infarct 6 months prior to study. He has topographagnosic symptoms. He noted altered color perception but only for the first few months: though he knew what each colour was, they lacked ‘emotional impact’.
2.1.2 Developmental Prosopagnosia
These were 9 subjects (7 female, mean age 43.3 years, range 31 to 61) recruited from www.faceblind.org. Diagnostic criteria were reported life-long difficulty in face recognition and objective confirmation of impaired face recognition (Dalrymple & Palermo, 2016), including a score at least 2 standard deviations below the control mean on the Cambridge Face Memory Test and a discordance between preserved word and impaired face memory on the Warrington Recognition Memory Test that was in the bottom 5th percentile. All had best corrected visual acuity of better than 20/60 and normal Goldmann perimetric results. To exclude autism spectrum disorders, all subjects scored less than 32 on the Autism Questionnaire (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001). All but one subject (DP033) in whom it was contraindicated had MRI with T1-weighted and FLAIR sequences to exclude structural lesions.
2.1.3 Control Subjects
For each prosopagnosic subject we recruited two healthy subjects of the same gender and within 5 years of age, creating a cohort of 42 subjects (26 female, mean age 41.3 years, range 17 to 65). All performed the Cambridge Face Memory test to exclude the possibility of unsuspected developmental prosopagnosia. Exclusion criteria were a history of neurologic or psychiatric conditions, including autism spectrum disorders, or of eye disease, including congenital dyschromatopsia, optic neuritis, macular degeneration, and cataracts. We excluded one subject with subjective claims of poor face recognition and low scores on the Cambridge Face Memory test, two subjects with episodes of unconsciousness lasting more than 10 minutes, and one with memory loss after electrocution. Two subjects with congenital dyschromatopsia were tested for illustrative purposes, a 46 year-old man with tritanopia and a 46 year-old man with protanopia.
2.2 Imaging
We obtained high-resolution (1mm3) T1-weighted three-dimensional structural images on a Philips Achieva 3.0 Tesla MR scanner, with an 8-channel head coil. We created a lesion mask for the 12 subjects with acquired prosopagnosia, using tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL) (Woolrich, et al., 2009). Images were preprocessed with lesion filling when necessary (Battaglini, Jenkinson, & De Stefano, 2012), and linear-registration (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) or non-linear registration to the MNI-152 stereotaxic space. Within the standard space, lesion masks of subjects with impaired hue discrimination were summed to give an overlap image1.
2.3 Protocol
The Farnsworth-Munsell 100-Hue Test (Farnsworth, 1943) probes hue discrimination by having subjects arrange 85 isoluminant coloured caps in a gradient, with unlimited time. The test was performed in an otherwise dark room, with illumination from a MacBeth Easel Lamp fitted with a Standard Illuminant C filter. Caps are divided into four racks, each presented to the subject with the caps in the same predetermined random order. Each cap has a hidden number, and the absolute difference between this number and those of the two caps placed to its immediate left and right by the subject is the score for that cap. If ordering were done perfectly, each cap would have a score of 2: thus the cap error score is the cap score minus 2. Overall performance is the sum of all 85 cap error scores, the Total Error Score. As recommended (Kinnear, 1970), we analyzed the √Total Error Score, because the Total Error Score skews towards higher values. To be classified as abnormal, a score must exceed 1) the 95% prediction interval calculated for our control cohort, and 2) published age-specific 95th percentiles (Kinnear & Sahraie, 2002).
2.4 Analysis
We examined for patterns of selective hue deficits with a Fourier analysis (Victor, 1988). First we converted the error score of each cap into a relative error score, which expresses error as a proportion of the error score that would result from chance performance. Because the caps are divided into four racks, the chance error score is not even but higher for caps near the end of each rack. If this correction were not made, spurious fourth Fourier components would result. The series of relative error scores was then subjected to a discrete Fourier transform (http://calculator.vhex.net/) to yield the coefficients of the sine and cosine terms of the Fourier series for each component, expressed as a complex number. The absolute value of this number represents the amplitude of the Fourier component, while the arctangent (sine term/cosine term) represents its phase in radians. The amplitude of the zero Fourier component is equal to the mean relative error score across all caps. Dividing the amplitude of each of the non-zero Fourier components by this term yields their harmonic indices. While we tabulated the amplitude, phase, and harmonic index for the first six Fourier components, as done elsewhere (Barton, Fong, & Knatterud, 2004), statistical tables are available only for the harmonic indices of the first and second Fourier components (Victor, 1988), on which we focused our analysis. A significant first Fourier component indicates difficulty with a single region of the hue spectrum, while a significant second Fourier component would indicate difficulty along a specific axis of colour space, with increased errors in two regions opposite each other on the hue circle.
