Abstract
While the cochlea is considered the primary site of the auditory response to bone conduction (BC) stimulation, the paths by which vibratory energy applied to the skull (or other structures) reaches the inner ear are a matter of continued investigation. We present acoustical measurements of sound in the inner ear that separate out the components of BC stimulation that stimulate the inner ear via ossicular motion (compression of the walls of the ear canal or ossicular inertia) from the components that act directly on the cochlea (cochlear compression or inertia, and extra-cochlear ‘third-window’ pathways). The results are consistent with our earlier suggestion that the inner-ear mechanisms play a large role in bone-conduction stimulation in the chinchilla at all frequencies. However, the data also suggest the pathways that conduct vibration to the inner ear via ossicular-motion make a significant contribution to the response to BC stimulation in the 1 to 3 kHz range, such that interruption of these path leads to a 5 dB reduction in total stimulation in that frequency range. The mid-frequency reduction produced by ossicular manipulations is similar to the ‘Carhart's notch’ phenomenon observed in otology and audiology clinics in cases of human ossicular disorders. We also present data consistent with much of the ossicular-conducted sound in chinchilla depending on occlusion of the ear canal.
Keywords: Bone conduction, chinchilla, intracochlear sound pressures, Carhart's notch
Introduction
Bone conduction (BC) describes a collection of mechanisms in which hearing sensation is initiated by vibrations of the skull bones (Bekesy 1960; Wever and Lawrence 1954; Tonndorf 1970). Like the conventional hearing mechanism termed air conduction (AC), the mechanisms in BC involve the outer ear, the middle ear, and the inner ear. Unlike AC, where the sound energy presented to the external ear is conducted along a single ‘series’ path (which includes the motion of the TM and ossicles) to the inner ear, BC sound transmission is parallel in nature, in that skull vibrations directly and simultaneously stimulate the external, middle, and inner ear. This parallel stimulation of multiple sound paths provides great complexity and challenges our ability to isolate and quantify the contribution of each individual mechanism.
Wever and Lawrence (1954) categorized the multiple bone conduction stimulus paths to the inner ear into two groups: The middle-ear conduction group that depends on ossicular conduction of sound to the inner ear—these include ear-canal compression and ossicular inertia (Tonndorf 1966)—and the direct inner ear stimulation group that involves the production of volume velocity in the cochlear fluids via the inertia of those fluids, compression of the cochlear capsule, and potential ‘third’ cochlear windows that couple sound volume velocity produced by compression of cranial soft-tissues (Yoshida et al. 1991; Freeman et al. 2000).
A series of studies that are central to our work are the demonstrations by Bekesy (1960) and others (e.g. Khanna et al. 1976, Stenfelt 2007) of cancellation of the perception of bone conduction tonal vibrations by air conducted tones of identical frequency and appropriate magnitude and phase. This result is consistent with the hypothesis that the cochlear acoustic and mechanical events that lead to stimulation of the sensory cells within the hearing organ are common to both AC and BC stimulation, regardless of the signal conduction pathway (e.g. Stenfelt et al. 2003; Chhan et al. 2013). Demonstrations of the similarity of BC induced cochlear sound pressures and cochlear potential are consistent with this hypothesis (Chhan 2013).
Previous work in our laboratory (Songer et al. 2008; Chhan et al. 2013) suggests inner ear mechanisms contribute significantly to bone conduction in chinchillas. We also observed an imbalance in the effect of ossicular interruption on the sound pressures produced in scala vestibuli PSV and tympani PST by vibration of the skull, which we hypothesized was due to the interruption-induced decrease in load impedance on the oval window that would impede the sound produced by inner-ear sources of BC stimulation (Chhan et al. 2013). In the present work, we directly test this hypothesis by measuring PSV and PST near the cochlear base in anesthetized chinchillas with AC and BC stimulation while decreasing the mobility of both the stapes and the round window (RW) membrane to increase the load impedance of the middle ear seen by any inner-ear based bone-conduction mechanisms. We also occlude the ear canal, which we demonstrate produces effects on BC stimulation that are similar to reducing the mobility of the footplate. This combination of manipulations together with our early reports demonstrate that: (1) an increase in the impedance of the stapes and round-window can have significant effects on BC-induced inner-ear sound pressures, but these effects are inconsistent with changes in the load of the windows on the inner ear, (2) The effects of impedances changes at the oval window on inner-ear sound pressures are more consistent with a decrease in ossicularly conducted bone-conducted sound, and (3) part of the effects of increasing the impedance at the stapes is to reduce the contribution of bone-conduction related ossicular motion producing an analog of Carhart's notch, a clinical finding associated with ossicular disorders in patients (Carhart 1952).
