Abstract
Introduction
Opioid analgesic use is a major cause of morbidity and mortality in the US, yet effective treatment programs have a limited ability to detect relapse. The utility of current drug detection methods is often restricted due to their retrospective and subjective nature. Wearable biosensors have the potential to improve detection of relapse by providing objective, real time physiologic data on opioid use that can be used by treating clinicians to augment behavioral interventions.
Methods
Thirty emergency department (ED) patients who were prescribed intravenous opioid medication for acute pain were recruited to wear a wristband biosensor. The biosensor measured electrodermal activity, skin temperature and locomotion data, which was recorded before and after intravenous opioid administration. Hilbert transform analyses combined with paired t-tests were used to compare the biosensor data A) within subjects, before and after administration of opioids; B) between subjects, based on hand dominance, gender, and opioid use history.
Results
Within subjects, a significant decrease in locomotion and increase in skin temperature were consistently detected by the biosensors after opioid administration. A significant change in electrodermal activity was not consistently detected. Between subjects, biometric changes varied with level of opioid use history (heavy vs. nonheavy users), but did not vary with gender or type of opioid. Specifically, heavy users demonstrated a greater decrease in short amplitude movements (i.e. fidgeting movements) compared to non-heavy users.
Conclusion
A wearable biosensor showed a consistent physiologic pattern after ED opioid administration and differences between patterns of heavy and non-heavy opioid users were noted. Potential applications of biosensors to drug addiction treatment and pain management should be studied further.
Keywords: Wearables, Opioids, Biosensors, Biometrics, Signal Processing
Introduction
Opioid overdose is a leading cause of accidental death in the USA, with death rates rising steadily over the last 20 years [1]. Of the over 22,000 deaths relating to pharmaceutical overdose in 2011, three quarters involved opioid analgesics [2]. Increases in problematic opioid use have paralleled a corresponding increase in drug treatment admissions [3]. Drug treatment programs currently focus on behavioral and pharmacologic interventions to sustain abstinence, and success is typically measured by self-reports or urine drug screening [4]. Both measurement methods are limited by such factors as recall bias, distortion, and lack of precision [5]. A detection method that accurately detects opioid use as it occurs in real time would provide several distinct advantages including the ability to obtain environmental and behavioral contexts surrounding relapse as well as an opportunity for targeted interventions.
Portable biosensors are small, wearable devices similar in size and appearance to a wristwatch that continuously monitor various physiologic parameters of the wearer. Biosensors designed for research applications commonly measure surrogate markers for sympathetic nervous system activity, namely electrodermal activity (EDA), skin temperature, and locomotion (Table 1). Similar devices have been utilized to monitor multiple physiologic and pathophysiologic conditions known to involve robust SNS changes such as stress, post-traumatic stress disorder (PTSD), and epilepsy [6–8]. They have also been applied to compliance monitoring for drug adherence and suicide risk [9, 10] and are able to detect cocaine use in natural environments [11].
Table 1.
Physiologic parameters commonly measured by portable biosensors
| Physiologic parameter | Definition |
|---|---|
| Electrodermal activity (EDA) | A measure of electrical conductance of the skin, which varies with the amount of cutaneous sweat production. Sweat production is mediated by the sympathetic nervous system, and therefore, EDA is an indirect measure of sympathetic nervous system activity [5], which may be heightened or depressed with exposure to various drugs |
| Skin temperature | A measure of the temperature of the skin/body surface. Skin temperature may differ from core temperature depending on the vasoconstriction or vasodilation effects of sympathetic nervous system activity that can be mediated by drug use |
| Locomotion | A measure of a patient’s motor activity with respect to the body location on which the sensor is worn, measured in three axes (x, y, and z). Movement patterns are expected to vary with sympathetic nervous system activity. The X-axis is the anterior-posterior direction; the Y-axis is the lateral direction; the Z-axis is the caudad-cephalad. |
Before biosensors can be deployed in natural environments to detect opioid use, a pattern of expected response must be established and contributing factors must be defined. We undertook the present study in a controlled environment with known doses, times, and routes of opioid exposure to determine if a standard response pattern of EDA, skin temperature, and locomotion occurs after intravenously administered opioids. We also examined the relationship between this response and patient variables such as opioid use history, gender, and location of measurement with respect to hand dominance.