3 RESULTS
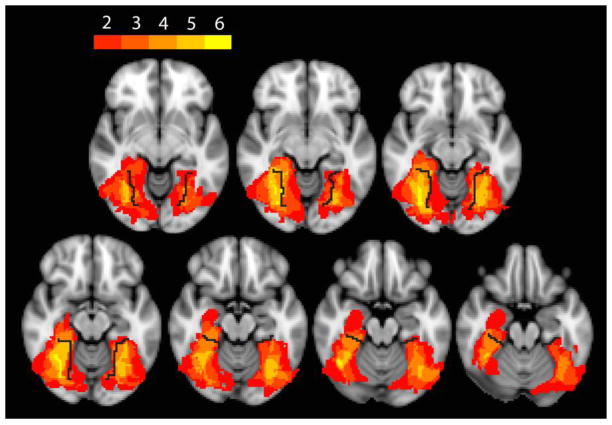
No subject with developmental prosopagnosia had an abnormal √Total Error Score, but six with acquired prosopagnosia did (Table 1). All but one of these six had bilateral occipitotemporal lesions. R-IOT4 had a unilateral right occipitotemporal lesion, and his √Total Error Score was the least of the six. The lesions of these patients overlapped maximally in the fusiform and, to a lesser extent, the lingual gyri, in the vicinity of the collateral sulcus (Figure 1).
Figure 1. Overlap analysis of lesions on MRI.
An axial image of lesions from the six subjects with impaired hue discrimination, shown as a color spectrum from red to yellow, with red indicating regions involved by the lesion in only two subjects, and yellow indicating regions where the lesions of all six subjects overlapped. Black line indicates collateral sulcus.
We examined the pattern of performance in our control subjects with two approaches. First we obtained the group mean relative error scores for each cap, and performed a Fourier analysis on these mean values. This showed no significant first Fourier component, but a borderline significant second Fourier component. To ensure against artifacts from averaging, our second approach performed a Fourier analysis on each control subject’s relative error scores, and then averaged the waveforms of the first and second Fourier components. Both approaches yielded similar components (Figure 2), with the phase of the second Fourier component indicating errors approximating the tritan (blue-yellow) axis.
Figure 2. Control analyses.
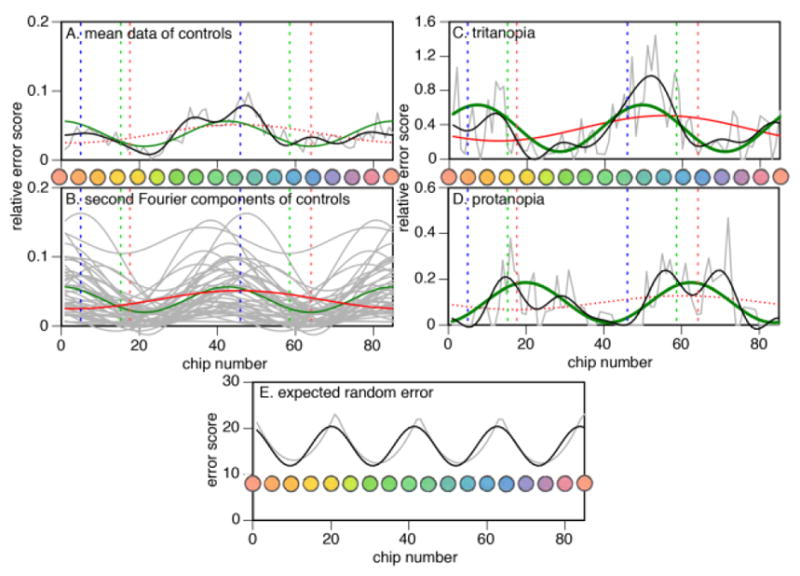
Error scores are plotted for each of the 85 caps, the hues of which are also shown. A) Mean relative error score for the group of 44 controls, indicated by grey jagged line. Shown are the first Fourier component (red curve), second Fourier component (green curve) and the curve produced by the sum of the first six Fourier components (black curve) as fitted to these mean data. B) Grey curves are the second Fourier components for each of the 44 controls, with the green curve showing the average of these curves. The red curve shows the average of the first Fourier components. C) A tritanopic subject. Jagged grey line indicates his relative error scores, with first Fourier component (red curve), second Fourier component (green curve) and sum of first six Fourier components (black curve) displayed. D) A similar plot for a protanopic subject. In each graph the vertical dashed lines indicate the axes for tritan (blue lines), deutan (green lines) and protan (red line) errors. Solid red or green curves indicate Fourier components that were significant, bold curves those with the largest amplitude of the first six components, while dotted red or green curves indicate those that were not significant. E) Plot of expected random error (grey curve), with the fourth Fourier component of its analysis superimposed (black curve).
