Abstract
Redox biology has evolved from studies of the pathology that involves oxidants to an understanding of how our oxidants participate in normal as well as aberrant signal transduction. Although the concept that signal transduction involved changes in the redox state dates from the 1930s, the modern history of redox biology began with the discovery of superoxide dismutase by McCord and Fridovich. The initial focus was on free radicals and damage of macromolecules, which remains an important topic. But, over time it was realized that hydroperoxides, especially H2O2 produced by NADPH oxidases, and electrophiles derived from lipid peroxidation or metabolism, played essential roles in physiologically relevant signaling. The mechanisms through which H2O2 and other electrophiles signal became an important area of study that provided insight into how these reactive molecules were involved in major signaling pathways and regulation of transcription factors. Thus, the field of redox signaling that is the overlap of signal transduction with redox biology was established. Alterations in redox signaling are observed in aging, but we also now know that redox signaling is essential in physiological homeostasis and that sustained deviation from redox homeostasis results in disease. This is a review of the history of redox biology from a personal perspective of nearly fifty years working in this field that hopefully provides some insights for the reader.
Keywords: oxidative stress: redox signaling, superoxide, hydrogen peroxide, NADPH oxidase, AP-1, Nrf2, aging, homeostasis, antioxidant, air pollution, thiolate, glutathione
Introduction
From the time of Joseph Priestly and the other co-discovers of oxygen in the eighteenth century, the potential toxicity of this essential gas has been known. But, understanding of the mechanism did not advance significantly until the 1950s when Rebecca Gerschman and coworkers noted that oxygen poisoning resembled the damage from X-rays and proposed that free radicals were involved [1]. Shortly after, Denham Harman proposed that aging could also involve free radicals based upon radiation chemistry [2]. The modern history of redox biology however, began with the discovery by Joe McCord and Irwin Fridovich of superoxide dismutase [3].
I was fortunate to become a postdoctoral fellow in the Fridovich laboratory at a time when the field, then known as free radical biology, was just beginning to expand. It is a very good thing to be in the right place at the right time. Is also good to be inspired by brilliant minds. My graduate mentor, Philip Feigelson, my postdoctoral mentor, Irwin Fridovich, and the person who gave me the opportunity to begin my independent career, Aron Fisher, are the scientists who most influenced my career.
Albert Szent-Gyorgyi, the Nobel Prize winning discover of vitamin C [4], proposed the involvement of free radicals in biological systems well before the discovery of superoxide dismutase [5]. Szent-Gyorgyi often quoted the statement, attributed to both Schopenhauer and Johnathan Swift in various forms, that, “Discovery consists of seeing what everybody has seen and thinking what nobody has thought.” That this statement itself evolved provides an example of what underlies most of the breakthroughs in science. We all depend upon what came before, and make advances by seeing things in different ways with the assistance of technologies that our predecessors did not have. Likewise, my contributions have depended on the work of many others in the field of redox biology, some of whom I have had the privilege to work with directly. They are listed at the end of this review. The lesson learned from the words of Szent-Gyorgyi that influenced my career is to keep your eyes and mind open.
Superoxide as a product or intermediate in enzymatic reactions – free radical biology begins Irwin Fridovich suggested that xanthine oxidase produced superoxide as an intermediate in the oxidation of sulfite [6], eight years before he and his student, Joe McCord discovered superoxide dismutase (SOD) [3]. In 1968, as a graduate student investigating the catalytic mechanism of tryptophan oxygenase with Philip Feigelson at Columbia University, we proposed that the enzyme formed a ferric heme-superoxide intermediate that did not release superoxide from the enzyme [7]. This began my interest in free radicals and I fell under the influence the old work of Szent-Gyorgyi. About that time, Frank Brady, who had done his graduate work at Duke University with Philip Handler and K.V. Rajagopalan in the laboratory next to the Fridovich lab, joined the Feigelson laboratory. Frank introduced us to the concept of using superoxide dismutase as a tool to study biological oxidations [8]. He also introduced me to Irwin Fridovich. Frank passed away at a relatively early age, but he had a great positive and lasting influence on those who knew him.
The Spring of 1968, my second semester in graduate school, was tumultuous. Early in the semester, a riot broke out and the police occupied the Columbia University campus where we attended most of our classes. In April, Martin Luther King was assassinated and riots broke out in several cities. During the summer of 1968, Robert Kennedy was assassinated. Not long after, a riot broke out outside the Democratic convention in Chicago. For a liberal New Yorker, it was a time that becoming radical could have happened easily. Fortunately, free radical biology was the clear choice over radical politics.
Superoxide dismutase
In 1969, Joe McCord and Irwin Fridovich published their classic paper on the discovery that erythrocuprein had SOD activity [3]. This discovery and the burst of publications from the Fridovich laboratory over the next few years (summarized in [9][10], provided a foundation for the field. A postdoctoral fellowship from the National Institutes of Health allowed me to work with Irwin and resulted in four publications [11][12][13][14]. These papers included determining the active site histidines in SOD1, the rate constants for different SODs, and the role of zinc in SOD 1. The findings regarding zinc in structural stability of SOD1 were cited fourteen years later in a ground-breaking paper suggesting a role for abnormal zinc binding in SOD1 that causes the enzyme to gain a nitration catalysis function and thereby participate in the pathology of amyotrophic lateral sclerosis [15]. The environment in the Fridovich laboratory was wonderful. It taught me that one should always try to take advantage of being in a place where new development is occurring.
Mitochondrial superoxide production
The budget consequences of the Vietnam War had a devastating economic impact on science. Under the circumstances, the best position offered was a Research Associate position in Kansas City. Without dwelling on how, at the age of twenty-six I nearly left academia, thanks to James Kennedy a position at the Veterans Administration Hospital, which led to a Research Assistant Professor appointment at the University of Kansas Medical Center developed. While at the VA, I began work with James on pyrimidine biosynthesis, which unexpectedly led to one of my most fortunate and important findings.
In the early 1970s, Alberto Boveris, Nozumo Oshino, and Britton Chance described the production of H2O2 by mitochondria [16]. In 1974, Loschen, Azzi, Richter and Flohe, discovered that succinate oxidation in in mitochondria produced O2·− and suggested that it was the source of the H2O2 [17]. At almost the same time, we discovered that the oxidation of dihydroorotate, which is the one step in pyrimidine biosynthesis that occurs in mitochondria, also produced O2·− [18]. But, the more important discovery in that study was that the reaction producing O2·− was thermodynamically unfavorable and actually pulled forward by SOD. We did not know what the exact reaction was that produced O2·−, but soon after a debate began about cytochrome b versus ubiquinone as the source. Work by Enrique Cadenas and Alberto Boveris [19] strongly indicated that ubisemiquinone oxidation was the source of O2·−. Christine Winterbourn showed that the reaction of semiquinones with oxygen to produce O2·− was thermodynamically unfavorable and that SOD could pull the reaction forward [20]. This work explained our previous finding that SOD promoted the production of O2·− in which is displaced to the right by SOD in the reaction . As later pointed out by Forman and Azzi, this results in mitochondrial O2·− concentration being negligible [21]. Only a reaction with a rate constant competitive with the near diffusion limited rate of SOD, such as the reaction of ·NO with O2·− to produce peroxynitrite [22], would be likely to involve O2·− directly.