2. Methods and Materials
2.1 Animal preparation
All animal procedures were reviewed and approved by the Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary. A detailed description of most of the surgical procedures and animal preparation can be found elsewhere (Songer and Rosowski, 2006). Briefly, each animal was anesthetized with pentobarbital (50 mg/kg) and ketamine (40 mg/kg), and their tracheas cannulated. The superior and posterior middle-ear cavities were opened. The tendon of the tensor tympani muscle was cut and the tympanic-portion of the VIIth nerve was sectioned to immobilize the stapedius muscle to prevent random contraction that can alter middle-ear sound transfer (see Rosowski et al. 2006). The bony outgrowth of the facial canal lateral and posterior to the round window (RW) was removed to visualize the surface of the round window and stapes footplate. The external ear was left intact, but a probe-tube microphone was sealed in the lateral end of the bony ear canal to measure ear canal sound pressure PEC within 1 to 2 mm of the umbo of the malleus. To measure scala vestibuli sound pressure PSV, a small hole (less than 250 μm in diameter) was made in the wall of the vestibule medial to the saccule after removal and retraction of a portion of the cerebellum. A hole made within 2 mm of the cochlear base near the round window was used to place a pressure sensor to measure scala tympani sound pressure PST. Both PSV and PST measurements were made with in-house built micro-optical pressure sensors (Olsen 1998).
2.2 Acoustic and vibration stimuli
Air-conducted sound was delivered to the ear canal via an ER-3A insert earphone (Etymotic) with a yellow foam plug probe tip placed within the cartilaginous ear canal. Bone conduction stimuli were delivered by a BAHA, Bone-Anchored Hearing Aid (Cochlea BAHA BP100) attached to a titanium screw implanted on the animal's skull near the vertex. The use of the BAHA stimulator allowed manipulation of the animals’ heads during experiments without affecting stimulus reproducibility. Since the chinchilla's skull is thin, the standard 3 mm titanium screw was inserted only 1 mm deep into the skull, and dental cement was used as a foundation and space filler to hold the screw in place. The BAHA was in its ‘direct audio input’ mode where electrical stimuli are presented directly to the vibration processor and the microphone input to the processor is muted. The BAHA was programmed for ‘linear’ operation over the range of stimulus levels used (0.1 to 100 mv). The stimulus voltage input to either the ER-3A earphone or the BAHA was generated by a National Instruments I/O board controlled by a custom-built LabVIEW program. A TDT-programmable attenuator was used to control the stimulus levels. The velocity of the head produced by the BAHA is bandpass in shape with a 10 dB bandwidth of 0.5 and 4.5 kHz, and a maximum around 1.6 kHz (see Supplemental Material). The measurement protocol called for simultaneous measurements of ear canal sound pressure PEC, scala vestibuli PSV, and scala tympani PST sound pressures during repeated presentations of stepped pure tones of 0.1 to 10 kHz at 24 points/octave.
2.3 Microphone and sensor calibration
The probe-tube microphone used to measure ear canal sound pressure PEC was calibrated against a 1/4” reference microphone (Larson-Davis 2530) using a broadband chirp stimulus (Ravicz and Rosowski 2013). Microphone calibration was performed at the beginning of each experiment.
Calibrations of the intra-cochlear pressure sensors were performed in water. The sensor tips were submerged 5 millimeters deep into a column of water sitting on a shaker head and accelerometer (Bruel and Kjaer 4290; see Olson, 1998). Measurements of sensor frequency response are determined from presentations of stepped sinusoids of the 0.1 to 10 kHz that are input to the accelerometer. To check the sensitivity and stability of each sensor probe, we performed pre- and post-measurement calibrations. The scheme is as follow:
-A pre-measurement calibration was made prior to insertion of the sensor into the vestibule to measure PSV or into the scala tympani, behind the round window membrane through the basal cochlear wall, to measure PST. The insertion depth of the sensors was between 100 and 300 μm.
-After insertion of the transducers, PSV and PST were measured with AC and BC stimulation with neither transducer sealed in place.
-The transducers were removed and a post-measurement calibration was made and compared to the pre-measurement calibration. If the sensitivity was within our stability criterion of ±3 dB, we proceeded with the same sensors, otherwise new sensors were introduced and the process repeated. At the end of this stage, we have a set of repeatable PSV and PST measurements in the normal condition with both AC and BC stimulation. The ±3 dB stability criteria we utilize introduces uncertainty in our quantification of the intra-cochlear pressure difference, ΔP = PSV - PST, which is central to our measurements strategy. These uncertainties are relatively small when the absolute value of the actual ΔP is larger than the calibration errors, but become sizeable when the actual ΔP is close in magnitude to the calibration uncertainties (see Supplemental Material).
- PSV and PST sensors were reinserted into their respective holes. The holes were then sealed with Jeltrate (an alginate-based dental impression material) and acrylic dental cement. Jeltrate sealed the PSV and PST holes around the transducer shafts preventing fluid egress from the inner ear, while dental cement affixed the sensor to the cochlear bone. The use of dental cement greatly reduced relative motions between the sensors and the ear during BC stimulation. Once the dental cement covering the holes firmed up, the sensor fibers were released from the manipulator so they could move freely with the bone. The sensors in the vestibule and scala tympani were placed within 100 to 300 μm of the boney wall of the inner ear, and were at least 300-500 μm from the cochlear partition. This sensor placement restricted the sound pressures to which they were exposed to the pressures associated with the ‘fast’ compressional wave of sound within the inner ear, with little effect of the ‘slower’ pressure changes associated with local motions of the cochlear partition (Peterson and Bogert 1950; Olsen 2001).
