Abstract
The importance of Zn ions (Zn) in regulating development and functions of the immune system is well established. However, recent years have witnessed a surge in our knowledge of how immune cells choreograph Zn regulatory mechanisms to combat the persistence of pathogenic microbes. Myeloid and lymphoid populations manipulate intracellular and extracellular Zn metabolism via Zn binding proteins and transporters in response to immunological signals and infection. Rapid as well as delayed changes in readily exchangeable Zn, also known as free Zn and the Zn proteome are crucial in determining activation of immune cells, cytokine responses, signaling and nutritional immunity. Recent studies have unearthed distinctive Zn modulatory mechanisms employed by specialized immune cells and necessitate an understanding of the Zn handling behavior in immune responses to infection. The focus of this review, therefore, stems from novel revelations of Zn intoxication, sequestration and signaling roles deployed by different immune cells, with an emphasis on innate immunity, to challenge microbial parasitization and cope with pathogen insult.
Keywords: Zinc, nutritional immunity, host defense, infection, immune response, innate immunity, adaptive immunity
Introduction
Zn is essential for all life forms as it regulates a multitude of processes extending from fundamental cell survival and proliferation to sophisticated mechanisms in differentiated cell types. For example, Zn controls a cardinal feature of proliferating cells, DNA synthesis, and also regulates complex signaling pathways in immune cells [1, 2]. In the context of an immune response, invasion by a pathogen creates a conflict in which Zn becomes a shared resource. The pathogen strives to utilize it for its biological functions at the expense of the host, while the host cell seeks to reserve Zn and render it inaccessible for pathogen uptake. While this strategy curtails the growth of some pathogens, others that resist these mechanisms possess robust Zn acquisition machineries that effectively compete with the host for the metal. However, excess free Zn can exert toxic effects on microbial survival. Immune cells have tapped this stratagem to localize and fuel excessive Zn at concentrations that intoxicate the pathogen without impacting host cells. Zn importers (Zips or SLC39A), Zn exporters (ZnTs or SLC30A) and Zn binding proteins such as calprotectin and metallothioneins serve to manipulate the concentration, distribution and availability of Zn in intracellular and extracellular environments. Mammalian cells express at least 14 Zips that import extracellular and intra-organelle Zn into the cytosol and 10 ZnTs that export cytosolic Zn into the extracellular space and into organelles. Some transporters are ubiquitously expressed, while others exhibit tissue-specific distribution, and are modulated by cytokines or pathogenic stimuli [3-5]. Zn transporters and binding proteins can therefore regulate the spatial and temporal distribution of Zn in immune cells and control localization of the labile Zn pool during infection.
The significance of Zn modulation in the immune response to infection stretches beyond the concept of nutritional immunity. In a previously uncharacterized role, Zn ions have emerged as signaling molecules that trigger pathways required for activation of innate and T cell mediated immunity. Thus, innate and adaptive defenses integrate Zn regulation to bolster pathogen clearance. Phagocytes such as neutrophils, macrophages and dendritic cells (DCs) are primary defenders at sites of pathogen encounter and must rapidly mount an immune response followed by cross-talk to elicit T cell activation. Under microbial stress, Zn is dynamically regulated and the Zn modulatory approach used by immune cells is contextual depending upon the cell type and pathogen in question.
For over 5 decades, studies on Zn deficiency in animals and humans have underscored the relationship between dietary Zn status and immunological competence. The profound impact of Zn deficiency on subdued immune responses and increased susceptibility to infections has been reviewed extensively [5-9]. The following sections of this review, will therefore, emphasize on emerging evidence about how specialized immune cell types execute unique and overlapping Zn modulatory arsenals at the molecular level to arrest microbial survival (summarized in Table 1). We pinpoint distinct myeloid and lymphoid populations and discuss the mechanisms they use to configure Zn homeostasis for crafting their inflammatory potential and ability to enforce infection control.
Table 1. Host innate strategies versus pathogen defense mechanisms that center on Zn regulation.
The table outlines Zn regulatory mechanisms deployed by innate immune cell types upon infection with different microbial classes and pinpoints counter regulatory defense strategies used by pathogens to establish infection.
| Immune cell type | Pathogen | Metal Regulated | Host Defense Mechanism | Pathogen Defense Mechanism |
|---|---|---|---|---|
| Macrophages | T. cruzi | Zn | Zn promotes pathogen internalization and killing [12] | |
| M. tuberculosis | Zn | ROS-dependent Zn intoxication and upregulation of MT1, MT2 and ZnT1 | P1B type Zn efflux ATPase CtpC [17] | |
| E. coli | Zn | Zn intoxication | P1B type Zn efflux ATPase ZntA [17] | |
| S. typhimurium | Zn | Zn intoxication | Salmonella pathogenicity island (SPI-1) [30] | |
| H. capsulatum | Zn | Zn sequestration by MT1, MT2 deprives fungal Zn and increases ROS production by NADPH oxidase [35] | ||
| *S. pneumoniae | Zn, Mn | Zn competes with bacterial Mn acquisition by PsaA and increases superoxide stress [31-33] | Mn acquisition by PsaA [31] Mn dependent SodA alleviates oxidative stress [33] |
|
| Neutrophils | S. aureus | Zn, Mn | Zn, Mn sequestration by calprotectin increases superoxide stress and SOD-independent killing mechanisms | Mn dependent superoxide dismutases, SodA and SodM [57] |
| S. typhimurium | Zn | Zn sequestration by calprotectin in inflamed gut | ZnuABC transporter overrides host Zn chelation and decreases commensal gut microbiota [73] | |
| A. fumigatus | Zn, Mn | Zn, Mn sequestration by calprotectin inhibits extracellular hyphal growth in cornea | ZafA promotes Zn uptake and virulence in a murine model of fungal keratitis [69] | |
| C. albicans | Zn, Mn | NETs contain calprotectin that sequesters Zn and Mn to inhibit C. albicans growth [68] | Pra-1 zincophore scavenges Zn and binds to Zrt1 to import Zn (endothelial invasion model) [74] | |
| S. pyogenes (Group A Streptococcus) | Zn | Rapid increase in intracellular Zn within 30 min of infection [72]Intracellular Zn signals required for NET formation [70] | Zn efflux by CzcD and GczA mediates resistance to Zn toxicity [72] | |
| Dendritic cells | TLR4 stimulants | Zn | Zn loss increases surface MHCII and drives DC maturation [77] | |
| Plasmacytoid DCs (pDCs) | A. fumigatus | Zn | Inhibition of fungal growth by pDC death and Zn sequestration by calprotectin [82, 83] | |
| Mast cells | H. polygyrus | Zn | Zn drives degranulation, FceR activation response and proliferation [90-93, 96] |
indicates that the mechanism has not been demonstrated in immune cells, but would hypothetically apply to the effect of Zn intoxication by macrophages during infection with S. pneumoniae.
Macrophages cast a Zn trap
Macrophages sense pathogenic cues, are highly phagocytic and kill extracellular and intracellular microbes [10]. They exhibit tissue-wide distribution as prenatally developed resident macrophages or differentiate from migrating monocytes [11]. With widespread distribution, macrophages are the primary cells to encounter pathogens and produce inflammatory mediators to attract other circulating myeloid populations such as monocytes, neutrophils and DCs.
Zn is essential for macrophage antimicrobial functions
Zn homeostasis controls monocyte chemotaxis, phagocytosis and cytokine production by macrophages [9]. Early evidence for a Zn requirement in macrophage antimicrobial functions came from studies on Trypanosoma cruzi infection [12]. Peritoneal macrophages from severely and moderately Zn-deficient mice weakly recognize and fail to kill internalized parasites. This effect is reversed by Zn, but not copper, manganese or nickel in vitro suggesting that Zn acts directly on macrophages to control infection rather than a consequence of elevated stress responses resulting from secondary effects of Zn deficiency. Although iron substitution was not assessed, the observations point to an indispensable role for Zn in macrophage parasite defenses.
Macrophages express a variety of pattern recognition receptors (PRRs) including carbohydrate antigen recognition and C-type lectin receptors. Alternatively activated or M2 macrophage surfaces are especially rich in scavenger mannose and galactose-type lectin receptors that bind and internalize parasites such as T. cruzi [13, 14]. Zn binds sulfhydryl groups and stabilizes the cell membrane that may promote parasite recognition and uptake via lectins [15]. While M2 macrophages are crucial in clearance of helminthic parasites, elimination of T. cruzi requires proinflammatory (M1) macrophage activation [16]. Thus, the restoration of parasite killing by Zn may be explained by an elevation in surface PRRs that recognize and internalize the pathogen and an increase in macrophage plasticity, potentially inclined to elevate the M1 mediators, nitric oxide, interleukin-6 (IL-6) and TNFα, during T. cruzi infection.
