Abstract
Concerns have been raised regarding the potential for endocrine disrupting compounds (EDCs) to alter brain development and behavior. Developmental exposure to bisphenol A (BPA), a ubiquitous EDC, has been linked to altered sociosexual and mood-related behaviors in various animal models and children but effects are inconsistent across laboratories and animal models creating confusion about potential risk in humans. Exposure to endocrine active diets, such as soy, which is rich in phytoestrogens, may contribute to this variability. Here, we tested the individual and combined effects of low dose oral BPA and soy diet or the individual isoflavone genistein (GEN; administered as the aglycone genistin (GIN)) on rat sociosexual behaviors with the hypothesis that soy would obfuscate any BPA-related effects. Social and activity levels were unchanged by developmental exposure to BPA but soy diet had sex specific effects including suppressed novelty preference, and open field exploration in females. The data presented here reinforce that environmental factors, including anthropogenic chemical exposure and hormone active diets, can shape complex behaviors and even reverse expected sex differences.
Key terms: endocrine disruption, neuroendocrine disruption, neuropeptides, social, diet, open field, affiliation, activity, environment
Introduction
Bisphenol A (BPA), a monomer used in epoxy sealants and plastics, has become ubiquitous in our environment and bodies, prompting concerns about its potential health impacts especially on neural development and behavior (Beronius et al., 2010; Chapin et al., 2008; FAO/WHO, 2011; FDA, 2012). Understanding how environmental factors, including endocrine disrupting chemicals (EDCs) such as BPA, contribute to impairments in affective and reciprocal social behaviors, are of considerable interest because they are features of psychosocial disorders for which incidence is rapidly rising, including autism spectrum disorders (ASDs) (Aguiar et al., 2010; Gore et al., 2014). Elevated gestational urinary BPA concentrations have been associated with adverse behavioral outcomes in children, including social deficits, hyperactivity, anxiety, and executive function deficits (Mustieles et al., 2015) suggesting the possibility that early life BPA exposure could impact behaviors related to emotionality. Numerous animal-based experiments have generated corroborating evidence for disruptions of sexual and mood-related behavior, most notably heightened anxiety, but supporting experimental evidence for impacts on social investigation and affiliation is extremely limited and highly discordant across animal models (Cox et al., 2010; Jasarevic et al., 2011; Sullivan et al., 2014; Wolstenholme et al., 2013). Diet is one factor thought to contribute to this variability, particularly hormonally active diets such as soy (Thigpen et al., 2013). Here we examined the impact of developmental, oral BPA exposure (at a dose considered human-relevant) alone and in combination with a soy rich diet or the individual soy phytoestrogen, genistein (GEN) on social investigation and exploratory behavior. A final group was exposed to BPA in peripubertal development and then placed on a soy diet at weaning to establish the impact of sequential exposure.
Soy contains numerous phytoestrogens, many of which are endocrine active but GEN is arguably the most well studied (Patisaul and Jefferson, 2010). That soy-based diets can confound EDC studies has been known for decades, but remains generally unrecognized by most researchers (Thigpen et al., 1999; Thigpen et al., 2013). The need to more comprehensively establish how hormonally active diets influence neural development and behavior (independently and in the presence of BPA and other ubiquitous anthropogenic EDCs) is pressing because of rapidly increasing soy consumption rates (Patisaul and Jefferson, 2010), particularly of soy-based infant formula.
The studies herein were undertaken to test the hypothesis that developmental, low dose BPA exposure has sex specific effects on social and exploratory behaviors, and that a soy rich diet or the isoflavone, GEN may modify these effects depending on timing of consumption. While the impacts of developmental BPA exposure on anxiety-related behaviors have been explored in some depth by us and others in a variety of species, impacts on social interactions have only been assessed in a handful of studies (with mixed results) and potential interactions with diet are virtually unknown. Thus the present studies fill a critical data gap and, importantly, incorporated research design recommendations for EDC studies on behavior and the sexual differentiation of the nervous system (Beronius et al., 2013; Li et al., 2008) including robust sample sizes (Chadman et al., 2009), controlling for possible litter effects, and minimizing exogenous EDC exposures which could confound study outcomes.