Although work on mitochondrial H2O2 and O2·− production continued, and has had a resurgence in the last few years [23], my work in that area ended when the Biochemistry Study Section of the National Institutes of Health told me that, “Superoxide production by mitochondria is an uninteresting artefact.” Moving on, I always have wondered whether my project would have been funded if the “artefact” was more interesting to those reviewers.
Pulmonary oxygen and paraquat toxicity
As mentioned at the beginning of this article, the toxicity of oxygen has been known since its discovery when Lavosier noted in 1783 that mice exposed to pure oxygen had damage to their lungs [24]. Probably the clearest example in terms of human experience is the toxicity of oxygen therapy for premature infants, which notably can cause lifelong consequences for lung function and blindness. But, for a century and a half, the underlying cause of oxygen toxicity remained unknown. While free radicals were proposed by Gerschman based on the resemblance to injury from X-rays [1], there was little direct evidence. Interest in the field increased during World War II when it was noticed that divers breathing pure oxygen had seizures. In 1945, Haugaard and coworkers however, demonstrated the loss of activity of enzymes after hyperbaric oxygen exposure [25].
Rats exposed to 100% oxygen at 1 atmosphere die within 3 days. Rats exposed for 5 days to 80% oxygen adapt and become able to survive indefinitely in 100% oxygen. In the 1970s, Crapo and Tierney [26], using this model of adaptation to hyperoxia, demonstrated that increased SOD activity in the lung correlated with adaptation to hyperoxia. About the same time, studies were being made of the toxicity of the herbicide, paraquat, which caused lethal lung damage through generation of O2·− continuously [27]. In 1978, I was recruited to the University of Pennsylvania by Aron Fisher, who introduced me to pulmonary physiology and toxicology. Investigating how different cells in the lungs of adapting rats changed in the activities of SOD1 and SOD2 along with other enzymes that contributed to antioxidant defense, we found that the cells that appeared to be most responsive in terms of increasing these activities were the granular type II epithelial cells [28]. With Tom Aldrich, we also explored how paraquat accumulates specifically into those cells making type II epithelial cells the target of the toxicity [29]. It was interesting to observe that the cells that adapted best to hyperoxia were the specific target of paraquat.
Phagocytic superoxide production
The signaling pathway and function we chose to focus on was the respiratory burst of alveolar macrophages. Bernie Babior discovered in 1973 that phagocytes generated O2·− [30]. We began our studies looking at how paraquat might cause loss of the ability of macrophages to produce O2·− when stimulated. Through serendipity and the careful notebook keeping of June Nelson, we found that in the absence of glucose, paraquat depleted the substrate, NADPH, for the phagocyte oxidase and only later became toxic to the cells in the presence of glucose [31]. But, paraquat did not deplete NADH. Babior learned about our results and said that there was an ongoing debate about whether NADPH or NADH was used by the phagocyte oxidase and that our work on toxicity contributed to resolution of this debate in favor of NADPH. Sometimes one gets very lucky.
In the 1980s free radical biological research was largely focused on mechanisms of cell injury and death. When I moved into my first fully independent position, we laboratory began to study the mechanisms whereby oxidants cause inhibition of signaling, as we observed changes in macrophage functions in exposure to hyperoxia and low exogenous concentrations of hydroperoxides both in vitro and in vivo without cell death [32,33]. This was important because we get sick more often than we die! Furthermore, if one is going to use in vitro models, it is essential needed to avoid using “the thermonuclear attack model of toxicology” in which cells die from doses of added hydroperoxides that has no resemblance to actual physiological or even pathological conditions.
Glutathione biosynthesis
Glutathione (GSH), γ-L-glutamyl-L-cysteinylglycine, is the reducing substrate for the glutathione peroxidases and peroxiredoxin 6 in removal of hydroperoxides, and for the glutathione S-transferases that help remove toxicants as well as participate in leukotriene C4 synthesis. GSH is synthesized and degraded in a cycle that was described by Alton Meister [34]. Helmut Sies, who coined the term oxidative stress [35], suggested that GSH oxidation to the disulfide (GSSG) and formation of mixed protein-glutathione disulfides was the essential determinant of that stress. Research in our laboratory on altered cell function led us into studies of glutathione redox cycling, primarily by Mark Sutherland, that were cited recently [36] in an issue of Archives of Biochemistry and Biophysics dedicated to Helmut Sies upon his retirement as Editor in Chief of the journal.
An increase in total GSH plus GSSG (GSH is almost all in the GSH form in unstressed cells) occurred in many situations where cells or animals were exposed to sublethal oxidative stress. We decided to pursue the question of how this occurred. Using redox cycling quinones, menadione that could be conjugated to GSH and another, 1,2-dimethoxy-naphthoquinone that could only redox cycle, Ming Shi, Li Tian, Rui-Ming, and Amir Kugelman demonstrated that both catalytic and modulatory subunits of the first enzyme in de novo GSH biosynthesis, glutamate cysteine ligase (GCL, also known as γ-glutamylcysteine synthetase) could be transcriptionally induced by mild oxidative stress [37–39]. We also showed that γ-glutamyltranspeptidase, which is involved in GSH recycling was also transcriptionally inducible by quinones [40].
The studies of GSH biosynthesis began at the Institute for Toxicology at the University of Southern California. Paul Hochstein, Alex Sevanian, Kelvin Davies, Enrique Cadenas, Joseph Landolph and others in this group that focused on free radical biology provided an outstanding environment for our work. I also was fortunate to become the Head of the Cell Biology Group at Children’s Hospital of Los Angeles, where we collaborated with Thomas Coates and Martine Torres among others. The latter became my main collaborator for many years until her retirement. Martine, an expert in signal transduction, influenced much of my thinking on the roles of oxidants in signaling. Retaining relationships over decades has been of great value to my career. With Tom Coates, we are currently looking at how sickle cell disease alters redox homeostasis in blood, and as described below, we currently collaborate with Kelvin Davies on studies of aging.
Redox signaling
In the 1930s Warburg and Szent-Gyorgyi proposed that redox reactions regulate cell function, but tools for studying this were absent as was the understanding that O2·− and H2O2 were generated in cells. In the 1970s a few studies showed that H2O2 could mimic insulin [41,42], but the mechanisms were unknown. Beginning in the late 1980s studies showed that exogenous H2O2 could modulate multiple signaling pathways, but specific targets were not clear with a few notable exceptions. Gopalakrishna and Anderson showed that classical protein kinase C activity could be elevated by H2O2 in the absence of calcium [43], while Lands and others showed that a lag in the activation of cyclooxygenase [44] and 5-lipoxygenase [45] was abolished by low concentrations of H2O2. Lands coined the term “peroxide tone” and suggested that the production of eicosanoids was regulated by the endogenous level of H2O2 in cells.
