Abstract
Purpose
The hypoxic environment around the lens is important for maintaining lens transparency. Lens epithelial cells (LECs) play a key role in lens metabolism. We measured oxygen consumption to assess the role of human LECs in maintaining hypoxia around the lens, as well as the impact of systemic and ocular diagnosis on these cells.
Methods
Baseline cellular respiration was measured in rabbit LECs (NN1003A), canine kidney epithelial cells (MDCK), trabecular meshwork cells (TM-5), and bovine corneal endothelial cells (CCEE) using a XF96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA), which measures oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in vitro. Following informed written consent, lens capsule epithelial cells were obtained from patients during cataract surgery and were divided into small explants in 96-well plates. Capsules were removed when LECs became confluent. OCR was normalized to the number of cells per well using rabbit LECs as a standard. The effect of patient age, sex, race, and presence of diabetes or glaucoma on oxygen consumption was assessed by using the Mann-Whitney U-test and multivariate regression analysis.
Results
Primary LECs were obtained from 69 patients. The OCR from donors aged 70 and over was lower than that of those under 70 years (2.21 ± 1.037 vs. 2.86 ± 1.383 fmol/min/cell; p<0.05). Diabetic patients had lower OCR than non-diabetic patients (2.02 ± 0.911 vs. 2.79 ± 1.332 fmol/min/cell; p<0.05), and glaucoma patients had lower OCR than non-glaucoma patients (2.27 ± 1.19 vs. 2.83 ± 1.286 fmol/min/cell; p<0.05). Multivariate regression analysis confirmed that donors aged 70 and over (p<0.05), diabetic patients (p<0.01), and glaucoma patients (p<0.05) had significantly lower OCR, independent of other variables. Gender and race had no significant effect on OCR.
Conclusions
The lower oxygen consumption rate of human LECs in older donors and patients with diabetes or glaucoma could contribute to cataract development. Diabetes and glaucoma are particularly important factors associated with decreased OCR, independent of age. Ongoing studies are examining pO2 at the anterior surface of the lens in vivo and oxygen consumption in the patient’s LECs.
Keywords: mitochondria, cataract, diabetes, glaucoma, lens epithelial cells, oxygen metabolism
Introduction
Cataract is the most common cause of reversible vision loss in the world. Several risk factors for cataract formation have been reported, with age being the most impactful.1 Age-related cataracts are responsible for nearly half of all blindness worldwide and half of all visual impairment in the United States.2 Other intrinsic factors aside from age, such as diabetes,3 glaucoma,4 and heritability5, 6 are also known to be significant risks for cataract development. In addition, ultraviolet B radiation (UV-B),7 body mass index (BMI),8 smoking,5, 9 diet,10 exposure to hyperbaric oxygen,11–13 and corticosteroids14 have been identified as extrinsic or environmental risk factors for cataract.
As mentioned above, heritability is a major contributing factor towards age-related cataracts but the genes involved have not yet been identified.2, 5, 6, 15 Nevertheless, many inheritable mitochondrial diseases have been associated with an elevated risk of cataract. In such mitochondrial cytopathies, cataracts are of particular interest due to the well-described MELAS (myopathy, encephalopathy, lactacidemia, and stroke-like episodes) syndrome,16 and many representative mitochondrial diseases such as mitochondrial myopathy17, mitochondriopathy,18 chronic progressive external ophthalmoplegia,19 mitochondrial encephalomyopathy,20 and autosomal dominant optic atrophy (OPA3)21 have all been associated with a higher risk of cataract.
Mitochondria provide energy generated by oxidative phosphorylation to the cell and at the same time play a central role in apoptosis and aging.22 It has been reported that mitochondria decay in number and function with increasing age.23–25 The number of mitochondria has been found to decrease in liver cells of aging mice24, 26 and humans,23, 27 and the age-related decline of mitochondrial function has long been recognized to occur concomitantly with the appearance of mitochondrial morphological alterations25 such as abnormal roundness which has been observed in aged mammals.28
In most tissues, oxygen partial pressures below 3% (~22 mmHg) are considered hypoxic. In humans, the oxygen level near the anterior lens epithelium is approximately 0.4% (~3 mmHg)29 indicating that the lens epithelium exists in a hypoxic environment.30, 31 The lens consumes oxygen32 and further studies have shown that most of the small amount of oxygen derived from the iris vasculature is consumed by the lens.30, 33
Mitochondria are abundant in the lens, but only within the epithelium and differentiating fibers, as mature fibers in the core of the lens lack mitochondria. Moreover, while mitochondria account for approximately 90% of the total oxygen consumption in the lens,34 those in the lens epithelium play the most important role in lens metabolism.35, 36 Interestingly, it has been proposed that aged mitochondria consume significantly less oxygen than young mitochondria,37 and the susceptibility of the older human lens to oxygen-induced damage was directly demonstrated in a study involving patients treated with long-term hyperbaric oxygen therapy.11 It is well known that nuclear cataracts are caused by excessive oxidation of proteins in the lens nucleus.38, 39
As described above, our previous study reported that the lens exists in a hypoxic environment which is important in maintaining lens transparency, and that oxygen levels are generally lower closer to the lens.29, 30 Exposure to increased oxygen has been identified as a risk factor for nuclear cataract, the most common type of age-related cataract.40 Therefore, we hypothesized that decreased mitochondrial function in lens epithelial cells (LECs) plays a key role in cataractogenesis.