Host driven Zn intoxication kills bacteria
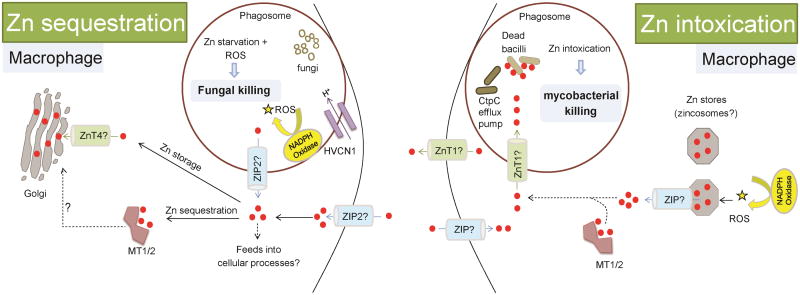
Two recent studies unveil evidence that macrophages cast a “Zn trap” resulting in strikingly opposing outcomes, one in which pathogens are trapped in an excessive Zn environment, and the other in which, Zn is sequestered away from pathogens within macrophages (Figure 1). Human macrophages intoxicate intracellular Mycobacterium tuberculosis with excess Zn, a unique defense strategy that is paradoxical to the well-established dogma of metal starvation as a major antimicrobial defense mechanism. Infection of macrophages with M. tuberculosis simultaneously triggers Zn intoxication signatures in the host and intracellular bacilli signifying that the host-pathogen interaction transforms Zn homeostasis in both organisms. Mycobacterial infection causes a “burst of free Zn” within macrophages and increases the Zn binding proteins, metallothioneins 1, 2 and the Zn exporter ZnT1 [17]. Metallothioneins contain ∼20 cysteines that tightly bind Zn, releasing it under oxidative conditions [18]. In line with this, rapid mobilization of free Zn stored in yet unknown intracellular compartment(s), possibly zincosomes, involves the reactive oxygen species (ROS) producing enzyme, NADPH oxidase [17, 19]. Macrophages poison bacilli by delivering Zn into phagolysosomes. Intracellular Zn stores may supply an immediate need for Zn intoxication, but how do infected macrophages prolong Zn delivery into phagolysosomes? Monocytes stimulated with Mycobacterium bovis BCG cell wall steadily induce Zip8 expression, suggesting that extracellular Zn could be drawn in to fuel the hosts' Zn poisoning strategy [20].
Figure 1. Intracellular Zn sequestration versus Zn intoxication in macrophages.
Zn regulation in innate antimicrobial defenses can be broadly classified into Zn sequestration, Zn intoxication and Zn loss (dendritic cells, see Figure 3).
i) Proinflammatory GM-CSF stimulated macrophages increase Zn import via ZIP2 upon fungal infection, likely indicating an increased Zn demand to drive macrophage activation. ZIP2 potentially diminishes phagosomal Zn by importing it into the cytosol. Imported cytosolic Zn is sequestered by metallothioneins 1 and 2 (MT1/2) and exported to the Golgi possibly via Zn exporter, ZnT4. Macrophages enter a free-Zn-deprived state that serves dual purpose: one, Zn-access to the pathogen is restricted and second, proton (H+) flux via HVCN1 augments superoxide production by the NADPH oxidase enzyme. Fungi fail to thrive in the hostile Zn-deprived environment and are susceptible to superoxide killing. Through Zn sequestration, macrophages bolster their defense mechanism, and ultimately arrest intracellular fungal growth.
ii) On the other hand, mycobacterial infection causes a free Zn-burst wherein, Zn is liberated from intracellular stores (possibly zincosomes) and is dependent on NADPH oxidase. Macrophages upregulate ZnT1, and MT1/2. Zn can be released from MT1/2 upon oxidation. Zn may also be imported from extracellular sources. Together, these represent a signature of Zn intoxication in the host as well as pathogen. Zn accumulates within phagosomes. A potential mechanism is ZnT1 mediated export from the cytosol. Mycobacteria experience a surge in phagosomal Zn and activate the CtpC efflux pump to expel Zn. In the “Zn intoxication battle” failure of Mycobacteria to detoxify excessive Zn results in death of bacilli. Red dots, Zn ions; Solid arrows and lines, established links; dotted arrows and lines, predicted links.
Bacterial defenses resist Zn intoxication within the host
M. tuberculosis is equipped with a counter-defense strategy that extrudes incoming Zn via the P1B-type ATPase efflux pump, CtpC to resist Zn toxicity. CtpC mutant bacilli are highly sensitive to Zn, but not other cations, rapidly accumulate the metal and are killed by human macrophages. Macrophages adopt a similar Zn intoxication mechanism to challenge non-pathogenic Escherichia coli further widening the scope of Zn poisoning as a general defense strategy against intracellular bacteria [17]. Avirulent M. smegmatis also encodes the cation diffusion facilitator protein, ZitA and mediates resistance to Zn intoxication [21]. These discoveries build upon a report by Wagner et. al. on M. tuberculosis containing phagosomal vacuoles in peritoneal macrophages. Infection with wild-type bacilli increases iron acquisition and lowers phagosomal Zn. Conversely, macrophages infected with siderophore-mutant bacilli contain minimal phagosomal iron, but accumulate Zn. Likewise, tumor necrosis factor (TNF)α or interferon (IFN)γ stimulation of macrophages decreases iron and augments phagosomal Zn, indicating that the host attempts to transport excessive Zn to disrupt iron-Zn homeostasis in engulfed Mycobacteria [22]. Together with studies on Zn intoxication, this phenomenon signifies the reciprocal regulation of Zn and iron homeostasis in Mycobacteria as a “thriving mechanism” within host phagosomes. Indeed, this organism can persist in phagosomes using evasive mechanisms such as precluding phagolysosomal fusion or even escaping into the cytosol [23, 24]. By doing so, bacilli could escape Zn overload and potentially gain access to essential metals like iron and manganese in the macrophage cytosol. Mycobacteria express a cysteine rich Cu-binding metallothionein, MymT that confers Cu resistance. Both Cu and Zn induce MymT expression, and this metallothionein binds Zn albeit with a lower affinity than Cu [25, 26]. These data raise the possibility that Mycobacteria may combine metallothioneins and Zn efflux mechanisms to dampen vulnerability to Zn poisoning within macrophages.
Clearly, Zn intoxication serves as a means of eliminating Mycobacteria within macrophages, but some questions remain. To elicit a free Zn burst, macrophages initially do not import extracellular Zn, but harbor Zn stores that are released and mobilized into phagocytic vacuoles. How is Zn liberated from intracellular stores? A common denominator in these studies is ROS, induced by mycobacterial infection or upon activation by IFNγ or TNFα which may facilitate the heightened release of free Zn into phagosomes. How does free Zn make its way to phagosomes while macrophages sustain superoxide production? The mammalian genome encodes 10 ZnTs that export cytosolic Zn extracellularly or into intracellular organelles [4, 27]. Although it is not clear whether phagosomes express dedicated Zn exporters for vacuolar Zn assimilation, plasma membrane-bound Zn exporters may passively associate with newly formed phagosomal membranes that were once a part of the cell surface. This hypothesis explicates an elevation in ZnT1 in Mycobacteria-infected macrophages (Figure 1) [17]. Despite an increase in this transporter, the host does not lose free Zn via export, but rather experiences a surge within an intracellular organelle. This possibility does not exclude active integration of newly synthesized ZnT1 or other Zn exporters onto phagosomal membranes following infection. The iron transporter, non-resistance associated macrophage protein (NRAMP)1 transports iron into the cytosol, but is thought to be bidirectional and can transport Zn [28]. In this view, NRAMP1 depletes phagosomal iron while delivering an excess of Zn. Further analyses are needed to reveal the relationship between iron efflux, Zn influx, and the directionality of transport by NRAMP1 in mycobacterial infection. Thus, in the host-pathogen battle, success of the host's metal-regulatory defense strategy may reside with simultaneous interference of iron acquisition and Zn detoxification mechanisms of the invading microbe. Phagosomal iron paucity may enforce substitution with Zn, rendering iron-dependent bacterial processes non-functional. To avert toxicity from incoming Zn, microbes have evolved to shuttle the metal back into the host milieu. A key requirement in the activation of this process in Mycobacteria is phosphorylation of the P1B-type ATPase efflux pump [29]. Whether Zn inhibits this process to eventually inactivate Zn efflux by Mycobacteria is elusive, but from an immunological perspective, executing a dual defense approach combining Zn poisoning with inactivation of mycobacterial P1B-type ATPases presents an efficient mechanism of limiting bacterial fitness. Recent studies continue to expand the repertoire of bacterial pathogens that are subjected to Zn poisoning by macrophages. Human macrophages infected with the gram negative bacteria, Salmonella typhimurium attempt to poison intracellular bacteria through vesicular Zn accumulation. S. typhimurium however, evades the host response through Salmonella pathogenicity island-1 dependent and independent mechanisms [30].
Zn competes for manganese acquisition in bacteria
Studies on Streptococcus pneumoniae reveal an interesting dimension of Zn intoxication in infection control. Zn competes with manganese accrual by the solute binding protein PsaA of S. pneumoniae in a dose dependent manner [31]. PsaA physiologically binds manganese but can interact with other divalent cations. Zn in particular, forms a stable complex with PsaA, ‘locking’ it to prevent manganese acquisition and transport [32]. Manganese acquisition by PsaA is essential to alleviate superoxide sensitivity, perhaps because the superoxide dismutase (SOD), SodA in S. pneumoniae is manganese-dependent [33, 34]. In the presence of excess Zn, S. pneumoniae fails to assimilate manganese and falls prey to oxidative stress. These findings exemplify Zn competition for manganese uptake and provide a molecular basis for the “intoxicating” effect of Zn on bacteria.