Methods
The study protocol was approved by the Institutional Review Board at the University of Massachusetts. Informed consent was obtained from all subjects prior to participation.
Biosensor
The Q sensor™ (Affectiva, Waltham, MA) was used to obtain all biometric data (Fig. 1). The sensor is approximately 4 × 5 cm and is secured via Velcro band to the volar aspect of the participant’s wrist. Electrodermal activity (in microSiemans), skin temperature (in degrees Celsius), and locomotion (in units g) in the X (anterior-posterior), Y (medial-lateral), and Z (caudad-cephalad) axes were continuously recorded at a sampling rate of 8 cycles per second.
Fig. 1.
Picture of Q sensor
Enrollment and Study Protocol
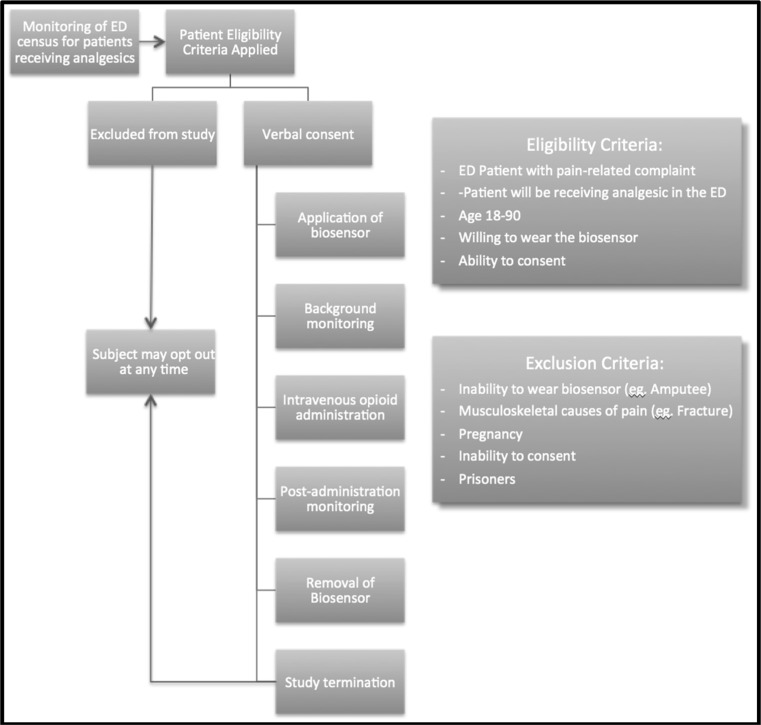
A total of 30 subjects who presented to the emergency department (ED) at a large tertiary care medical center with a pain-related complaint were recruited for this study. A toxicology fellow and a research assistant enrolled a convenience sample of subjects during in-house call hours (two weekdays per week, 9 am–5 PM). Patients who were likely to receive intravenous analgesics were identified by the ED electronic tracking board and approached to participate. Inclusion criteria were age between 18 and 90 years old, ability to consent, and willingness to wear the biosensor on their wrist before, during, and after the administration of opioid analgesics. Exclusion criteria were inability to wear the biosensor (e.g., amputation), musculoskeletal causes of pain limiting motion (e.g., acute fractures), pregnancy, inability to consent, and incarceration.
As the study was designed not to interfere with patient care or delay treatment, the duration of biosensor monitoring pre-opioid administration varied between subjects. A minimum of 5 min of data prior to receiving the opioid analgesics was obtained to allow sufficient time to establish a physiologic baseline, and 30-min post-administration data was obtained to capture the peak physiologic effect of the opioid. To correct for this variability in duration of baseline monitoring, only the 5 min immediately prior to opioid administration was used for the baseline biometric characteristics. Study subjects were able to discontinue their participation in the study at any time. Figure 2 demonstrates the enrollment and participation of participants through the study.
Fig. 2.