Age correlated with the amplitude of the zero (r = .45, F(1,41) = 10.3, p < .004), first (r = .33, F(1,41) = 4.96, p < .033), and second (r = .52, F(1,41) = 15.5, p < .001) Fourier components, and with the first (r = .45, F(1,41) = 10.8, p < .003), but not the second harmonic index (r = .10, F(1,41) = .38, p = .54). Thus there is a general age-related decline in hue discrimination that is accentuated in the blue-green region, but not relatively increased along the tritan axis.
For illustrative purposes, we analyzed two healthy subjects with congenital dyschromatopsia. As expected these showed strong second Fourier components with phase relationships consistent with the axes of their deficits (Figure 2).
Among our six acquired prosopagnosic subjects with elevated √Total Error Scores, four had a significant first Fourier component, in all of whom this tended to peak around cap 40, in the blue region (Table 2, Figure 3). All six had significant second Fourier components, which in four had the largest amplitude of any of the first six Fourier components. The phase of this second Fourier component approximated the tritan axis in subjects B-IOT1, L-IOT2, B-IOT2, and R-IOT4, similar to our controls, but was advanced to a phase intermediate between protanopic and tritanopic axes in B-ATOT1, and in B-ATOT2 in whom the second Fourier component was only marginally significant.
Table 2.
Results of Fourier analysis for 6 subjects with impaired hue discrimination
| subject | R-IOT4 | B-IOT1 | L-IOT2 | B-ATOT1 | B-IOT2 | B-ATOT2 |
|---|---|---|---|---|---|---|
|
| ||||||
| sqrt(TES) | 15.10 | 16.00 | 17.78 | 19.18 | 20.30 | 33.00 |
| amplitude | ||||||
| A0 | 0.175 | 0.188 | 0.231 | 0.284 | 0.313 | 0.785 |
| A1 | 0.061 | 0.049 | 0.027 | 0.016 | 0.123 | 0.208 |
| A2 | 0.092 | 0.069 | 0.119 | 0.081 | 0.166 | 0.088 |
| A3 | 0.031 | 0.020 | 0.078 | 0.059 | 0.078 | 0.091 |
| A4 | 0.069 | 0.045 | 0.037 | 0.137 | 0.108 | 0.070 |
| A5 | 0.040 | 0.025 | 0.063 | 0.007 | 0.018 | 0.078 |
| A6 | 0.053 | 0.043 | 0.054 | 0.012 | 0.030 | 0.019 |
| harmonic index | ||||||
| A1 | 0.352** | 0.261* | 0.117 | 0.058 | 0.395*** | 0.265*** |
| A2 | 0.526*** | 0.365*** | 0.518*** | 0.287** | 0.530*** | 0.112* |
| A3 | 0.177 | 0.108 | 0.341 | 0.208 | 0.251 | 0.115 |
| A4 | 0.398 | 0.243 | 0.159 | 0.484 | 0.346 | 0.089 |
| A5 | 0.229 | 0.136 | 0.273 | 0.027 | 0.059 | 0.099 |
| A6 | 0.305 | 0.228 | 0.236 | 0.044 | 0.098 | 0.024 |
| phase | ||||||
| A1 | 0.484 | 0.335 | 0.929 | −0.58 | 0.165 | −0.07 |
| A2 | −0.06 | −0.57 | −0.55 | −1.42 | −0.73 | −1.43 |
| A3 | −0.82 | 0.410 | −1.23 | 0.917 | −1.14 | −1.47 |
| A4 | −0.00 | 1.146 | 1.539 | 0.023 | −0.11 | 0.378 |
| A5 | 0.688 | 0.816 | 0.268 | −1.19 | −1.26 | 0.153 |
| A6 | 0.493 | −1.51 | −0.38 | −0.75 | 1.138 | −0.12 |
p <.05;
p <.01;
p < .0001
bold = component with the largest harmonic index.