Zn sequestration starves fungi and bolsters oxidative defenses
We demonstrated that mouse and human macrophages infected with Histoplasma capsulatum sequester “free” Zn away from the fungus [35]. This study emerged from our previous finding that granulocyte-macrophage colony stimulating factor (GM-CSF) activated macrophages deplete fungal Zn [36]. In a paired stimulus involving GM-CSF activation and fungal infection, macrophages import extracellular Zn via the importer, ZIP2. Rather than merely supplementing the intracellular free Zn pool, this influx of Zn in macrophages is accompanied by an upregulation in metallothioneins 1 and 2 that bind Zn and restrict its availability [35]. Given that numerous host signaling processes depend on Zn for their functions, heightened Zn acquisition likely reflects a precautionary step to avert microbial hijacking of the cell signaling cascade. Cells contain micromolar concentrations of Zn, a majority of which is bound to macromolecules, but free Zn occurs in picomolar to femtomolar quantities and is tightly regulated [37]. By sequestering this fraction and channeling it to the Golgi, macrophages safeguard the readily exchangeable Zn pool and limit its exploitation as a shared reservoir between the pathogen and host [35]. What transport mechanisms deprive the yeast-containing phagosomes of Zn? A theory analogous to plasma membrane Zn transporters surfacing on newly formed phagosomes can, in principle, be applied to macrophages infected by fungi. In this case, the likely candidate would be ZIP2 that imports extracellular or intra-organelle Zn into the cytosol.
The consequence of this defense mechanism is two-fold. First, the host superoxide defenses are strengthened and second, invading fungi are Zn-starved and succumb to superoxide mediated damage. Efficient generation of ROS by NADPH oxidase requires H+ flux into phagosomes by the proton channel, HVCN1 [38]. Zn inhibits HVCN1 and sequestration of the metal rescues this effect to promote NADPH oxidase activity [35, 39]. Fungal susceptibility to killing by ROS is enhanced in a Zn deprived milieu, but what cripples them under these conditions is unclear. H. capsulatum encodes four Zn/Cu-dependent SODs [40]. It is plausible that Zn starvation impairs SOD function among other essential Zn-requiring cell processes rendering fungi incompetent within the host. Contrary to mycobacterial infection, in fungi infected macrophages, superoxide burst does not release free Zn from metallothioneins. Heightened metallothionein expression, Zn sequestration and compartmentalization of Zn in the Golgi argue against Zn release even under oxidative conditions [35]. Zn mobilization from metallothioneins requires binding to an “effector molecule”, such as glutathione disulfide. In the absence of effector interactions, metallothioneins liberate only one zinc ion, pointing at their restrictive Zn release properties [41]. Moreover, GM-CSF activated macrophages have a high metallothionein:Zn ratio, enough to sequester the free Zn pool, and also exhibit a two-three fold shift in their molecular mass signifying clustering of the protein through oxidation [35]. This manifestation may suggest that metallothioneins confer protection against oxidative stress to the host during infection [42]. Taken together, in “hitting two with one stone” approach, by sequestering Zn through metallothioneins, macrophages simultaneously starve fungi of an essential metal and fortify oxidative defenses. GM-CSF is essential for the induction in metallothioneins in macrophages in vivo during infection [35], supporting the premise that this cytokine is at the peak of, and emerges as a major metallothionein regulator during fungal infection amidst a complex inflammatory process involving several cytokines.
Deciding a defense path – To sequester or to intoxicate?
Factors that dictate macrophage decisions on Zn intoxication versus sequestration remain enigmatic. At least two parameters potentially influence Zn homeostasis in phagocytes. One, the cytokine milieu determines subsequent signaling mechanisms that control Zn flux by import, export and binding. Second, the host may mold Zn homeostatic mechanisms contingent upon the pathogen it encounters. GM-CSF and macrophage-colony stimulating factor (M-CSF) differentiated macrophages exhibit classical and alternative activation characteristics respectively [43]. The former engages signal transducer and activator of transcription (STAT)5 and STAT3 [35, 44]; M-CSF activates the phosphoinositide-3-kinase (PI3K)/Akt and extracellular-signal-regulated-kinase (ERK) pathways [45]. GM-CSF-driven Zn influx and sequestration by metallothioneins depends on STAT5/STAT3 signaling. Multiple lines of evidence suggest that Zn promotes PI3K and ERK signaling [46-48], but whether these molecules in turn regulate cellular Zn homeostasis is unknown. The underlying difference here is from the pathogen standpoint - how much Zn is too much? Diverse classes of microbes may differ vastly in their “Zn quota”, i.e. the total Zn content required for optimal growth and threshold concentrations above which Zn may adversely affect survival [49]. For example, the zinc quota of E. coli and Saccharomyces cerevisiae differs approximately over two orders of magnitude (105 vs. 107 Zn atoms per cell) [49, 50]. Thus, bacteria and yeasts may display distinct Zn requirements and therefore, the host preferentially confronts one class of pathogens by Zn intoxication and the other by sequestration. However, although the zinc quota is apparently greater for S. cerevisiae, differences in cell size and volume between these microbial classes results in comparable zinc concentrations of 0.1 – 0.5mM in bacteria and yeasts [3]. It is plausible that both the host's cytokine environment and the invading pathogen converge to influence defensive strategies adopted by macrophages that center on Zn handling.
Neutrophil Zn choreography: Calprotectin, NETs and Zn signals
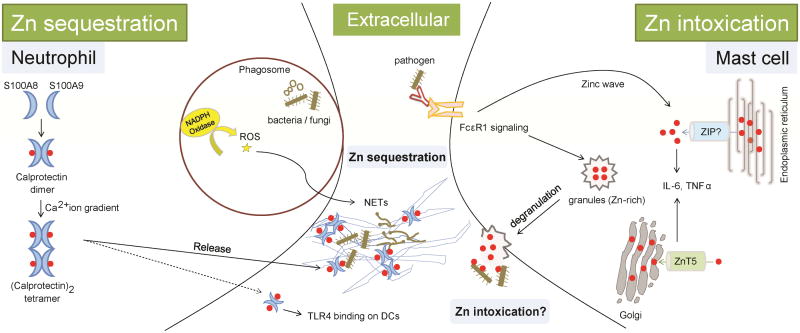
Neutrophils are short-lived polymorphonuclear leukocytes with a prominent role in acute inflammation. Immunological surveillance by neutrophils enables detection of invading pathogens and rapid recruitment to the site of infection. Neutrophils synthesize various defense molecules including azurophilic granules that contain defensins, cationic antimicrobial peptides, proteolytic enzymes and metal chelators [51]. About 40% of the neutrophil cytosol is composed of calprotectin, a calcium binding heterodimer of S100A8 and S100A9 [52]. Calprotectin was identified in 1980 as leukocyte derived protein L1 that exerts antimicrobial activity against a broad range of pathogens including E. coli, Klebsiella pneumoniae, Staphylococcus aureus, S. epidermidis, Candida spp., Aspergillus fumigatus and Cryptococcus neoformans [53, 54]. Though calprotectin binds calcium, it soon became evident that Zn and/or manganese, but not calcium is responsible for its biostatic defense functions [54]. Monomeric subunits lack Zn binding, but the calprotectin complex binds Zn at two sites via histidines. Neutrophils may utilize physiological calcium ion gradients to modulate the Zn binding affinity of calprotectin, wherein, binding of calcium decreases the dissociation constant of the calprotectin-Zn interaction [55]. The protein is subject to second-tier regulation to maintain an adequate free Zn pool and restrict it upon activation by external stimuli such as pathogen insult. Consistent with this idea, calcium binding promotes assembly of the heterotetrameric (S100A8/A9)2 complex with augmented Zn binding affinity [56]. Thus, cytosolic calprotectin may weakly bind Zn under homeostasis, while an influx of calcium in neutrophils caused by infection triggers the Zn chelating effector function of the protein (Figure 2).
Figure 2. Extracellular Zn sequestration by neutrophils and potential intoxication by mast cells.
i) Neutrophils drive Zn sequestration by calprotectin. Infection by bacteria or fungi increases expression of S100A8 and S100A9 that dimerize to form calprotectin. Calprotectin senses a Ca2+ ion gradient in activated neutrophils, wherein elevated Ca2+ causes tetramerization of calprotectin subunits with enhanced Zn binding affinity. Upon NETosis, an NADPH oxidase dependent process, neutrophils expel calprotectin, nuclear content and defensive granules to capture extracellular bacteria and fungi. Calprotectin sequesters Zn in NETs and is crucial for antimicrobial action of neutrophils.
ii) Mast cell degranulation is a potential extracellular Zn intoxication mechanism. Degranulation is crucial for antimicrobial defenses of mast cells. When antigen-bound IgE binds to FcεR1, Zn-rich mast cell granules are transported from cytosol to extracellular space. Zn is required for this process. Along with other microbicidal components, degranulation expels Zn that plausibly results in Zn poisoning of extracellular pathogens. Mast cell activation generates a ‘Zn wave’, i.e., a burst in intracellular free Zn, released from endoplasmic reticulum and Zn is exported by ZnT5 to the Golgi. Both, Zn wave and Zn export by ZnT5 are required for production of cytokines, IL-6 and TNFα that facilitate proinflammatory activation for pathogen clearance. Red dots, Zn ions; Solid arrows and lines, established links; dotted arrows and lines, predicted links.
Calprotectin sequesters Zn and manganese to inhibit microbial growth
Microbial success during host invasion demands combating superoxide-mediated damage. For example, manganese-dependent SODs shield S. aureus, S. epidermidis, and methicillin resistant S. aureus from superoxides [57]. A key prerequisite for the staphylococcal SOD defense system is manganese. In a cleverly aimed defensive mechanism, the host combines harmful oxidative radicals with elevated calprotectin to drive manganese chelation, thus precluding formation of bacterial SOD-manganese complexes. Calprotectin impairs both SOD-dependent and SOD-independent bacterial defenses. SOD deficient S. aureus are ROS sensitive, but chelation of manganese by calprotectin further escalates killing of mutant bacteria [57]. Intriguingly, in this case, Zn protects bacteria from killing, suggesting that either Zn saturates calprotectin to augment manganese availability, or that manganese or Zn are interchangeably utilized by staphylococcal SODs for their function. This host mechanism can be envisioned in parallel with metallothionein-Zn sequestration in macrophages that cripples fungi by triggering ROS while simultaneously enhancing susceptibility to damage caused by it.