-After sealing the sensors in the ear, another set of PSV and PST measurements were made in the normal condition before manipulations (i.e. stapes immobilization and incudostapedial joint interruption) occurred. These measurements were repeated several times and compared to the earlier set of measurements with the holes unsealed to make sure that we had consistent PSV and PST measurements before we proceeded with the manipulations. Sealing the sensor holes generally caused a slight increase in the PSV produced by AC stimulation (Slama et al. 2010), but had little effect on the PST produced by AC or either pressure produced by BC stimulation.
-Manipulation of the ear canal and middle ear were performed and PSV and PST were measured repeatedly.
-If possible, we calibrated the sensors after the experiment. Post-measurement calibrations at the end of the experiment were not always possible: sometimes the sensors broke because of the quality of the dental cement seal to the inner-ear bone. Thus we sometimes relied on the pre-measurement calibration and the time course of the repeated in-the-ear measurements to identify and track any changes in sensor sensitivity.
2.4 Experimental procedure
Measurements of PEC, PSV, and PST were made with both AC and BC before and after ear canal occlusion and manipulation of the middle ear. Measurements were made at a variety of stimulus levels (over a 20 dB range), and were consistent with linear responses. Once we established a good series of stable pressure sensor measurements in the normal condition, we proceeded to the manipulations. The manipulations include using a tendon knife to separate the incudo-stapedial joint (ISJ), using acrylic cement to decrease the mobility of the stapes by affixing both the posterior stapes crus and posterior part of the stapes footplate to the wall of the oval window, and using acrylic cement to immobilize the round window membrane by capping it with a sheet of cement affixed to the bony surround of the window. After these manipulations, measurements of PEC, PSV, and PST were repeated in both AC and BC. The animal remained anesthetized throughout the whole procedure and was euthanized after completion of the experiment. In order to separate the potential effects of ear-canal compression from ossicular inertia, we also made BC measurements in the normal and post-manipulation conditions before and after removing the ER3 earphone with its yellow foam plug that served to occlude the lateral end of the ear canal.
2.5 Data analysis
For the most part, data reported in this paper only includes those measurements in which the sensitivity of the pressure sensor varied by less than ±3 dB between pre- and post-measurement calibrations. In some cases, adjustments to the sensitivity of the sensors were made based on small changes in response seen during repeated measurements, e.g. the change in the sensor sensitivity post-measurement calibration was similar to a change in the measured pressure observed over repeated measurements. The pre-measurement calibration was also used for measurements in which the sensors were glued to the bones by dental cement and unrecovered at the end of the experiment where there was a significant record of data stability (i.e. very repeatable measurements except at the instant of the introduction of an ossicular manipulation). All data were gathered as frequency-dependent functions, which were smoothed in the frequency-domain using the Savitzky-Golay function in MATLAB. The smoothing used a cubic-polynomial fit to windows of 7 data points. After smoothing, only data gathered with a signal-to-noise ratio of 10 dB or higher are included in the analysis (signal to noise was estimated by observation of the magnitude of off-stimulus frequency components determined when we computed Fourier transforms of the responses to sinusoidal stimulation). Furthermore, data are only plotted between 200 Hz to 8 kHz, the range corresponding to the pass band of the BAHA stimulator (See supplementary information). For comparison with measurements taken in different ears, the AC data were normalized by ear canal sound pressure PEC. BC magnitude data were normalized by the voltage used to drive the BAHA (generally no larger than 100 mv rms), while the phases of the BC evoked data were normalized by the phase of the BC excited velocity of the cochlear bone (Stenfelt et al. 2004).
3. Results
3.1 Intracochlear sound pressures in normal ears with AC and BC stimulation
Intracochlear differential sound pressure ΔP (the difference between scala vestibuli and scala tympani sound pressures defined by ΔP = PSV - PST), the acoustic input drive to the cochlea, is a useful tool to study sound transmission with different conditions of the auditory conductive apparatus (the tympanic membrane and the middle ear) and different types of stimuli (e.g. AC and BC). It has been demonstrated that ΔP is proportional to the sensory output of the cochlea, and is a good indicator of hearing sensitivity (Dancer & Franke, 1980; Lynch et al. 1982).
Figure 1 shows the magnitude and phase of scala vestibuli sound pressure PSV, scala tympani sound pressure PST, and differential sound pressure ΔP in a normal ear with air conduction and bone conduction stimulation from one representative experiment. In air conduction, the two intracochlear sound pressures differ largely at low frequency, but the differences narrow as frequency increases. The PSV and PST are affected by the mostly-resistive cochlear input impedance and the highly compliant round window impedance (Ravicz et al. 2010). The notch at around 3 kHz results from a maximum in the middle-ear input impedance related to a parallel resonance between the middle ear cavity and the opening in the cavity wall (Guinan and Peake, 1967; Rosowski et al. 2006). At most frequencies the intracochlear differential sound pressure ΔP resembles PSV both in magnitude and phase, because PSV magnitude is larger than PST. Only at higher frequencies where PSV and PST are more similar do we see ΔP differ significantly from PSV. In bone conduction, PSV and PST differed by about 10 dB across a wide range of frequency. The difference in frequency responses of the intracochlear sound pressures in AC and BC is related to the relatively broad-band AC output of the ER3A earphone that is modulated by the impedance of the chinchilla middle ear, and the band pass output of the BAHA BC source (see supplemental material).