But, almost all the early work on the activation of cell signaling by H2O2 was done through addition of H2O2 to cells. This included our own work on the respiratory burst of alveolar macrophages [46]. In those studies, we had switched from looking at how hydroperoxides inhibited the respiratory burst without killing the cells to investigating the mechanism underlying an serendipitous discovery that low concentrations of hydroperoxides increased subsequent stimulation of the respiratory burst [47], which we eventually found was due to an elevation of intracellular calcium [48] and activation of phosphatidylcholine specific phospholipase C (PC-PLC) [49]. This work was carried out by two graduate students, Judith Murphy and Carolyn Hoyal, and Julio Giron-Calle, a postdoc.
One of the most interesting and influential studies of signaling caused by the addition of exogenous H2O2 was the activation of the NF-κB transcription factor [50]. Realizing that our experimental model cells, macrophages, generated H2O2 upon stimulation, my colleague Martine Torres and I wondered if the amount of H2O2 generated would be sufficient to activate NF-κB in these cells. It was [51]. Working in collaboration with Rayadu Gopalakhrisna, Nalini Kaul from our laboratory found that the activation of NF-κB by endogenously produced H2O2 was be due to the activation of protein kinase C [52]. We also showed that increased tyrosine phosphorylation of many proteins and activation of the ERK pathway by stimulants of the respiratory burst in macrophages was H2O2 dependent [53]. At the time we discovered this, it was widely believed that the only cells that produced H2O2 in response to stimulation were phagocytes. Thus, despite our being able to publish this work and even get funded by the NIH to study it, the activation of signaling by endogenously generated H2O2 was considered an oddity of macrophages and other phagocytes. That changed dramatically in 1999 when David Lambeth and coworkers discovered that other cells had a related NADPH oxidase [54] and then that there were seven mammalian NADPH oxidases labeled as NOXs and DuOXs [55] distributed in every cell type. With that discovery, the field of redox signaling now clearly had identified a major source in all cell types of H2O2 produced upon stimulation. While mitochondria, other organelles and some oxidoreductases generate H2O2, it remains to be determined whether anything other than the NOX and DuOX proteins are truly involved in stimulated signaling. The work on redox signaling resulted in one of the first reviews [56] and two edited books on the topic [57,58]. Going back to the suggestion of Lands regarding peroxide tone [59], it seems the lipoxygenases and cyclooxygenases have the best chance of fitting that description by providing lipid hydroperoxides in response to stimulation. That hydroperoxides rather than other so-called reactive oxygen species are the mediators of signaling has been addressed in detail in recent reviews [60,61].
In 1999, we moved to the University of Alabama at Birmingham where I became the Chair of the Department of Environmental Health Sciences and joined the Center for Free Radical Biology headed by Bruce Freeman and Victor Darley-Usmar. It was fortunate that almost all of the graduate students and postdocs in my laboratory at USC moved to UAB. Both USC and UAB were great environments in which we interacted with some of the best investigators in the free radical biology field.
At UAB, we continued to investigate how H2O2 generated by cells acts as a signaling molecule, particularly on the activation of the AP-1 transcription factor [62]. Ichijo and colleagues demonstrated that oxidation of thioredoxin (Trx) by addition of H2O2 to cells activated ASK1, a kinase that is upstream of both p38MAPK and JNK [63]. Honglei Liu, a postdoc in our laboratory, showed that the activation of H2O2 production in macrophages led to activation of the JNK pathway through the Trx mediated activation of ASK1 [64]. Subsequently, Im and coworkers showed that the oxidation of Trx in this system is through the activity of peroxiredoxin [65].
Mechanisms of signaling by electrophiles
The non-enzymatic oxidation of polyunsaturated lipids leads to the production of a large variety of products. Hermann Esterbauer was the discoverer of 4-hydroxy-2-nonenal (HNE), one of the more abundant and biologically active products [66,67]. Along with Dianzani, Comporti, Poli, Slater, and Esterbauer showed that HNE appeared to be responsible for pathology of several diseases [68–71]. Poli then demonstrated that low concentrations of HNE could act as stimulants of signaling [72–74]. We first learned about HNE when we began to examine the mechanism through which exposure to the atmospheric pollutant, nitrogen dioxide, caused loss of macrophage function. Working with Michael J. Thomas, Tim Robison and I showed that NO2 exposure of macrophages resulted in the formation of many aldehydes, and that HNE was the most abundant [75]. Then Rui-Ming Liu, Dale Dickinson, Karen Iles, and Hongqiao Zhang showed that HNE could induce GSH biosynthesis through transcriptional elevation of GCL and GGT gene expression [76,77]. Expression of GCL genes appeared to be under the control of the AP-1 transcription factor [78].
It was becoming clear that while generation of H2O2 could induce GCL, other electrophiles were actually stronger inducers. Nrf2 is the transcription factor that appears to be a master rheostat in transcriptional regulation of a large number of enzymes involved in protection of cells from stress. The regulatory element, first called the antioxidant response element (ARE) was discovered by Cecil Pickett [79]. Shortly after, a more accurate name for these group of related sequences, the electrophile response element (EpRE) was supplied by Violet Daniel [80]. Around the time we began to look at HNE-induced GCL expression, Tim Mulcahy demonstrated that the two subunits of GCL were regulated by Nrf2 in response to electrophiles [81,82]. So, we examined whether the electrophiles, HNE, curcumin, and 15-deoxy-Δ12,14-prostaglandin J2, the latter in collaboration with Anna-Liisa Levonen and Victor Darley-Usmar, induced GCL gene expression through Nrf2 [83–86]. What was clear from our studies and those of others was that the GCL genes are dually regulated by both AP-1 and Nrf2 [84].
In 2003, we moved to the new University of California campus that was being built in Merced, a small city in the agricultural heartland of the Central Valley of California. Once again, several members of the UAB laboratory made the move to UC Merced. There, surrounded by cows, we continued studies on how both hydroperoxides and HNE activated the ERK, JNK pathways.
Two new postdocs, Alessandra Rinna and Smadar Levy joined the lab along with a graduate student, Chris Mahaffey. Smadar discovered that c-Myc played a role in regulation of Nrf2 by apparently binding to Nrf2 in the Nrf2-EpRE complex and thereby inhibiting transcription of several phase II genes. Moreover, c-Myc also appeared to cause degradation of the nuclear pool of Nrf2 [87]. Chris showed that HNE could induce multidrug resistance protein 3 through activation of Nrf2 in human bronchial cells and non-small cell lung carcinoma cell lines, where many of the latter have defective Keap1 that causes constitutively high MRP3 expression [88][89].