Materials and methods
Subjects
Animal Care and Use
Complete study design details are described in a prior study in which we used the same animals (Cao et al., 2015). Briefly, 54 pairs of Wistar rats were obtained and their offspring tested for these experiments. Rats were housed at the Biological Resource Facility of North Carolina State University (NCSU; 23°C, 50% average relative humidity and 12:12 h reverse light: dark cycle; lights off at 10:00), according to the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services Guide for the Care and use of Laboratory Animals. All aspects of this study were approved by the Institutional Animal Care and Use Committee of NCSU. As in our prior studies (Cao et al., 2012; Patisaul et al., 2009), and in accordance with recommended practices for EDC research (Beronius et al., 2013; Li et al., 2008), rats were housed in conditions specifically designed to minimize unintended EDC exposure including thoroughly washed polysulfone (BPA-free) caging with glass water bottles and wood chip (not corn cob) bedding.
Exposure
On gestational day 1 (GD 1), dams were randomly assigned to six exposure groups (n = 9 per group; Table 1): Soy-Free (soy-free diet; Teklad 2020), Soy (soy diet; custom soy diet, Harlan), BPA+Soy-Free (soy-free diet plus water containing BPA), BPA+Soy (soy diet plus water containing BPA), BPA/GIN+Soy-Free (soy-free diet plus water containing BPA and genistin (GIN) daily via food treat) and BPA/Soy-Free/Soy PND 21 (soy-free diet plus water containing BPA then pups switched to soy diet on PND 21). Exposure continued until weaning (postnatal day (PND) 21), with the exception of the group switched from soy-free (Soy-Free) to soy-based diet on PND 21 (BPA/Soy-Free/Soy PND 21). The soy diet was a custom diet (Harlan Laboratories, Madison, WI) with maximum similarity to Teklad 2020 with isoflavone levels ~400 mg/kg diet. Effects on growth, body weight, age at puberty and other developmental parameters for these animals are detailed in a prior publication (Cao et al., 2015).
Table 1.
Experimental Design and Groups
| Water (through PND 21) | GIN Through PND 21 | Soy-Free Diet | Soy Diet | ||||
|---|---|---|---|---|---|---|---|
| Exposure Group | Vehicle | BPA | Before PND 21 | After PND 21 | Before PND 21 | After PND 21 | |
| Soy-Free | + | − | − | + | + | − | − |
| Soy | + | − | − | − | − | + | + |
| BPA+Soy-Free | − | + | − | + | + | − | − |
| BPA+Soy | − | + | − | − | − | + | + |
| BPA/GIN+Soy-Free | − | + | + | + | + | − | − |
| BPA/Soy at PND 21 | − | + | − | + | − | − | + |
BPA (Sigma-Aldrich, St. Louis, MO) was administered via drinking water, as described previously (Cao et al., 2012), at 2 mg/L of water; to produce serum levels in the human range (<4 ng/ml serum) (Cao et al., 2015). GEN levels (dams and pups) in the soy-fed group were 3 – 31 ng/ml (Cao et al., 2015). Genistin (GIN; the glycosylated form of GEN found in food (Jefferson et al., 2009)) was administered (40 mg GIN (LC Laboratories, Woburn, MA)) on a peanut butter (Skippy Natural Creamy, Englewood Cliffs, NJ) covered Mini Nilla Wafer (Nabisco, East Hanover, NJ) (1 wafer/day/dam), daily through PND 21 (resulting serum GEN levels in the range of 18 – 48 ng/ml) (Cao et al., 2015). This range is approximately equivalent to vegetarians but below that of soy formula-fed infants (Patisaul and Jefferson, 2010). The detected range was wide but not atypical for a single measurement taken from animals given free access to the diet. This assessment was intended to be a survey of internal levels and not a formal pharmacokinetic-pharmacodynamic (PPK)-type analysis.