Despite its importance in restraining infection, inadequate calprotectin metabolism poses serious consequences to Zn homeostasis. Hyperaccumulation of calprotectin in patient plasma increases susceptibility to infections and may be attributed to impaired clearance of the protein [58]. These patients have increased plasma Zn, but suffer recurrent infections because nearly all of the calprotectin is Zn bound, yielding a free-Zn depleted state. Thus, Zn restriction is an important nutritional immunity strategy, but prolonged free Zn paucity may preclude availability to cells for physiological processes and attenuate the capacity to mount a strong immune response.
Calprotectin magnifies antimicrobial inflammatory responses
Aside from its direct antimicrobial action calprotectin amplifies immunological processes during inflammation [59]. Calprotectin deficient mice exhibit attenuated granulocyte migration. By associating with tubulin, calprotectin causes rapid cytoskeletal rearrangements, a process likely designed to meet the high migratory demand of neutrophils to infection sites [60, 61]. A receptor for calprotectin was identified in a study wherein calprotectin deficient mice exhibited diminished toll-like receptor 4 (TLR4) activation and were protected from lethality during septic shock [62]. This led to the finding that calprotectin directly interacts with TLR4 and endogenously activates this pathway, designating it as a danger associated molecular pattern. That TLR4 recognizes lipopolysaccharide (LPS) as well as mammalian divalent cation-binding calprotectin is an intriguing finding. Whether unbound or Zn-bound calprotectin forms differentially regulate the TLR4 response in phagocytes is undetermined. Importantly, calprotectin binding to TLR4 alone does not induce inflammation but requires a secondary stimulus, in this case, LPS [62]. Thus, physiological release of calprotectin leaves immune homeostasis undisturbed, but prepares the system to rapidly counter microbial insult. Through TLR4 signaling, calprotectin increases S100a8/a9 gene expression in neighboring cells and stimulates IL-6, TNFα, and IL-1β production [59]. Thus, calprotectin release into extracellular space magnifies the inflammatory response that would prove beneficial in microbial elimination. Endogenous signaling by calprotectin raises the possibility that mechanisms other than NET formation may exist for calprotectin release, that do not require neutrophil death or a small number of neutrophils die at inflammatory sites to seed subsequent calprotectin mediated inflammatory cascade.
Neutrophil extracellular traps starve ensnared pathogens of Zn
In a remarkable, and perhaps, ultimate attempt to control infection, neutrophils expel nuclear chromatin that combines with cytosolic defense granules and is released as neutrophil extracellular traps (NETs). In this process called ‘NETosis’, neutrophils produce a complex network where DNA, nuclear proteins and antimicrobial molecules formulate an extracellular snare to immobilize pathogens (Figure 2) [63]. Calprotectin, a major NET component is abundant (∼1mg/ml) in tissue abscesses [64]. NETosis releases calprotectin extracellularly, where it colocalizes with myeloperoxidase and DNA. NADPH oxidase is required for neutrophil disintegration and NET formation [63]. It is plausible that calprotectin sequesters Zn to augment HVCN1-driven NADPH oxidase activity analogous to that of metallothioneins. Indeed, neutrophils lacking H+ charge compensation fail to maintain electron transport for superoxide generation and do not kill pathogens [65]. Whether neutrophils employ intracellular Zn sequestration by calprotectin to mediate this function requires further investigation.
NETs have been found to inhibit the growth of several bacteria and fungi [64, 66, 67]. Infection with C. albicans releases about one-third of the neutrophil calprotectin into NETs. Calprotectin depletion from extracellular traps ablates antimicrobial activity of NETs and deficient mice poorly restrain infection [68]. In a murine model of corneal infection caused by A. fumigatus, Zn and manganese chelation by neutrophil-derived calprotectin is crucial in curtailing extracellular hyphal growth [69]. Together, these data support an undisputed role for calprotectin in pathogen control. The protein exerts its function without establishing physical contact with pathogens, suggesting that through calprotectin release, NETs create a hostile free-Zn deficient “milieu” unfavorable to microbial survival. Localized calprotectin in NETs, however, permits direct attack on microbial surfaces. Addition of Zn or manganese obliterates the biostatic activity of calprotectin [68]. What is the half-life of calprotectin in NETs? What factors clear or recycle metal-bound calprotectin? Does this have implications in shaping tissue repair functions? These remain unanswered. Nonetheless, it is apparent that inhibiting pathogen accessibility to the two essential divalent cations, Zn and manganese, marks calprotectin as a crucial ‘metal-restrictive’ nutritional defense mechanism deployed by neutrophils.
Zn signals arm neutrophil antimicrobial defenses
Zn signals have direct impact on neutrophil antimicrobial functions independent of calprotectin. Activation of neutrophils with the protein kinase C activator, phorbol 12-myristate 13-acetate results in an ROS-dependent increase in intracellular Zn that is required for NET formation. Chelation of Zn with a membrane permeable Zn chelator blocks NETosis without impacting ROS production, suggesting a role for Zn downstream of NADPH oxidase function in neutrophils [70]. This finding appears contrary to the idea that calprotectin mediated sequestration of Zn potentially augments NADPH oxidase activity. But, a rise in intracellular Zn may be required to support very early stages of NET formation, as chelation of the metal an hour post neutrophil activation fails to inhibit NETosis [70]. The duration for which neutrophils experience an intracellular elevation in free Zn is unknown, but it can be proposed this pool is eventually sequestered to sustain ROS production by NADPH oxidase. Supporting an antimicrobial role for the Zn signal, neutrophils increase intracellular Zn upon exposure to Group A streptococcus plausibly impairing bacterial glycolytic metabolism [71, 72]. In line with this, Streptococcus pyogenes lacking an intact CzcD and GczA Zn efflux machinery are sensitive to killing by neutrophils [72]. The source of a heightened Zn pool in neutrophils and more importantly, the mechanisms by which intracellular Zn fluctuations dictate the formation of extracellular traps remain undetermined. Analogous to the ROS-dependent Zn burst in Mycobacteria infected macrophages, Zn is mobilized by superoxides plausibly from intracellular Zn stores such as metallothioneins or zincosomes to mediate NET formation [17].
Pathogen Zn acquisition overrides the neutrophil biostatic machinery
Bacteria possess versatile tools to strive for metal acquisition within the host's restrictive environment. The gut is a classic example where Zn is a resource that the host and the gut microbiota must compete for. Thus, for intestinal colonization, pathogenic bacteria must overcome at least two barriers. The first is to disrupt host nutritional immunity to establish a safe niche, and second, compete with commensal gut flora to exploit a portion of Zn in the host nutrient reservoir. In mice infected with S. typhimurium, a common cause of inflammatory diarrhea, infiltrating neutrophils highly upregulate calprotectin [73]. S. typhimurium is equipped with the high affinity ZnuABC transporter for Zn acquisition under limiting conditions that renders bacteria resistant to Zn chelation by intestinal calprotectin. ZnuA mutant bacteria poorly colonize and fail to override the gut microbiota in wildtype but not in calprotectin deficient mice [73]. Bearing the complex gut microbiological environment, Zn chelation by calprotectin can potentially jeopardize thriving commensal microbiota while supporting growth of pathogens such as S. typhimurium that resist Zn starvation.
How do pathogen Zn transporters compete with calprotectin for chelated Zn? While Zn release by metallothioneins under oxidative stress is well documented [18], the release or exchange of Zn from calprotectin, though possible, remains unknown. One postulate is, by killing resident gut microbiota and subsequently reducing competition for Zn, S. typhimurium increases its share of Zn in the host reservoir and acquires it through the ZnuABC transporter. This strategy overrides Zn chelation mediated by calprotectin. Fungi also possess soluble Zn scavengers to cope with Zn deprivation. C. albicans releases the pH-regulated antigen 1 (Pra1) dubbed as a ‘zincophore’ under alkaline conditions to chelate Zn [74]. Each Pra1 molecule acquires 3 Zn atoms in the host milieu and binds to the Zrt1 transporter for Zn uptake during endothelial infection. Interestingly, exposure to Pra1 activates neutrophils that would be followed by localized accumulation of Zn-rich calprotectin, raising the supposition that fungi exploit neutrophil activation to scavenge the metal from calprotectin or other host Zn-bound proteins [75]. Similarly, the Zn sensitive transcription factor, ZafA in A. fumigatus upregulates Zn transporters in response to Zn deprivation by calprotectin [69]. Whether bacterial ZnuABC or fungal Pra1 acquire free Zn or chelate it from Zn-bound proteins is unclear. With especially high concentrations of calprotectin and metal sequestration mechanisms in place, free Zn abundance is unlikely, leading to the postulate that interactions between microbial Zn scavengers and host calprotectin may exist to enforce the release or “transfer” of Zn to pathogens. For microbes that successfully survive Zn deprivation mechanisms, this strategy could ablate sensitivity to nutritional immunity to increase persistence. Evidence for microbial Zn scavengers interacting with calprotectin is lacking and is an open ground for investigation. Nonetheless, clearly, therapeutic approaches targeting Zn restriction must combine chelation with inhibition of microbial Zn acquisition systems to efficaciously disrupt pathogen defenses.