underline = large fourth Fourier component
sqrtTES = square root of Total Error Score
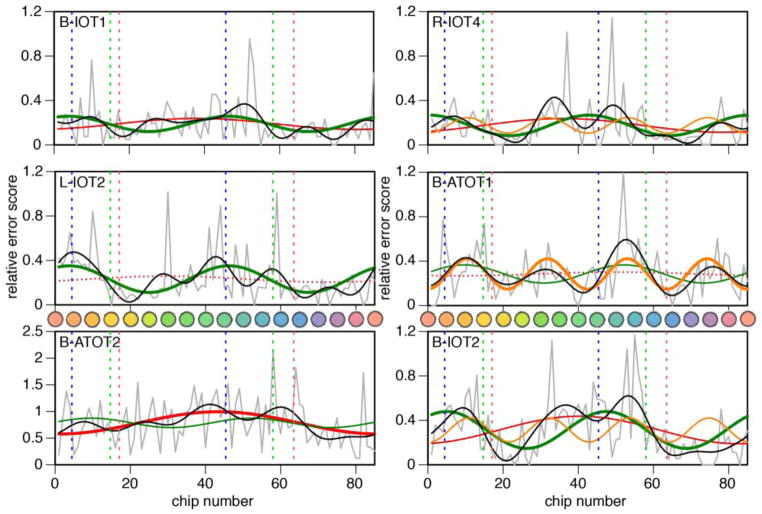
Figure 3. Fourier analysis for 6 subjects with impaired hue discrimination.
Jagged grey lines show relative error score plotted against cap number, the hues of which are also shown. Superimposed are the first Fourier component (red curve), the second Fourier component (green curve), and the sum of the first six Fourier components (black curve). For three subjects, the fourth Fourier component is also shown (orange curve). In each graph the vertical dashed lines indicate the axes for tritan (blue lines), deutan (green lines) and protan (red line) errors. Solid red or green curves indicate Fourier components that were significant, bold curves those with the largest amplitude of the first six components, while dotted red or green curves indicate those that were not significant.
A novel finding was a large fourth Fourier component in three subjects (Figure 3). Although tables of significance for this component have not been provided (Victor, 1988), by approximation to those for the first two components these are almost certainly significant. This fourth component had a nearly identical phase in all three subjects and does not correspond to a mixture of protanopic/deuteranopic and tritanopic deficits.
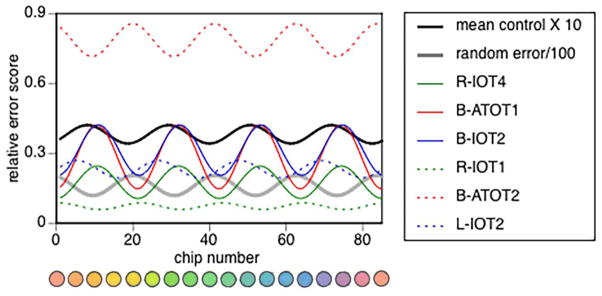
While this unexpected finding might reflect some novel distortion of colour space, it is also important to consider whether it reflects an artifact of the testing procedure. Note that the division of the caps into four trays generates a non-uniform random error pattern that is reflected in a fourth Fourier component (Figure 2), for which Victor devised a correction procedure that we used (Victor, 1988). However, this correction may be excessive in the case of non-random sorting, as with subjects with very good colour vision. Indeed, the mean control data show a fourth Fourier component in counterphase to that of the random error (Figure 4), which is the anticipated result of over-correction.
Figure 4. Fourth Fourier component analysis.
The components of all six subjects with dyschromatopsia are shown. Solid coloured lines indicate those subjects with large fourth Fourier components, and dashed lines those with more modest components. For comparison, the thick black line indicates the fourth Fourier component from the analysis of the mean control data, multiplied by 10 to facilitate the comparison. The thick grey line shows that from the analysis of random error, divided by 100 for facilitated viewing. The fourth Fourier component of the control data is in counterphase to that of the random error, indicating a slight over-correction by the procedure of Victor (1988).
If we examine the data of all our dyschromatopsic subjects, we find that the peaks for the three with large fourth Fourier components – R-IOT4, B-ATOT1, and B-IOT2 – have locations that approximate that of the control data, suggesting the possibility of an over-correction artifact (Figure 4). However, the fourth Fourier components of R-IOT1, with the lowest error score, and of B-ATOT2, with the highest error score, align more in phase with that of the random error, which is more consistent with an under-correction artifact. In L-IOT2, the phase of the fourth Fourier component is offset from those of both the random error and the control data, indicating neither under- nor over-correction. Hence, while an artifact of the procedure to correct for nonuniform random error could explain the fourth Fourier components in R-IOT4, B-ATOT1, and B-IOT2, two points give us pause. First, there is no consistent relationship between the severity of dyschromatopsia and whether the fourth Fourier component fits with either under- or over-correction. Second, the phase of their fourth Fourier components are not precisely aligned with the control data, but offset to the right.
