Abstract
The American College of Medical Toxicology established the Toxicology Investigators Consortium (ToxIC) Case Registry in 2010. The Registry contains all medical toxicology consultations performed at participating sites. The Registry has continued to grow since its inception, and as of December 31, 2015, contains 43,099 cases. This is the sixth annual report of the ToxIC Registry, summarizing the additional 8115 cases entered in 2015. Cases were identified by a query of the Registry for all cases entered between January 1 and December 31, 2015. Specific data reviewed for analysis included demographics (age, race, gender), source of consultation, reason for consultation, agents and agent classes involved in exposures, signs, symptoms, clinical findings, fatalities, and treatment. By the end of 2015, there were 50 active sites, consisting of 101 separate health-care facilities; 51.2 % of cases involved females. Adults between the ages of 19 and 65 made up the majority (64.2 %) of Registry cases. Caucasian race was the most commonly reported (55.6 %); 9.6 % of cases were identified as Hispanic ethnicity. Inpatient and emergency department referrals were by far the most common referral sources (92.9 %). Intentional pharmaceutical exposures remained the most frequent reason for consultation, making up 52.3 % of cases. Of these intentional pharmaceutical exposures, 69 % represented an attempt at self-harm, and 85.6 % of these were a suicide attempt. Nonopioid analgesics, sedative-hypnotics, and antidepressant agents were the most commonly reported agent classes in 2015. Almost one-third of Registry cases involved a diagnosed toxidrome (32.8 %), with a sedative-hypnotic toxidrome being the most frequently described. Significant vital sign abnormalities were recorded in 25.3 % of cases. There were 98 fatalities reported in the Registry (1.2 %). Adverse drug reactions were reported in 4.3 % of cases. Toxicological treatment was given in 65.3 % of cases, with 33.0 % receiving specific antidotal therapy. Exposure characteristics and trends overall were similar to prior years. While treatment interventions were required in the majority of cases, fatalities were rare.
Electronic supplementary material
The online version of this article (doi:10.1007/s13181-016-0580-6) contains supplementary material, which is available to authorized users.
Keywords: Poisonings, Overdose, Surveillance, Epidemiology, Medical toxicology
Introduction
The Toxicology Investigators Consortium (ToxIC) Case Registry was created by the American College of Medical Toxicology (ACMT) in 2010 as a tool for clinical toxicology research and toxico-surveillance [1]. Unlike other poisoning databases, ToxIC is a prospective registry based on medical toxicologists’ experience conducting consultations in both inpatient and ambulatory settings. Cases where a formal consultation was not conducted, such as where advice was given over the telephone, are not included in the database.
The registry was started in 2010 with four sites and has continuously expanded to its current level. Four new sites were added in 2015. As of December 31, 2015, the total number of active sites was 50, comprised of 101 separate health-care facilities. Currently, 83 % of active accredited medical toxicology fellowship programs in the USA participate in the ToxIC registry (“ToxIC”). The objective of this report is to summarize the Registry’s 2015 data and activity. Cases entered in 2015 are described in this sixth annual report.
Since its inception, several supplemental or subregistries have been created within ToxIC. These are designed to collect more detailed information in specific areas. Two new subregistries were added in 2015: one collecting clinical, clearance, and pharmacokinetic data on patients undergoing extracorporeal substance removal, and another assessing the use and efficacy of sodium bicarbonate as an antidote. There are currently nine subregistries. Other changes in 2015 include enhanced data collection providing more detailed information about the circumstances and reasons for the toxic agent exposures, as well as more detailed assessments of the frequencies of attempts at self-harm, drug use/abuse, and adverse drug reactions as well as medication errors and patient outcomes.
In 2015, six manuscripts using ToxIC data were published in the peer-reviewed literature (Finkelstein et al. [2]; Froberg et al. [3]; Rhyee et al. [4]; Smith and Farmer [5]; Watkins et al. [6]; Zelner et al. [7]), and 25 published abstracts based on ToxIC data were presented at three national and international meetings.
In 2015, ToxIC was supported from extramural funds obtained from both government and industry sources. Government funding was provided by grants from the U.S. National Institute of Drug Abuse. Industry funding was in the form of an unrestricted grant from BTG International Inc. (North America), utilized for the support of the North American Snakebite Registry.
Methods
To be part of the consortium, all medical toxicologists at participating institutions agree to enter data into the registry on all medical toxicology consultations performed. Cases are entered into a password-protected, encrypted, online data collection form. The site is maintained by ACMT with oversight by the ToxIC Registry Steering Committee. The registry is compliant with the Health Insurance Portability and Accountability Act (HIPAA) and does not collect any protected health information or otherwise identifying fields. Registry participation is pursuant to a participating institution’s Independent Review Board (IRB) approval and is compliant with their policies and procedures. The registry is also independently reviewed by the Western Institutional Review Board (WIRB). WIRB has determined that the collection protocol based on submission of de-identified data from a clinical visit under the maintenance and control of the medical toxicologist does not meet the threshold of human subjects research under federal regulation 45 CFR 46 and associated guidance.
Data collected include presenting signs and symptoms, clinical course, treatments, patient demographics (age, gender, pregnancy status, race/ethnicity), outcomes, laboratory values, and types and reasons for toxicological exposures. The term “consultation” is used in this report to describe any encounter with a medical toxicologist for which a formal evaluation was conducted and placed in the medical record. Such encounters may include admission to a medical toxicology inpatient service or evaluation by a medical toxicologist, as a consulting physician in an emergency department, inpatient unit, or outpatient clinic. The online data collection form is formatted to ensure that data entry remains organized and easily searchable. Free text entry fields allow caregivers to provide further detail or supplementary information. As part of ToxIC’s mission to provide near-real time toxico-surveillance, a sentinel detection field signaling novel or unusual cases is included on the data entry form.