Behavior
Two tests were conducted: social choice (juveniles) and open field (adults). All testing was conducted within the first four hours of the dark cycle under red-light, video recorded, and scored using TopScan (Clever Sys Inc) software as we have done previously (Rebuli et al., 2015; Sullivan et al., 2014) using published testing methodologies (Crawley et al., 2007; Insel et al., 1999; Winslow and Insel, 2002). Test order was randomized among subjects and a randomly selected subset of videos was scored by hand by an independent observer to validate the computer scoring (Pearson Correlation r = 0.98). Juveniles (PND 24–28) were tested for 20 min in a three-chambered social choice apparatus (Sullivan et al., 2011). Familiar animals were same-sex sibling cage-mates and novel animals were age and sex-matched conspecifics. No more than two animals per sex per litter were used and sample size was within the range recommended for behavioral studies (Chadman et al., 2009). Final animal numbers were as follows: Females: Soy-Free n = 15, Soy n = 10, BPA + Soy-Free n = 15, BPA + Soy n = 16, BPA/GIN + Soy-Free n = 11, BPA+Soy-Free/Soy PND 21 n = 12. Males: Soy-Free n = 9, Soy n = 16, BPA + Soy-Free n = 12, BPA + Soy n = 19, BPA/GIN + Soy-Free n = 11, BPA+Soy-Free/Soy PND 21 n = 13.
Beginning on PND 60, the rats were then subjected to a standard 20 minute open field (OF) test (Sullivan et al., 2014). Adult females were tested in estrus (assessed by vaginal cytology (Becker et al., 2005)) by PND 110. Distance traveled, entries and time in the center, edges and corners were quantified using TopScan and validated by an independent observer (Pearson Correlation r = 0.98). No more than two animals per sex per litter were used. Final animal numbers were as follows: Females: Soy-Free n = 17, Soy n = 16, BPA + Soy-Free n = 17, BPA + Soy n = 15, BPA/GIN + Soy-Free n = 13, BPA+Soy-Free/Soy PND 21 n = 15. Males: Soy-Free n = 16, Soy n = 19, BPA + Soy-Free n = 15, BPA + Soy n = 15, BPA/GIN + Soy-Free n = 12, BPA+Soy-Free/Soy PND 21 n = 14.
Statistical Analysis
To probe for baseline behavioral sex differences, t-tests were used to compare males and females within the Soy-Free and Soy groups (not exposed to BPA). For each task, evidence of expected sex differences (as discerned from prior, published literature) in the soy-free group was considered validation that the testing protocol was successful. If sex differences were not found in both groups, diet was interpreted to have played an intervening role. Effect size was estimated using Cohen’s d.
For all endpoints, the four core exposure groups (Soy-Free, Soy, BPA+Soy-Free, and BPA+Soy) were then analyzed (Sigmaplot 13) by a three-way ANOVA with exposure, diet and sex as factors as we did for our prior study examining different endpoints in the same animals (Cao et al., 2015). If any main effects or interactions were significant (P ≤ 0.05) they were followed up with a protected Fisher’s least significant differences (PLSD) post-hoc test. ANOVA effect size was calculated by Eta squared (η2). PLSD effect size was calculated using Cohen’s d.
The BPA/GIN+Soy-Free group and BPA/Soy-Free/Soy PND 21 group were included in the experiment only to test specific hypotheses related to the capacity of soy phytoestrogens to interact with BPA, and thus not included in the main statistical analysis. Instead, two-way ANOVA was used to compare the BPA+Soy-Free group to the BPA/GIN+Soy-Free group (sex and GIN as factors) and the BPA+Soy-Free group to the BPA+Soy-Free/Soy PND 21 group (sex and Soy as factors). If main effects or interactions were significant (P ≤ 0.05) then a PLSD post-hoc test was performed to evaluate pair-wise differences. Effect size was estimated using η2 or Cohen’s d as appropriate. Results were graphed using Prism 6. As per Cohen’s guidelines, effects for η2 were considered small at 0.01, medium at 0.06 and large at 0.14, effects for Cohen’s d were considered small at 0.2, medium at 0.5 and large at 0.81 (Cohen, 1988; Lakens, 2013).