Figure 1.
Magnitudes and phase-angles of PSV, PST and ΔP in AC and BC in a normal ear (Experiment DC9_141106). In AC stimulation, the intracochlear sound pressures are normalized by the ear canal sound pressure, while in bone conduction, they are normalized by the voltage input drive to the BAHA sound processor. Effective stimulation produced by this voltage is quantified in the supplemental material by the velocity of the petrous-bone produced by BAHA stimulation. Because the BAHA sound processor introduces a large phase delay the phase of the BC generated intracochlear sound pressure measurements are normalized by the phase of the BC generated velocity of the petrous bone. The BC measurements were made while the ear canal was occluded by the AC source.
3.2 Effects of stapes immobilization in AC and BC
The mobility of the stapes was significantly reduced by applying dental cement between the posterior crus of the stapes, posterior part of the footplate, and the cochlear bone. (The reduction in mobility occurs due to an increase in the stiffness of the stapes in the oval window and a potential small increase in the stapes mass.) Because of the wet environment around the stapes, ‘fixing’ the stapes is a challenge. Careful removal of serous middle-ear fluid enabled curing of the cement and prevented the flow of cement to unwanted areas (e.g. the round window membrane). The post-cement AC measurements quantify the degree of immobilization. Figure 2 shows the magnitude of the intracochlear sound pressures PSV and PST before and after stapes immobilization from one experiment with AC and BC stimulations. In Figure 3 we plot the ratio of the sound pressure measured after immobilization of the stapes to the sound pressure measured in the normal ear (this ratio is illustrated as a dB difference and denoted by Δ). Figure 3 plots the mean and standard deviations of these ratios measured with AC and BC stimulation in 8 ears. In AC stimulation, the 25 to 30 dB decreases in PSV and PST at frequencies below 2 kHz and the 5 to 10 dB decreases at higher frequencies are the result of a reduction in the transmission of sound from the ear canal to the inner ear due to the more-impedant stapes. In BC, the immobilization induced changes in PSV and PST are different from each other (Figure 2 & 3) and much smaller than the changes in AC. Decreases in stapes mobility induced only small changes (5 to 10 dB) in the BC induced intracochlear sound pressures, but in general the effects were larger on PSV than PST : Paired Student-t-tests comparing 315 ΔPSV and ΔPST measurements made at 40 frequencies between 1 and 3.2 kHz in 8 ears (not all measurements in each ear had a signal-to-noise ratio greater than 10 dB) yield a mean dB difference (ΔPSV (dB) – ΔPST (dB)) of - 4.5 dB with a standard deviation of ± 7.76 dB and a probability that the difference is 0 dB of p < 0.0001. Similar comparisons of the 139 measurements made at 27 frequencies between 3.2 and 10 kHz in the 8 ears yield a mean difference of 5.41 ± 6.03 dB with a probability that the difference is 0 dB of p < 0.0001). The Carhart-notch-like mean 4.1 ± 4.94 dB decrease in the BC evoked differential intracochlear sound pressure ΔP between 1 and 3 kHz measured at 315 frequency-ear combinations was significantly different from 0 dB at p < 0.001.
Figure 2.
Magnitudes of PSV and PST with AC and BC before and after stapes fixation in a single ear. Only data gathered at frequencies where signal to noise was better than 10 dB are included in the plots. The BC measurements were made while the ear canal was occluded by the AC source. Note the stapes fixation induced 10 dB drop in PSV in the 1 to 3 kHz frequency range.
Figure 3.
The change in intracochlear sound pressures PSV, PST and ΔP produced by immoblizing the stapes in AC and BC plotted as means and standard deviations (n=8 or 9). The BC measurements were made while the ear canal was occluded by the AC source. The broad horizontal bars in the BC plots demark the 1 to 3 kHz range in PSV and ΔP in regions where the mean of all of the measurements made in that range was at least – 4 dB re the control condition and significance testing showed the chance the mean difference was 0 dB is p < 0.0001.
3.3 Effects of interrupting the incudostapedial joint in AC and BC
Interrupting the incudostapedial joint significantly reduced the sound conduction pathway from the outer- to the inner-ear and resulted in a significant (20 to 40 dB) reduction in the AC-generated intracochlear sound pressures (Figure 4). The effect of this interruption on BC-generated scala pressures was much smaller. A decrease of about 10 dB between 1 and 3 kHz in scala vestibule sound pressure PSV and ΔP suggests either a direct contribution of the middle ear to the inner-ear pressures, which was removed by joint interruption, or a decrease in middle-ear ear load on the oval window (Chhan et al. 2013). Again, the effect of the manipulation was significantly larger on PSV than PST (p< 0.001) when averaged over the 461 data points at frequencies between 1 and 3.1 kHz in the 11 ears.
Figure 4.
Effects of incudostapedial joint interruption on the intracochlear sound pressures PSV, PST and ΔP in AC and BC plotted as means and standard deviations (n=13). The BC measurements were made while the ear canal was occluded by the AC source. The broad horizontal bars in the BC plots demark the 1 to 3 kHz range in PSV and ΔP in regions where the mean of all of the measurements made in that range was at least – 8 dB re the control condition and significance testing showed the chance the mean difference was 0 dB is p < 0.0001.