In this report, we summarize demographic data, source and location of consultation, reasons for encounter, toxicological agents, and treatments provided for all ToxIC cases recorded from January 1, 2015, through December 31, 2015. Summary statistics for cases involving fatalities and adverse drug reactions are also presented. In the summary tables, unless otherwise indicated, individual agent or agent classes with frequency counts of less than 5 are grouped into a “miscellaneous” category. Percentages noted in the tables for individual agents represent their relative proportion within their respective agent class. For clinical signs or symptoms, the tables provide the percentage of any individual sign or symptoms relative to the total number of cases. In the detailed treatment tables, percentages for each treatment modality represent the relative frequency among the subset of cases receiving at least one type of treatment. In instances of limited data for an entire class or clinical effects (e.g. such as 50 or fewer cases overall, or the majority of a class having less than 5 entries per individual agent), a detailed table is not presented but information may be described in the text section or available in the Supplementary material.
Results
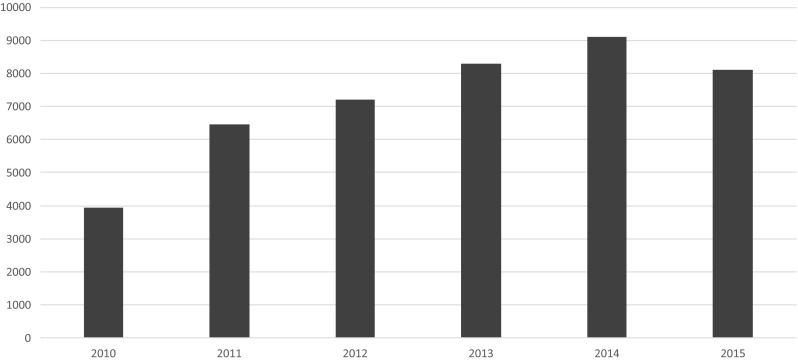
Tables 1 and 2 list the individual sites entering cases in ToxIC during 2015. The total number of cases in 2015 was 8115, down slightly from 2013 with 8598 cases and 2014 with 9172 cases [4, 8]. The number of cases contributed by individual sites ranged from 2 to 1163. Figure 1 shows the total case counts since the beginning of the registry in 2010.
Table 1.
Participating institutions providing cases to ToxIC in 2015—USA
| Arizona | Massachusetts |
| Phoenix | Boston |
| Banner-University Medical Center Phoenix | Beth Israel Boston |
| Phoenix Children’s Hospital | Children’s Hospital Boston |
| California | Worcester |
| Fresno | University of Massachusetts Memorial Medical Center |
| UCSF Fresno Medical Center | Michigan |
| Loma Linda | Grand Rapids |
| Loma Linda University Medical Center | Spectrum Health Hospitals |
| Los Angeles | Minnesota |
| University of Southern California Verdugo Hills | St. Paul |
| San Diego | Regions Hospital |
| Rady Children’s Hospital | Missouri |
| University of California San Diego - Hillcrest | Kansas City |
| University of California San Diego - Thornton | Children’s Mercy Hospitals & Clinics |
| San Francisco | St. Louis |
| San Francisco General Hospital | Washington University School of Medicine |
| Colorado | Nebraska |
| Denver | Omaha |
| Children’s Hospital Colorado | University of Nebraska Medical Center |
| Denver Health | New Mexico |
| Porter and Littleton Adventist Hospital | Albuquerque |
| Swedish Medical Center | University of New Mexico |
| University of Colorado Medical Center | New Jersey |
| Connecticut | Morristown |
| Hartford | Morristown Medical Center |
| Connecticut Children’s Medical Center | New Brunswick |
| Hartford Hospital | Robert Wood Johnson University Hospital Newark |
| John Dempsey Hospital | Newark |
| Florida | Newark Beth Israel Medical Center |
| Tampa | New Jersey Medical School |
| Tampa General Hospital | New York |
| Georgia | Manhasset |
| Atlanta | Long Island Jewish |
| Children’s Healthcare of Atlanta Egelston | North Shore University Hospital |
| Children’s Healthcare of Atlanta Hughes Spalding | New York |
| Emory University Hospital | Bellevue Medical Center |
| Emory University Hospital Midtown | Elmhurst Medical Center |
| Grady Health System | Mount Sinai Hospital |
| Grady Memorial Hospital | NYU Langone Medical Center |
| Illinois | Staten Island |
| Chicago | Staten Island University Hospital |
| Cook County Hospital | Rochester |
| Evanston | Highland Hospital |
| Evanston North Shore University Health System | Strong Memorial Hospital |
| Indiana | Syracuse |
| Indianapolis | Upstate Medical University-Downtown Campus |
| IU-Eskenazi Hospital | North Carolina |
| IU-Indiana University Hospital | Charlotte |
| IU-Methodist Hospital-Indianapolis | Carolinas Medical Center |
| IU-Riley Hospital for Children | |
| Oregon | Texas |
| Portland | Dallas |
| Doernbecher Children’s Hospital | Children’s Medical Center Dallas |
| Oregon Health & Science University Hospital | Parkland Memorial Hospital |
| Pennsylvania | University of Texas Southwestern Clinic |
| Harrisburg | William P Clements University Hospital |
| Harrisburg Hospital | Houston |
| PinnacleHealth-Community General Osteopathic | Ben Taub General Hospital |
| PinnacleHealth-Harrisburg Hospital | Texas Children’s Hospital |
| PinnacleHealth-West Shore | San Antonio |
| Philadelphia | San Antonio Military Medical Center |
| Children’s Hospital of Philadelphia | Utah |
| Einstein Medical Center–Montgomery | Salt Lake City |
| Einstein Medical Center–Main | Primary Children’s Hospital |
| Hahnemann University Hospital | University of Utah Hospital |
| Mercy Fitzgerald Hospital | Virginia |
| Mercy Hospital of Philadelphia | Charlottesville |
| St. Christopher’s Hospital for Children | University of Virginia Health Systems |
| Pittsburgh | Richmond |
| UPMC Children’s Hospital of Pittsburgh | Virginia Commonwealth University Medical Center |
| UPMC Magee Women’s Hospital | Wisconsin |
| UPMC Mercy Hospital | Milwaukee |
| UPMC Presbyterian/Shadyside | Froedtert Memorial Lutheran Hospital |
| South Carolina | |
| Greenville | |
| Vidant Medical Center |
Table 2.