Zn takes a “Toll” on dendritic cell activation
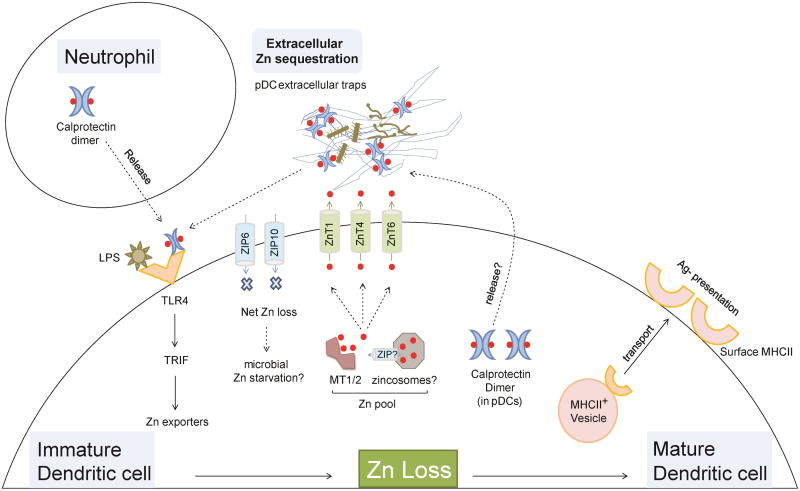
Apart from bolstering overall immunological competence, Zn homeostasis in individual cell populations, in particular, DCs, is an imperative step in defining the nature of cellular activation. DCs express small amounts of surface major histocompatibility complex (MHC)II. Activation via PRRs boosts surface MHCII levels leading to maturation and DC-T cell interactions, crucial in scaling T cell responses [76]. Kitamura et. al. demonstrated that “free Zn loss” is required for DC activation (Figure 3) [77]. Stimulation with the TLR4 ligand, LPS, shrinks the intracellular Zn pool to mobilize vesicular MHCII to cell surface and alters the Zn transport machinery via TIR-domain-containing adapter-inducing interferon-β (TRIF) signaling. Zn loss in DCs facilitates vesicular transport from MHCII+LAMP2+ to MHCII+LAMP2- followed by MHCII appearance on the surface. Likewise, Zn deficiency increases surface MHCII expression on the human monocyte cell line, THP1, in resting state and in response to LPS [78]. The exact mechanism is elusive, but can be projected in parallel with augmented receptor endocytosis caused by Zn abundance. Maturing DCs elevate ZnT1, ZnT4 and ZnT6 exporters and reduce ZIP6 and ZIP10 Zn importers, signifying a preparative mechanism to drive net intracellular Zn loss. At least one downregulated importer, ZIP6, is responsible for reduced Zn and guides the DC activation process [77]. Although a role for MTs was not identified, Zn modulation possibly results from combined decreased influx, increased efflux and sequestration.
Figure 3. Orchestration of Zn loss in dendritic cell activation.
Zn loss drives maturation of dendritic cells. TLR4 activation by pathogen stimuli such as LPS increases Zn export (ZnT1,4,6) and decreases Zn import (ZIP6,10) in a TRIF dependent manner. The result is net “Zn loss” from DCs that potentially starves phagocytosed microbes of Zn. Loss of Zn drives vesicular transport of MHCII to the plasma membrane and reduces receptor endocytosis to elevate surface MHCII expression. Mature DCs efficiently present antigens via MHCII, critical in eliciting a T cell response to clear pathogens. Zn released in extracellular space may be sequestered by caprotectin (neutrophil-derived and/or DC-derived). pDCs express calprotectin to sequester Zn, and release it into pDC extracellular traps to inhibit hyphal growth. Red dots, Zn ions; Solid arrows and lines, established links; dotted arrows and lines, predicted links.
DCs poorly phagocytose, but efficiently kill several intracellular microbes [79]. From this standpoint, loss of DC Zn may serve a dual purpose, one, driving maturation and second, restricting Zn as a means to inhibit pathogen growth. The fact that neutrophil calprotectin binds TLR4 and can activate DCs offers an intriguing inference in this context. Paracrine signaling by calprotectin released from neutrophils together with TLR4 stimulation by an invading pathogen could potentially link the neutrophil calprotectin response to DC activation unraveling a communication thread between the two populations. TLR4 not only recognizes gram-negative bacterial antigens, but also viral glycoproteins [80]. Downstream activation of this receptor engages myeloid differentiation primary response 88 (MyD88) dependent and independent NF-κB transcriptional programs that prepare DCs to elicit a proinflammatory response through production of cytokines and chemokines [81]. Thus, neutrophil-derived calprotectin can plausibly drive an inflammatory cascade that magnifies DC activation and subsequently enhances antigen presentation to T cells via MHCII. Upon encountering microbial insult, the host may employ this apparently beneficial “calprotectin communication module” between two innate populations to facilitate rapid elimination of foreign invaders (Figure 3).
Whether the loss of Zn by DCs into extracellular space is subsequently captured by calprotectin is an interesting prospect to be determined. If true, this mechanism may work against extracellular microbes that would otherwise gain exposure to a milieu, rich in exchangeable Zn. Analogous to neutrophils, plasmacytoid DCs (pDCs) form extracellular traps, express calprotectin albeit to a lesser extent, and chelate Zn to limit A. fumigatus hyphal growth [82, 83]. Thus, DCs may also express endogenous calprotectin as a modifier of intracellular Zn status to promote maturation (Figure 3).
It is reasonable to predict that an altered extracellular Zn pool emerging from the DC activation process would impact other immune populations undergoing activation in the same environment. Can Zn released by an innate cell type dictate activation of adaptive immunity? Studies on early Zn flux in T cell activation provide captivating insights into why this may be a potential mechanism (discussed later in this review). It is not known whether DCs prolong the Zn deprived state post-maturation and if adjusting the free Zn pool in DCs guides subset differentiation. Systematic Zn fluctuation assessments in DCs over time are needed to decipher these questions. Prevailing data indicate that Zn regulation is highly dynamic. LPS stimulation of leukocytes drives rapid free Zn release from intracellular stores within 2 minutes perhaps yielding an urgent free Zn demand to initiate Zn-dependent cellular mechanisms [48]. The released Zn may be utilized by these processes and eventually sequestered or exported over 6 hours. Early Zn fluctuation is TRIF or MyD88 independent in leukocytes, but TRIF dependent in DCs during later stages hinting that signaling pathways take over to diverge the course of Zn homeostasis [48, 77].
In conjunction with these findings, a Zn-dependent transcription factor, Zbtb46, impedes DC maturation to maintain them in steady state [84]. One postulate is that upon microbial attack, maturing DCs must turn off this transcription factor. Zn ions stabilize Zn finger proteins, but whether Zn is removed from Zbtb46 leading to destabilization and degradation of the transcription factor is unknown [85]. Nonetheless, TLR stimulation downregulates Zbtb46 protein in vivo to increase surface MHCII on DCs [84]. Perhaps, nuclear Zn fluctuation integrates to yield DC activation, but transporters that facilitate it are undefined. Taken together, Zn interweaves a complex network of mechanisms that unleash the potential of DCs to dictate a full-blown activation response upon pathogen encounter.
Zn degranulates mast cell sentinels
Mast cells (MCs) are distributed in nearly all tissues and differentiate from granulocyte/monocyte progenitors upon exposure to IL-3 or stem cell factor. MC responses have best been dissected in allergies, but expanding evidence underlines their role in manipulating host defenses against pathogens. These cells are pre-equipped with a diverse array of inflammatory mediators including histamines, TNFα and proteases packed in cytosolic granules that are rapidly released during infection [86]. The primary mechanism of activation is via FcεRI clustering when antigen-bound IgE associates with the FcεRI receptor driving degranulation. MC degranulation confers resistance to numerous species of parasites, bacteria and possibly also fungi [86, 87]. In this capacity, MCs arm innate immunity by mounting an early attack, especially in barrier tissues vulnerable to environmental contact.
MCs derived from the peritoneal cavity of rats are Zn-rich, harboring 40% more Zn than other leukocytes; their Zn content increases as the cells mature [88]. Animal models of Zn deprivation suggest that the metal has a potential role in maturation of MCs [89]. Kabu et. al. demonstrated an indispensable role for Zn in MC degranulation (Figure 2) [90]. Chelation of Zn, but not iron, copper or manganese impairs the release of MC granules containing β-hexosaminidase, TNFα, histamine and leukotrienes. Cytosolic granules must make their way to the plasma membrane preceding release, in a Zn-dependent manner. Zn deficiency inhibits granule translocation, arresting them within the cytosol. Importantly, this effect is calcium-independent, whose influx is crucial in FcεRI driven degranulation. The “Zn wave” was first described in MCs identifying Zn as an intracellular second messenger [91]. Activated MCs liberate free Zn from intracellular stores, likely the endoplasmic reticulum into the cytosol. The requirement of Zn extends beyond rapid degranulation, in that, it transcriptionally regulates TNFα and IL-6 in response to FcεRI clustering by driving nuclear translocation of nuclear factor NF-κB (Figure 2) [90, 91]. Thus, these studies substantiate a pivotal role for Zn in MC degranulation and antimicrobial immunity.