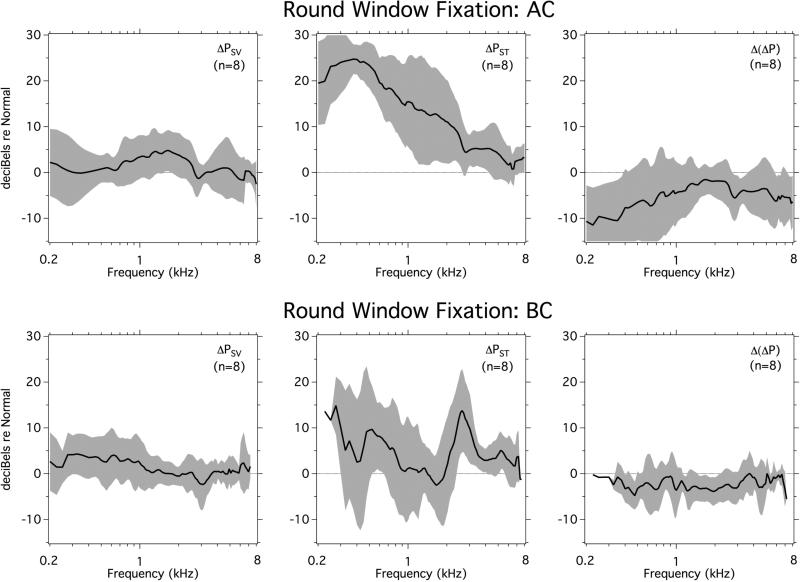
3.4 Effects of round window membrane fixation
Round window membrane fixation should increase the impedance looking out the round window from the inner ear, and is expected to lead to an increase in the PST produced by both AC and BC stimulation. The middle panels in Figure 5 are consistent with this prediction, where the increase in PST seen in AC stimulation at 0.5 kHz is about 25 dB, which is larger than the 8-10 dB increase in PST seen in BC stimulation near that frequency. The increase in AC conducted PST makes this sound pressure more similar to PSV and leads to a decrease in AC induced ΔP at frequencies below 1 kHz, where an equalization of PSV and PST is consistent with the simple two-window cochlear model, in which the terminating impedance at the round window is increased from negligible to significant. In contrast, while BC induced PST is also increased by round-window immobilization, PSV is little changed, and we see little change in ΔP in BC.
Figure 5.
Effects of round window fixation on the intracochlear sound pressures PSV, PST and ΔP in AC and BC plotted as means and standard deviations (n=8).
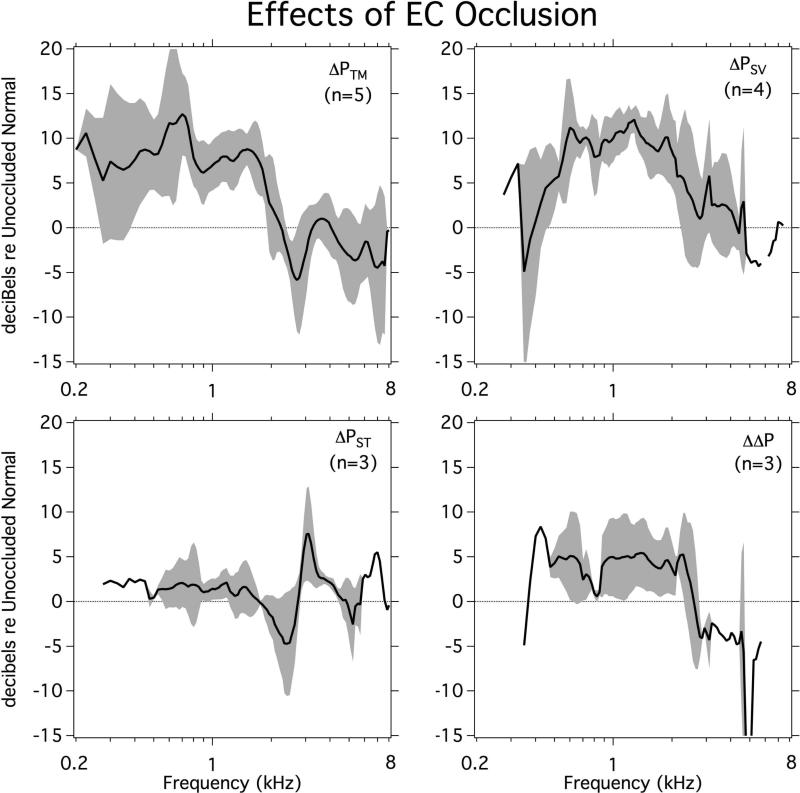
3.5 Effects of occluding the ear canal in BC stimulation
As a control for the presence of the AC sound source in the ear canal of the chinchilla we also performed a few measurements where we stimulated with BC in normal chinchilla ears before and after occluding the ear canal by placement of the AC source. Figure 6, illustrates the average and standard deviation of the change in PEC, PSV, PST and ΔP after occluding the ear canal. As might be expected, occlusion was related to a significant increase in BC generated PEC, especially at frequencies below 2 kHz. It also produced a 5 to 10 dB bandpass increase (0.4 to 2.5 kHz) in PSV, a 0 to 5 dB increase in ΔP, and less of an effect on PST.