Participating institutions—international ToxIC sites
| Canada |
| Toronto |
| Hospital for Sick Children |
| Israel |
| Haifa |
| Rambam Health Care Campus |
| Saudi Arabia |
| Riyadh |
| King Abdulaziz Medical City |
Fig. 1.
ACMT ToxIC Registry total case count by year, 2010–2015
Demographics
The ToxIC 2015 demographic data are summarized in Tables 3 and 4. Similar to 2014, there were slightly more female (51.2 %) than male (48.8 %) cases [4]. Fifty-two cases involved pregnant women, representing 0.6 % of the total 2015 cases. The majority (64.2 %) of cases involved adults (age 19–65), with adolescents (age 13–18) the next most common (19.3 %). Race and ethnicity data are summarized in Table 4. “Unknown/uncertain” was reported for race in 24.0 % of cases, and for Hispanic ethnicity in 25.9 % of cases. These percentages were lower than those recorded in 2014 (32.8 % for race and 29.5 % for ethnicity) [4]. In 2015, the most commonly reported races were Caucasian (55.6 %) and Black/African (13.1 %), while 9.6 % of cases reported Hispanic ethnicity.
Table 3.
ToxIC case demographics—age and gender
| N (%) | |
|---|---|
| Gender | |
| Male | 3957 (48.8) |
| Female | 4158 (51.2) |
| Pregnant | 52 (0.6) |
| Age (years) | |
| <2 | 266 (3.3) |
| 2–6 | 386 (4.8) |
| 7–12 | 209 (2.6) |
| 13–18 | 1565 (19.3) |
| 19–65 | 5208 (64.2) |
| 66–89 | 431 (5.3) |
| >89 | 21 (0.3) |
| Unknown | 29 (0.4) |
| Total | 8115 (100) |
Table 4.
ToxIC case demographics—race and Hispanic ethnicity
| N (%) | |
|---|---|
| Race | |
| Caucasian | 4511 (55.6) |
| Unknown/uncertain | 1951 (24.0) |
| Black/African | 1063 (13.1) |
| Other | 327 (4.0) |
| Asian | 127 (1.6) |
| American Indian/Alaska Native | 70 (0.9) |
| Mixed | 58 (0.7) |
| Native Hawaiian or Pacific Islander | 7 (0.1) |
| Not listed | 1 (0.0) |
| Total | 8115 (100) |
| Hispanic ethnicitya | |
| Hispanic | 779 (9.6) |
| Non-Hispanic | 5231 (64.5) |
| Unknown | 2104 (25.9) |
| Not listed | 1 (0.0) |
| Total | 8115 (100) |
aHispanic ethnicity as indicated exclusive of race
Source of Referral and Primary Reason for Encounter
Table 5 describes the sources of referrals, stratified by encounter location. The overwhelming majority (92.9 %) of cases were inpatient (IP) or emergency department (ED) referrals with the remaining 7.1 % outpatient (OP), clinic, or office referrals. Only one case did not have a referral source listed. Hospital EDs were the largest referral source overall of IP or ED encounters, making up 57.4 % of this group. Admitting services, outside hospital transfers, and requests from other hospital services made up most other IP or ED encounters with 28.8, 7.9, and 5.6 % of cases, respectively. In the OP, clinic, or office setting, the majority of encounters were self-referrals (45.0 %), followed by primary care or other OP physician referrals (42.7 %), and employer/independent medical exams (5.0 %).
Table 5.
ToxIC registry case referral sources by inpatient/outpatient status
| N (%) | |
|---|---|
| Emergency department (ED) or inpatient (IP)a | |
| ED | 4322 (57.4) |
| Admitting service | 2168 (28.8) |
| Outside hospital transfer | 595 (7.9) |
| Request from another hospital service (not ED) | 423 (5.6) |
| Poison Center | 22 (0.3) |
| Primary care provider or other | 4 (0.1) |
| Self-referral | 2 (0.0) |
| ED/IP total | 7536 (100) |
| Outpatient (OP)/clinic/office consultationb | |
| Self-referral | 260 (45.0) |
| Primary care provider or other OP physician | 247 (42.7) |
| Employer/independent medical eval | 29 (5.0) |
| Poison Center | 24 (4.2) |
| ED | 13 (2.3) |
| Admitting service | 3 (0.5) |
| Request from another hospital service (not ED) | 2 (0.4) |
| Outpatient total | 578 (100) |
| Not recorded outpatient or inpatient | 1 (0.0) |
aPercentage based on the total number of cases (N = 7536) seen by a medical toxicologist as consultant (ED or IP) or as attending (IP)
bPercentage based on the total number of cases (N = 578) seen by a medical toxicologist as outpatient, clinic visit, or office consultation
Table 6 summarizes data for primary reason for medical toxicology encounter. Similar to past years, intentional pharmaceutical exposures were the most frequent primary reason for an encounter (52.3 %). Intentional pharmaceutical exposures include all intentional ingestions, whether the intent was self-harm, misuse or abuse, or therapeutic use. The intentional nonpharmaceutical exposure category was the second most common reason for encounter (11.0 %). These include all nonmedications taken purposefully for any reason, such as abusing heroin, or ingesting a caustic for self-harm. The unintentional pharmaceutical category captures exposures such as pediatric exploratory behavior and other accidental medication ingestions and was the third most common reason for encounter (8.2 %). Withdrawal from opioids continued to increase from past years (4.0 % vs. 2.9 % in 2014), while ethanol withdrawal cases remained stable (2.5 % in both years) [4]. Snake envenomation cases also remained stable in 2015 at 2.8 % of total cases.
Table 6.