Recently, a noncontact method of measuring cellular respiration with an extracellular flux analyzer, the Seahorse Bioscience XF96 (North Billerica, MA, USA), has been described.43 To test our hypothesis, we measured the oxygen consumption rate (OCR) in ex vivo human LECs to assess whether age, diabetes, glaucoma and other intrinsic factors contribute to mitochondrial function and, potentially, cataractogenesis.
Methods
Cell Culture
In order to measure baseline cellular respiration, established cell lines from rabbit LECs (NN1003A), canine kidney epithelial cells (MDCK) and human trabecular meshwork cells (TM-5) were utilized. In addition, early-passage cells from bovine corneal endothelium (CCEE) were established from fresh bovine eyes. In brief, the cornea was removed and placed in a small petri dish with media and the endothelial tissue layer was dissected to small segments. In a T25 Corning flask, a media channel was made with 1.0 mL growth media (described in detail below) and one small segment of endothelium was submerged in the media. The flask was then placed in a 37°C/5% CO2 incubator for approximately one week until the cells grew from the tissue to a 250 mm2 area. At that point, the tissue was slowly lifted and placed in another T25 flask for additional cell growth and eventual harvesting; this process may be repeated up to a total of three times. For cell collection each flask was trypsinized at a ratio of 1:1 with media (0.25% Trypsin-EDTA, Gibco® #25200, Life Technologies, Grand, NY). Once the cells dissociated from the flask, three times the amount of media was added followed by slow pipetting and reallocation of the cells and media into a 15 mL tube. The tube was spun at 700 rpm in a 4°C centrifuge for 5–7 minutes, after which the supernatant was discarded, and the pellet re-suspended in media. The cells and media were aliquoted into a T75 flask and subculture was done at 65–75% confluence. The first five subcultures (passage 1 – passage 5) were used for this study.
Growth media
NN1003A were cultured in 1 g/L D-Glucose Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies, #11885, Grand Island, NY) with 10% fetal calf serum (FCS, Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin and streptomycin (Life Technologies, Grand Island, NY). MDCK cells were cultured in Minimum Essential Medium (MEM #11090, Life Technologies, Grand Island, NY) with 10% FBS and 1% penicillin and streptomycin (Life Technologies, Grand Island, NY). TM-5 cells were cultured in DMEM (Gibco® #10566, Grand Island, NY) with 10% fetal calf serum (FCS, Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin and streptomycin. CCEE cells were cultured in DMEM (Gibco® #10566, Grand Island, NY) with 10% FBS, 1% non-essential amino acid-100x (Gibco® #11140), 2% essential amino acid-50x (Gibco® #11130-051, Grand Island, NY),1ug/ml-Fungizone (Gibco® #15290-018, Grand Island, NY) and 2.5ug/ml-Gentamycin (Gibco® #15750-060, Grand Island, NY).
Visualization of mitochondria by confocal laser microscopy
MitoTracker® Red CMXRos (M7512, Red CMXRos Life Technologies, Grand Island, NY) is a red-fluorescent dye (abs/em ~579/599 nm) that stains mitochondria in live cells and its accumulation is dependent upon membrane potential. MitoTracker® Red CMXRos was used to stain mitochondria in NN1003A, MDCK, TM-5 and CCEE cells. Staining of mitochondria was performed by the method described by Wolf and colleagues.41, 42 Cells growing on chambered slide-glass (Lab-Tek™ II Nunc Thurmo Scientific Inc., USA) were incubated with MitoTracker® Red CMXRos at a final concentration of 200 nM for 20 minutes in a 37°C/5% CO2 culture incubator. The chambered slide-glasses were then rinsed with culture medium followed by fixation with 4% PFA and permeabilized with PBS containing 0.2% Triton™ X-100 (Sigma-Aldrich, St. Louis, MO). Specimens were examined under a confocal laser microscope (Zeiss LSM 510 META, Jena, Germany).
Primary culture of human lens epithelial cells from donors
The human research protocol was approved by the Washington University Institutional Review Board and Human Research Protection Office, according to the tenets of the Declaration of Helsinki. Informed written consent was obtained from the subjects undergoing cataract extraction and intraocular lens implantation, as well as intraocular oxygen measurements, as described elsewhere.29 After continuous curvilinear capsulorhexis, the capsular specimens were passed off the surgical field via forceps and immediately placed into a vial of DMEM with 1 g/L D-Glucose DMEM with 25% fetal calf serum and 1% penicillin and streptomycin. The specimens were immediately brought to the laboratory and divided into 4 to 6 explants on a petri dish, and each piece (HLECs along with the capsule) was placed cell-side-down, centered at the bottom of the wells of XF 96-well plates. In two to three days, the lens epithelial cells migrated under the capsule with expansion beyond the capsule. After seven to ten days, the HLECs became confluent and the capsules were carefully removed so that the cells could migrate and fill in the spaces previously occupied by capsule. Two days post-capsule removal, the confluent and monolayered cells were measured by the XF96 analyzer with the XF Cell Mito Stress Test Kit (Seahorse Bioscience, North Billerica, MA). For this study, human primary lens epithelial cells were obtained from 69 patients over a 17 month time frame (September 2012 to April 2014) with a mean age of 69.7 ± 10.8 years. Comparative analysis for mitochondrial function was performed by age stratification (≤69 years vs. ≥70 years), gender (male vs. female), self-reported race (Caucasian vs. African-American), diagnosis of open angle glaucoma and diagnosis of diabetes. (Table 1)
Table 1.