Alessandra demonstrated that activation of H2O2 production caused transient inactivation of protein tyrosine phosphatase 1B (PTP1B) through glutathionylation stimulated by the endogenous generation of H2O2 [90]. PTP1B is the enzyme that prohibits activation of the ERK pathway by dephosphorylating Raf1. Another of her studies demonstrated that HNE could activate the JNK pathway through inhibition of the protein tyrosine phosphatase, SHP-1 [91].
Studies on the pathophysiological role of HNE have continued in a collaboration with Giuseppe Valacchi [92,93]. We first met on the tennis courts of the University of California at Davis when Giuseppe was a postdoc in Carroll Cross’s laboratory.
Air pollution
Collaborations with people at UC Davies led to development of a joint training program with UC Merced in air pollution. During this time, I was appointed by the Governor of California to the Governing Board of the San Joaquin Valley Air Pollution Control District. Meeting a movie star and the niece of a president, Arnold Schwarzenegger and Maria Shriver was nice, but the appointment was a unique opportunity to see how research could be helpful in formulating policy that affects the health of the population.
Over many decades, there have been a great many studies on the effect of airborne particulates and manufactured nanoparticles on health [94,95]. In terms of free radical biology, some of the most notable work has been done by Andre Nel and coworkers [96]. Studies by Andrij Holian on silica induced toxicity interested us as well [97]. At UC Merced, Honglei Liu and Hongqiao Zhang began our studies of the mechanism through which respirable silica activates the production of pro-inflammatory cytokines. Their studies implicated activation of PC-PLC in the signaling [98]. Gayatri Premasekharan, a graduate student working in materials science with Valerie Leppert, took this work further by demonstrating an essential role for surface iron and the role of lipid raft disruption in the mechanism [99].
Aging
In 1999, Kelvin Davies asked me if I was interested in returning to the University of Southern California and offered to share space in his laboratory in the Leonard Davis School of Gerontology. For the following six years, my laboratory was at USC, while all of my teaching responsibilities remained at UC Merced. This was an undergraduate organic chemistry and a graduate level course in signal transduction.
Hongqiao Zhang and Honglei Liu, who moved from UAB to UC Merced also moved with the laboratory to USC. A very positive part of coming back to USC was the opportunity to collaborate with Kelvin along with Caleb (Tuck) Finch, leaders in aging research. USC is also the home of the Southern California Environmental Health Sciences Center, where my lab can interact with many experts in air pollution, including Costas Sioutas and Frank Gilliland. In 2015, I became a Distinguished Emeritus Professor of Biochemistry at UC Merced and the next day went to USC to my current position, which involves almost full time research.
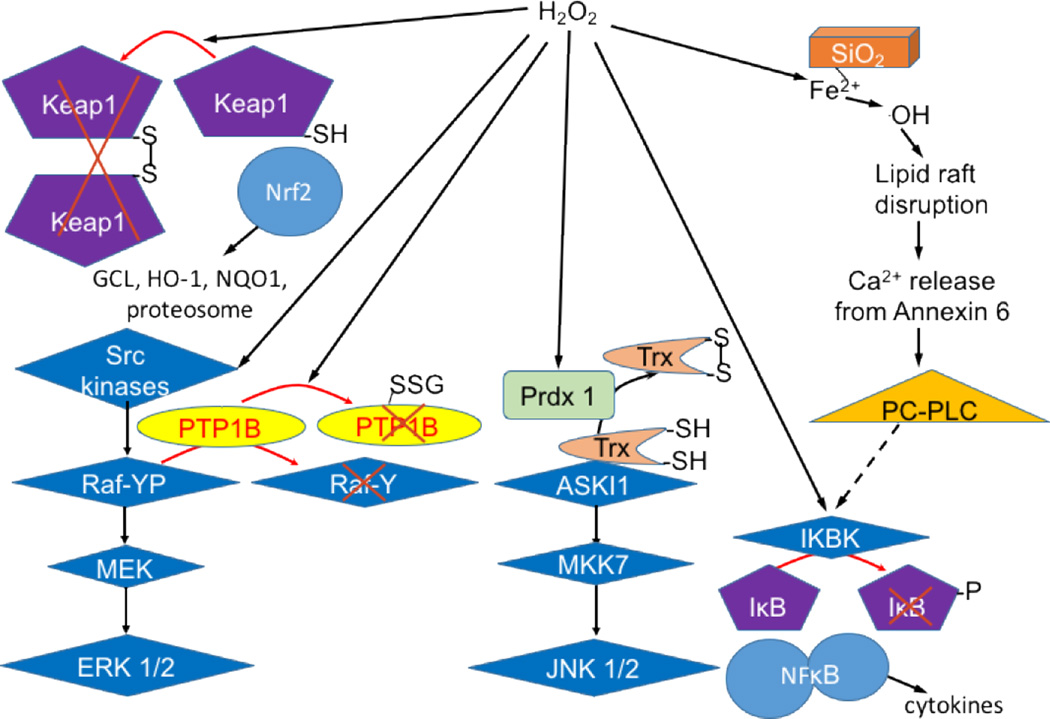
At USC, we began a collaboration with Davies and Finch to try to understand the mechanisms that cause increased susceptibility to the deleterious consequences of air pollution in aging. We suspected that this involved a decrease in responsiveness of Nrf2 signaling that had been reported by Tory Hagen and my former postdoc and colleague, Rui-Ming Liu [100]. Our studies demonstrated that the age-related loss in Nrf2-regulated transcription due to chronic exposure to air pollution was observed not only in lungs, but in the cerebellum and liver as well [101]. The results also suggested that an increase during aging in c-Myc and Bach 1, another inhibitor of Nrf2 transcription, may be responsible for the loss of inducibility of Nrf2-regulated genes [101]. This work is currently being pursued in our laboratory. We continue also to collaborate with colleagues at UC Merced, particularly Valerie Leppert and Peggy O’Day, who are analyzing the surface chemistry of air pollution nanoparticles and synthesizing model nanoparticles to allow for a more uniform material for the mechanistic studies we pursue at USC. Our work on signaling in which H2O2 production by cells is involved is illustrated in Figure 1.
Figure 1. Studies on H2O2 signaling from the Forman laboratory.
The studies are for the endogenous production of H2O2. H2O2 produced by Nox2 can activate Src kinases [105] and cause inactivation of PTP1B through glutathionylation [90]. Together the activation of a Src kinase and inactivation of PTP1B cause ERK activation [53]. H2O2 produced by Nox2 is also used to oxidize thioredoxin, which allows activation of the ASK1 to JNK pathway. Stimulation of Nox2 also leads to H2O2-dependent NF-κB activation [51]. H2O2 produced by redox cycling of quinones activates Nrf2 and Phase 2 gene expression [37]. Silica activates Nox2 generation of H2O2 that is then used in the Fenton reaction to initiate localized lipid peroxidation that results in lipid raft disruption, activation of PC-PLC, and NF-κB-dependent cytokine production.