Results
Juvenile Novel Social Task
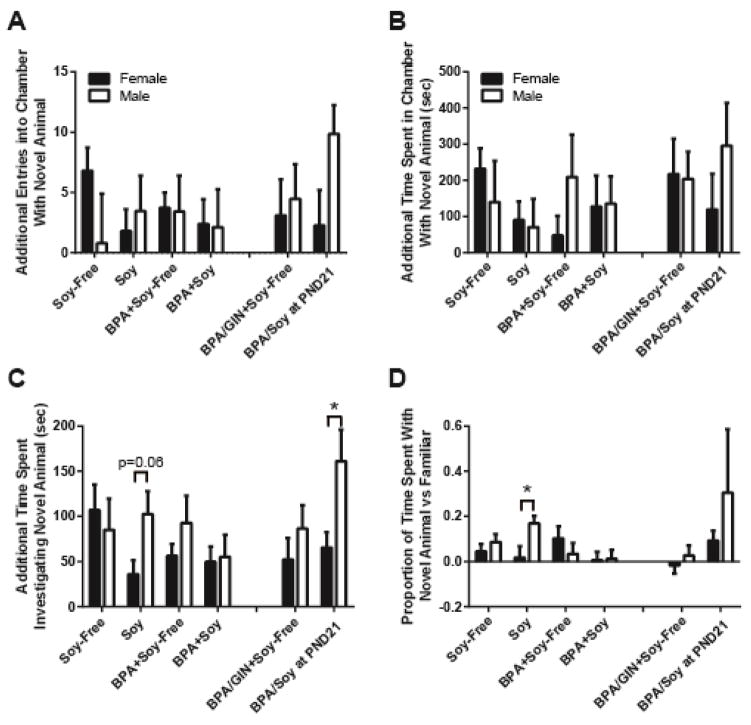
Because rats of both sexes prefer and spend more time with the novel animal in the novel social task, for each subject the difference between time spent with the novel animal versus the familiar was calculated to quantify the strength of novelty preference, and those differences were used for the statistical analysis and depicted in the graphs (Figure 1). No main effects of sex, diet or BPA exposure were found for entries made into the novel animal chamber, time spent in the chamber with the novel animal, time spent investigating (in contact with) the novel animal, or the overall proportion of time spent in contact with the novel animal versus the familiar animal (Figure 1). Behavioral sex differences were only observed in the Soy group with males spending a greater proportion of time with the novel animal versus the familiar (t = 2.42; P ≤ 0.02; d = 1.03). There was also evidence for increased time investigating the novel animal (t = 1.95; P = 0.06; d = 0.85) but although effect size fit Cohen’s classification of “large,” it did not reach statistical significance (Figure 1C and D). GIN had no effect on social behavior in either sex and although males reared on BPA and a Soy-Free diet and then switched to a soy diet at weaning (BPA/Soy-Free + Soy PND21) appeared to spend a greater proportion of time with the novel animal versus the familiar (Figure 1D), than the animals reared on Soy-Free and BPA (diet not switched), inter-individual variability in this trait was too high to reach statistical significance. Time spent investigating the novel animal, however, was sexually dimorphic in the group switched to soy at PND 21 (t = 2.08; P ≤ 0.05; d = 0.91; Figure 1C).
Figure 1.
Novel social behavior as juveniles. BPA had no effect on social interactions with a novel conspecific including exploration of the chamber (A, B) or contact with the conspecific (C, D). Diet impacted the capacity to detect sex differences and there was some evidence that soy diet (C, D) including the introduction of soy diet at weaning (C) heightened male sociality. Data are shown as the mean ± SE. Significant difference from control indicated by *P ≤ 0.05.