Reason for medical toxicology encounter
| N (%) | |
|---|---|
| Intentional exposure—pharmaceutical | 4244 (52.3) |
| Intentional exposure—nonpharmaceutical | 891 (11.0) |
| Unintentional exposure—pharmaceutical | 664 (8.2) |
| Organ system dysfunction | 344 (4.2) |
| Withdrawal—opioid | 327 (4.0) |
| Unintentional exposure—nonpharmaceutical | 279 (3.4) |
| Unknown | 258 (3.2) |
| Envenomation—snake | 230 (2.8) |
| Withdrawal—ethanol | 205 (2.5) |
| Interpretation of toxicology data | 156 (1.9) |
| Environmental evaluation | 138 (1.7) |
| Occupational evaluation | 126 (1.6) |
| Ethanol abuse | 102 (1.3) |
| Withdrawal—sedative/hypnotic | 59 (0.7) |
| Envenomation—spider | 31 (0.4) |
| Malicious/criminal | 24 (0.3) |
| Withdrawal—other | 10 (0.1) |
| Envenomation—scorpion | 10 (0.1) |
| Envenomation—other | 7 (0.1) |
| Marine | 5 (0.1) |
| Withdrawal—cocaine/amphetamine | 4 (0.1) |
| Not documented | 1 (0.0) |
| Total | 8115 (100) |
Table 7 further describes the intentional pharmaceutical group, classifying exposures according to the presence of self-harm, and by whether or not the exposure represented suicidal intent. This descriptor was added in 2014, so this report represents the first year with data on all exposures. In 2015, 69.0 % of intentional pharmaceutical exposures were a self-harm attempt. Of these, 85.6 % represented exposures with at least some component of true suicidal intention.
Table 7.
Detailed reason for encounter—intentional pharmaceutical exposure
| N (%) | |
|---|---|
| Reason for intentional pharmaceutical exposure subgroupa | |
| Attempt at self-harm | 2927 (69.0) |
| Misuse/abuse | 678 (16.0) |
| Therapeutic use | 383 (9.0) |
| Unknown | 229 (5.4) |
| None listed | 27 (0.6) |
| Total | 4244 (100) |
| Attempt at self-harm—suicidal intent subclassificationb | |
| Suicidal intent | 2506 (85.6) |
| No suicidal intent | 115 (3.9) |
| Suicidal intent unknown | 276 (9.4) |
| No data entered for suicidal intent | 30 (1.0) |
| Total | 2927 (100) |
aPercentage of total number of cases (N = 4244) indicating primary reason for encounter due to intentional pharmaceutical exposure
bPercentage of number of cases indicating attempt at self-harm (N = 2927)
Agent Classes
Each agent entry into the ToxIC database is classified into one of 40 agent categories to improve organization and ease of searching. In 2015, 10,740 agent entries accompanied the 8115 total cases. Twenty-eight percent of cases had multiple agents entered. Table 8 lists the 40 agent classes reported in 2015 in order of frequency. The top 20 agents have remained relatively constant in frequency since 2013. Analgesics were the leading agent class, making up 12.9 % of 2015 agent entries. Sedative-hypnotic and muscle relaxant agents were the second most common class (12.1 %). In 2015, antidepressants were more commonly reported than opioids (10.8 vs. 8.8 % of total cases). In the ToxIC 2011–2014 annual reports, opioids typically comprised a slightly larger proportion of total cases than antidepressants [4, 8–10].
Table 8.
Agent classes involved in medical toxicology consultation
| N (%)a | |
|---|---|
| Analgesic | 1386 (12.9) |
| Sedative-hypnotic/muscle relaxant | 1297 (12.1) |
| Antidepressant | 1159 (10.8) |
| Opioid | 943 (8.8) |
| Anticholinergic/antihistamine | 718 (6.7) |
| Ethanol | 669 (6.2) |
| Cardiovascular | 665 (6.2) |
| Antipsychotic | 620 (5.8) |
| Sympathomimetic | 606 (5.6) |
| Anticonvulsant | 369 (3.4) |
| Psychoactive | 355 (3.3) |
| Envenomation and marine | 248 (2.3) |
| Diabetic medication | 154 (1.4) |
| Lithium | 143 (1.3) |
| Herbal products/dietary supplements | 141 (1.3) |
| Metals | 134 (1.2) |
| Toxic alcohol | 122 (1.1) |
| Gases/irritants/vapors/dusts | 116 (1.1) |
| Cough and cold products | 101 (0.9) |
| Hydrocarbon | 94 (0.9) |
| Unknown agents | 82 (0.8) |
| Antimicrobial | 82 (0.8) |
| Caustic | 82 (0.8) |
| Household products | 78 (0.7) |
| Plants and fungi | 67 (0.6) |
| Endocrine | 42 (0.4) |
| Other nonpharmaceutical product | 41 (0.4) |
| Gastrointestinal agents | 39 (0.4) |
| Chemotherapeutic/immunological | 32 (0.3) |
| Insecticide | 31 (0.3) |
| Anticoagulant | 30 (0.3) |
| Other pharmaceutical product | 27 (0.3) |
| Anesthetic | 24 (0.2) |
| Rodenticide | 11 (0.1) |
| Pulmonary | 9 (0.1) |
| Herbicide | 9 (0.1) |
| Anti-parkinsonism drugs | 8 (0.1) |
| Ingested foreign body | 5 (0.0) |
| WMD/riot agent/radiological | <5 (0.0) |
| Fungicide | NR |
| Total | 10,740 (100) |
NR no cases reported
aPercentages are out of total number of reported agent entries per year; 2271 cases (32.7 %) reported multiple agents
Individual Agents by Class
Tables 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, and 26 summarize data regarding each specific agent category. There are two exceptions to the agent categories for unique agents which are given their own class: ethanol and lithium. Summary data for each of these is presented in tables along with toxic alcohols for ethanol and anticonvulsants/mood stabilizers for lithium, although the entries do not contribute toward the total counts for those classes. For agent classes with fewer case entries, or with little diversity in agents, more detailed information can be found in the Supplemental Material.
Table 9.
Analgesics
| N (%) | |
|---|---|
| Acetaminophen | 941 (67.9) |
| Aspirin | 214 (15.4) |
| Ibuprofen | 172 (12.4) |
| Naproxen | 27 (1.9) |
| Acetylsalicyclic acid | 7 (0.5) |
| Meloxicam | 6 (0.4) |
| Salicylamide | 5 (0.4) |
| Miscellaneousa | 14 (1.0) |
| Class total | 1386 (100) |
aIncludes aminophenazone, unspecified analgesic, diclofenac, indomethacin, magnesium salicylate, methylsalicylate, unspecified NSAID, oil of wintergreen, phenazopyridine, salicylic acid, and sulindac
Table 10.