Redox signaling chemistry and redox homeostasis
In 1990, Fulvio Ursini and Matilde Maiorino invited me to come to the University of Padova for a month long visit. The purpose was to begin a collaboration on the effect of hydroperoxides on signaling. Over the past 26 years, the collaboration has evolved, as did the field, toward attempting to understand how hydroperoxides work as signaling molecules, understanding how antioxidants work, and how redox signaling contributes to homeostasis. Too many people have contributed significantly to these areas to mention all of them here. But, the work of many of those contributors are cited in our recent articles.
In two reviews [60,61], we have argued that among the so-called reactive oxygen species, only H2O2 and other hydroperoxides fulfill the requirements for functioning as a second messenger. The important points we made in these reviews were based on understanding of kinetic constraints that are frequently ignored. The targets for hydroperoxide signaling are protein cysteine in their thiolate form (−S−). But, non-enzymatic oxidation of thiolates by H2O2 is, in most cases, too slow to be involved in signaling. Indeed, the rate of non-enzymatic oxidation of GS−, which is 105 times as great as the protein thiolate. Thus, the intermediate formation of a sulfenic acid through oxidation of a protein thiolate must involve enzymatic catalysis of thiol oxidation.
In another review with Kelvin Davies [102], we described how the kinetics of antioxidant defenses requires that, with the exception of vitamin E scavenging of hydroperoxyl radicals, rules out a role for scavenging of oxidants including free radicals by non-enzymatic mechanisms. Instead, enzymes with fast rate constants remove superoxide and hydroperoxides. This then prevents formation of hydroxyl radical, which no enzyme or small molecule can do efficiently as ·OH reacts with all molecules with rate constants near the limit of diffusion. The likely reason why many dietary compounds, including isocyanates and flavonols that have been called antioxidants work is because they or their metabolites can alkylate Keap1. By activating Nrf2, transcription of many antioxidant enzymes as well as the enzymes that provide the substrates for the antioxidant enzymes are elevated. This maintains a nucleophilic tone in cells. This brings the discussion back to Albert Szent-Gyorgyi. In 1936, he proposed that the dietary phytochemicals, flavonols, should be called vitamin P foreshadowing again an important component of redox biology [103]. Indeed, as with other vitamins, it is difficult to demonstrate any positive effect for health with high doses. It remains to be seen whether an insufficiency of electrophiles in the diet (if that can actually be achieved without general malnutrition) would reveal that they are indeed vitamins.
Our most recent publication [104] concerns the maintenance of redox homeostasis, which involves signaling by electrophiles including HNE along with hydroperoxide signaling to maintain nucleophilic tone. The principle arguments we made, besides reiterating what we described in the prior reviews, are: that redox signaling acts as a rheostat in a continuous dynamic process of oxidant production balanced by reduction rather than an on-off switch; redox signaling is determined by reaction kinetics and does not rely on reaching thermodynamically defined ratios; redox signaling involves reactions of specific electrophiles with specific protein thiolates, primarily through enzyme catalyzed reactions; and that challenges to redox homeostasis generally stay within the bounds of a physiological defined range of electrophiles and nucleophiles, but that a condition of prolonged and/or enhanced exposure to environmental stressors in either the oxidized (pro-inflammatory) or reductive direction results in a new steady state that is abnormal. Thus, while adaptation may preserve function, it results a shift away from physiological redox homeostasis.
Key publications in the field
Table 1 is a biased list of key publications in the field. These are some of the very many key citations that influenced me. Citations on nitric oxide have been included in this table although not described in the text. This is because the author worked in this field only as a collaborator, and had the pleasure of Lou Ignarro as a frequent visitor during a period of several years when a group of LA radicals met in his home for monthly chalk talks. Studies on H2S and other important areas are not cited. While that work certainly influenced my thinking, I did not want to have thousands of references here. My Mendeley database has over 10,000 entries, most of which could have been included. But, I warned you that this was biased! The table ends a decade ago because history has taught me that one needs the perspective of time to see what is the most significant work.
Table 1.
Key papers in the history of redox biology - a biased view.
| Year | Key finding | Reference |
|---|---|---|
| 1954 | Gerschman and coworkers notes oxygen poisoning resembles X-ray damage and propose free radical involvement |
[1] |
| 1956 | Harman proposes the free radical theory of aging | [2] |
| 1958 | Szent-Gyorgyi proposed involvement of free radicals in biological systems | [5] |
| 1967 | Esterbauer describes the production of 4-hydroxy-2-nonenal (HNE) | [66] |
| 1969 | McCord and Fridovich discover superoxide dismutase | [3] |
| 1970 | Meister describes the γ-glutamyl cycle in glutathione biosynthesis | [34] |
| 1972 | Boveris Oshino, and Chance discover production of H2O2 by mitochondria | [16] |
| 1973 | Babior and coworkers demonstrate O2 production by phagocytes | [30] |
| 1974 | Two labs demonstrate O2 production by mitochondrial respiratory chain | [17,18] |
| 1974 | Crapo and Tierney demonstrate adaptation to hyperoxia in rats correlates with increased superoxide dismutase |
[26] |
| 1976 | Cadenas and Boveris show that ubisemiquinone oxidation is the source of mitochondrial O2·− |
[19] |
| 1977 | Murad demonstrates nitric oxide induction of cyclic GMP | [106] |
| 1979 | Ignarro demonstrates muscle relaxation by nitro compounds including NO | [107] |
| 1981 | Furchgott describes endothelial derived relaxing factor (EDRF) | [108] |
| 1983 | Lands demonstrates peroxide tone regulates cyclooxygenase | [44] |
| 1984- 1985 |
Comporti, Dianzani, Poli, Slater, and Esterbauer demonstrate pathology due to HNE | [66,67] [70] |
| 1985 | Sies defines oxidative stress | [35] |
| 1987 | Poli demonstrates that HNE acts as a stimulant of signaling | [72] |
| 1989 | Gopalakrishna and Anderson demonstrate H2O2 activation of classical protein kinase C | [43] |
| 1991 | Schreck and coworkers demonstrate NF-κB activation by addition of exogenous H2O2 to lymphocytes |
[50] |
| 1991 | Rushmore and Pickett discover the antioxidant response element (aka EpRE) | [79] |
| 1994 | Forman and coworkers demonstrate that quinones induce glutamate cysteine ligase (GCL) | [38] |
| 1995 | Mulcahy and coworkers show that EpRE regulates | [81] |
| 1996 | Kaul and Forman demonstrate activation of NFκB by endogenously generated H2O2 in macrophages |
[51] |
| 1996 | Venugopal and Jaiswal identify Nrf2 as regulator of EpRE | [109] |
| 1998 | Denu and Tanner demonstrate reversible inactivation of PTP1B by H2O2 | [110] |
| 1999 | Lambeth and coworkers discover the NOX DuOX family of enzymes is found in almost all cells |
[55] |
| 1999 | Chock demonstrates glutathionylation of PTP1B | [111] |
| 2001 | Kensler, Yamamoto and coworkers demonstrate link of Nrf2 deficiency to carcinogenesis | [112] |
| 2002 | Talalay, Yamamoto, and coworkers demonstrate Keap1 modification by electrophiles | [113] |
| 2003 | Nel and coworkers demonstrate a role for oxidants in signaling by air pollution particle exacerbation of asthma |
[114] |
| 2003 | Yamamoto, Hayes and coworkers demonstrate Keap1 facilitates Nrf2 degradation | [115] |
| 2004 | Levonen, Darley-Usmar and coworkers show HNE conjugates to Keap1 | [116] |
| 2004 | Hagen and coworkers demonstrate loss of Nrf2 signaling in aging | [100] |
| 2006 | Forman and coworkers demonstrate selective and reversible PTP1B glutathionylation during signaling |
[90] |
Summary of lessons learned
Keep your eyes and mind open.