Adult Open Field (OF)
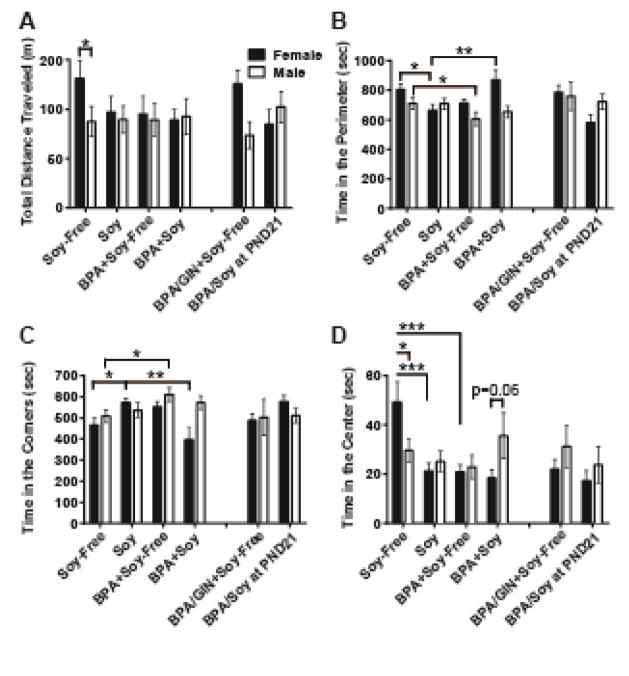
Total distance traveled (t = 2.01; P ≤ 0.05; d = 0.64), and time spent in the center chamber (t = 2.06; P ≤ 0.05; d = 0.52) were sexually dimorphic in the Soy-Free group (Figure 2A and D) as expected based on a large prior literature (ex. Masur et al., 1980; Slob et al., 1986; Valles, 1976), but not the Soy group, with females more active and less anxious than males. Given those sex differences, plus a main effect of sex for time in the perimeter (F(1,129) = 10.65; P ≤ 0.009; η2 =0.07), all OF data were further analyzed within sex. Within females, there was an exposure by diet interaction (F(1,64) = 8.41, P ≤ 0.006; η2 = 0.04) with BPA having opposite effects on time in the perimeter (Figure 2B) depending on diet (increased on soy diet, decreased on Soy-Free). Within males, there was a main effect of BPA exposure (F(1,63) = 4.78; P ≤ 0.03; η2 =0.02), with decreased time in the perimeter reaching significance in the Soy-Free (P ≤ 0.05; d = 1.04), but not the Soy, group. Outcomes on time spent in the corners (Figure 2C) were similarly complex. Again, because a main effect of sex was identified (F(1,129) = 7.08; P ≤ 0.009; η2 =0.05), the data were analyzed within sex. Within females, there was a significant interaction between diet and exposure (F(1,64) = 10.39; P ≤ 0.002; η2 =0.14) with BPA increasing time in the corners only within the soy-reared group (P ≤ 0.002; d = 1.64). Among unexposed females, soy reared females spent more time in the corners (P ≤ 0.05; d = 1.02) while within BPA exposed females, soy-fed females spent less time in the corners (P ≤ 0.02; d = 1.21). In males, there was a main effect of BPA exposure on time spent in the corners (F(1,64) = 4.18; P ≤ 0.04; η2 = 0.06), an effect almost entirely attributable to the Soy-Free group (P ≤ 0.05; d = 1.05). Center activity was also influenced by sex and diet. There were no main effects in males. Among females, there were main effects of diet (F(1,64) = 8.71; P ≤ 0.004; η2 =0.14) and exposure (F(1,64) = 9.32; P ≤ 0.003; η2 =0.10) as well as a significant interaction between the two (F(1,64) = 6.23; P ≤ 0.02; η2 =0.09) on time in the center. Both BPA and soy significantly reduced time in the center, but the effect of BPA was only observed in females on the Soy-Free diet (P ≤ 0.001; d = 1.38). Within the Soy-Free group, females spent more time in the center (t = 2.16; P ≤ 0.05; d = 0.89) and this sex difference was either lost or trended towards reversal in the other groups, particularly the BPA+Soy group (P = 0.06; d = 0.68). Compared to the BPA+Soy-Free group, time in the center was not affected by GIN or the switch to a soy diet on PND 21 in either sex. For each region, number of entries was not affected by sex, diet or BPA (data not shown).