Sedative-hypnotics/muscle relaxants by subtype
| N (%) | |
|---|---|
| Benzodiazepines | 680 (52.4) |
| Clonazepam | 217 (16.7) |
| Alprazolam | 213 (16.4) |
| Lorazepam | 132 (10.2) |
| Diazepam | 54 (4.2) |
| Benzodiazepine unspecified | 34 (2.6) |
| Temazepam | 7 (0.5) |
| Chlordiazepoxide | 7 (0.5) |
| Midazolam | 6 (0.5) |
| Miscellaneousa | 10 (0.8) |
| Muscle relaxants | 262 (20.2) |
| Cyclobenzaprine | 100 (7.7) |
| Baclofen | 77 (6.0) |
| Carisoprodol | 36 (2.8) |
| Tizanidine | 33 (2.5) |
| Methocarbamol | 10 (0.8) |
| Miscellaneousb | 6 (0.5) |
| Other sedatives | 220 (17.0) |
| Gabapentin | 145 (11.2) |
| Pregabalin | 35 (2.7) |
| Buspirone | 27 (2.1) |
| Sedative-hypnotic/muscle relaxant unspecified | 5 (0.4) |
| Miscellaneousc | 8 (0.6) |
| Nonbenzodiazepine agonists (“Z” drugs) | 89 (6.9) |
| Zolpidem | 84 (6.5) |
| Miscellaneousd | 5 (0.4) |
| Barbiturates | 46 (3.5) |
| Butalbital | 28 (2.2) |
| Phenobarbital | 11 (0.9) |
| Miscellaneouse | 7 (0.5) |
| Class total | 1297 (100) |
aIncludes etizolam, flubromazepam, clorazepate, oxazepam, and triazolam
bIncludes metaxolone, atracurium, chlorzoxazone, orphenadrine, and vecuronium
cIncludes chloral hydrate, phenibut, acamprosate, dichloralphenazone, tropicamide, and propofol
dIncludes eszopiclone and zaleplon
eIncludes butabarbital, barbiturate unspecified, and pentobarbital
Table 11.
Opioids
| N (%) | |
|---|---|
| Heroin | 243 (25.8) |
| Oxycodone | 167 (17.7) |
| Tramadol | 99 (10.5) |
| Hydrocodone | 81 (8.6) |
| Methadone | 77 (8.2) |
| Buprenorphine | 71 (7.5) |
| Opioid unspecified | 61 (6.5) |
| Morphine | 41 (4.3) |
| Fentanyl | 39 (4.1) |
| Hydromorphone | 26 (2.8) |
| Codeine | 11 (1.2) |
| Naloxone | 7 (0.7) |
| Oxymorphone | 7 (0.7) |
| Loperamide | 5 (0.5) |
| Miscellaneousa | 8 (0.8) |
| Class total | 943 (100) |
aIncludes diphenoxylate, tapentadol, and MT-45 (N,N-disubstituted piperazine)
Table 12.
Antidepressants
| N (%) | |
|---|---|
| Other antidepressants | 480 (41.4) |
| Bupropion | 258 (22.3) |
| Trazodone | 167 (14.4) |
| Mirtazapine | 37 (3.2) |
| Tranylcypromine | 5 (0.4) |
| Miscellaneousa | 13 (1.1) |
| Selective serotonin reuptake inhibitors (SSRIs) | 428 (36.9) |
| Sertraline | 115 (9.9) |
| Citalopram | 105 (9.1) |
| Fluoxetine | 103 (8.9) |
| Escitalopram | 76 (6.6) |
| Paroxetine | 29 (2.5) |
| Tricyclic antidepressants (TCAs) | 145 (12.5) |
| Amitriptyline | 104 (9.0) |
| Doxepin | 17 (1.5) |
| Nortriptyline | 17 (1.5) |
| Miscellaneousb | 7 (0.6) |
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | 106 (9.1) |
| Venlafaxine | 51 (4.4) |
| Duloxetine | 45 (3.9) |
| Fluvoxamine | 5 (0.4) |
| Miscellaneousc | 5 (0.4) |
| Class total | 1159 (100) |
aIncludes antidepressant unspecified, phenelzine, vortioxetine, and vilazodone
bIncludes clomipramine, imipramine, and desipramine
cIncludes desvenlafaxine and levomilnacipran
Table 13.
Anticholinergics and antihistamines
| N (%) | |
|---|---|
| Diphenhydramine | 418 (58.2) |
| Hydroxyzine | 104 (14.5) |
| Benztropine | 35 (4.9) |
| Chlorpheniramine | 33 (4.6) |
| Doxylamine | 33 (4.6) |
| Promethazine | 32 (4.5) |
| Loratadine | 9 (1.3) |
| Antihistamine unspecified | 6 (0.8) |
| Oxybutynin | 6 (0.8) |
| Pyrilamine | 6 (0.8) |
| Anticholinergic unspecified | 5 (0.7) |
| Cyproheptadine | 5 (0.7) |
| Dicyclomine | 5 (0.7) |
| Miscellaneousa | 21 (2.9) |
| Class total | 718 (100) |
aIncludes cetirizine, atropine, brompheniramine, clinidium, fexofenadine, meclizine, tolterodine, glycopyrrolate, homatropine, hyoscyamine, and trospium chloride
Table 14.