Dysregulation of Zn homeostasis can be inferred to profoundly impair the IgE mediated immune response to parasites. Indeed, Zn is necessary for resistance to Heligmosomoides polygyrus nematode in the gastrointestinal tract [92]. Zn-deficient animals exhibit attenuated IgE responses, MC proliferation, and degranulation [93]. Disease exacerbation under Zn scarcity may stretch farther than defects in degranulation and involve suboptimal Th2 immunity. Zn starvation diminishes IL-4 production by Th2 cells and possibly MCs, negatively impacting IgE synthesis [93]. However, Zn has an immediate effect on release of cytosolic contents that occurs within minutes post-activation signifying that Zn is required for both rapid and sustained MC activation. Since the granules contain a high Zn concentration, the extracellular space likely experiences a surge of Zn when MCs degranulate. Intracellular Zn is lost from MCs upon degranulation, somewhat analogous to Zn loss during DC activation [90]. How Zn release influences the action of MCs post-degranulation is unknown. Two reasonable suppositions arise, one that the surge in Zn serves as a potential mechanism to intoxicate pathogens in the extracellular space, and second, Zn is required for adequate function of the released enzymes and antimicrobial peptides (Figure 2). However, the former possibility would likely require additional mechanisms that mobilize exchangeable Zn into the bacterial niche, because, the availability of extracellular free Zn is typically minimized through cellular transport and sequestration. The relative contribution of direct intoxicating and indirect effects of Zn through action on enzymes requires further investigation.
Apart from FcεRI activation, MCs degranulate in response to pathogen ligation of TLRs, C-type lectins Dectin-1, and NOD-like receptors [86]. MCs express TLR4 and release proinflammatory cytokines in response to LPS stimulation [94]. In principle, TLR4 on MCs may bind neutrophil-derived calprotectin to endogenously activate MCs upon pathogen encounter and escalate inflammatory responses against infection. Zn is likely non-specifically involved in degranulation, irrespective of the stimulus that leads to it. This hypothesis needs to be explored, but would imply that Zn homeostasis dictates the MC reaction to diverse pathogen classes, including bacteria, fungi and viruses.
Data on Zn transport mechanisms in MCs are limited. It is apparent that MCs must express ZIP(s) to assimilate Zn during maturation. The Zn transporter, ZnT4 localizes on MC plasma membrane and likely on granule surfaces [95]. However, whether this transporter delivers Zn into MC granules is unclear. Bone-marrow derived MCs upregulate ZnT5 that localizes to the Golgi membrane, perhaps exporting cytosolic Zn into this organelle [96]. ZnT5 deficiency causes cytosolic Zn accumulation and inhibits translocation of protein kinase C to the plasma membrane, in effect, attenuating NF-κB signaling and downstream cytokine production. Intracellular Zn distribution is altered in Znt5-/- MCs, but degranulation is unaffected. In the Golgi, Zn is loaded onto proteins leading to the postulate that although degranulation stays intact, ZnT5 controls the potency of inflammatory mediators contained in MC granules. An aberration in Zn homeostasis will therefore disrupt the degranulation and cytokine activation response, ultimately compromising MC defenses against pathogens. Thus, Zn transport is dynamically integrated into the process of MC activation and reflects upon its crucial role in driving antimicrobial effector functions of these cells.
Collectively, animal studies demonstrate that MCs are assigned a higher Zn quota in the innate compartment. What transport mechanisms allocate high Zn concentrations to MCs? Are MCs a preferred Zn storage site in the innate compartment? Are MC granules the equivalent of zincosomes? How does Zn potentiate the antimicrobial activity of MCs? We may have just scratched the surface of Zn homeostasis in MC biology, but a lot remains to be discovered about how Zn regulates MC antimicrobial immunity.
Zn ropes in T cell activation for infection control
Pathogen clearance is potentiated further by initiating robust T cell responses. Athymic mice lacking T cell immunity display high propensity to infections and mortality [97]. Zn starvation causes thymic atrophy unequivocally drawing attention to the importance of Zn in maintaining T cell homeostasis [98]. Early studies on Zn-deficient animals and human subjects have underscored the significance of Zn in thymic development, and maintenance of T cells in peripheral lymphoid tissues [99]. Zn deficiency predisposes to bacterial and fungal infections that primarily rely on T cell immunity for clearance. A classic example is the genetic disorder, acrodermatitis enteropathica mostly caused by mutation in ZIP4 resulting in malabsorption of dietary Zn. ZIP4 deficiency leads to poor circulating numbers of CD4 T cells and recurrent bacterial and fungal infections [98]. A general consensus from whole body animal and human Zn deficiency analyses has emerged – lower Zn levels broadly associate with poor T cell responses and decreased resistance to infection. However, T cells constitute diversified subsets including Th1, Th2, Th17 and regulatory T cells (Tregs) with distinct roles in infection control. Therefore, aberrant Zn homeostasis during adaptive responses to bacteria, viruses, fungi or parasites may alter T cell functions at multiple levels including activation, proliferation and lineage differentiation. Fortunately, in most cases, the disparities in T cell responses caused by Zn deficiency can be rescued by adequate Zn supplementation [9].
Research by Prasad et. al. on Zn-deficient humans yielded fundamental revelations about profound roles of Zn on T cell homeostasis [98]. Restricted dietary Zn intake skews the CD4:CD8 T cell ratio with a reduction in the CD4 population. T cells from these subjects respond weakly to mitogenic stimuli and exhibit selective decrease in IL-2, IFNγ and TNFα, but not IL-6, IL-10 and IL-4 production elucidating impaired potency to mount a Th1 response and also a probable imbalance in Th1:Th2 ratios. Eliciting Th1 immunity is broadly essential to the clearance of numerous bacteria including E. coli, Salmonella, Staphylococcus, Pseudomonas, Yersinia, Klebsiella spp., fungi, H. capsulatum, C. neoformans, C. albicans, A. fumigatus, the protozoan parasite L. major and viruses [100, 101]. Therefore, the consequence of Zn deficiency on resistance to infections dominated by Th1 immunity can be dramatic. For example, the incidence and duration of diarrhea episodes caused by pathogens that elicit Th1 immunity is high in Zn-deficient children [102]. Although data are lacking to support a role for improved Th1 responses, Zn supplementation is a simple and efficacious approach in controlling diarrheal infections and is in fact the WHO recommendation for management of the disease.
The above human analyses revealed no apparent dysregulation in Th2 immunity, but animal models point to a role for Zn in regulating Th2-dependent IL-4 and IL-5 production and downstream signaling via STAT6 [103]. Zn-deficient animals fail to exert Th2 defenses against H. polygyrus nematode infection [104]. As discussed above, parasites thrive readily in Zn-deficient hosts. Additional data are required to substantiate the role of Zn in regulating Th2 immunity and to expand the repertoire of pathogens whose clearance may rely on Zn-dependent functions of Th2 cells.
Zn inhibits STAT3 activation and subsequent Th17 differentiation from naïve T cells in a model of collagen-induced arthritis [105]. Zn supplemented mice are protected from Th17 induced inflammation. Mounting data indicate that Zn supplementation favors naïve T cell differentiation into Foxp3+ Tregs [106, 107]. Tregs and Th17 populations strike a balance, where Th17 induced inflammation is suppressed by the regulatory functions of Foxp3+ Tregs. Thus, Zn-driven differentiation of the T cell response towards Tregs will in effect, influence the scale of Th17 defenses during infection. Th17 cells produce IL-17, a cytokine essential in neutrophil recruitment and control of bacterial and fungal infections [108]. Although there are no data, the consequences of skewing the Treg-Th17 balance by Zn supplementation may dampen immunity against infections that must be cleared by a dominant Th17 response. Analogous to antimicrobial function of metallothioneins in macrophages, their induction in T cells may indirectly control infections. Metallothioneins 1 and 2 negatively regulate Tr1 differentiation, a subtype of IL-10 producing Tregs by inhibiting STAT1 and STAT3 signaling and suppress IL-10 production [109]. The induction of metallothionein by Zn raises an added mechanistic explanation for why the metal curtails STAT3-driven Th17 differentiation. Through its immunosuppressive functions, IL-10 deactivates macrophages and diminishes IL-17, causing intracellular pathogens, parasites and viruses to escape proinflammatory control [110]. In the setting of infection, T cell-associated metallothionein would therefore sustain proinflammatory effector responses while suppressing Tregs. Thus, the induction of metallothioneins by Zn or cytokine signals may well be involved in striking the effector – regulatory T cell balance, in effect, bolstering pathogen clearance when adaptive immunity sets in.
Intriguing questions arise as a result of the complex balance between Th1/Th17/Treg cells and the immunomodulatory role of Zn in shaping their proportions. Though Zn deficiency ameliorates Th1 immunity, it may favor the development of Th17 cells that could potentially take over infection control. Studies on Zn supplementation suggest restored Th1 functions, but, how does Zn fine-tune the balance between Th1 and Th17 cells while promoting Treg differentiation? Skewing of T cell subset proportions by Zn deserves further examination especially considering that promoting Tregs by Zn supplementation would, in general, downmodulate inflammation irrespective of the contributing T effector population. Answers to these questions might reside with the dynamic regulation of Zn by these T cell subsets pertinent to the duration and concentration of Zn exposure.
Zn deficiency also attenuates cytotoxic T cell (CTL) responses as a result of diminished proportions of CD8+CD73+ CTL precursors [98]. CTLs bind MHCI on virus infected cells and kill them. Does the “Zn loss” phenomenon augment surface MHCI during viral infection? If true, Zn regulation in virus-invaded cells would be crucial in driving a CTL response. In conjunction with its role in antiviral immunity, Zn administration is efficacious for the common cold, caused by infection with rhinoviruses. Zn ions compete for intracellular adhesion molecule 1 (ICAM1) binding site on T cells, thereby blocking rhinoviral entry into T cells for replication [111].