4 DISCUSSION
We found impaired hue discrimination in all five subjects with acquired prosopagnosia due to bilateral occipitotemporal lesions. Overlap lesion analysis confirmed that the area of commonality involved the fusiform and lingual gyri bilaterally. However, one subject with a right occipitotemporal lesion also had impaired hue discrimination, though with a Total Error Score that was less than those of all subjects with bilateral lesions. Hue discrimination was normal in six other subjects with bilateral anterior temporal, right anterior temporal, or right occipitotemporal lesions. Fourier analysis of the hue deficits confirmed a tritanopic-like pattern in most subjects with impairments, which approximated similar milder patterns in healthy controls. A novel finding was a large fourth Fourier component in three subjects, with nearly identical phase.
Functional imaging suggests that colour processing involves bilateral regions of occipitotemporal cortex ranging from the lingual gyrus to the collateral sulcus (Bartels & Zeki, 2000; Beauchamp, et al., 1999). A meta-analysis of previous cases showed overlap in these areas, particularly on the right (Bouvier & Engel, 2006). However, meta-analyses have limitations, including potential selection or reporting bias, variable diagnostic criteria, variations in scanning protocols and input images, and challenges in image registration and alignment, among others. Our study has the advantage of a single cohort assessed with uniform protocols for colour perception testing and neuroimaging. Our results agree with that of the meta-analysis, indicating that dyschromatopsia localizes to a region surrounding the collateral sulcus bilaterally. Lesions limited to anterior temporal regions did not cause subtle impairments.
The consistent lack of hue deficits in developmental prosopagnosia indicates an important difference from acquired prosopagnosia. One simple explanation may be that both face and colour processing deficits are less severe in developmental prosopagnosia, so that they fail to reach criteria for dyschromatopsia. However, the evidence that the face processing deficit is less severe in our developmental subjects is mixed: while their scores on the face component of the Warrington Recognition Memory Test are lower (mean 27.1, s.d. 6.3 for the developmental form versus mean 34.9, s.d. 2.9 for the acquired form, p < 0.003), the difference between scores on the Cambridge Face Memory Test was not significant (mean 32.3, s.d. 8.3 for the developmental form versus mean 36.0, s.d. 4.3 for the acquired form, p = 0.241). A second explanation may relate to the anatomic correlations in the cohort with acquired prosopagnosia. Normal colour processing in the developmental cohort may suggest that in most cases of developmental prosopagnosia the underlying defect is either more selective in its effects on the fusiform gyrus or located in more anterior regions of cortex.
While cerebral dyschromatopsia is generally thought to require bilateral lesions, with unilateral lesions leading instead to an often asymptomatic hemi-achromatopsia (Paulson, Galetta, Grossman, & Alavi, 1994; Short & Graff-Radford, 2001), we found one subject with a right occipitotemporal lesion who had impaired hue discrimination, though less severe than that following bilateral lesions. This is not without precedent. As noted (Meadows, 1974), older surveys of patients with unilateral lesions find occasional cases with mild colour deficits (Assal, Eisert, & Hecaen, 1969; Lhermitte, Chain, Aron, Leblanc, & Souty, 1969), particularly on the sensitive Farnsworth-Munsell 100-Hue Test and in subjects with right-sided lesions (Scotti & Spinnler, 1970). A more recent study found impaired hue discrimination in three of 27 subjects with unilateral cerebral lesions (Ruttiger, et al., 1999), while another found poor discrimination of red, green and blue caps in a subject with a lesion of the right lingual and fusiform gyri (Clarke, Walsh, Schoppig, Assal, & Cowey, 1998). The reasons why a colour processing deficit usually found with bilateral damage can sometimes occur with a unilateral lesion are not clear, but one possibility is natural variability in functional lateralization. Thus an accentuation of the normally mild right predominance for colour processing noted above (Bouvier & Engel, 2006) may render some individuals prone to colour deficits after right-sided damage.
Reports, almost all of single cases, have been mixed concerning whether there is a selective hue impairment in cerebral dyschromatopsia. Many claim there is not. This is understandable for severe complete achromatopsia, when Farnsworth-Munsell 100-Hue error scores are so large ( 1200) that they are equivalent to random sorting (Heywood, Nicholas, & Cowey, 1996). Even with less elevated Farnsworth-Munsell 100-Hue error scores, though, it was claimed that there was no well-defined axis for patients BL (Kennard, Lawden, Morland, & Ruddock, 1995)or A32660 (Meadows, 1974), that EH had equal impairment in all 3 axes (Shuren, Brott, Schefft, & Houston, 1996), and that another’s deficits were ‘sans axes’ (Duvelleroy-Hommet, et al., 1997). On the D-15 test, Mme D showed no definite confusion axis (Bartolomeo, Bachoud-Levi, & Denes, 1997). MS, who seemed more accurate for red-purple hues, did not show significant Fourier components (Victor, et al., 1989). Two other patients showed no particular axis on the Farnsworth-Munsell 100-Hue Test, but one had a tritanopic pattern on the D-15 and the Lanthony New Color Test (Rizzo, et al., 1993).