Cardiovascular agents by subtype
| N (%) | |
|---|---|
| Beta blockers | 181 (27.2) |
| Metoprolol | 68 (10.2) |
| Propranolol | 46 (6.9) |
| Atenolol | 32 (4.8) |
| Carvedilol | 17 (2.6) |
| Labetalol | 9 (1.3) |
| Nadolol | 5 (0.8) |
| Miscellaneousa | <5 (<0.8) |
| Sympatholytics | 175 (26.3) |
| Clonidine | 132 (19.8) |
| Guanfacine | 43 (6.5) |
| Calcium channel antagonists | 97 (14.6) |
| Amlodipine | 50 (7.5) |
| Verapamil | 22 (3.3) |
| Diltiazem | 20 (3.0) |
| Miscellaneousb | 5 (0.8) |
| Cardiac glycosides | 50 (7.5) |
| Digoxin | 46 (6.9) |
| Digitoxin | <5 (<0.8) |
| ACE inhibitors | 46 (6.9) |
| Lisinopril | 39 (5.9) |
| Miscellaneousc | 7 (1.1) |
| Other antihypertensives and vasodilators | 42 (6.3) |
| Prazosin | 16 (2.4) |
| Tamsulosin | 8 (1.2) |
| Hydralazine | 5 (0.8) |
| Miscellaneousd | 13 (2.0) |
| Diuretics | 26 (3.9) |
| Hydrochlorothiazide | 14 (2.1) |
| Furosemide | 5 (0.8) |
| Miscellaneouse | 7 (1.1) |
| Other cardiovascular agents | 18 (2.7) |
| Atorvastatin | 11 (1.7) |
| Miscellaneousf | 7 (1.1) |
| Antidysrhythmics | 16 (2.4) |
| Flecainide | 6 (1.0) |
| Sotalol | 5 (0.8) |
| Miscellaneousg | 5 (0.8) |
| Angiotensin receptor blockers | 14 (2.1) |
| Losartan | 8 (1.2) |
| Miscellaneoush | 6 (1.0) |
| Class total | 665 (100) |
aIncludes nebivolol and bisoprolol
bIncludes nifedipine and felodipine
cIncludes enalapril, captopril, and ramipril
dIncludes isobutyl nitrate, alkyl nitrite, antihypertensive unspecified, nitroglycerin, minoxidil, nitroprusside, isosorbide, and terazosin
eIncludes spironolactone, triamterene, acetazolamide, pamabrom, and torsemide
fIncludes simvastatin, fenofibrate, midodrine, and pravastatin
gIncludes amiodarone, propafenone, and quinidine
hIncludes valsartan, olmesartan, and irbesartan
Table 15.
Antipsychotics
| N (%) | |
|---|---|
| Quetiapine | 287 (46.3) |
| Olanzapine | 83 (13.4) |
| Risperidone | 65 (10.5) |
| Aripiprazole | 50 (8.1) |
| Haloperidol | 46 (7.4) |
| Ziprasidone | 18 (2.9) |
| Chlorpromazine | 14 (2.3) |
| Clozapine | 14 (2.3) |
| Lurasidone | 14 (2.3) |
| Paliperidone | 7 (1.1) |
| Miscellaneousa | 22 (3.5) |
| Class total | 620 (100) |
aIncludes fluphenazine, asenapine, antipsychotic unspecified, loxapine, prochlorperazine, trifluoperazine, brexpiprazole, perphenazine, and thioridazine
Table 16.
Sympathomimetics
| N (%) | |
|---|---|
| Cocaine | 180 (29.7) |
| Methamphetamine | 140 (23.1) |
| Amphetamine | 70 (11.6) |
| Methylphenidate | 47 (7.8) |
| Dextroamphetamine | 39 (6.4) |
| Lisdexamfetamine | 25 (4.1) |
| Methylenedioxy-N-methamphetamine | 24 (4.0) |
| 25I-NBOMe | 13 (2.1) |
| Pseudoephedrine | 12 (2.0) |
| Sympathomimetic unspecified | 10 (1.7) |
| Cathinone | 8 (1.3) |
| Atomoxetine | 7 (1.2) |
| Phentermine | 7 (1.2) |
| Dexmethylphenidate | 5 (0.8) |
| Phenylephrine | 5 (0.8) |
| Miscellaneousa | 14 (2.3) |
| Class total | 606 (100) |
aIncludes clenbuterol, alpha-pyrrolidinopentiophenone, propylhexedrine, 2C series drugs, methylenedioxypyrovalerone (MDPV), phenethylamine, phenethylamine designer drug unspecified, methedrone, and tetrahydrozoline
Table 17.
Anticonvulsants and mood stabilizers
| N (%) | |
|---|---|
| Lithiuma | 143 (100) |
| Valproic acid | 101 (27.4) |
| Lamotrigine | 97 (26.3) |
| Carbamazepine | 46 (12.5) |
| Topiramate | 33 (8.9) |
| Phenytoin | 29 (7.9) |
| Oxcarbazepine | 28 (7.6) |
| Levetiracetam | 18 (4.9) |
| Divalproex | 7 (1.9) |
| Miscellaneousb | 10 (2.7) |
| Class total | 369 (100) |
aLithium is considered a separate agent class
bIncludes primidone, lacosamide, clobazam, fosphenytoin, and zonisamide
Table 18.
Psychoactives
| N (%) | |
|---|---|
| Cannabinoid synthetic | 182 (51.3) |
| Marijuana | 85 (23.9) |
| Gamma hydroxybutyrate | 18 (5.1) |
| Phencyclidine | 17 (4.8) |
| Cannabinoid nonsynthetic | 11 (3.1) |
| LSD | 9 (2.5) |
| Cannabinoid synthetic (AB FUBINACA) | 4 (1.1) |
| Ketamine | 4 (1.1) |
| Miscellaneousa | 25 (7.0) |
| Class total | 355 (100) |
aIncludes donepezil, hallucinogen unspecified, methoxetamine, nicotine, psychoactive unspecified, disulfiram, dimethyltryptamine (DMT), 1,4-butanediol, ibogaine, methylone, pharmaceutical tetrahydrocannabinol (THC), tryptamine, and varencicline
Table 19.
Synthetic cannabinoid historical trends
| Year | N (%)a |
|---|---|
| 2015 | 182 (51.3) |
| 2014 | 87 (27.4) |
| 2013 | 56 (18.2) |
| 2012 | 70 (18.8) |
| 2011 | 66 (21.9) |
| 2010 | 13 (14.4) |
aPercentage within agent class (psychoactive) for that year
Table 20.