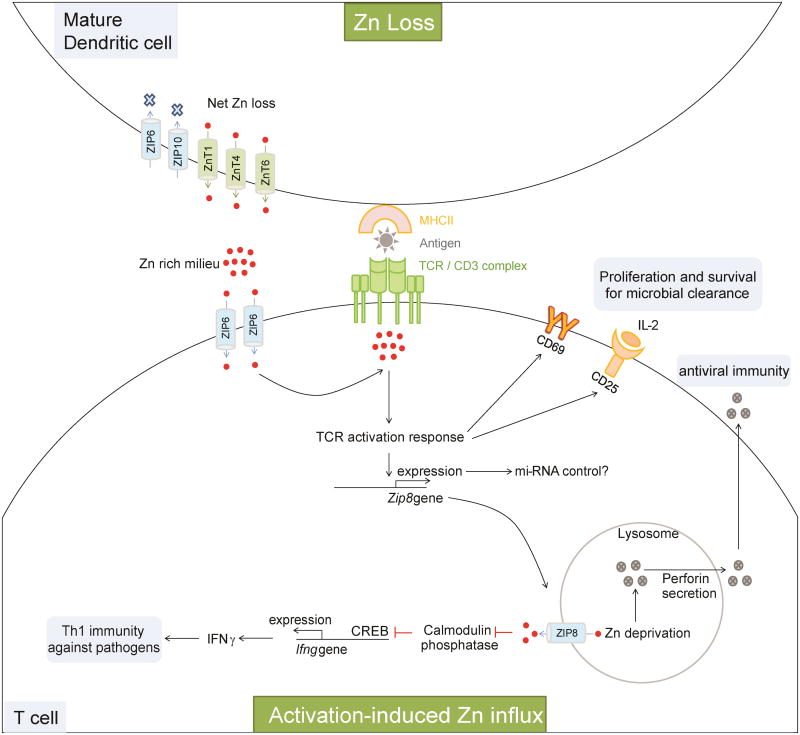
Zn is intricately tied to innate immunity and activation of this arm is pivotal in shaping adaptive defense mechanisms. Thus, an aberration in Zn homeostasis at any stage can potentially strike the entire inflammatory cascade leading to an immunological collapse that would prove detrimental during microbial attack. Yu et. al. demonstrated that Zn is drawn into T cells within seconds upon activation and Zn accumulation at the TCR synapse occurs only in T cells that establish physical contact with DCs [112]. This unique observation draws the premise that Zn efflux from maturing innate cells, in this case, DCs, can “feed” into T cells undergoing activation while still in contact with the innate population (Figure 4). Zn influx is driven by ZIP6 in activating T cells; coincidentally, DCs downregulate ZIP6 to drive net Zn loss [77]. As an ionic signaling molecule, Zn stimulates formation of TCR activation complexes to drive multiple processes – sustained calcium influx, increased CD69, a T cell activation marker, and an elevation in the IL-2 receptor, CD25 [112]. Mechanistically, this would mean that once antigen-presenting DCs and T cells have laid the “Zn communication” signal, T cell mediated immunity is roped in to accelerate microbial elimination.
Figure 4. Zn regulation in T cell mediated antimicrobial defenses.
Activation of T cells is essential for proliferation, cytokine production and clearance of bacteria, fungi and viruses. Zn is exported from DCs to drive Zn loss. This process matures them and increases surface MHCII. When T cells establish physical contact with an antigen-presenting MHCII, Zn is imported from extracellular space via ZIP6. The Zn lost from DCs into the extracellular milieu can potentially be drawn into T cells. Imported Zn accumulates in the sub-synaptic region at the point of DC-T cell contact to drive the TCR activation response leading to an increase in CD69 and CD25. These molecules support survival and proliferation of T cells required to elicit T cell antimicrobial immunity. Activation enhances Zip8 expression, which may be negatively controlled by mi-RNAs. ZIP8 imports lysosomal Zn into the cytosol. Cytosolic Zn inhibits Ca2+/calmodulin phosphatase to sustain CREB mediated IFNγ production to bolster Th1 immunity against microbes. Lysosomal Zn deprivation caused by ZIP8 induces perforin secretion from lysosomes that is essential in CD8 T cell responses to viral infection. Red dots, Zn ions; Solid arrows and lines, established links; dotted arrows and lines, predicted links.
A thorough analysis of Zn transport mechanisms in T cells is impending. IFNγ produced by Th1 and to some extent by Th17 cells is a hallmark cytokine in resistance to bacterial and fungal infections [113]. Upon activation, T cells upregulate ZIP8 to transfer lysosomal Zn into cytosol, that impacts at least two processes i) elevated cytosolic Zn increases IFNγ; ii) reduced lysosomal Zn promotes perforin secretion important for most antiviral CD8 T cell responses (Figure 4) [114]. An increase in cytosolic Zn by ZIP8 inhibits dephosphorylation of c-AMP response element binding protein (CREB) by calcium/calmodulin-regulated phosphatase, in effect, increasing Ifng transcription by CREB. Thus, the cytosol draws both extracellular and lysosomal Zn via ZIP6 and ZIP8 respectively for cohesive TCR activation followed by cytokine production. In monocytes, however, ZIP8 thwarts excessive NF-κB activation to preclude hyperinflammation caused by sepsis [115]. These observations underscore that a single Zn transporter can generate divergent outcomes contingent upon the cell type that expresses it. The Zn transport system in mammalian cells may be subject to three-tier regulation involving transcription, translation and regulation by microRNAs (miRNAs). miR-488 downregulates ZIP8 expression in chondrocytes [116]. Several miRNAs have been found to regulate CD4 and CD8 T cell responses to pathogens. For example, miR-155 highly expressed in activated CD8+ T cells, is required to mount an optimal proliferation and memory response against influenza virus and the bacterium, Listeria monocytogenes [117]. The control of ZIP8 or other Zn transporters in T cells by miRNAs has not been elucidated, but offers an interesting prospect reflecting the complexity involved in maintaining immunological Zn homeostasis.
Concluding insights
Zn orchestration in the immune system is a tightly knit circuit wherein this unique divalent cation levies a global impact on immunological performance against infection, but also acts as a “micromanager” of the activation mechanisms in individual immune cell types. This feature of Zn homeostasis ensures maximal potency of a coherent immune response that resists and protects the host from microbial pathogenesis. Recent revelations pertaining to Zn biology of immune cells has gravitated increasing curiosity towards the Zn modulatory mechanisms in play during pathogen attack. A lot remains to be discovered in the field of “Zn immunology” especially considering that nature has chosen this non-redox metal to be incorporated in the evolution of all forms of life. The indispensable involvement of Zn in cellular processes across prokaryotes, lower eukaryotes and mammals has a fundamental implication in how the immune system chooses to resolve the battle with invading microbes. Zn sequestration, Zn intoxication and Zn signaling, have thus far, emerged as the “three prime foci” of immunological strategies deployed to protect the host from infections. Thus, immune cells operate with a robust arsenal of multitude Zn modulatory approaches to counter invading pathogens, illuminating the versatility that Zn regulation has in scaling the nature of immune signals.
Highlights.
Immunological orchestration of Zn homeostasis challenges microbial parasitization
Specialized immune cells execute unique and overlapping Zn modulatory arsenals
Zn intoxication, sequestration and signaling are three prime foci of host defenses
Pathogens manipulate host Zn environment to battle immunological resistance
Acknowledgments
This work is supported by NIH grant AI106269. K.S.V is supported by American Heart Association 15POST25700182, 2015 and CEG NIEHS P30ES006096. The authors declare no conflicts of interest.