Patient Characteristics
| Age (years) | Gender | Race | Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ≤69 | ≥70 | Female | Male | Caucasian | African American | Diabetes | Glaucoma | ||
| No | Yes | No | Yes | ||||||
|
| |||||||||
| 36 | 33 | 47 | 22 | 44 | 25 | 48 | 21 | 35 | 34 |
Measurement of oxygen consumption rate and extracellular acidification rate of cells
The XF96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) was utilized to measure the extracellular flux changes of oxygen and protons in the media surrounding the adherent cells cultured onto the poly-D-lysine coated XF96-well microplates. This technology allows for real time measurements of cellular bioenergetics including oxygen consumption rates, glycolysis, ATP production and respiratory capacity in a noninvasive format.43 In order to identify unique characteristics of lens epithelial cells, four other types of cells were seeded in the microplate at a density of 2.5 × 104 cells/well for rabbit LECs (NN1003A), 4.8×104 cells/well for kidney epithelial cells (MDCK), 9.6×104 cells/well for trabecular meshwork cells (TM-5), 9.6×104 cells/well for corneal endothelial cells (CCEE) in each 80 μl of DMEM. After 24 hours of incubation at 37 °C in 5% CO2, baseline cellular respiration was measured. This device measures oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in 96-well plates after sequentially adding to each well 20 μl of an ATP coupler, oligomycin, 22 μl of an uncoupling agent, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), 12.5 μl of Complex1 inhibitor, Antimycin A, and 12.5 μl of Rotenone to reach working concentrations of 1.26 μM, 1 μM, 0.5 μM, and 0.5 μM, respectively. OCR and ECAR were normalized to the number of cells per well using rabbit LECs (NN1003A) as a standard, as described below.
Cell normalization and viability assay
To normalize the cell number, 6 wells of NN1003A were counted by Automated Cell Counter (Countess, Life Technologies) with direct use of 0.25% Trypsin-EDTA (25200-Gibco®, Grand Island, NY), and a WST-1 assay was performed on the remaining wells according to the manufacturer’s protocol as previously described.45 After completion of the mitochondria stress test, the wells were carefully washed with PBS and incubated in fresh medium containing WST-1 assay solution (Cell Proliferation Reagent WST-1; Roche Applied Science, Indianapolis, IN) at 37°C for 1 hour. Absorbance was measured at 450 nm with a reference wavelength of 650 nm using a microplate reader (SpectraMax 190 Absorbance Microplate Reader; Molecular Devices, LLC, Sunnyvale CA).
XF Cell mitochondria stress test
The XF Cell Mitochondria Stress Test Kit assesses the mitochondrial activity of cells. In this assay, During the XF mitochondria stress test, three different compounds are added in succession, shifting the bioenergetics profile of the cells.46 Basal respiration is predominantly controlled by ATP synthase and proton leak. Addition of the ATP coupler, oligomycin, blocks ATP synthase (ATP production) and the residual respiration is due to the proton leak, which results in a significant decrease in OCR. The electron transport chain accelerator FCCP induces a high artificial proton conductance into the membrane. Finally, electron transport chain inhibitors Rotenone and Antimycin A are added to inhibit total mitochondrial respiration.
Calculating baseline OCR for human primary lens epithelial cells
With regard to basal respiration of mitochondria, the baseline value in OCR represents the oxygen consumption rate under quiescent conditions. Baseline measurements were taken for a total of 30 minutes, with values at the 15-minute interval used for the following analysis. OCR and ECAR values were normalized to the number of cells per well, using rabbit LECs (NN1003A) as a standard as described above.
Statistics
The data was analyzed by the Mann-Whitney U test and multivariate regression analysis with SPSS software version 17.0 (SPSS, Chicago, Illinois, USA). Statistical significance was defined as a p value of < 0.05. Results are expressed as mean ± SD.
Results
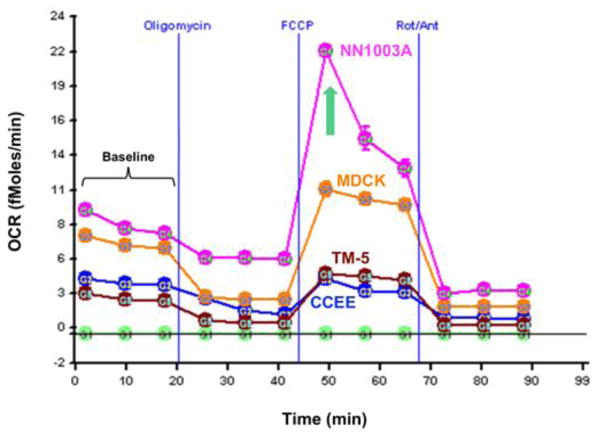
During baseline measurements, that is, while the cells were at a quiescent state prior to the addition of the metabolism-altering compounds, NN1003A demonstrated the highest oxygen consumption rate (7.64 ± 0.37 fmol/min/cell; p<0.0001) compared to each of the other three cell lines of MDCK (6.56 ± 0.19 fmol/min/cell), TM-5 (2.54 ± 0.09 fmol/min/cell and CCEE (3.77 ± 0.09 fmol/min/cell) (Figure 1, Table 2) NN1003A was also found to have the highest baseline ECAR (2.19 ± 0.49 μpH/min/cell) during basal respiration in comparison to MDCK (0.78 ± 0.28 μpH/min/cell; p <0.001), and also trended higher compared to TM-5 (0.61 ± 0.13 μpH/min/cell) and CCEE (0.56 ± 0.14 μpH/min/cell).
Figure 1. Line graph representation of oxygen consumption rate (OCR) of cell lines as measured by the XF Mitochondrial Stress Test.