Flow diagram for study participants
Evaluation of Opioid Use History
In order to determine differences in physiologic responses to intravenous opioid administration based on use history, patients were classified into two categories: heavy and non-heavy users. Heavy opioid users were defined as having chronic daily opioid use, being on opioid maintenance therapy (e.g., methadone, buprenorphine), or having a diagnosis of opioid abuse. Subjects that did not meet these criteria were classified as non-heavy users. The patient’s opioid use history was determined by the patient’s self-report, as well as data obtained through the patient’s electronic medical record at the same medical center for the previous 10 years, looking specifically at the patient’s prescription drug use history and documented social history. Two reviewers (SC and KW) reviewed all data and assigned each patient to a category, and results were compared for agreement. In the event of difference in classification, a third reviewer (EB) was asked to review.
Statistical Analysis
Signal Processing of Locomotion Data
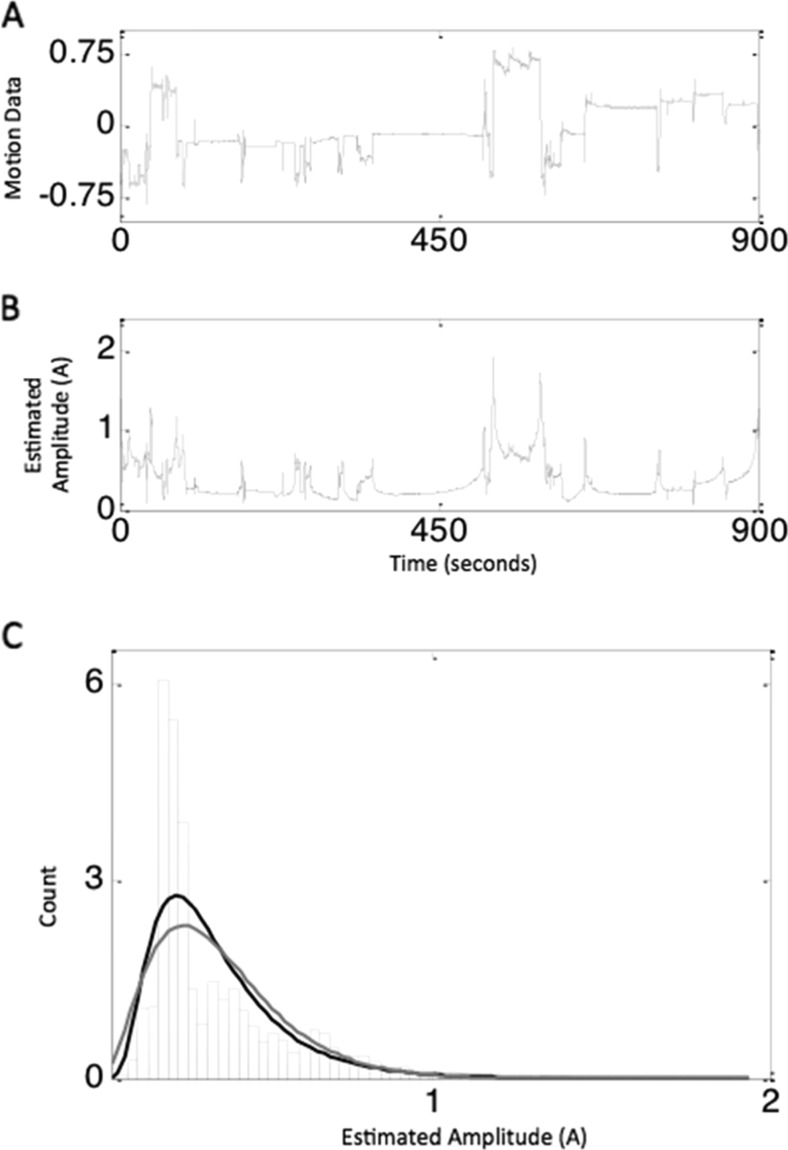
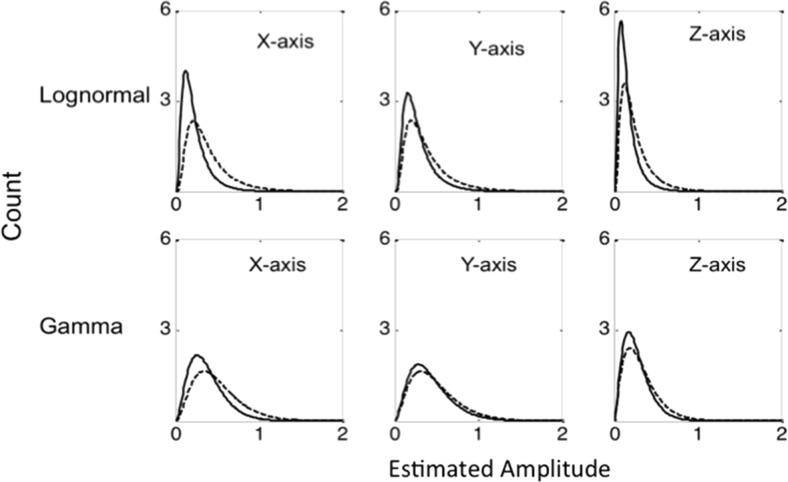
To better understand the rapid fluctuations and heterogeneity inherent in the measurement of locomotion, we estimated the amplitude of the fluctuations using Hilbert transform method [12]. This method is widely used in signal processing to estimate the amplitude of the data that rapidly changes with time (non-stationary). We applied Hilbert transform to the data at each of the axes and obtained the amplitude, which reflected the fluctuations in the data. To check whether such fluctuations were random in nature, we plotted the distribution of amplitudes. This showed a skew distribution rather than a Gaussian (normal) distribution suggesting that the amplitude fluctuations were not random. To characterize the distribution of amplitudes, we used gamma function as well as lognormal function, as these functions are both generally used for skew distributions [13]. Figure 3 shows how the raw data from Z-axis (a) is converted to the estimated amplitude plot via the Hilbert transform method (b) from a single subject. A density plot of the amplitudes was then generated (c). Figure 4 shows how the distribution of amplitudes for all subjects was then displayed as density plots along with the functions used to characterize the data.
Fig. 3.
a, b, c Sample from a single subject, showing the raw data from Z-axis (a) along with the estimated amplitude obtained from the Hilbert transformation (b). Distribution of amplitudes from individual above represented as density plots (black is lognormal, and gray is gamma function) (c)
Fig. 4.