Take advantage of being in a place where new development is taking place.
Study mechanisms rather than phenomena.
Use experimental designs that mimic reality rather than produce spectacular results.
Thermodynamics predict what can happen eventually, but kinetics tell us what is happening.
Retain good relationships with your colleagues when you move. It isn’t true in science that you can’t go home again.
Highlights.
Free radical biology began with Szent-Gyorgyi and became modern with Fridovich.
First focus was damage and oxidative stress and sources, then alteration of signaling.
H2O2 is a second messenger used by peroxidases to oxidize signal protein thiolates.
Redox signaling pathways include tyrosine protein kinases and transcription factors.
Redox homeostasis involves Nrf2 activity, while aging causes decreased Nrf2 activation.
Acknowledgments
As stated at the beginning, my contributions to the field of redox biology would not have occurred without the help of a great number of people. My primary mentors were Philip Feigelson, Irwin Fridovich, and Aron B. Fisher.
My Ph.D. students were Judith K. Murphy, Michael Ming Shi, Evelyne Gozal, Carolyn Hoyal, Li Tian, Xiaobo Qiu, Chang-Jun Yue, Jinah Choi, Lin Gao, Nobuo Watanabe, Hongqiao Zhang, David Krzywanski, Christopher Mahaffey, and Gayatri Premasekharan.
My postdocs and fellows were Ilan D. Arad, Yuen (Lyen) Huang, Thomas K. Aldrich, Mark W. Sutherland, Mitchell Glass, Timothy W. Robison, George A. Loeb, Jill E. Ryer-Powder, Ewa Rajpert-De Meyts, Minyuen Chang Enger, Floyd R. Livingston, David Shoseyov, Rui-Ming Liu, Huanfang Zhou, Nalini Kaul, Julio Girón-Calle, Beth Schomer, Dale A. Dickinson, Karen Iles, Honglei Liu, Alessandra Rinna, Smadar Levy, and Lulu Zhou.
My collaborators also included Frank Brady, James Kennedy, Ronald Coburn, Neils Haaguard, Ray Dorio, Tom Coates, Takeo Iwamoto, Mike Thomas, Alex Sevanian, Pat Reynolds, Martine Torres, Julie Andersen, Zea Borok, Rayadu Gopalakhrishna, Kwang Jin Kim, Victor Darley-Usmar, Doug Moellering, Volker Blank, Jay West, Charles Plopper, Charles Venglarik, Sadis Matalon, Ed Postlethwait, John Tomich, Doug Spitz, Bob Floyd, Terry Kavanagh, Andrew Thomas, Jon Fukuto, Jon Detterich, Gloria Yepiz-Plascencia, Philip Mack, Rudy Ortiz, Matilde Maiorino, Fulvio Ursini, Jose Pablo Vázquez-Medina, David Ann, Kelvin J.A. Davies, Valerie J. Leppert, Peggy O’Day, Giuseppe Valacchi, Nicolas Chepelev, Tuck Finch, Todd Morgan, and Corinne Spickett.
Important contributions were also made by several undergraduate students, notably Mark Posner, Eric Rotman, Kim Foldenauer, Natalie Court, Albert Shih, and Sam Chung.
A number of scientists with whom I never published a research paper, but whose work inspired mine included Paul Hochstein, Helmut Sies, Dean P. Jones, Alton Meister, Leopold Flohé, Regina Brigelius-Flohé, Christine Winterbourn, Sue Goo Rhee, Leslie Poole, Ron Mason, Bruce Freeman, James Crapo, Barry Fanburg, Joe McCord, Hara Misra, Giuseppe Poli, Lars Ernster, Garry Buettner, Herman Esterbauer, Timothy Mulcahy, Britton Chance, Giovanni Mann, Balaraman Kalyanaraman, Giuseppi Poli, Meg Tarpey, Shannon Bailey, Rakesh Patel, Joe Beckman, Doug Ruden, and Albert Szent-Gyorgyi. Of course, there are thousands of other scientists whose work has influenced the field of redox chemistry and biology and I apologize to all whose work should have been acknowledged here, but where my memory failed.
Finally, I thank the National Institutes of Health, the California Tobacco Smoke Research Program, and the Berger Foundation for providing the resources for our work. Current major support is from NIH grant ES023864.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science (80-.) 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 4.Svirbely JL, Szent-Gyorgyi A. The chemical nature of vitamin C. Biochem J. 1932;26:865–870. doi: 10.1042/bj0260865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isenberg I, Szent-Gyorgyi A. Free Radical Formation in Riboflavin Complexes. Proc Natl Acad Sci U S A. 1958;44:857–862. doi: 10.1073/pnas.44.9.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridovich I, Handler P. Detection of free radicals generated during enzymic oxidations by the initiation of sulfite oxidation. J Biol Chem. 1961;236:1836–1840. [PubMed] [Google Scholar]
- 7.Forman HJ, Feigelson P. Kinetic evidence indicating the absence during catalysis of an unbound ferroprotoporphyrin form of tryptophan oxygenase. Biochemistry. 1971;10:760–763. doi: 10.1021/bi00781a006. [DOI] [PubMed] [Google Scholar]
- 8.Brady FO, Forman HJ, Feigelson P. The role of superoxide and hydroperoxide in the reductive activation of tryptophan-2,3-dioxygenase. J. Biol. Chem. 1971;246:7119–7124. [PubMed] [Google Scholar]
- 9.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. The biology of oxygen radicals. Science (80-.) 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 11.Forman HJ, Fridovich I. Electrolytic univalent reduction of oxygen in aqueous solution demonstrated with superoxide dismutase. Science (80-.) 1972;175:339. doi: 10.1126/science.175.4019.339. [DOI] [PubMed] [Google Scholar]
- 12.Forman HJ, Fridovich I. Superoxide dismutase: A comparison of rate constants. Arch. Biochem. Biophys. 1973;158:396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- 13.Forman HJ, Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J. Biol. Chem. 1973;248:2645–2649. [PubMed] [Google Scholar]
- 14.Forman HJ, Evans HJ, Hill RL, Fridovich I. Histidine at the active site of superoxide dismutase. Biochemistry. 1973;12:823–827. doi: 10.1021/bi00729a006. [DOI] [PubMed] [Google Scholar]
- 15.Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 16.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loschen G, Azzi A, Richter C, Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 18.Forman HJ, Kennedy JA. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem. Biophys. Res. Commun. 1974;60:1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- 19.Boveris A, Cadenas E, Stoppani AOM. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winterbourn CC, French JK, Claridge RF. Superoxide dismutase as an inhibitor of reactions of semiquinone radicals. FEBS Lett. 1978;94:269–272. doi: 10.1016/0014-5793(78)80953-3. [DOI] [PubMed] [Google Scholar]
- 21.Forman HJ, Azzi A. On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J. 1997;11:374. doi: 10.1096/fasebj.11.5.9141504. [DOI] [PubMed] [Google Scholar]
- 22.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 23.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bean JW. Effects of oxygen at increased pressure. Physiol. Rev. 1945;25:1–147. [Google Scholar]
- 25.Stadie WC, Haugaard N. Oxygen poisoning; the effect of high oxygen pressure upon enzymes; succinicdehydrogenase and cytochrome oxidase. J Biol Chem. 1945;161:153–174. [PubMed] [Google Scholar]