The addition of oligomycin at 20 minutes inhibits ATP production resulting in a decrease in OCR. OCR increases significantly in all cell lines following the addition of carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), an electron transport chain accelerator. Rotenone and Antimycin A, electron transport chain inhibitors, decrease oxygen consumption levels to very low levels inhibiting total mitochondrial respiration. Compared to the four different cell lines measured, rabbit lens epithelial cells (NN1003A) demonstrated the highest maximal oxygen consumption rate (green arrow).
Table 2.
Mitochondria Stress Test Results from Established Cell Lines
| Cell Line | Median Age (passage) |
Baseline OCR ± SD (fmol/min/cell) |
Maximal OCR ± SD (fmol/min/cell) |
O2-dependent ATP Production (%) |
Proton Leak (%) |
Spare Resp. Capacity (fmol/min/cell) |
Baseline ECAR ± SD (μpH/min/cell) |
|---|---|---|---|---|---|---|---|
| NN1003A | 10 | 7.64 ± 0.37 | 21.60 ± 0.42 | 26% | 74% | 13.93 | 2.19 ± 0.49 |
| MDCK | 8 | 6.56 ± 0.19**** | 11.04 ± 0.60**** | 61% | 39% | 4.48 | 0.78 ± 0.28** |
| TM-5 | 7 | 2.54 ± 0.09**** | 4.57 ± 0.37**** | 67% | 33% | 2.03 | 0.61 ± 0.13** |
| CCEE | 4 | 3.77 ± 0.09**** | 4.17 ± 0.22**** | 60% | 40% | 0.40 | 0.56 ± 0.14** |
OCR = Oxygen Consumption Rate, ECAR = Extracellular Acidification Rate, Resp. = respiratory
In relation to NN1003A, p<0.001
In relation to NN1003A, p<0.0001
Inhibition of mitochondrial ATP synthesis via the addition of Oligomycin reduced the OCR of all four cell lines, and NN1003A had the lowest oxygen-dependent ATP production compared to the other cell lines. These differences represent the extent of oxygen-dependent ATP production for each cell line. Proton leak rates were calculated by subtracting oxygen-dependent ATP production from 100%. To that end, the values for proton leak were found to be highest in the NN1003A cell line (Table 2). FCCP was used to uncouple mitochondria, stimulating maximal oxygen consumption. Despite having the lowest oxygen-dependent ATP production during the baseline measurement, rabbit LECs demonstrated the highest maximal OCR (21.60 ±SD 0.42 fmol/min/cell; p<0.0001) (Table 2) compared to the other cell lines when their mitochondria were uncoupled.
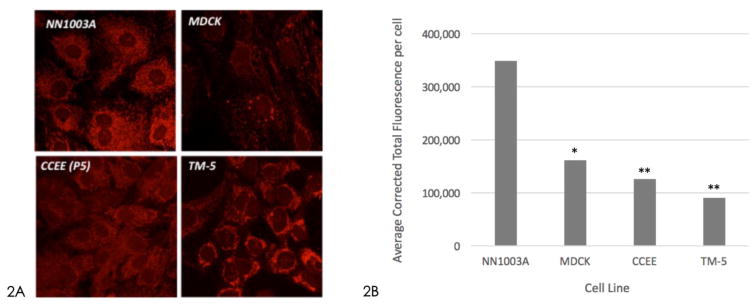
Samples from all four cell lines were stained with MitoTracker® Red CMXRos, and rabbit lens epithelial cells appeared to have a higher density of mitochondria compared to other cells.
Human primary lens epithelial cells
As shown in Table 3, the average OCR values from the cell mitochondria stress test for all 69 patients were 2.55 ± 1.264 fmol/min/cell for baseline respiration, 33% for oxygen-dependent ATP production, 67% for Proton Leak, 4.82 ± 2.723 fmol/min/cell for Maximal Respiration, 2.27 ± 1.718 fmol/min/cell for Spare Respiratory Capacity and 1.91 ± 2.068 μpH/min/cell for ECAR. The oxygen-dependent ATP -production of human primary LECs was 33%, similar to the rabbit lens epithelial cells (26%), was lower in comparison to other cell lines.
Table 3.
Mitochondria Stress Test Results from Primary Culture Human Lens Epithelial Cells
| Patient Characteristic | Baseline OCR (fmol/min/cell) |
Maximal OCR (fmol/min/cell) |
O2-dependent ATP Production (%) |
Proton Leak (%) |
Spare Resp. Capacity (fmol/min/cell) |
Baseline ECAR (μpH/min/cell) |
|---|---|---|---|---|---|---|
| Age ≤69 years | 2.86* | 5.28 | 32% | 68% | 2.37 | 2.04 |
| Age ≥70 years | 2.21 | 4.33 | 35% | 65% | 2.12 | 1.76 |
| Non-Diabetic | 2.79* | 5.20 | 33% | 67% | 2.41 | 1.93 |
| Diabetic | 2.02 | 3.96 | 34% | 66% | 1.94 | 1.86 |
| No Glaucoma | 2.83* | 5.46* | 33% | 67% | 2.59 | 2.13 |
| Glaucoma | 2.27 | 4.17 | 33% | 67% | 1.90 | 1.68 |
| Female | 2.63 | 4.85 | 32% | 68% | 2.19 | 2.09 |
| Male | 2.39 | 4.76 | 35% | 65% | 1.57 | 1.51 |
| Caucasian | 2.57 | 4.69 | 32% | 68% | 2.10 | 1.68 |
| African American | 2.53 | 5.05 | 36% | 64% | 2.53 | 2.31 |
| Total (n=69) | 2.55 | 4.82 | 33% | 67% | 2.27 | 1.91 |
p<0.05
OCR = Oxygen Consumption Rate, ECAR = Extracellular Acidification Rate
The baseline OCR of donors aged 70 and over was lower than that of donors under 70 years of age (2.21 ± 1.037 vs. 2.86 ± 1.383 fmol/min/cell; p<0.05). Diabetic patients had lower OCR than non-diabetic patients (2.02 ± 0.911 vs. 2.79 ± 0.133 fmol/min/cell; p<0.05), and glaucoma patients had lower OCR than non-glaucoma patients (2.27 ± 1.193 vs. 2.83 ± 1.286 fmol/min/cell; p<0.05). Further analysis using multivariate logistic regression analysis confirmed that donors aged 70 and over (p<0.05), diabetic patients (p<0.01), and glaucoma patients (p<0.05) had significantly lower OCR, independent of other variables. Gender and race had no significant effect on OCR.