Lognormal (top panels) and gamma distribution function (bottom panels) obtained from the average values (mu) of all subjects of X, Y, and Z axes of locomotion data during pre (black line) and post (dotted line) opioid administration
The signal processing technique included an estimation of mu (average) and sigma (standard deviation) of lognormal distribution as well as shape (of the curve) and scale (absolute) parameters of the gamma distribution for each axis of dimension. Changes in these parameters indicate changes in each distribution. Thus, an increase in mu of lognormal distribution or an increase in scale of gamma distribution indicates a decrease of short amplitudes on that axis. An increase in sigma of lognormal distribution or decrease in shape of gamma distribution indicates more right skewness of the distribution. Once pre- and post-administration values were obtained for each parameter (mu, sigma scale and shape for each of the three axes), they were compared using paired t tests.
Skin Temperature and EDA
Skin temperature and EDA data inherently demonstrate less rapid fluctuations compared to locomotion and thus did not require the application of signal transform methods described above. Instead, paired t tests were used to compare the difference between pre- and post-opioid administration body temperature and EDA.
Effect of Subject Characteristics
To examine the effects of opioid use history, age, gender, type of opioid, antiemetic use, and hand dominance on the changes in the temperature, EDA, and locomotion, a generalized estimating equation approach that assumed an exchangeable correlation between two hands (dominant hand and non-dominant hand) was used. The difference in parameters of temperature, X, Y, and Z were the outcome variables, as these were found to be significant in the original analysis. A separate model was run for each outcome variable. Age, gender, type of opioid, antiemetic use, history of opioid use (classified into two groups, heavy users and non-heavy users), and dominant hand versus non-dominant hand were the independent variables in all the models. All analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Demographics
Thirty participants were enrolled over a 4-month period. Of the 35 potential participants screened for enrollment, 2 participants declined to participate and 3 were disqualified because they did not receive opioids due to a change in treatment plan. The demographic data are depicted in Table 2. The numbers of female and male participants were approximately equal. The majority of participants were right-hand dominant which is reflective of the general population [14]. Morphine was the most common opioid administered, which is reflective of local practice. The duration of baseline monitoring ranged from 5 to 48 min, with a mean duration of 16.5 min.
Table 2.