Figure 6.
The effect of unoccluding the ear canal on PEC and intracochlear pressures PSV, PST and ΔP produced by BC Stimulation plotted as means and standard deviation (n=3 to 5).
4. Discussion
4.1 Contribution of the outer and middle ear in bone conduction in the mid frequency range
The three middle ear ossicles—the malleus, incus, and stapes—are suspended in the middle-ear cavity. During bone conduction stimulation, vibration of the head allows the middle-ear ossicles to act as free masses that are loosely attached to the head by the spring-like action of the tympanic membrane, ligaments and tendons. At low frequencies, inertia would cause the middle-ear ossicles to vibrate in-phase with the surrounding bone. At higher frequencies, the presence of the compliant coupling between the ossicles and skull would result in inertia related relative motions of the ossicles and inner ear that stimulate the cochlea much like the ossicular motions produced by AC stimulation. Consistent with this picture, Stenfelt and coworkers experimentally showed that in human cadaveric specimens the ossicles vibrated with the surrounding bone at low frequencies and showed large relative motion around the middle ear resonance frequency of 1.5 kHz (Stenfelt et al. 2002). These observations suggest that middle-ear inertia can be significant for human BC perception at frequencies between 1 and 3 kHz (Stenfelt 2006).
Our demonstration of a 5 to 10 dB decrease in the differential intracochlear sound pressure ΔP measured in live chinchillas at frequencies between 1 and 3 kHz after stapes fixation and incudostapedial joint interruption is consistent with a contribution of the middle ear to BC activation of the inner ear. However, our manipulation data were performed with an occluded ear canal, and it is possible that the BC related ossicular motions that were reduced by our ossicular manipulations resulted from sound pressures produced in the ear canal by ear-canal compression (Tonndorf 1966; Khanna et al. 1976). Indeed, the removal of the 5 to 10 dB increase in PSV and ΔP produced in the 1 to 3 kHz range by ear-canal occlusion (Figure 6) can explain much of the mid-frequency ‘Carhart-notch-like’ decrease in PSV and ΔP seen after stapes fixation and incudostapedial joint interruption when the ear canal is occluded (Figures 3 & 4).
These data suggest that ear-canal occlusion and the concurrent increase in the sound pressure produced by ear-canal compression have a big effect on bone conduction hearing in chinchillas. This interpretation is consistent with our observations that unoccluding the ear canal, interrupting the ossicular chain or immobilizing the stapes all produce a 5 to 10 dB Carhart's notch-like decrease in ΔP between 1-3 kHz. Therefore, it appears the Carhart's notch-like change in BC sensitivity observed in the chinchilla with occluded ear canals results primarily from a loss of the occlusion effect and not a result of a decrease in middle-ear inertia.
We previously suggested the ossicular-interruption induced decrease in BC-induced PSV and ΔP might be mediated by a decrease in the load of the middle ear on inner-ear sound sources; however, our new observations that immobilizing the stapes produces a similar decrease in PSV and ΔP argues against this suggestion. Immobilization of the footplate increases the load of the middle ear on the oval window, and should have lead to an increase in inner-ear sound pressure produced by inner-ear sources. Furthermore, our observation that fixation of the round window did lead to increases in PST, demonstrate that our methods were sensitive to such an effect. Therefore, the most likely interpretation of our present results is that manipulations that interfere with the generation and ossicular conduction of bone-conducted sound generated within the middle and external ear (e.g. ossicular fixation or interruption, as well as unocclusion of the ear canal) leads to a decrease in PSV, irrespective of whether the manipulation increases or decreases the middle-ear load on the inner ear. Regarding the relative size of the ossicularly conducted BC sound to the inner ear, the small size (~5 dB) and limited frequency range of the reductions in BC-induced PSV that we observed, even with total ossicular interruption, suggest ossicularly conducted BC sound in chinchilla is generally smaller than the BC sound generated by inner-ear sources. Stenfelt and coworkers (2005, 2015) have come to similar conclusions in humans.
4.2 A possible role of cochlear compressibility in the unequal change between BC-generated PSV and PST after manipulations
Our AC data is generally consistent with the two-window model that describes the generation of the differential cochlear pressure ΔP = PSV – PST by sound transfer between the oval and round windows in an otherwise rigid-walled cochlea: The points of consistency include (a) the roughly parallel decreases in PSV, PST and ΔP when the ossicular system is manipulated and (b) the low-frequency increase in PST, decrease in ΔP and little change in PSV when the round-window was immobilized. These changes are predicted by a series combination of the window and cochlear impedances, with a normally low round-window impedance (e.g. Ravicz and Rosowski 2013).