Basal respiration measures ATP synthase and proton leak. The addition of Oligomycin blocks ATP synthase (ATP production) and the residual respiration is due to the proton leak. This results in a significant decrease in OCR. The donors aged 70 and over had lower proton leak than those less than 70 years of age in their OCR (1.45 ±0.915 vs. 2.02 ± 1.237 fmol/min/cell; p<0.05). This decreased proton leak conferred decreased basal respiration in the older group. Age had no significant effect on oxygen utilization for ATP production, maximal OCR, spare respiratory capacity or ECAR.
Effect of diabetes and glaucoma diagnosis on basal respiration and proton leak
Diabetic patients had a lower baseline OCR than non-diabetic patients (2.02 ± 0.911 vs. 2.79 ± 1.332 fmol/min/cell; p<0.05) and lower proton leak than non-diabetic patients (1.31 ± 0.686 vs. 1.94 ± 1.226 fmol/min/cell; p<0.05). Diabetes had no significant effect on oxygen utilization for ATP production, maximal OCR, spare respiratory capacity or ECAR.
Glaucoma patients had a lower baseline OCR than non-glaucoma patients (2.27 ± 1,193 vs. 2.83 ± 1.286 fmol/min/cell; p<0.05) and lower oxygen utilization for ATP production than non-glaucoma patients (0.72 ± 0.505 vs. 0.932 ± 0.452 fmol/min/cell; p<0.05). This decrease in oxygen utilization for ATP production accounted for the decreased baseline OCR in glaucoma patients. Glaucoma patients also had a lower maximal respiration than non-glaucoma patients (4.17 ± 2.294 vs. 5.46 ± 2.978 fmol/min/cell; p<0.05). Glaucoma had no significant effect on proton leak, spare respiratory capacity, or ECAR. Glaucoma patients trended to lower ECAR, but this difference did not reach statistical significance.
Gender and racial effects
Although females tended to have a higher baseline OCR than males, this difference was not statistically significant. Gender also had no effect on ECAR. Race also had no significant effect on OCR and ECAR.
Multivariate logistic regression analyses
Further analysis using multivariate logistic regression analysis confirmed that donors aged 70 and over (p<0.05), diabetic patients (p<0.01), and glaucoma patients (p<0.05) had significantly lower baseline OCR, independent of other variables. Gender and race had no significant effect on OCR. Multivariate logistic regression analysis confirmed that donors aged 70 and over (p<0.05), diabetic patients (p<0.01) had significantly less proton leak, independent of other variables. Glaucoma, gender and race had no significant effect on proton leak. Glaucoma patients had a lower baseline OCR than non-glaucoma patients (2.27 ± 1.193 vs. 2.83 ± 1.286 fmol/min/cell; p<0.05) and lower oxygen utilization for ATP production than non-glaucoma patients (0.72 ± 0.505 vs. 0.93 ± 0.451 fmol/min/cell; p<0.05) by Mann-Whitney U test. However, multivariate logistic regression analysis showed that glaucoma patients had no significant difference in oxygen utilization for ATP production (p=0.079). Moreover, age, diabetes, gender and race also had no significant effect on oxygen utilization for ATP production.
Discussion
This study revealed decreased mitochondrial function in lens epithelial cells (LECs) in older patients, glaucoma patients and diabetic patients – factors which may play a key role in cataractogenesis.
Mitochondria have been found in abundance in the lens epithelium and in differentiating fibers, and are known to play the most important role in lens metabolism.35, 36 Since LECs live in a hypoxic environment without vasculature, aqueous humor is the only source of oxygen and nutrients. Thus, we began our study by using rabbit LECs to investigate the specialization of LEC metabolism in comparison to other types of cells. In our analysis of oxygen consumption rate (OCR), we found that rabbit LECs showed the highest basal respiration, the lowest oxygen-dependent ATP production and highest proton leak as compared to other types of cells tested in this study. In addition, rabbit LECs had the highest maximal respiration among the cells evaluated. From this result, we hypothesized the mitochondria in LECs were more adaptable than mitochondria from other cell types. Remaining relatively quiescent under the baseline conditions, these cells are able to achieve maximum mitochondrial respiration when exposed to stimulation or stress. The high OCR, high proton leak and relatively low oxygen consumption for ATP production found in LECs, compared to other cell types, raises the possibility that lens mitochondria are specialized to consume oxygen. In addition, the increased number of mitochondria in the rabbit LECs compared to the other cell types tested seems to be consistent with the highest OCR measured during baseline respiration and maximal respiration.