Figure 2.
Open field performance as adults. As anticipated, total distance traveled and time in the center was sexually dimorphic (A, D). Neither diet nor BPA affected overall activity (A). Impacts of BPA on exploration were diet and sex specific and generally indicative of heightened anxiety. In Soy-Free fed females, time in the center was decreased by BPA (D). Soy was also decreased time in the center and BPA did not modify this effect (D). Accordingly, as time in the center decreased, time in the perimeter (B) and/or corners (C) was increased. Data are shown as the mean ± SE. Significant difference from control indicated by *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Discussion
Consistent with prior studies by us and others, these experiments provided confirmatory evidence that developmental BPA exposure is anxiogenic. As we have reported previously (Patisaul et al., 2012), effects were sex specific, modest, and obfuscated by hormonally active diet. By contrast, we found no compelling evidence that BPA impacts social interactions. Juvenile sociality was not affected by BPA in either sex but soy diet influenced some parameters, including potential sex differences for some measures. In females, soy diet also suppressed novelty preference, and exploration of the OF, results which collectively suggest heightened anxiety and a tentativeness to explore novel individuals and spaces. Males placed on a soy-rich diet at weaning had enhanced novelty preference to some degree, an outcome which supports the hypothesis that the peripubertal period could be a sensitive window for hormonal influences on sociality in males. The results presented here emphasize that hormonally active diets, such as soy, can be endocrine disrupting, even more so than anthropogenic compounds like BPA, and emphasize the importance of considering diet when assessing environmental or other impacts on sexually dimorphic, hormone-sensitive behaviors.
As expected in the juvenile novel social task, both sexes showed heightened investigation of novel animals compared to familiar cage-mates (Crawley et al., 2007; Gabor et al., 2012), but sex differences in time spent investigating the novel animal and the proportion of time in contact with the novel animal was only detected in the soy-reared animals. Compared to the Soy-Free animals, this resulted from a combination of decreased novelty preference in females and slightly elevated novelty preference in males. A sex difference in investigation time was also apparent in the animals switched to soy at PND 21 suggesting that hormonally active diets can also impact behavior during adolescence. This result is confounded by the fact that the animals were also given BPA, but concern about the potential impact of BPA is minimal because there is no evidence that BPA affected any aspect of sociality in this experiment. Although concerns have been raised that BPA and other EDCs may contribute to disorders of social behavior (de Cock et al., 2012; Grandjean and Landrigan, 2014; Kalkbrenner et al., 2014), the experimental and epidemiological literature is sparse, and discordant (Mustieles et al., 2015; Wolstenholme et al., 2013). For example, a study in deer mice reported limited evidence of demasculinization of some social behaviors by dietary BPA, such as mate choice (Jasarevic et al., 2011), and Wolstenholme et al. reported evidence of transgenerational effects on sociability in mice, but the direction of the effect differed across generations, making the overall outcome difficult to interpret. A few studies have found no main effects of developmental BPA exposure on social interactions but an abrogation of expected sex differences (Cox et al., 2010; Kundakovic et al., 2013). We observed some evidence for effects in the prairie vole (Microtus ochrogaster), a species which naturally displays more “human typical” social behaviors than rats including partner preference, paternal care, and pair boding, including the loss of sex differences in social investigation (Sullivan et al., 2014). Collectively these available data suggest that any effect of BPA on social behavior is small and thus unlikely to be meaningfully contributory to human disorders of social interaction, such as ASD. Going forward, animal models which are naturally more prosocial may be more utilitarian for examining the impacts of other EDCs and toxicants on social behaviors.