- 26.Crapo JD, Tierney DF. Superoxide dismutase and pulmonary oxygen toxicity. Am. J. Physiol. 1974;226:1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- 27.Bus JS, Aust SD, Gibson JE. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem. Biophys. Res. Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 28.Forman HJ, Fisher AB. Antioxidant enzymes of rat granular pneumocytes. Constitutive levels and effect of hypoxia. Lab. Investig. 1981;45:1–6. [PubMed] [Google Scholar]
- 29.Forman HJ, Aldrich TK, Posner MA, Fisher AB. Differential paraquat (PO) accumulation and redox kinetics in rat lung cells. Fed. Proc. 1982;41 [PubMed] [Google Scholar]
- 30.Babior BM, Kipnes RS, Curnutte JT. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman HJ, Nelson J, Fisher AB. Rat alveolar macrophages require NADPH for superoxide production in the respiratory burst. Effect of NADPH depletion by paraquat. J. Biol. Chem. 1980;255:9879–9883. [PubMed] [Google Scholar]
- 32.Sutherland MW, Glass M, Nelson J, Lyen Y, Forman HJ. Oxygen toxicity: Loss of lung macrophage function without metabolite depletion. J. Free Radic. Biol. Med. 1985;1:209–214. doi: 10.1016/0748-5514(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland MW, Nelson J, Harrison G, Forman HJ. Effects of t-butyl hydroperoxide on NADPH, glutathione, and the respiratory burst of rat alveolar macrophages. Arch. Biochem. Biophys. 1985;243:325–331. doi: 10.1016/0003-9861(85)90509-0. [DOI] [PubMed] [Google Scholar]
- 34.Orlowski M, Meister A. y-Glutamyl Cycle : A Possible Transport System for amino acids. Proc Natl Acad Sci. 1970;67:1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadenas E, Sies H. Oxidative stress: excited oxygen species and enzyme activity. Adv. Enzym. Regul. 1985;23:217–237. doi: 10.1016/0065-2571(85)90049-4. [DOI] [PubMed] [Google Scholar]
- 36.Forman HJ. Glutathione - From antioxidant to post-translational modifier. Arch. Biochem. Biophys. 2016;595:64–67. doi: 10.1016/j.abb.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced Oxidative Stress Elevates Glutathione and Induces γ-Glutamylcysteine Synthetase Activity in Rat Lung Epithelial L2 Cells. J. Biol. Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- 38.Shi MM, Iwamoto T, Forman HJ. γ-Glutamylcysteine synthetase and GSH increase in quinone-induced oxidative stress in BPAEC. Am. J. Physiol. - Lung Cell. Mol. Physiol. 1994;267 doi: 10.1152/ajplung.1994.267.4.L414. [DOI] [PubMed] [Google Scholar]
- 39.Tian L, Shi MM, Forman HJ. Increased transcription of the regulatory subunit of gamma-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch. Biochem. Biophys. 1997;342:126–133. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- 40.Kugelman A, Choy HA, Liu R, Shi MM, Gozal E, Forman HJ. gamma-Glutamyl transpeptidase is increased by oxidative stress in rat alveolar L2 epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994;11:586–592. doi: 10.1165/ajrcmb.11.5.7946387. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 42.Czech MP. Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system. J Biol Chem. 1976;251:1164–1170. [PubMed] [Google Scholar]
- 43.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. USA. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulmacz RJ, Lands WE. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins. 1983;25:531–540. doi: 10.1016/0090-6980(83)90025-4. [DOI] [PubMed] [Google Scholar]
- 45.Bryant RW, She HS, Ng KJ, Siegel MI. Modulation of the 5-lipoxygenase activity of MC-9 mast cells: activation by hydroperoxides. Prostaglandins. 1986;32:615–627. doi: 10.1016/0090-6980(86)90043-2. [DOI] [PubMed] [Google Scholar]
- 46.Hoyal CR, Gozal E, Zhou H, Foldenauer K, Forman HJ. Modulation of the rat alveolar macrophage respiratory burst by hydroperoxides is calcium dependent. Arch. Biochem. Biophys. 1996;326:166–171. doi: 10.1006/abbi.1996.0061. [DOI] [PubMed] [Google Scholar]
- 47.Murphy JK, Livingston FR, Gozal E, Torres M, Forman HJ. Stimulation of the rat alveolar macrophage respiratory burst by extracellular adenine nucleotides. Am J Respir Cell Mol Biol. 1993;9:505–510. doi: 10.1165/ajrcmb/9.5.505. [DOI] [PubMed] [Google Scholar]
- 48.Hoyal CR, Thomas AP, Forman HJ. Hydroperoxide-induced Increases in Intracellular Calcium Due to Annexin VI Translocation and Inactivation of Plasma Membrane Ca2+-ATPase. J. Biol. Chem. 1996;271:29205–29210. doi: 10.1074/jbc.271.46.29205. [DOI] [PubMed] [Google Scholar]
- 49.Giron-Calle J. Priming of Alveolar Macrophage Respiratory Burst by H2O2 Is Prevented by Phosphatidylcholine-Specific Phospholipase C Inhibitor Tricyclodecan-9-yl-xanthate (D609) J. Pharmacol. Exp. Ther. 2002;301:87–94. doi: 10.1124/jpet.301.1.87. [DOI] [PubMed] [Google Scholar]
- 50.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaul N, Forman HJ. Activation of NFκB by the respiratory burst of macrophages. Free Radic. Biol. Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 52.Kaul N, Gopalakrishna R, Gundimeda U, Choi J, Forman HJ. Role of protein kinase C in basal and hydrogen peroxide-stimulated NF-kappa B activation in the murine macrophage J774A.1 cell line. Arch. Biochem. Biophys. 1998;350:79–86. doi: 10.1006/abbi.1997.0487. [DOI] [PubMed] [Google Scholar]
- 53.Torres M, Forman HJ. Activation of several MAP kinases upon stimulation of rat alveolar macrophages: role of the NADPH oxidase. Arch. Biochem. Biophys. 1999;366:231–239. doi: 10.1006/abbi.1999.1225. [DOI] [PubMed] [Google Scholar]
- 54.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 55.Arnold WP, Lambeth JD. The Nox enzymes and the regulated generation of reactive oxygen species. In: Forman HJ, Fukuto J, Torres M, editors. Signal Transduct. by React. Oxyg. Nitrogen Species Pathways Chem. Princ. Boston: Kluwer Academic Publishers, Dordrecht; 2003. pp. 102–118. [Google Scholar]
- 56.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as Stimulators of Signal Transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 57.Forman HJJ, Cadenas E. Oxidative Stress and Signal Transduction. New York: Springer Verlag; 1997. [Google Scholar]
- 58.Forman HJ, Fukuto J, Torres M. Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles. Kluwer Academic Publishers; 2003. [Google Scholar]
- 59.Culp BR, Titus BG, Lands WE. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979;3:269–278. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- 60.Forman HJ, Maiorino M, Ursini F. Signaling Functions of Reactive Oxygen Species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H2O2 generation by alveolar macrophages. Free Radic. Biol. Med. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 63.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H, Zhang H, Iles KE, Rinna A, Merrill G, Yodoi J, Torres M, Forman HJ. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic. Res. 2006;40:865–874. doi: 10.1080/10715760600758514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Im JY, Lee KW, Junn E, Mouradian MM. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci. Res. 2010;67:203–208. doi: 10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esterbauer H, Wegner W. Uber die wirkungen von aldehyden auf gesunde und maligne zellen,3.mitt:synthese von homologen 4-hydroxy-2-alkenalen, II. Monatsh Chem. 1967;98:1994–2000. [Google Scholar]