Participant characteristics
| Characteristic | Percent (N = 30) |
|---|---|
| Age | |
| 18–50 | 63 % |
| 50–82 | 37 % |
| Sex | |
| Male | 47 % |
| Female | 53 % |
| Hand dominance | |
| Right | 90 % |
| Left | 10 % |
| Chief complaint | |
| Abdominal pain | 87 % |
| Flank pain | 7 % |
| Back pain | 3 % |
| Other | 3 % |
| Opioid analgesia | |
| Morphine | 70 % |
| Hydromorphone | 23 % |
| Fentanyl | 7 % |
| Opioid user class | |
| Non-heavy | 70 % |
| Heavy | 30 % |
Biophysiometric Parameters
Within Subjects Pre- and Post-Opioid Changes
Pre- and post-opioid delta values for all parameters are summarized in Table 3. EDA did not show a significant difference post administration compared to baseline. Average patient skin temperature was significantly higher after opioid admission (p < 0.001). Average units in overall movement parameters associated with the mean of X, Y and Z axes decreased significantly after opioid administration (all p values range <0.05), shown in Fig. 4.
Table 3.
Within subjects pre- and post-opioid results
| Parameter | Average delta | p value | |
|---|---|---|---|
| Skin temp (°C) | N/A | 2.62 | <0.0001 |
| EDA (microS) | N/A | 0.67 | 0.11 |
| Locomotion (G) | |||
| X-axis | Mu | −0.54 | 0.00 |
| Sigma | 0.01 | 0.70 | |
| Shape | 0.00 | 1.00 | |
| Scale | −0.04 | 0.00 | |
| Y-axis | Mu | −0.29 | 0.04 |
| Sigma | −0.04 | 0.40 | |
| Shape | 0.11 | 0.75 | |
| Scale | −0.02 | 0.08 | |
| Z-axis | Mu | −0.46 | 0.02 |
| Sigma | −0.01 | 0.82 | |
| Shape | 0.29 | 0.29 | |
| Scale | −0.03 | 0.00 | |
Between Subjects Comparison Groups (Table 4)
Table 4.
Between subjects comparison variables
| Temperature | X mu | Y mu | Z mu | X scale | Y scale | Z scale | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | Chi-square | p | DF | Chi-square | p | DF | Chi-square | p | DF | Chi-square | p | DF | Chi-square | p | DF | Chi-square | p | DF | Chi-square | p | |
| Age | 1.00 | 0.05 | 0.82 | 1.00 | 0.03 | 0.86 | 1.00 | 0.09 | 0.76 | 1.00 | 0.04 | 0.84 | 1.00 | 4.35 | 0.04 | 1.00 | 0.84 | 0.36 | 1.00 | 1.23 | 0.27 |
| Gender | 1.00 | 0.00 | 0.94 | 1.00 | 0.60 | 0.44 | 1.00 | 0.49 | 0.49 | 1.00 | 0.11 | 0.74 | 1.00 | 0.06 | 0.81 | 1.00 | 0.00 | 0.95 | 1.00 | 0.39 | 0.53 |
| Type of opioid med | 2.00 | 2.20 | 0.33 | 2.00 | 3.66 | 0.16 | 2.00 | 1.89 | 0.39 | 2.00 | 2.77 | 0.25 | 2.00 | 3.35 | 0.19 | 2.00 | 0.43 | 0.80 | 2.00 | 5.92 | 0.05 |
| Anti emetics | 1.00 | 0.40 | 0.53 | 1.00 | 0.25 | 0.62 | 1.00 | 1.11 | 0.29 | 1.00 | 1.61 | 0.20 | 1.00 | 2.71 | 0.10 | 1.00 | 0.08 | 0.78 | 1.00 | 0.78 | 0.38 |
| Hand dominance | 1.00 | 0.26 | 0.61 | 1.00 | 0.15 | 0.70 | 1.00 | 0.13 | 0.72 | 1.00 | 0.61 | 0.43 | 1.00 | 2.05 | 0.15 | 1.00 | 0.27 | 0.60 | 1.00 | 1.00 | 0.32 |
| Use history | 1.00 | 1.60 | 0.21 | 1.00 | 1.57 | 0.21 | 1.00 | 5.88 | 0.02 | 1.00 | 1.57 | 0.21 | 1.00 | 3.18 | 0.07 | 1.00 | 1.34 | 0.25 | 1.00 | 0.31 | 0.58 |
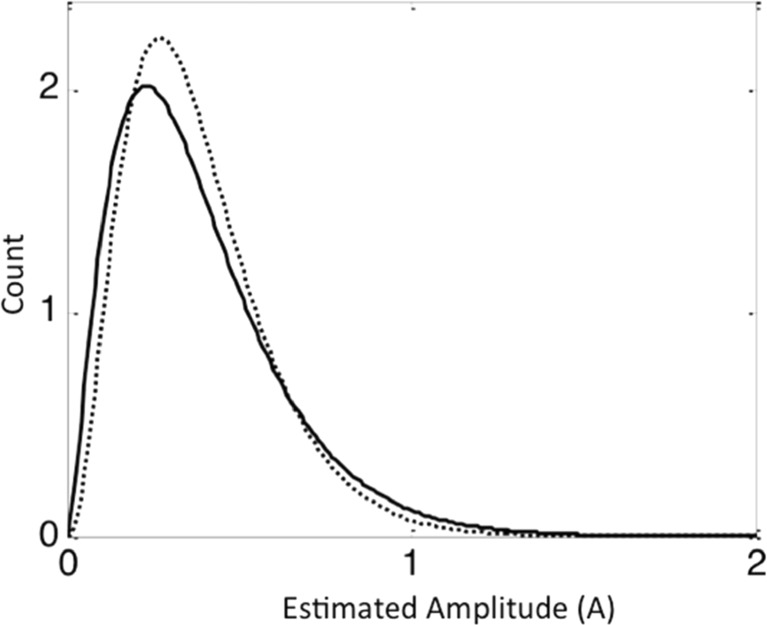
Heavy Versus Non-Heavy Users: Heavy users had a greater relative decrease of short amplitude signals on the Y-axis compared to non-heavy users, suggesting a specific decrease in small movement immediately after opioid administration (Fig. 5). There was no significant difference between heavy and non-heavy users on skin temperature change or EDA.