Among reports of selective deficits, PM showed a mild protanomaly on Hardy-Rand and Rittler test, but also found blues and greens difficult to distinguish (Critchley, 1965). Likewise, another subject struggled with blue-green hues (Fine & Parker, 1996) and a third subject had more difficulty in the blue sector (Rondot, et al., 1967). A fourth subject had strong blue-yellow versus medium red-green defects on Standard Pseudo-isochromatic plates (Adachi-Usami, et al., 1995). On the other hand, CB had diffuse deficits that were worse for red and green-yellow (Heywood, Wilson, & Cowey, 1987), and anomaloscope testing of another subject showed better recovery of blue-green than red-yellow discrimination (Jaeger, Krastel, & Braun, 1988). JPC had a large error score of 888 on the Farnsworth-Munsell 100-Hue Test but showed protanopic-like performance with the anomaloscope (D'Zmura, Knoblauch, Henaff, & Michel, 1998).
With one exception (Victor, et al., 1989), all of the studies above characterized hue-sorting scores solely on the basis of inspection, and in only one or two cases. We advance upon this by studying a relatively large cohort with a quantitative mathematical analysis, which is particularly useful at detecting underlying patterns when errors are diffuse and scattered. Our results show a consistent tendency to tritanopic-like errors, sometimes augmented by a first-Fourier component showing increased errors in the blue-green range, validating some of the prior subjective observations (Adachi-Usami, et al., 1995; Critchley, 1965; Rizzo, et al., 1993; Rondot, et al., 1967).
We also noted similar tendencies in our controls. Numerous prior studies using the Farnsworth-Munsell 100-Hue Test have documented that both young and older healthy subjects have greater difficulty in the blue-green region or along the blue-yellow axis (Kinnear & Sahraie, 2002; Scotti & Spinnler, 1970; Smith, Pokorny, & Pass, 1985). It has been asserted that this may be accentuated by age, but the evidence is inconsistent (Kinnear & Sahraie, 2002; Smith, et al., 1985). Our correlation analysis supports a relatively greater increase in blue-green errors with aging, but not in the tritanopic-like second Fourier component. A normal tritanopic tendency has been attributed to retinal or lenticular factors, but studies have cast doubt upon this assertion (Smith, et al., 1985).
Regardless, our results suggest that, when partial, cerebral dyschromatopsia is characterized primarily by an accentuation of the normal blue-green and tritanopic-like patterns evident in controls, rather than a distortion of colour space. In half of our dyschromatopsic subjects, though, the emergence of a large fourth Fourier component may indicate a new anomaly unrelated to traditional red-green and blue-yellow axes, with error peaks in green-yellow and purple dimensions of hue space. However, the implications of this observation for cortical colour mechanisms are not clear, the result requires replication in other dyschromatopsic subjects, and it is possible that this secondary observation derives from an over-correction artifact. Nevertheless, these findings illustrate the value of applying formal mathematical analyses to complex behavioural results, to reveal patterns that are otherwise difficult to discern.
Highlights.
We investigated hue discrimination in acquired and developmental prosopagnosia.
Lesions to the fusiform gyri but not anterior temporal cortex caused dyschromatopsia.
Developmental prosopagnosia is not associated with impaired hue discrimination.
Cerebral dyschromatopsia is mainly an accentuation of normal tritanopic-like tendencies.
Acknowledgments
FINANCIAL SUPPORT:
This work was supported by CIHR operating grant MOP-102567 to JB. JB was supported by a Canada Research Chair and the Marianne Koerner Chair in Brain Diseases. BD was supported by grants from the Economic and Social Research Council (UK) (RES-062-23-2426) and the Hitchcock Foundation. SC was supported by National Eye Institute award F32 EY023479-02 and the Loan Repayment Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank C Hills, A Sekunova, M Abegg, CJ Fox, R Pancaroglu and J Davies-Thompson for assistance with data collection.
Footnotes
CONFLICT OF INTEREST:
no conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel Moroz, Email: daniel.moroz@ucalgary.ca.
Sherryse L Corrow, Email: sherryse.corrow@icloud.com.
Jeffrey C Corrow, Email: corrowje@gmail.com.
Alistair R S Barton, Email: alistair.barton@mail.mcgill.ca.
Brad Duchaine, Email: bradley.c.duchaine@dartmouth.edu.
Jason J S Barton, Email: jasonbarton@shaw.ca.