In the two-window cochlear model AC stimulation produces volume displacements of the oval and round windows that are of equal magnitude but opposite direction. This model is consistent with measurements of equal oval and round window volume displacement made in human temporal bones during AC stimulation (Stenfelt et al. 2004). In BC, however, the same study found there was a difference of 5 to 15 dB in the volume displacements of the two windows, where the round window volume displacement is larger at frequencies below 2 kHz and the oval window volume displacement becomes larger at higher frequencies. Stenfelt attributed the differences in the windows’ volume displacements to inner-ear mechanisms including: cochlear bone compression, compressibility of the fluid or content of the inner ear and third-window pathways. Our observations that our manipulations consistently had different effects on the BC induced PSV measured near the oval window and PST measured near the round window are consistent with Stenfelt's observations of a difference in window displacement as well as with the model of Kim et al. (2011).
Our manipulations of ossicularly conducted sound (ossicular interruption, stapes immobilization and unoccluding the ear canal) produced small but significant reductions in the BC generated sound pressure at the oval window PSV with smaller effects on PST. What mechanism causes differential effects on PSV and PST? This difference cannot be explained by the two-window rigid-walled incompressible-fluid cochlear model because in such a model the sound flow at the cochlear windows must be equal and opposite. Instead we must invoke a ‘compressible’ cochlear model, in which either (a) the cochlear-walls deform with the BC stimulus, producing an alteration in cochlear volume, (b) there are either leaks from the cochlea or ‘compressible’ structures or fluids within the cochlea that allow a change in volume while the cochlear walls remain rigid, or (c) a combination of (a) and (b).
The observations that our ossicular manipulations had less effect on PST are consistent with some leak, compressible structure or BC sound source that preferentially affects the sound pressure near the round window. One possibility is the cochlear aqueduct, a small bony channel that connects the scala tympani near the round window to the cerebrospinal fluid within the meninges in the brain stem. While the dimensions of the cochlear aqueduct of the chinchilla are not well described, the aqueduct is known to be of small caliber in guinea pigs (0.02 mm2 in diameter and 1 mm in length, Ghiz et al. 2001), such that the duct may allow bulk fluid flow at near static frequencies, but should have a high-impedance for sound flow (e.g. Gopen, et al. 1997; Stenfelt 2015). Indeed our measurements demonstrating the significant increase in PST with round window fixation and with either AC or BC stimulation are consistent with a relatively large cochlear aqueduct impedance with limited flow through the aqueduct. Therefore, the source of the ‘cochlear compressibility’ is likely elsewhere.
5. Summary and Conclusions
- In air conduction, as expected, middle ear manipulations (incudostapedial joint interruption and stapes fixation) produced a 30-40 dB decrease across a wide range of frequencies in the intracochlear sound pressures. In bone conduction; however, these manipulations produced small effects on the intracochlear sound pressures, suggesting inner-ear sources are dominant at most frequencies.
-Contributions of the outer- and middle-ear are significant at frequencies between 1-3 kHz, and interference with ossicular conduction produced a 5 db Carhart's notch-like reduction in ear stimulation in those middle frequencies.
-The existence of compressible cochlear structures or third window pathways is needed to explain why we see differences in the change between PSV and PST in bone conduction stimulation after our manipulations. Possibilities include Sohmer's idea of non-osseous mechanisms in bone conduction (Freeman et al. 2000), and/or other third-window mechanisms (Stieger et al 2013); however, the cochlear aqueduct is not a likely candidate for such a pathway.
Supplementary Material
Highlights.
- Middle-ear manipulations affect the chinchilla's response to vibration.
- The effect of these manipulations are generally small.
- Interruption of ossicular conduction results in a notch in BC sensitivity.
- The presence of the notch depends on ear-canal occlusion.
- The inner ear paths are the primary determinant of BC response in chinchilla.
Acknowledgement
The authors would like to thank the staff of the Eaton-Peabody Laboratory for their technical support. This work was carried out in part through the use of MIT's Microsystems Technology Laboratories and the help of Kurt Broderick. This work was supported by the USA National Institutes of Health: NIDCD R01DC000194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Békésy G von. Experiments in Hearing. McGraw-Hill; New York: 1960. pp. 127–203. Chap 6. [Google Scholar]
- Carhart R. Bone conduction advances following fenestration surgery. Trans Am Acad Opthamol Otolaryngol. 1952;56:621–629. [PubMed] [Google Scholar]
- Chhan D, Röösli C, Mckinnon ML, Rosowski JJ. Evidence of inner ear contribution in bone conduction in chinchilla. Hear Res. 2013;301:66–71. doi: 10.1016/j.heares.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhan D. MS Thesis in Electrical Engineering and Computer Science. Massachusetts Institute of Technology; Jun, 2013. Role of middle ear inertial component of bone conduction in chinchilla. [Google Scholar]