Fig. 5.
Gama distribution function from the non-dominant hand of heavy users (black line) compared to the non-dominant hand of non-heavy users (dotted line) in the Y-axis of the movement data. The low amplitudes in heavy users are significantly reduced compared to non-heavy users. The parameters are heavy users, Y-scale = 0.15 ± 0.11 and Y-shape = 2.49 ± 1.18, and non-heavy users Y-scale = 0.11 ± 0.09 and Y-shape = 3.41 ± 2.16
Age: Advanced age was associated with a greater decrease in movement on the X-axis after opioid administration when compared to younger patients.
Other Parameters: No significant effect on the change in skin temperature or locomotion were found based on gender, type of opioid (synthetic versus non-synthetic), hand dominance, and concomitant antiemetic use.
Discussion
In this preliminary study, wearable biosensors detected an increase in skin temperature and decrease in locomotion immediately following opioid administration, which is consistent with the physiologic effect of this drug class. Interestingly, heavy users as defined in our study had a significant reduction of short amplitude signals (i.e., fidgeting) compared to non-heavy users, particularly in the Y-axis (medial-lateral direction). In general, we expect heavy users to have a less pronounced effect when given an equivalent dose of opioid analgesic as a subject with less historical opioid exposure; however, these data suggest that their physiologic response is qualitatively different. Fidgeting or restless is described on multiple opioid withdrawal scoring systems [15] and is typically qualified subjectively by the subject or an observer. Our study was not designed to address craving or withdrawal or their relationship to opioid administration; however, we hypothesize that this phenomenon of short amplitude shifting may be related to subclinical opioid withdrawal in dependent subjects. The Y-axis predominance of this effect may be due to the physical limitations imposed by the ED stretcher in these participants. This should be explored further as it may have significant implications in the application of this technology.
The observed biometric patterns exhibited by our participants are unlikely to be reflective of other processes such as the relief of pain or the onset of sleep. Previous literature on biometric response to pain has demonstrated significant rise in EDA during painful stimuli and to a lesser degree decrease in skin temperature [16, 17]. We would expect that if the changes were simply due to relief of pain, a marked decrease in EDA would occur, which was not observed in our study population. Biometric monitoring of sleep is complex, and the expected profile depends on the stage of sleep. Skin temperature does rise with sleep as seen in our study group. However, sleep onset has been associated with a gradual decrease in EDA amplitude [18], while high-frequency EDA activity is noted in multiple other sleep stages including REM sleep [19]. These characteristic EDA changes were not seen in our participants.