References
- Adachi-Usami E, Tsukamoto M, Shimada Y. Color vision and color pattern visual evoked cortical potentials in a patient with acquired cerebral dyschromatopsia. Doc Ophthalmol. 1995;90:259–269. doi: 10.1007/BF01203861. [DOI] [PubMed] [Google Scholar]
- Assal G, Eisert HG, Hecaen H. Analysis of Farnsworth D15 test results in 155 patients with left or right hemisphere lesions. Acta Neurol Psychiatr Belg. 1969;69:705–717. [PubMed] [Google Scholar]
- Avidan G, Tanzer M, Hadj-Bouziane F, Liu N, Ungerleider LG, Behrmann M. Selective dissociation between core and extended regions of the face processing network in congenital prosopagnosia. Cerebral Cortex. 2014;24:1565–1578. doi: 10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur J Neurosci. 2000;12:172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Bachoud-Levi AC, Denes G. Preserved imagery for colours in a patient with cerebral achromatopsia. Cortex. 1997;33:369–378. doi: 10.1016/s0010-9452(08)70012-1. [DOI] [PubMed] [Google Scholar]
- Barton FB, Fong DS, Knatterud GL. Classification of Farnsworth-Munsell 100-hue test results in the early treatment diabetic retinopathy study. Am J Ophthalmol. 2004;138:119–124. doi: 10.1016/j.ajo.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33:2062–2071. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA. An fMRI version of the Farnsworth-Munsell 100-Hue test reveals multiple color-selective areas in human ventral occipitotemporal cortex. Cereb Cortex. 1999;9:257–263. doi: 10.1093/cercor/9.3.257. [DOI] [PubMed] [Google Scholar]
- Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb Cortex. 2006;16:183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- Clarke S, Walsh V, Schoppig A, Assal G, Cowey A. Colour constancy impairments in patients with lesions of the prestriate cortex. Exp Brain Res. 1998;123:154–158. doi: 10.1007/s002210050556. [DOI] [PubMed] [Google Scholar]
- Critchley M. Acquired anomalies of colour perception of central origin. Brain. 1965;88:711–724. doi: 10.1093/brain/88.4.711. [DOI] [PubMed] [Google Scholar]
- D'Zmura M, Knoblauch K, Henaff MA, Michel F. Dependence of color on context in a case of cortical color vision deficiency. Vision Res. 1998;38:3455–3459. doi: 10.1016/s0042-6989(97)00407-0. [DOI] [PubMed] [Google Scholar]
- Dalrymple KA, Palermo R. Guidelines for studying developmental prosopagnosia in adults and children. Wiley Interdiscip Rev Cogn Sci. 2016;7:73–87. doi: 10.1002/wcs.1374. [DOI] [PubMed] [Google Scholar]
- Davies-Thompson J, Pancaroglu R, Barton J. Acquired prosopagnosia: structural basis and processing impairments. Front Biosci (Elite Ed) 2014;6:159–174. doi: 10.2741/e699. [DOI] [PubMed] [Google Scholar]
- Duvelleroy-Hommet C, Gillet P, Cottier JP, de Toffol B, Saudeau D, Corcia P, Autret A. Achromatopsie cérébrale sans prosopagnosie ni alexie ni agnosie des objets. Revue Neurologique. 1997;153:554–560. [PubMed] [Google Scholar]
- Farnsworth D. The Farnsworth-Munsell 100-Hue and Dichotomous Tests for Color Vision. Journal of the Optical Society of America. 1943;33:568–574. [Google Scholar]
- Fine RD, Parker GD. Disturbance of central vision after carbon monoxide poisoning. Aust N Z J Ophthalmol. 1996;24:137–141. doi: 10.1111/j.1442-9071.1996.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Heywood CA, Nicholas JJ, Cowey A. Behavioural and electrophysiological chromatic and achromatic contrast sensitivity in an achromatopsic patient. J Neurol Neurosurg Psychiatry. 1996;60:638–643. doi: 10.1136/jnnp.60.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood CA, Wilson B, Cowey A. A case study of cortical colour “blindness” with relatively intact achromatic discrimination. J Neurol Neurosurg Psychiatry. 1987;50:22–29. doi: 10.1136/jnnp.50.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills CS, Pancaroglu R, Duchaine B, Barton JJ. Word and text processing in acquired prosopagnosia. Ann Neurol. 2015;78:258–271. doi: 10.1002/ana.24437. [DOI] [PubMed] [Google Scholar]