In the OF, expected sex differences were observed in the Soy-Free controls including greater OF exploration by females and time in the center (indicative of lower anxiety) (Frye et al., 2000; Gould, 2010; Jefferson et al., 2009; Valles, 1976). These sex differences were not observed in the soy-fed animals, and, in the BPA+Soy group, reversed for time in the center. These observations corroborate prior work by us and others showing that hormonally active diets can obfuscate sexually dimorphic behaviors and other neuroendocrine traits (Cao et al., 2012; Patisaul and Jefferson, 2010; Thigpen et al., 2013). Loss of sex differences by soy diet was primarily attributable to behavioral changes in females (Figure 2A and D). Soy reduced female exploration of the OF, and exploration shifted from the center and perimeter to the corners, a pattern indicative of higher anxiety. BPA had minimal impact on males, with only time in the perimeter slightly reduced, but measurable anxiogenic effects on females regardless of diet.
Published data regarding BPA-related effects in rodents generally suggests heightened anxiogenic activity (Wolstenholme et al., 2011), a factor which contributed to the conclusion by the National Toxicology Program and the World Health Organization that there is some concern for BPA-related effects on brain and behavior, particularly anxiety (Beronius et al., 2010; Chapin et al., 2008; FAO/WHO, 2011; FDA, 2012). Sex-specific effects are typical but inconsistent. For example, two mouse studies published since the WHO assessment reported evidence of increased anxiety in juvenile C57BL/6J males, but not in females (Cox et al., 2010; Matsuda et al., 2012), while another reported the opposite in CD1 mice (Gioiosa et al., 2013). Using an experimental design similar to the one used for the present studies, we previously reported heightened anxiety in BPA-exposed juvenile Wistar rats (Cao et al., 2012) using the elevated plus maze and the light/dark box. This effect was not sex-specific and abrogated by soy diet. Here, we did not observe a protective effect of soy-rich diet. This difference between study findings could be due to several factors including time of testing (juvenile versus adult), dietary composition (the prior study used a standard commercial diet and this one used a custom diet where isoflavone levels were controlled) and behavioral assays employed. Collectively these data provide yet further evidence supporting the concern that developmental BPA exposure can heighten anxiety, and also emphasize that species, strain, and diet are all factors which likely contribute to outcome variability in the literature. Finally, although BPA is associated with hyperactivity in humans and some animal models (Anderson et al., 2013; Kinch et al., 2015; Mustieles et al., 2015; Sullivan et al., 2014; Wolstenholme et al., 2011), we observed no supporting evidence of that here.
Conclusions
Consistent with what has been previously reported in multiple animal models and humans, here we show that developmental exposure to BPA and soy can affect anxiety-related behaviors and that there may be sexually dimorphic aspects to vulnerability. Confirmatory information is critically important when considering the potential health impacts of BPA, and these studies enhance available evidence for behavioral impacts in that regard. Social and activity levels were unchanged by developmental exposure to BPA, a result which was not entirely unexpected based on the discordance of the available literature and the known modes of action for BPA. Social investigation and recognition is not widely reported as being sexually dimorphic in rats, and we did not find strong evidence for that here either. As we have observed previously (Patisaul et al., 2012), soy diet can influence the ability to detect behavioral sex differences and obfuscate BPA-related behavioral outcomes, emphasizing the importance of considering diet when assessing hormone-sensitive outcomes in neuroendocrine systems. The data presented here reinforce the long-recognized concept that environmental factors, including anthropogenic chemical exposure and hormone active diets, can shape complex behaviors and potentially eliminate or reverse expected sex differences.
Highlights.
Environmental chemicals can affect brain development and behavior.
Hormonally active diets may also affect behavior and sexual dimorphisms.