- 67.Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP- iron in isolated rat hepatocytes and rat liver microsomal suspension. Biochem. J. 1985;227:629. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curzio M, Esterbauer H, Poli G, Biasi F, Cecchini G, DiMauro C, Capello N, Dianzani MU. Possible role of aldehydic lipid peroxidation products as chemoattractants. Int. J. Tissue React. 1987;9:295–306. [PubMed] [Google Scholar]
- 69.Grune T, Siems WG, Kowalewski J, Esterbauer H. Postischemic accumulation of the lipid peroxidation product 4-hydroxynonenal in rat small intestine. Life Sci. 1994;55:693–699. doi: 10.1016/0024-3205(94)00676-8. [DOI] [PubMed] [Google Scholar]
- 70.Benedetti A, Comporti M, Fulceri R, Esterbauer H. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1984;792:172. doi: 10.1016/0005-2760(84)90219-4. [DOI] [PubMed] [Google Scholar]
- 71.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 72.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogl S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor b1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 73.Leonarduzzi G, Arkan MC, Basaga H, Chiarpotto E, Sevanian A, Poli G. Lipid oxidation products in cell signaling. Free Radic Biol Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 74.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J. Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 75.Robison TW, Forman HJ, Thomas MJ. Release of aldehydes from rat alveolar macrophages exposed in vitro to low concentrations of nitrogen dioxide. Biochim. Biophys. Acta - Lipids Lipid Metab. 1995;1256:334–340. doi: 10.1016/0005-2760(95)00041-a. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, Dickinson DA, Liu R-M, Forman HJ. 4-Hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic. Biol. Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 79.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 80.Friling RS, Bergelson S, Daniel V. Two adjacent AP-1 binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proc. Natl. Acad. Sci. USA. 1992;89:668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5’-flanking region of the human g-glutamylcycteine synthetase heavy subunit gene. Biochem. Biophys. Res. Commun. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- 82.Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 83.Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu R-M, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch. Biochem. Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 85.Levonen AL, Dickinson DA, Moellering DR, Timothy Mulcahy R, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-Δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 86.Dickinson DA, Levonen A-L, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Levy S, Forman HJ. C-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahaffey CM, Zhang H, Rinna A, Holland W, Mack PC, Forman HJ. Multidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinoma. Free Radic. Biol. Med. 2009;46:1650–1657. doi: 10.1016/j.freeradbiomed.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahaffey CM, Mahaffey NC, Holland W, Zhang H, Gandara DR, Mack PC, Forman HJ. Aberrant regulation of the MRP3 gene in non-small cell lung carcinoma. J. Thorac. Oncol. 2012;7:34–39. doi: 10.1097/JTO.0b013e318233d753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic. Biol. Med. 2006;41:86–91. doi: 10.1016/j.freeradbiomed.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am. J. Respir. Cell Mol. Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sticozzi C, Belmonte G, Pecorelli A, Arezzini B, Gardi C, Maioli E, Miracco C, Toscano M, Forman HJ, Valacchi G. Cigarette smoke affects keratinocytes SRB1 expression and localization via H 2O 2 production and HNE protein adducts formation. PLoS One. 2012;7:e33592. doi: 10.1371/journal.pone.0033592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pecorelli A, Belmonte G, Meloni I, Cervellati F, Gardi C, Sticozzi C, De Felice C, Signorini C, Cortelazzo A, Leoncini S, Ciccoli L, Renieri A, Forman HJ, Hayek J, Valacchi G. Alteration of serum lipid profile, SRB1 loss, and impaired Nrf2 activation in CDKL5 disorder. Free Radic. Biol. Med. 2015;86:156–165. doi: 10.1016/j.freeradbiomed.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamilton RF, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic. Biol. Med. 2008;44:1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Zhang H, Forman HJ. Silica induces macrophage cytokines through phosphatidylcholine-specific phospholipase C with hydrogen peroxide. Am. J. Respir. Cell Mol. Biol. 2007;36:594–599. doi: 10.1165/rcmb.2006-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Premasekharan G, Nguyen K, Contreras J, Ramon V, Leppert VJ, Forman HJ. Iron-mediated lipid peroxidation and lipid raft disruption in low-dose silica-induced macrophage cytokine production. Free Radic. Biol. Med. 2011;51:1184–1194. doi: 10.1016/j.freeradbiomed.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 100.Suh JH, V Shenvi S, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM, Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis. which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Liu H, Davies KJA, Sioutas C, Finch CE, Morgan TE, Forman HJ. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic. Biol. Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forman HJ, Davies KJA, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bentsath A, Rusznyak S, Szent-Gyorgyi A. Vitamin P. Nature. 1937;139:326–327. [Google Scholar]
- 104.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H, Davies KJA, Forman HJ. TGFβ1 rapidly activates Src through a non-canonical redox signaling mechanism. Arch. Biochem. Biophys. 2015;568:1–7. doi: 10.1016/j.abb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cycl. Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- 108.Furchgott RF, Cherry PD, V Zawadzki J, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J. Cardiovasc. Pharmacol. 1984;6(Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- 109.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. J. Clin. Invest. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochem. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 111.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochem. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 112.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165:2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- 115.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 116.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]