It has been found that mitochondria account for approximately 90% of the total oxygen consumption in the lens.34 Furthermore, as mentioned, our previous study reported that the lens exists in a hypoxic environment which is important in maintaining lens transparency, and that oxygen levels are generally lowest closer to the lens.29, 30 Increased oxygen exposure has been identified as a risk factor for nuclear cataract, the most common type of age-related cataract.40 However, to our knowledge, mitochondrial function has not been evaluated directly using primary LECs from patients undergoing cataract surgery.
In the next stage of this study, we therefore established the human primary LEC culture, and measured OCR to assess whether age, diabetes, glaucoma and other intrinsic factors alter mitochondrial function and cataractogenesis. We found human primary LECs had higher oxygen-dependent ATP production and lower proton leak in basal respiration, as compared to rabbit LECs. That said, both rabbit and human LECs have lower oxygen-dependent ATP production than the other cell lines. This suggests that human LECs are also maintaining a high level of respiration without greatly depending on oxygen for ATP synthesis under non-stressful circumstances.
Our finding of lower OCR in older donors corroborates the fact that mitochondria decay in number and function with increasing age.23–25 From gross microscopic observations of these cells, there was no significant difference between younger cases and older cases in terms of estimated number of viable fresh LECs on the capsule (data not shown). We chose not to count the cell number or cell density on the fresh capsules since we prioritized the initiation of primary cultures as soon as possible following harvesting from the operating room. As noted above, the data was normalized per cell after mitochondrial function was measured using the Seahorse XF96 analyzer.
Diabetic patients had lower OCR than non-diabetic patients (p<0.05). Diabetes is notably associated with oxidative stress.47 Hyperglycemia-induced intracellular reactive oxygen species (ROS) are produced by the proton electrochemical gradient generated by the mitochondrial electron transport chain.48 It has also been shown in humans that the glucose concentration of the aqueous humor is 70% of that found in blood,49 and a direct correlation exists between the glucose concentration in the eye and blood glucose concentration with an average time lag of less than 5 minutes.50 On the other hand, it is known that sorbitol accumulates in the lens by the polyol pathway, and the excessive accumulation of sorbitol causes an increased osmotic load within the lens causing swelling, fiber cell breakdown, and opacification.51 We postulate that hyperglycemia in the aqueous humor caused a decrease in the OCR in diabetes patients.
Glaucoma patients had lower OCR than non-glaucoma patients (p<0.05). During lens capsule isolation and culture, the LECs from glaucoma patients tended to appear to have irregular edges and rough on the capsule under the microscope, and detached from the capsule easily during primary culture. Some of the primary cultures from glaucoma patients did not succeed as performed under the identical protocol as with cataract patients without glaucoma. As glaucoma patients commonly require topical eye medication to reduce intraocular pressure, the LECs of glaucoma patients may have sustained damage from high intraocular pressure as well as side effects of these various topical medications and preservatives. These drugs are applied topically to the ocular surface with relatively high concentration in the aqueous humor as compared with drugs administered systemically. Long term use of topical eye medications and associated preservatives are known to cause corneal epithelial disorders. These medications may also damage the LECs following penetration into the anterior chamber. Future studies may elucidate specific effects of these medications.
We demonstrated that age, diabetes and glaucoma were associated with significantly lower OCR during basal respiration, independent of other variables. Interestingly, the mechanism that causes the lower OCR in glaucoma was decreased proton leak, in contrast to the effects of increased age and diabetes, which had decreased oxygen utilization for ATP production. This finding suggests an alternative mechanism for decreased OCR in patients with glaucoma.
We also evaluated other intrinsic factors such as gender and race. Although males tended to have lower OCR and lower proton leak than females, and African-Americans tended to have lower OCR and lower proton leak than Caucasians, neither gender nor race had a statistically significant effect on the OCR of LECs. The trend toward lower OCR in African-Americans is consistent with our previous results, which found higher oxygen levels (reflecting lower oxygen consumption) in the anterior segment of African-Americans as compared to Caucasian patients.52
A recent study of the metabolic activity of human corneal endothelial cells obtained from donor corneal tissue was performed to compare the central and peripheral regions of the tissue to assess biochemical viability for corneal transplantation.53 Notably, there were no differences in the two regions and the measured metabolic activity of the corneal endothelial cells were comparable to our data utilizing a bovine corneal endothelial cell line (CCEE). Their data utilizing primary cell culture demonstrated lower basal respiration and higher maximal respiration, but remained consistently significantly lower than our data of primary LECs. We propose that the differences in our corneal data may be due to the fact that Greiner et al utilized primary human tissue culture and we utilized a cell line which had been isolated and sub-cultured several times.
In summary, oxygen consumption and proton production differ in cells derived from different tissues, with the highest oxygen consumption rate in a LEC line. Most of the oxygen consumption in lens cells is not used for ATP synthesis and the mitochondria in these cells have high “reserve capacity.” The lower O2 consumption in older donors and patients diagnosed with diabetes or glaucoma could contribute to cataract formation.
Conclusions
As it is well known that nuclear cataracts are caused by excessive oxidation of proteins in the lens nucleus, it is important to further understand the mechanism of cataract formation, the most common cause of reversible blindness worldwide. Our previous study reported that the lens exists in a hypoxic environment which is important in maintaining lens transparency, and that exposure to increased oxygen has been identified as a risk factor for nuclear cataract. Mitochondria account for approximately 90% of the total oxygen consumption in the lens, and those in the lens epithelium play the most important role in lens metabolism. These unique epithelial cells contain a high density of mitochondria with specialized energy metabolism as compared to other cells in our study. Our results in this study of mitochondrial function of lens epithelial cells show that the lower oxygen consumption rate of human LECs in older donors and patients with diabetes or glaucoma, independent of age, could contribute to cataract formation. Ongoing studies are examining the oxygen environment at the surface of the lens in vivo and oxygen consumption in the LECs of patients with glaucoma and diabetes.