Non-invasive, wearable devices are rapidly becoming popular due to commercially available versions marketed as health tracking tools [20, 21]. Various patient populations have reported the devices as acceptable for wear in both controlled and natural environments, further supporting their feasibility for use in outpatient therapy [8, 22]. The sensors are small and user friendly and provide continuous data streams that can be stored for retrieval and review at a later time point or transmitted wirelessly for real-time review and analysis. Substance abusing populations would benefit significantly from the ability to extend therapeutic support into natural environments. Before these devices can be deployed clinically however, more data is needed to identify a variety of states related to drug exposure (use, toxicity, withdrawal) and to more precisely distinguish these profiles from behaviors associated with daily life (i.e., sleeping or prolonged sedentary states). Multiple studies are underway to examine the biometric profiles associated with opioid administration in a variety of clinical scenarios to better define our parameters and develop identification profiles that are both sensitive and specific to the events of interest. This preliminary study works toward that goal.
The ability to identify instances of opioid use and opioid tolerance in real time could have a significant impact in multiple clinical settings including pain management and substance abuse treatment. In patients starting opioid therapy, biosensors could be used to monitor for developing opioid tolerance and to identify individuals at risk for substance abuse/addiction. In patients undergoing substance abuse treatment, biosensors can be applied to detect episodes of drug use. Relapse data can either be reviewed retrospectively to or transmitted wirelessly to trigger an intervention (for example, an alert to a family member or a community support system). Mobile platform-based applications can be programmed to recognize these patterns and can record contextual data (i.e., timing, location, circumstance) of events, which can be ultimately used to tailor behavioral interventions. Future studies should focus on utilization of biosensors to gauge response to behavioral therapy, to evaluate opioid tolerance and narcotic seeking behaviors, and to integrate with mobile technology platforms to deliver real-time interventions.
Limitations
The main limitations is the inability to generalize these results due to small sample size (N = 30) and the controlled healthcare facility setting where dose and route of administration are highly standardized. We expect that patients who engage in recreational self-administration of opioids would use higher opioid equivalent doses based on their tolerance and history. The particular opioid and dose administered in our study was based on treating physician discretion, and differences in agent may also influence results. Given that our study population was presenting for acute pain, this may have influenced both the baseline data and the response to opioids observed. Our duration of baseline data was limited, as we were not permitted to prolong the subjects’ wait time for medication. Patient use history was based on self-report and review of the electronic medical record, raising the possibility of inadvertent misclassification. Motion measurements in natural environments may be different than those of subjects lying on a stretcher in the ED.
Conclusion
Wearable biosensors show a consistent physiologic pattern after opioid administration in an ED population. This biometric response shows some distinguishing features between heavy and non-heavy opioid users in a controlled ED setting. This pattern may be useful to detect episodes of opioid use in real time. Further study is needed to evaluate the potential diagnostic and interventional applications of these devices in drug abuse treatment and pain management.
Acknowledgments
This work was generously supported by NIH National Institute on Drug Abuse grant R01DA033769-01 (EWB), the NIH National Institute on Drug Abuse Loan Repayment Program L30 DA038357 (SC), and partly supported by NIH National Institute on Drug Abuse grant 1R01DA033323-01 (JF) and NIH National Center for Advancing Translational Sciences 5UL1TR000161-04 pilot study award (UMass CTCS).
Compliance with Ethical Standards
Funding
NIH NIDA R01DA033769-01, L30 DA038357, NIH NIDA 1R01DA033323-01, and NIH NCATS 5UL1TR000161-04.
Conflicts of Interest
The authors have no conflicts to disclose.
References
- 1.Center for Disease Control and Prevention. (Last updated January 9, 2015). Prescription overdose in the United States: fact sheet. Retrieved 14 Jan 2015 from http://www.cdc.gov/homeabdrecreationalsafety/overdose/facts.html (2015).
- 2.Fletcher R, Tam S, Omojola O, Redemske R, Kwan J. Wearable sensor platform and mobile application for use in cognitive behavioral therapy for drug addiction and PTSD. Conf Proc IEEE Eng Med Biol Soc. 2011;1802–5. doi:10.1109/IEMBS.2011.6090513. [DOI] [PubMed]
- 3.Services DOHH. Addressing prescription drug abuse in the United States. 2014, p. 1–36.