Abbreviations
- Zn
Zinc ions or Zn2+
- DCs
dendritic cells
- pDCs
plasmacytoid dendritic cells
- PRR
pattern recognition receptors
- IL-
interleukin
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor α
- IFNγ
interferon γ
- ZnT
Zn transporter
- NRAMP1
non-resistance associated macrophage protein 1
- GM-CSF
granulocyte-macrophage colony stimulating factor
- M-CSF
macrophage-colony stimulating factor
- STAT
signal transducer and activator of transcription (STAT)
- HVCN1
H+ channel
- Zip or SLC39A
Zn importer
- ZnT or SLC30A
Zn exporter
- SOD
superoxide dismutase
- PI3K
phosphoinositide-3-kinase
- S100A8/A9
calprotectin complex
- Pra1
pH-regulated antigen-1
- NET
neutrophil extracellular traps
- TLR4
toll-like receptor 4
- TRIF
TIR-domain-containing adaptor-inducing-interferon β
- MyD88
myeloid differentiation primary response gene 88
- MHC
major histocompatibility complex
- MC
mast cells
- NF-κB
nuclear factor-κB
- Treg
regulatory T cell
- CTL
cytotoxic T cell
- ICAM1
intracellular adhesion molecule 1
- miRNA
microRNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDonald RS. The Journal of Nutrition. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 2.Haase H, Rink L. Biofactors. 2014;40:27–40. doi: 10.1002/biof.1114. [DOI] [PubMed] [Google Scholar]
- 3.Eide DJ. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Liuzzi JP, Cousins RJ. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS., Jr PLoS Pathog. 2013;9:e1003815. doi: 10.1371/journal.ppat.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rink L, Gabriel P. Proc Nutr Soc. 2000;59:541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 7.Fraker PJ, King LE. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 8.Haase H, Rink L. Immun Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar AH, Prasad AS. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 10.Mosser DM. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 11.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirth JJ, Fraker PJ, Kierszenbaum F. Immunology. 1989;68:114–119. [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon S. Nature Reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 14.de Araujo-Jorge TC, de Souza W. Z Parasitenkd. 1986;72:153–171. doi: 10.1007/BF00931143. [DOI] [PubMed] [Google Scholar]
- 15.O'Dell BL. J Nutr. 2000;130:1432S–1436S. doi: 10.1093/jn/130.5.1432S. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Fernandez MA, Fernandez MA, Fresno M. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 17.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maret W. J Biol Inorg Chem. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 19.Wellenreuther G, Cianci M, Tucoulou R, Meyer-Klaucke W, Haase H. Biochem Biophys Res Commun. 2009;380:198–203. doi: 10.1016/j.bbrc.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 20.Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- 21.Grover A, Sharma R. J Bacteriol. 2006;188:7026–7032. doi: 10.1128/JB.00643-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 23.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 24.Vergne I, Chua J, Singh SB, Deretic V. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 25.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C. Nat Chem Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowland JL, Niederweis M. Tuberculosis (Edinb) 2012;92:202–210. doi: 10.1016/j.tube.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevo Y, Nelson N. Biochim Biophys Acta. 2006;1763:609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Smith AT, Smith KP, Rosenzweig AC. J Biol Inorg Chem. 2014;19:947–960. doi: 10.1007/s00775-014-1129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapetanovic R, Bokil NJ, Achard ME, Ong CY, Peters KM, Stocks CJ, Phan MD, Monteleone M, Schroder K, Irvine KM, Saunders BM, Walker MJ, Stacey KJ, McEwan AG, Schembri MA, Sweet MJ. FASEB J. 2016 doi: 10.1096/fj.201500061. [DOI] [PubMed] [Google Scholar]
- 31.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. PLoS Pathog. 2011;7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Counago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. Nat Chem Biol. 2014;10:35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 33.Eijkelkamp BA, Morey JR, Ween MP, Ong CL, McEwan AG, Paton JC, McDevitt CA. PLoS One. 2014;9:e89427. doi: 10.1371/journal.pone.0089427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng HJ, McEwan AG, Paton JC, Jennings MP. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS., Jr Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters MS, Chan Q, Caruso JA, Deepe GS., Jr J Infect Dis. 2010;202:1136–1145. doi: 10.1086/656191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colvin RA, Holmes WR, Fontaine CP, Maret W. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 38.DeCoursey TE. Physiology (Bethesda) 2010;25:27–40. doi: 10.1152/physiol.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeCoursey TE, Morgan D, Cherny VV. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 40.Youseff BH, Holbrook ED, Smolnycki KA, Rappleye CA. PLoS Pathog. 2012;8:e1002713. doi: 10.1371/journal.ppat.1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maret W. The Journal of Nutrition. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 42.Haase H. Immunity. 2013;39:623–624. doi: 10.1016/j.immuni.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehtonen A, Matikainen S, Miettinen M, Julkunen I. J Leukoc Biol. 2002;71:511–519. [PubMed] [Google Scholar]
- 45.Martinez FO, Gordon S. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaltenberg J, Plum LM, Ober-Blobaum JL, Honscheid A, Rink L, Haase H. Eur J Immunol. 2010;40:1496–1503. doi: 10.1002/eji.200939574. [DOI] [PubMed] [Google Scholar]
- 47.Barthel A, Ostrakhovitch EA, Walter PL, Kampkotter A, Klotz LO. Arch Biochem Biophys. 2007;463:175–182. doi: 10.1016/j.abb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 49.Outten CE, O'Halloran TV. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 50.MacDiarmid CW, Gaither LA, Eide D. The EMBO Journal. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nauseef WM. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 52.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. J Biol Chem. 1991;266:13462–13467. [PubMed] [Google Scholar]
- 53.Fagerhol MK, Dale I, Anderson T. Scandinavian Journal of Haematology. 1980;24:393–398. [Google Scholar]
- 54.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 55.Brophy MB, Hayden JA, Nolan EM. J Am Chem Soc. 2012;134:18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korndorfer IP, Brueckner F, Skerra A. J Mol Biol. 2007;370:887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 57.Kehl-Fie Thomas E, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro Kim A, Chazin Walter J, Skaar Eric P. Cell Host & Microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, Kovar IZ, Beattie JH, Wolska-Kusnierz B, Saito Y, Roth J. Lancet. 2002;360:1742–1745. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- 59.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 60.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. Blood. 2004;104:4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 61.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Int Immunol. 2000;12:1593–1604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 62.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 63.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 65.Segal AW. Novartis Found Symp. 2006;279:92–98. discussion 98-100, 216-109. [PubMed] [Google Scholar]
- 66.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 67.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 68.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. The Journal of Immunology. 2015 doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasan R, Rink L, Haase H. Innate Immun. 2013;19:253–264. doi: 10.1177/1753425912458815. [DOI] [PubMed] [Google Scholar]
- 71.Ong CL, Walker MJ, McEwan AG. Sci Rep. 2015;5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. J Infect Dis. 2014;209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 73.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Citiulo F, Jacobsen ID, Miramon P, Schild L, Brunke S, Zipfel P, Brock M, Hube B, Wilson D. PLoS Pathog. 2012;8:e1002777. doi: 10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Losse J, Svobodova E, Heyken A, Hube B, Zipfel PF, Jozsi M. Mol Immunol. 2011;48:2135–2143. doi: 10.1016/j.molimm.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Mildner A, Jung S. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 78.Wong CP, Rinaldi NA, Ho E. Mol Nutr Food Res. 2015;59:991–999. doi: 10.1002/mnfr.201400761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savina A, Amigorena S. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 80.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 81.Akira S, Takeda K. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 82.Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM. Cell Host Microbe. 2011;9:415–424. doi: 10.1016/j.chom.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loures FV, Rohm M, Lee CK, Santos E, Wang JP, Specht CA, Calich VL, Urban CF, Levitz SM. PLoS Pathog. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meredith MM, Liu K, Kamphorst AO, Idoyaga J, Yamane A, Guermonprez P, Rihn S, Yao KH, Silva IT, Oliveira TY, Skokos D, Casellas R, Nussenzweig MC. J Exp Med. 2012;209:1583–1593. doi: 10.1084/jem.20121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krishna SS, Majumdar I, Grishin NV. Nucleic Acids Research. 2003;31:532–550. doi: 10.1093/nar/gkg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urb M, Sheppard DC. PLoS Pathog. 2012;8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wernersson S, Pejler G. Nat Rev Immunol. 2014;14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 88.Angyal AM, Archer GT. Aust J Exp Biol Med Sci. 1968;46:119–121. doi: 10.1038/icb.1968.10. [DOI] [PubMed] [Google Scholar]
- 89.Belanger LF. J Nutr. 1978;108:1315–1321. doi: 10.1093/jn/108.8.1315. [DOI] [PubMed] [Google Scholar]
- 90.Kabu K, Yamasaki S, Kamimura D, Ito Y, Hasegawa A, Sato E, Kitamura H, Nishida K, Hirano T. J Immunol. 2006;177:1296–1305. doi: 10.4049/jimmunol.177.2.1296. [DOI] [PubMed] [Google Scholar]
- 91.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi HN, Scott ME, Stevenson MM, Koski KG. J Nutr. 1998;128:20–27. doi: 10.1093/jn/128.1.20. [DOI] [PubMed] [Google Scholar]
- 93.Scott ME, Koski KG. J Nutr. 2000;130:1412S–1420S. doi: 10.1093/jn/130.5.1412S. [DOI] [PubMed] [Google Scholar]
- 94.Sandig H, Bulfone-Paus S. Front Immunol. 2012;3:185. doi: 10.3389/fimmu.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ho LH, Ruffin RE, Murgia C, Li L, Krilis SA, Zalewski PD. J Immunol. 2004;172:7750–7760. doi: 10.4049/jimmunol.172.12.7750. [DOI] [PubMed] [Google Scholar]
- 96.Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, Hirano T. The Journal of Experimental Medicine. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.North RJ. Cell Immunol. 1973;7:166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- 98.Prasad AS. J Infect Dis. 2000;182(Suppl 1):S62–68. doi: 10.1086/315916. [DOI] [PubMed] [Google Scholar]
- 99.Fraker PJ, Gershwin ME, Good RA, Prasad A. Fed Proc. 1986;45:1474–1479. [PubMed] [Google Scholar]
- 100.Spellberg B, Edwards JE. Clinical Infectious Diseases. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 101.Allendoerfer R, Biovin GP, Deepe GS., Jr J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 102.Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. N Engl J Med. 1995;333:839–844. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- 103.Gruber K, Maywald M, Rosenkranz E, Haase H, Plumakers B, Rink L. J Biol Regul Homeost Agents. 2013;27:661–671. [PubMed] [Google Scholar]
- 104.Shi HN, Scott ME, Stevenson MM, Koski KG. Parasite Immunol. 1994;16:339–350. doi: 10.1111/j.1365-3024.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 105.Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T, Ueda N, Azuma I, Hirota H, Murakami M, Hirano T. Int Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 106.Rosenkranz E, Metz CH, Maywald M, Hilgers RD, Wessels I, Senff T, Haase H, Jager M, Ott M, Aspinall R, Plumakers B, Rink L. Mol Nutr Food Res. 2015 doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 107.Rosenkranz E, Hilgers RD, Uciechowski P, Petersen A, Plumakers B, Rink L. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 108.Jin W, Dong C. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu C, Pot C, Apetoh L, Thalhamer T, Zhu B, Murugaiyan G, Xiao S, Lee Y, Rangachari M, Yosef N, Kuchroo VK. Proc Natl Acad Sci U S A. 2013;110:7802–7807. doi: 10.1073/pnas.1211776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Couper KN, Blount DG, Riley EM. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 111.Hulisz D. J Am Pharm Assoc (2003) 2004;44:594–603. doi: 10.1331/1544-3191.44.5.594.Hulisz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 114.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song J, Kim D, Lee CH, Lee MS, Chun CH, Jin EJ. J Biomed Sci. 2013;20:31. doi: 10.1186/1423-0127-20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]