Supplementary Material
Figure 2.
Figure 2A – Confocal microscopy of Mito Tracker Red CMXRos staining of different cell lines. 2B – Average fluorescence of mitochondrial staining. (* p<0.05, ** p<0.001 compared to NN1003A). Rabbit lens epithelial cell (NN1003A) staining revealed the highest density of mitochondria within the cells compared to canine kidney epithelial cells (MDCK; p<0.05), bovine corneal endothelial cells (CCEE; p<0.001) and human trabecular meshwork cells (TM-5; p<0.001).
HIGHLIGHTS.
Measurement of oxygen consumption rate (OCR) in four mammalian cell lines
Lens epithelial cells (LEC) exhibit higher OCR indicating specialized metabolism
Mitochondrial function measurement of human LECs taken from surgical patients in vivo
Older age, diabetes and glaucoma were associated with significantly lower OCR
Decreased mitochondrial function in LECs may contribute to cataractogenesis
Acknowledgments
We thank Drs. Shunsuke Kubota, Gokul Kumar, Ian Pitha, and Arsham Sheybani for their assistance in collecting patient samples and data analysis. We especially appreciate the contributions of Teri Davidson and Laura Luecking of the Tissue Culture Support Center for their assistance with the Seahorse XF Analyzer. Research was supported by NIH grant EY021515 to Dr. Carla Siegfried, Core Grant EY02687 to the Department of Ophthalmology and Visual Sciences and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M Kubota, Email: myu.kubota@gmail.com.
YB Shui, Email: shui@vision.wustl.edu.
M Liu, Email: margs.liu@gmail.com.
F Bai, Email: baifang@hotmail.com.
AJ Huang, Email: huanga@vision.wustl.edu.
N Ma, Email: manan840808@163.com.
CJ Siegfried, Email: siegfried@vision.wustl.edu.
References
- 1.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80(5):709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, Broman KW, Lai H, et al. Nuclear cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Invest Ophthalmol Vis Sci. 2004;45(7):2182–6. doi: 10.1167/iovs.03-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnier VM, Sell DR, Nagaraj RH, et al. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 4.Harding JJ, Egerton M, van Heyningen R, Harding RS. Diabetes, glaucoma, sex, and cataract: analysis of combined data from two case control studies. Br J Ophthalmol. 1993;77(1):2–6. doi: 10.1136/bjo.77.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342(24):1786–90. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 6.Iyengar SK, Klein BE, Klein R, et al. Identification of a major locus for age-related cortical cataract on chromosome 6p12-q12 in the Beaver Dam Eye Study. Proc Natl Acad Sci U S A. 2004;101(40):14485–90. doi: 10.1073/pnas.0400778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty CA, Taylor HR. Recent developments in vision research: light damage in cataract. Invest Ophthalmol Vis Sci. 1996;37(9):1720–3. [PubMed] [Google Scholar]
- 8.Hutnik CM, Nichols BD. Cataracts in systemic diseases and syndromes. Curr Opin Ophthalmol. 1999;10(1):22–8. doi: 10.1097/00055735-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Foster PJ, Wong TY, Machin D, et al. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: the Tanjong Pagar Survey. Br J Ophthalmol. 2003;87(9):1112–20. doi: 10.1136/bjo.87.9.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mares JA, Voland R, Adler R, et al. Healthy diets and the subsequent prevalence of nuclear cataract in women. Arch Ophthalmol. 2010;128(6):738–49. doi: 10.1001/archophthalmol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68(2):113–7. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freel CD, Gilliland KO, Mekeel HE, et al. Ultrastructural characterization and Fourier analysis of fiber cell cytoplasm in the hyperbaric oxygen treated guinea pig lens opacification model. Exp Eye Res. 2003;76(4):405–15. doi: 10.1016/s0014-4835(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 13.Simpanya MF, Ansari RR, Suh KI, et al. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46(12):4641–51. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst P, Baltzan M, Deschenes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006;27(6):1168–74. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- 15.Klein AP, Duggal P, Lee KE, et al. Polygenic effects and cigarette smoking account for a portion of the familial aggregation of nuclear sclerosis. Am J Epidemiol. 2005;161(8):707–13. doi: 10.1093/aje/kwi102. [DOI] [PubMed] [Google Scholar]
- 16.Latkany P, Ciulla TA, Cacchillo PF, Malkoff MD. Mitochondrial maculopathy: geographic atrophy of the macula in the MELAS associated A to G 3243 mitochondrial DNA point mutation. Am J Ophthalmol. 1999;128(1):112–4. doi: 10.1016/s0002-9394(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 17.Servidei S, Zeviani M, Manfredi G, et al. Dominantly inherited mitochondrial myopathy with multiple deletions of mitochondrial DNA: clinical, morphologic, and biochemical studies. Neurology. 1991;41(7):1053–9. doi: 10.1212/wnl.41.7.1053. [DOI] [PubMed] [Google Scholar]
- 18.Finsterer J, Bittner R, Bodingbauer M, et al. Complex mitochondriopathy associated with 4 mtDNA transitions. Eur Neurol. 2000;44(1):37–41. doi: 10.1159/000008190. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso M, Filosto M, Bellan M, et al. POLG mutations causing ophthalmoplegia, sensorimotor polyneuropathy, ataxia, and deafness. Neurology. 2004;62(2):316–8. doi: 10.1212/wnl.62.2.316. [DOI] [PubMed] [Google Scholar]