- Dancer A, Franke R. Intracochlear sound pressure measurements in guinea pigs. Hear Res. 1980;2:191–205. doi: 10.1016/0378-5955(80)90057-x. [DOI] [PubMed] [Google Scholar]
- Freeman S, Sichel JY, Sohmer H. Bone conduction experiments in animals-evidence for a non-osseous mechanism. Hear Res. 2000;146:72–80. doi: 10.1016/s0378-5955(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Ghiz AF, Salt AN, DeMott JE, Henson MM, Henson OW, Jr, Gewalt SL. Quantitative anatomy of the round window and cochlear aqueduct in guinea pigs. Hear Res. 2001;162:105–112. doi: 10.1016/s0378-5955(01)00375-6. [DOI] [PubMed] [Google Scholar]
- Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Peake WT. Middle-ear characteristics of anesthetized cats. J Acoust Soc Am. 1967;41:12371261. doi: 10.1121/1.1910465. [DOI] [PubMed] [Google Scholar]
- Khanna SM, Tonndorf J, Queller J. Mechanical parameters of hearing by bone conduction. J Acoust Soc Am. 1976;60:139–154. doi: 10.1121/1.381081. [DOI] [PubMed] [Google Scholar]
- Kim N, Homma K, Puria S. Inertial bone conduction: symmetric and anti-symmetric components. J Ass Res Otolaryngol. 2011;12:261–279. doi: 10.1007/s10162-011-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, III, Nedzelnitsky V, Peake WT. Input impedance of the cochlear in cat. J Acoust Soc Am. 1982;72:108–130. doi: 10.1121/1.387995. [DOI] [PubMed] [Google Scholar]
- Olson ES. Observing middle and inner ear mechanics with novel intracochlear pressure sensors. J Acoust Soc Am. 1998;103:3445–3463. doi: 10.1121/1.423083. [DOI] [PubMed] [Google Scholar]
- Olson ES. Intracochlear pressure measurements related to cochlear tuning. J. Acoust. Soc. Am. 2001;110:349–367. doi: 10.1121/1.1369098. [DOI] [PubMed] [Google Scholar]
- Peterson LC, Bogert BP. A dynamical theory of the cochlea. J. Acoust. Soc. Am. 1950;22:369–381. [Google Scholar]
- Ravicz ME, Slama MC, Rosowski JJ. Middle-ear pressure gain and cochlear partition differential pressure in chinchilla. Hear Res. 2010;263:16–25. doi: 10.1016/j.heares.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ. Inner-ear sound pressures near the base of the cochlea in chinchilla: Further investigation. J Acoust Soc Am. 2013;133:2208–23. doi: 10.1121/1.4792139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Ravicz ME, Songer JE. Structures that contribute to middle-ear admittance in chinchilla. J Comp Physiol. 2006;192(12):1287–1311. doi: 10.1007/s00359-006-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama MCC, Ravicz ME, Rosowski JJ. Middle ear function and cochlear input impedance in chinchilla. J Acoust Soc Am. 2010;127(3):1397–410. doi: 10.1121/1.3279830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JE, Rosowski JJ. The effect of superior-canal opening on middle-ear input admittance and air-conducted stapes velocity in chinchilla. J. Acoust. Soc. Am. 2006;120:258–269. doi: 10.1121/1.2204356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JE, Rosowski JJ. A mechano-acoustic model of the effect of superior canal dehiscence of hearing in chinchilla. J Acoust Soc Am. 2008;122(2):943–51. doi: 10.1121/1.2747158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfelt S. Middle ear ossicles motion at hearing thresholds with air conduction and bone conduction stimulation. J Acoust Soc Am. 2006;119:2848–2858. doi: 10.1121/1.2184225. [DOI] [PubMed] [Google Scholar]
- Stenfelt S. Simultaneous cancellation of air and bone conduction tones at two frequencies: Extension of the famous experiment by von Békésy. Hear Res. 2007;225:105–116. doi: 10.1016/j.heares.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Stenfelt S. Model predictions for bone conduction perception in the human. Hear Res. 2016 doi: 10.1016/j.heares.2015.10.014. in press. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Håkansson B, Tjellström A. Vibration characteristics of bone conducted sound in vitro. J. Acoust. Soc. Am. 2000;107:422–431. doi: 10.1121/1.428314. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Hato N, Goode RL. Factors contributing to bone conduction: The middle ear. J Acoust Soc Am. 2002;111:947–959. doi: 10.1121/1.1432977. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Hato N, Goode R. Fluid volume displacement at the oval and round windows with air and bone conduction stimulation. J Acoust Soc Am. 2004;115(2):797–812. doi: 10.1121/1.1639903. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Puria S, Hato N, Goode RL. Basilar membrane and osseous spiral lamina motion in human cadavers with air and bone conduction stimuli. Hear Res. 2003;181:131–143. doi: 10.1016/s0378-5955(03)00183-7. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Reinfeldt S. A model of the occlusion effect with bone-conducted stimulation. International Journal of Audiology. 2007;46:595–608. doi: 10.1080/14992020701545880. [DOI] [PubMed] [Google Scholar]
- Stieger C, Rosowski JJ, Nakajima HH. Comparison of forward (ear-canal) and reverse (round-window) sound stimulation of the cochlea. Hearing Research. 2013;301:105–114. doi: 10.1016/j.heares.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonndorf J. Bone conduction. Studies in experimental animals. Acta Otolaryngol. 1966;(suppl):1–132. [PubMed] [Google Scholar]
- Wever EG, Lawrence M. Physiological Acoustics. Princeton University Press; Princeton, NJ: 1954. [Google Scholar]
- Yoshida M, Uemura T. Transmission of cerebrospinal fluid pressure changes to the inner ear and its effect on cochlear microphonics. Eur Arch Otorhinolaryngol. 1991;246:139–143. doi: 10.1007/BF00178923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.