- 4.Principles of Drug Addiction Treatment [Internet]. 2012. 44 p. Available from: http://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/principles-effective-treatment
- 5.Fishman SM, Wilsey B, Yang J, Reisfield GM. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manag. 2000;20(4):293–307. doi: 10.1016/S0885-3924(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 6.Poh M-Z, Swenson NC, Picard RW. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans Biomed Eng. 2010;57(5):1243–1252. doi: 10.1109/TBME.2009.2038487. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher R, Tam S, Omojola O, Redemske R, Kwan J. Wearable sensor platform and mobile application for use in cognitive behavioral therapy for drug addiction and PTSD. 2011, p. 1–4. [DOI] [PubMed]
- 8.Boyer EW, Fletcher R, Fay RJ, Smelson D, Ziedonis D, Picard RW. Preliminary efforts directed toward the detection of craving of illicit substances: the iHeal project. J Med Toxicol. 2012;8(1):5–9. doi: 10.1007/s13181-011-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer EW, Smelson D, Fletcher R, Ziedonis D, Picard RW. Wireless technologies, ubiquitous computing and mobile health: application to drug abuse treatment and compliance with HIV therapies. J Med Toxicol. 2010;6(2):212–216. doi: 10.1007/s13181-010-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indic P, Murray G, Maggini C, Amore M, Meschi T, Borghi L, et al. Multi-scale motility amplitude associated with suicidal thoughts in major depression. de Erausquin GA, editor. PLoS ONE. 2012;7(6):e38761. [DOI] [PMC free article] [PubMed]
- 11.Carreiro S, Fang H, Zhang J, Wittbold K, Weng S, Mullins R, et al. iMStrong: deployment of a Biosensor System to Detect Cocaine Use. J Med Syst. 2015. [DOI] [PMC free article] [PubMed]
- 12.Hahn S. “The instantaneous complex phase and complex frequency” in Hilbert transforms in signal processing. Boston, MA: Arthech House; 1996. p. 48.
- 13.Johnson NL, Kotz S, Balakrishnan N. “Order statistics” in continuous univariate distributions. 2nd ed. Wiley series in probability and statistics. New York, NY: Wiley and Sons; 1994. p 10.
- 14.Johnston DW, Nicholls MER, Shah M, Shields MA. Nature’s experiment? Handedness and early childhood development. Demography. 2009;46(2):281–301. doi: 10.1353/dem.0.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Substance Abuse Treatment. Appendix B Assessment and Screening Instruments. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2004. Available from: http://www.ncbi.nlm.nih.gov/books/NBK64244/
- 16.Jang E-H, Park B-J, Park M-S, Kim S-H, Sohn J-H. Analysis of physiological signals for recognition of boredom, pain, and surprise emotions. J Phys Anthropol. 2015;34:25. doi: 10.1186/s40101-015-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle BN, McNeil DW. Autonomic arousal and experimentally induced pain: a critical review of the literature. Pain Res Manag. 2014;19(3):159–167. doi: 10.1155/2014/536859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Seo S, Yoon HN, Jung, DW, Baek, HJ, Cho, J, et al. Sleep period time estimation based on electrodermal activity. IEEE J Biomed Health Inform. 2015. doi:10.1109/JBHI.2015.2490480. [DOI] [PubMed]
- 19.Sano A, Picard RW, Stickgold R. Quantitative analysis of wrist electrodermal activity during sleep. Int J Psychophysiol. 2014;94(3):382–389. doi: 10.1016/j.ijpsycho.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiordelli M, Diviani N, Schulz PJ. Mapping mHealth research: a decade of evolution. J Med Internet Res. 2013;15(5):e95. doi: 10.2196/jmir.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. Am Assoc Adv Sci. 2015;7(283):283rv3–283rv3. [DOI] [PMC free article] [PubMed]
- 22.Carreiro S, Smelson D, Ranney M, Horvath KJ, Picard RW, Boudreaux ED, et al. Real-time mobile detection of drug use with wearable biosensors: a pilot study. J Med Toxicol. 2014;11(1):1–7. doi: 10.1007/s13181-014-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]