Envenomations and marine poisonings
| N (%) | |
|---|---|
| Crotalus spp. | 108 (43.5) |
| Agkistrodon spp. | 64 (25.8) |
| Snake unspecified | 21 (8.5) |
| Loxosceles spp. | 19 (7.7) |
| Scorpion unspecified | 7 (2.8) |
| Latrodectus spp. | 5 (2.0) |
| Miscellaneousa | 24 (9.7) |
| Class total | 248 (100) |
aIncludes envenomation unspecified, hymenoptera, Vipera palaestinae, Centuroides spp., Micrurus spp., spider unspecified, Mygelopyge opercularis, stingray, and ciguatera poisoning
Table 21.
Diabetic medications
| N (%) | |
|---|---|
| Metformin | 50 (32.5) |
| Insulin | 39 (25.3) |
| Glipizide | 34 (22.1) |
| Glimepiride | 12 (7.8) |
| Glyburide | 10 (6.5) |
| Miscellaneousa | 9 (5.8) |
| Class total | 154 (100) |
aIncludes sulfonylurea unspecified, sitagliptin, liraglutide, pioglitazone, and tolbutamide
Table 22.
Ethanol and toxic alcohols
| N (%) | |
|---|---|
| Ethanola | 669 (100) |
| Nonethanol alcohols and glycols | |
| Ethylene glycol | 50 (41.0) |
| Isopropanol | 38 (31.1) |
| Methanol | 14 (11.5) |
| Acetone | 6 (4.9) |
| Miscellaneousb | 14 (11.4) |
| Class total | 122 (100) |
aEthanol is considered a separate agent class
bIncludes diethylene glycol, propylene glycol, butyl ethylene glycol, methyl ethyl ketone, toxic alcohol unspecified, furfuryl alcohol, and glycol ethers
Table 23.
Plants and fungi
| N (%) | |
|---|---|
| Mold unspecified | 25 (37.3) |
| Mushroom, other/unknown | 12 (17.9) |
| Mitragyna speciosa (kratom) | 4 (6.0) |
| Mushroom, psilocibin | 4 (6.0) |
| Miscellaneousa | 22 (32.8) |
| Class total | 67 (100) |
aIncludes Amanita phalloides, Taxus (yew), valerian root, Amanita pantherina, Datura stramonium (jimson weed), Amanita bisporigera, barley, Datura inoxia (moonflower, thornapple), forskolin extract, Herdera helix (common ivy), hordenine, Piper methysticum (kava), Ricinus communis (castor beans), and Solanum dulcamara (bitter nightshade)
Table 24.
Metals
| N (%) | |
|---|---|
| Lead | 35 (26.1) |
| Iron | 27 (20.1) |
| Mercury | 15 (11.2) |
| Chromium | 13 (9.7) |
| Cobalt | 10 (7.5) |
| Arsenic | 6 (4.5) |
| Miscellaneousa | 28 (20.9) |
| Class total | 134 (100) |
aIncludes vanadium, aluminum, barium, copper, manganese, nickel, silver, titanium, beryllium, cadmium, gadolinium, potassium dichromate, metal unspecified, uranium, and strontium
Table 25.
Gases, irritants, vapors, and dusts
| N (%) | |
|---|---|
| Carbon monoxide | 53 (45.7) |
| Chlorine | 11 (9.5) |
| Hydrogen sulfide | 11 (9.5) |
| Cyanide | 7 (6.0) |
| Smoke | 6 (5.2) |
| Polyurethane vapors | 5 (4.3) |
| Miscellaneousa | 23 (19.8) |
| Class total | 116 (100) |
aIncludes carbon dioxide, nitrogen oxides, duster (canned air), helium, petroleum vapors, argon, asbestos, chloramine, diesel exhaust, unspecified gases, phosgene, soot, triphosgene, wood dusts, and ethylene
Table 26.
Household products
| N (%) | |
|---|---|
| Laundry detergent pod | 21 (26.9) |
| Sodium hypochlorite <6 % | 21 (26.9) |
| Cleaning solutions and disinfectants | 14 (17.9) |
| Soaps and detergents | 5 (6.4) |
| Hand sanitizer unspecified | 4 (5.1) |
| Miscellaneousa | 13 (16.7) |
| Class total | 78 (100) |
aIncludes household product unspecified, ammonia ≤10 %, dishwasher detergent, paint, dental adhesives, hair product, phenylenediamine (hair dye), and windshield washer fluid
Table 9 presents data for nonopioid analgesics, the most common agent class reported in 2015. Acetaminophen was by far the most commonly reported analgesic, as well as the most common agent overall, with 941 cases. Analogous to 2014, acetaminophen comprised 67.9 % of the analgesic class and was involved in 11.6 % of total registry cases in 2015 [4]. Aspirin (15.4 %), ibuprofen (12.4 %), and naproxen (1.9 %) followed as the next most common analgesics.
Table 10 presents the sedative-hypnotic/muscle relaxant class, which was again the second largest group reported in the Registry in 2015. Benzodiazepines were the leading subgroup, constituting 52.4 % of the agent class. Clonazepam and alprazolam, the top reported benzodiazepines, together made up 33.1 % of the agent class. Muscle relaxants, such as cyclobenzaprine (7.7 %) and baclofen (6.0 %), made up 20.2 % of the sedative-hypnotic class. Gabapentin made up the majority of the “other sedatives” class, at 11.2 % of the 17.0 % this class contributed. Nonbenzodiazepine agonists and barbiturates were relatively uncommon (6.9 and 3.5 %). Zolpidem was by far the most commonly reported nonbenzodiazepine, constituting 84 of the 89 cases. No barbiturates contributed more than 5 % to the sedative-hypnotic agent class.
The opioid agent class, summarized in Table 11, provides data for all natural opiates, as well as semisynthetic and synthetically derived opioid agonists and antagonists. The number of opioid cases was slightly decreased from last year, representing 8.8 % of Registry cases [4]. Heroin remained the most common agent in the class at 25.8 %. Following this, oxycodone was responsible for an additional 17.7 % of the opioid class entries. In prior years, oxycodone had been the leading agent in the class but was surpassed by heroin in 2013, and this trend has continued [4, 8–11]. This year, the most common synthetic opioid was tramadol (10.5 %), closely followed by methadone (8.2 %). The synthetic opioid fentanyl was less common (4.1 %) but increased slightly from 2014 (2.9 %) [4]. The opiates morphine and codeine had a relatively low combined frequency at 5.5 %. Reported loperamide exposures were infrequent but stable from last year at 0.5 % of all opioid agents reported [4].