- Jaeger W, Krastel H, Braun S. Cerebral achromatopsia (symptoms, course, differential diagnosis and strategy of the study). I. Klin Monbl Augenheilkd. 1988;193:627–634. doi: 10.1055/s-2008-1050309. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kennard C, Lawden M, Morland AB, Ruddock KH. Colour identification and colour constancy are impaired in a patient with incomplete achromatopsia associated with prestriate cortical lesions. Proc Biol Sci. 1995;260:169–175. doi: 10.1098/rspb.1995.0076. [DOI] [PubMed] [Google Scholar]
- Kinnear PR. Proposals for scoring and assessing the 100-Hue test. Vision Res. 1970;10:423–433. doi: 10.1016/0042-6989(70)90123-9. [DOI] [PubMed] [Google Scholar]
- Kinnear PR, Sahraie A. New Farnsworth-Munsell 100 hue test norms of normal observers for each year of age 5–22 and for age decades 30–70. Br J Ophthalmol. 2002;86:1408–1411. doi: 10.1136/bjo.86.12.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer-Sousa R, Conway BR. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat Neurosci. 2013;16:1870–1878. doi: 10.1038/nn.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte F, Chain F, Aron D, Leblanc M, Souty O. Color vision disorders in posterior lesions of the brain a propos of 42 observations. Rev Neurol (Paris) 1969;121:5–29. [PubMed] [Google Scholar]
- Liu RR, Pancaroglu R, Hills CS, Duchaine B, Barton JJ. Voice Recognition in Face-Blind Patients. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows JC. Disturbed perception of colours associated with localized cerebral lesions. Brain. 1974;97:615–632. doi: 10.1093/brain/97.1.615. [DOI] [PubMed] [Google Scholar]
- Paulson HL, Galetta SL, Grossman M, Alavi A. Hemiachromatopsia of unilateral occipitotemporal infarcts. Am J Ophthalmol. 1994;118:518–523. doi: 10.1016/s0002-9394(14)75806-4. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Smith V, Pokorny J, Damasio AR. Color perception profiles in central achromatopsia. Neurology. 1993;43:995–1001. doi: 10.1212/wnl.43.5.995. [DOI] [PubMed] [Google Scholar]
- Rondot P, Tzavaras A, Garcin R. Sur un cas de prosopagnosie persistant depuis quinze ans. Revue Neurologique. 1967;117:424–428. [Google Scholar]
- Ruttiger L, Braun DI, Gegenfurtner KR, Petersen D, Schonle P, Sharpe LT. Selective color constancy deficits after circumscribed unilateral brain lesions. J Neurosci. 1999;19:3094–3106. doi: 10.1523/JNEUROSCI.19-08-03094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Watanabe E, Onodera Y, Uchida I, Kato H, Yamamoto E, Koizumi H, Miyashita Y. Functional mapping of the human colour centre with echo-planar magnetic resonance imaging. Proc Biol Sci. 1995;261:89–98. doi: 10.1098/rspb.1995.0121. [DOI] [PubMed] [Google Scholar]
- Scotti G, Spinnler H. Colour imperception in unilateral hemisphere-damaged patients. J Neurol Neurosurg Psychiatry. 1970;33:22–28. doi: 10.1136/jnnp.33.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short RA, Graff-Radford NR. Localization of hemiachromatopsia. Neurocase. 2001;7:331–337. doi: 10.1093/neucas/7.4.331. [DOI] [PubMed] [Google Scholar]
- Shuren JE, Brott TG, Schefft BK, Houston W. Preserved color imagery in an achromatopsic. Neuropsychologia. 1996;34:485–489. doi: 10.1016/0028-3932(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J, Pass AS. Color-axis determination on the Farnsworth-Munsell 100-hue test. Am J Ophthalmol. 1985;100:176–182. doi: 10.1016/s0002-9394(14)75002-0. [DOI] [PubMed] [Google Scholar]
- Song S, Garrido L, Nagy Z, Mohammadi S, Steel A, Driver J, Dolan RJ, Duchaine B, Furl N. Local but not long-range microstructural differences of the ventral temporal cortex in developmental prosopagnosia. Neuropsychologia. 2015;78:195–206. doi: 10.1016/j.neuropsychologia.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD. Evaluation of poor performance and asymmetry in the Farnsworth-Munsell 100-hue test. Invest Ophthalmol Vis Sci. 1988;29:476–481. [PubMed] [Google Scholar]
- Victor JD, Maiese K, Shapley R, Sidtis J, Gazzaniga MS. Acquired central dyschromatopsia: analysis of a case with preservation of color discrimination. Clin Vision Sci. 1989;4:183–196. [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zeki S. A century of cerebral achromatopsia. Brain. 1990;113(Pt 6):1721–1777. doi: 10.1093/brain/113.6.1721. [DOI] [PubMed] [Google Scholar]