- 20.Atay Z, Bereket A, Turan S, et al. A novel homozygous TMEM70 mutation results in congenital cataract and neonatal mitochondrial encephalo-cardiomyopathy. Gene. 2013;515(1):197–9. doi: 10.1016/j.gene.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Reynier P, Amati-Bonneau P, Verny C, et al. OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract. J Med Genet. 2004;41(9):e110. doi: 10.1136/jmg.2003.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babizhayev MA. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem Funct. 2011;29(3):183–206. doi: 10.1002/cbf.1737. [DOI] [PubMed] [Google Scholar]
- 23.Tauchi H, Sato T. Age changes in size and number of mitochondria of human hepatic cells. J Gerontol. 1968;23(4):454–61. doi: 10.1093/geronj/23.4.454. [DOI] [PubMed] [Google Scholar]
- 24.Herbener GH. A morphometric study of age-dependent changes in mitochondrial population of mouse liver and heart. J Gerontol. 1976;31(1):8–12. doi: 10.1093/geronj/31.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123(3):951–7. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stocco DM, Hutson JC. Quantitation of mitochondrial DNA and protein in the liver of Fischer 344 rats during aging. J Gerontol. 1978;33(6):802–9. doi: 10.1093/geronj/33.6.802. [DOI] [PubMed] [Google Scholar]
- 27.Yen TC, Chen YS, King KL, et al. Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun. 1989;165(3):944–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]
- 28.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegfried CJ, Shui YB, Holekamp NM, et al. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51(11):5731–8. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shui YB, Fu JJ, Garcia C, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47(4):1571–80. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 31.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–10. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Ely LO. Metabolism of the crystalline lens; respiration of the intact lens and its separated parts. Am J Ophthalmol. 1949;32 Pt 2(6):220–4. [PubMed] [Google Scholar]
- 33.Barbazetto IA, Liang J, Chang S, et al. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78(5):917–24. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Bantseev VL, Herbert KL, Trevithick JR, Sivak JG. Mitochondria of rat lenses: distribution near and at the sutures. Curr Eye Res. 1999;19(6):506–16. doi: 10.1076/ceyr.19.6.506.5279. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, Yappert MC, Jumblatt MM, Borchman D. Hyperoxia and thyroxine treatment and the relationships between reactive oxygen species generation, mitochondrial membrane potential, and cardiolipin in human lens epithelial cell cultures. Curr Eye Res. 2008;33(7):575–86. doi: 10.1080/02713680802167554. [DOI] [PubMed] [Google Scholar]
- 36.Kinsey VE, Reddy DV. STUDIES ON THE CRYSTALLINE LENS. XI. THE RELATIVE ROLE OF THE EPITHELIUM AND CAPSULE IN TRANSPORT. Invest Ophthalmol. 1965;4:104–16. [PubMed] [Google Scholar]
- 37.Hagen TM, Wehr CM, Ames BN. Mitochondrial decay in aging. Reversal through supplementation of acetyl-L-carnitine and N-tert-butyl-alpha-phenyl-nitrone. Ann N Y Acad Sci. 1998;854:214–23. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 38.Spector A. Oxidative stress-induced cataract: mechanism of action. Faseb j. 1995;9(12):1173–82. [PubMed] [Google Scholar]
- 39.Dische Z, Zil H. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. Am J Ophthalmol. 1951;34(5:2):104–13. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- 40.Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19(2):125–33. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf HK, Buslei R, Schmidt-Kastner R, et al. Neu N: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44(10):1167–71. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 42.Wolf N, Penn P, Pendergrass W, et al. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81(3):276–85. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13(5–6):268–74. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Invernizzi F, D’Amato I, Jensen PB, et al. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 2012;12(2):328–35. doi: 10.1016/j.mito.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubota M, Shimmura S, Miyashita H, et al. The anti-oxidative role of ABCG2 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(11):5617–22. doi: 10.1167/iovs.10-5463. [DOI] [PubMed] [Google Scholar]
- 46.Inc. SB; Inc. SB, editor. User Manual XF cell mito stress test kit. 2012. [Google Scholar]
- 47.Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347(8999):444–5. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 49.Pohjola S. The glucose content of the aqueous humour in man. Acta Ophthalmol (Copenh) 1966;(Suppl 88):1–0. [PubMed] [Google Scholar]
- 50.Cameron BD, Baba JS, Cote GL. Measurement of the glucose transport time delay between the blood and aqueous humor of the eye for the eventual development of a noninvasive glucose sensor. Diabetes Technol Ther. 2001;3(2):201–7. doi: 10.1089/152091501300209552. [DOI] [PubMed] [Google Scholar]
- 51.Datta V, Swift PG, Woodruff GH, Harris RF. Metabolic cataracts in newly diagnosed diabetes. Arch Dis Child. 1997;76(2):118–20. doi: 10.1136/adc.76.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegfried CJ, Shui YB, Holekamp NM, et al. Racial differences in ocular oxidative metabolism: implications for ocular disease. Arch Ophthalmol. 2011;129(7):849–54. doi: 10.1001/archophthalmol.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greiner MA, Burckart KA, Wagoner MD, et al. Regional assessment of energy-producing metabolic activity in the endothelium of donor corneas. Invest Ophthalmol Vis Sci. 2015;56(5):2803–10. doi: 10.1167/iovs.15-16442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.