Here we tested the individual and interactive effects BPA, soy diet and genistein.
Behaviors examined included juvenile sociality and open field behavior.
Soy diet but not BPA had sex specific effects.
Impacted behaviors included suppressed novelty preference, and open field exploration in females.
These data reinforce the importance of considering environmental factors when examining brain and behavior.
Acknowledgments
These experiments constitute the undergraduate research project of K.D.H and the authors gratefully acknowledge the support of the NCSU undergraduate research program. This work was also supported by NIEHS R21ES021233. We also thank Brian Horman and Emily Cox for their assistance with various aspects of data collection and analysis. We also appreciate Kylie Rock for assisting with the Cleversys analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar A, Eubig PA, Schantz SL. Attention deficit/hyperactivity disorder: a focused overview for children’s environmental health researchers. Environ Health Perspect. 2010;118:1646–1653. doi: 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 2013;27:1784–1792. doi: 10.1096/fj.12-223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Beronius A, Johansson N, Ruden C, Hanberg A. The influence of study design and sex-differences on results from developmental neurotoxicity studies of bisphenol A, implications for toxicity testing. Toxicology. 2013 doi: 10.1016/j.tox.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Beronius A, Ruden C, Hakansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A. Reprod Toxicol. 2010;29:132–146. doi: 10.1016/j.reprotox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cao J, Echelberger R, Liu M, Sluzas E, McCaffrey K, Buckley B, Patisaul HB. Soy but Not Bisphenol A (BPA) or the Phytoestrogen Genistin Alters Developmental Weight Gain and Food Intake in Pregnant Rats and Their Offspring. Reprod Toxicol. 2015 doi: 10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. L. Erlbaum Associates; Hillsdale, N.J: 1988. [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Cock M, Maas YG, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review Acta Paediatr. 2012;101:811–818. doi: 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. World Health Organization; 2011. [Google Scholar]
- FDA; Administration, F.a.D, editor Bisphenol A (BPA): use in food contact application. 2012. [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral neuroscience. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm Behav. 2013;63:598–605. doi: 10.1016/j.yhbeh.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35:961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TDD, DT, Kovacsics CE. The Open Field Test. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice. Humana Press; 2010. [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet neurology. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biological psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A. 2011;108:11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–1889. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current problems in pediatric and adolescent health care. 2014;44:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–519. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Masur J, Schutz MT, Boerngen R. Gender differences in open-field behavior as a function of age. Dev Psychobiol. 1980;13:107–110. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:273–279. doi: 10.1016/j.pnpbp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Mustieles V, Perez-Lobato R, Olea N, Fernandez MF. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, Gore AC. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30:350–357. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor D, Patisaul HB. Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on Juvenile and Adult Rat Exploratory and Anxiety Behavior: A CLARITY-BPA Consortium Study. Toxicol Sci. 2015 doi: 10.1093/toxsci/kfv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, Huizer T, Van der Werff ten Bosch JJ. Ontogeny of sex differences in open-field ambulation in the rat. Physiol Behav. 1986;37:313–315. doi: 10.1016/0031-9384(86)90239-8. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster) Endocrinology. 2014;155:3867–3881. doi: 10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AW, Hamilton P, Patisaul HB. Neonatal agonism of ERbeta impairs male reproductive behavior and attractiveness. Horm Behav. 2011;60:185–194. doi: 10.1016/j.yhbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Environmental Health Perspectives. 1999;107:A182–183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Kissling GE, Locklear J, Caviness GF, Whiteside T, Belcher SM, Brown NM, Collins BJ, Lih FB, Tomer KB, Padilla-Banks E, Camacho L, Adsit FG, Grant M. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. Journal of the American Association for Laboratory Animal Science : JAALAS. 2013;52:130–141. [PMC free article] [PubMed] [Google Scholar]
- Valles FP. Age factors in sex differences in open-field activity of rats. Animal Learning and Behavior. 1976;4:457–460. [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]