Of the 243 case entries involving heroin, 163 (67.1 %) were related to intoxication or overdose, and 74 (30.5 %) were related to withdrawal. One hundred forty-six (64 %) of the cases involved males and 84 (36 %) were female. The mean age was 33.4 years. Heroin was a single agent in 140 cases although, in addition to heroin, many of these cases included other agents. Sixty-two cases included two agents and 40 had more than 3 drugs involved. Stimulants were the most common co-occurring class of substances, being reported in 69 cases (28.4 %). Cocaine was reported most frequently with 46 cases (18.9 %), followed by amphetamines/ methamphetamine in 14 (5.8 %). Benzodiazepines and other sedative/hypnotics were the second most common type of substance reported occurring in 41 cases (16.9 %). The most common benzodiazepine reported was alprazolam (N = 18) followed by clonazepam (N = 14). Other opioids were reported in 26 cases (10.7 %) with the most common opioid reported being buprenorphine (N = 8) followed by methadone (N = 4). Ethanol was a coingestant in nine cases.
When looking specifically at the 163 heroin intoxication/overdoses, simulants were reported along with heroin in 45 (27.6 %), benzodiazepines were reported in 29 (17.8 %), and other opioids were reported in 17 (10.4 %).
Naloxone was administered in 92 cases and 31 individuals were intubated. A greater proportion of the mixed drug overdoses involving heroin were intubated (23 %) compared to heroin alone (15 %). A specific route was reported in 81 % of the cases with the most common route being parenteral (53 %). Heroin was insufflated in 12 %, ingested orally in 16 %, and used rectally in <1 %. Other or “unknown” routes account for the rest of the cases (17 %).
Table 12 organizes the antidepressants into subgroups based on pharmacologic class: selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and other antidepressants. Antidepressants were the third most common agent class in 2015, with 1159 entries (10.8 % of Registry entries). Consistent with past years 2013 and 2014, bupropion was again the most frequently reported agent in the antidepressant class [4, 8]. Bupropion on its own accounted for 22.3 % of the antidepressant category and represented 2.4 % of reported agent entries overall (N = 258). Trazodone was the next most commonly reported agent at 14.4 % of the agent class. Together, bupropion and trazodone constituted the majority of the “other antidepressant” category. Along with mirtazapine and several additional agents, the “other antidepressant” class made up 41.4 % of the antidepressant agent class. Among the SSRIs, sertraline (9.9 %), citalopram (9.1 %), and fluoxetine (8.9 %) were reported with similar frequency. This is the first time that citalopram was not the most commonly reported SSRI in the Registry [4, 8–11]. The SSRI subgroup accounted for more than one-third of the antidepressant class. The TCA subgroup was overwhelmingly made up of amitriptyline entries (71.7 % of the subgroup). Doxepin and nortriptyline were equally represented (1.5 %). The TCAs made up 12.5 % of antidepressant exposures. The SNRIs were the least common antidepressant subgroup (9.1 %). Venlafaxine and duloxetine together accounted for 90.6 % of the subgroup.
Table 13 presents the anticholinergics and antihistamine agents. Anticholinergics and antihistamines made up 6.7 % of total agent entries into the Registry in 2015. The predominant agent in this class was diphenhydramine (58.2 %), with hydroxyzine being second (14.5 %), which is consistent with past Registry years. Benztropine, chlorpheniramine, doxylamine, and promethazine were all reported in relatively equal numbers (N = 32 to N = 35), and together comprised 18.6 % of the anticholinergic/antihistamine agent class.
Table 14 presents data on cardiovascular agents. There were 665 total cardiovascular agents reported in the Registry in 2015. This agent class was subdivided based on pharmacologic class into 10 groups: beta blockers, sympatholytics, calcium channel antagonists, cardiac glycosides, angiotensin converting enzyme (ACE) inhibitors, diuretics, antidysrhythmics, angiotensin receptor blockers (ARBs), other antihypertensives and vasodilators, and other cardiovascular agents. The highest reported single agent in the cardiovascular agent class was clonidine, at 19.8 % of the class. Clonidine, together with guanfacine, made up the sympatholytic subgroup which accounted for 26.3 % of the total agent class. Beta blockers were only slightly more frequently encountered than the sympatholytics at 27.2 % of the agent class. Metoprolol (10.2 %) and propranolol (6.9 %) were the top two reported beta blockers in 2015, as they have been since 2011 [4, 8–10]. Amlodipine was the most common calcium channel antagonist, comprising 7.5 % of the cardiovascular agent class, and totaling greater than all other calcium channel antagonists combined. Digoxin was the predominant cardiac glycoside reported, at 92.0 % of the subgroup. ACE inhibitors were uncommonly reported (6.9 %); lisinopril made up the great majority of this group. The cardiovascular subgroups of diuretics, antidysrhythmics, ARBs, and other cardiovascular agents each accounted for less than 5 % of the agent class.
Table 15 presents data on antipsychotic agents reported in the Registry. This year, as in each prior year, quetiapine was the most frequent antipsychotic agent, making up 46.3 % of the agent class in 2015 [4, 8–11]. Quetiapine alone accounted for 2.7 % of agents in the total Registry. Olanzapine (13.4 %), risperidone (10.5 %), aripiprazole (8.1 %), and haloperidol (7.4 %) were the next most commonly entered. These five drugs constitute 85.7 % of the antipsychotic agent class.
Table 16 shows the 606 sympathomimetics entered, which included both prescription medications as well as illicit substances. Cocaine and methamphetamine were the two most numerous agents (29.7 and 23.1 %). Amphetamine followed at 11.6 %. Methylphenidate, dextroamphetamine, and lisdexamfetamine were the next most common medications, together responsible for 18.3 % of the sympathomimetic agent class. Less common designer drugs—methylenedioxy-N-methamphetamine, 25I-NBOMe, and cathinone—contributed a total of 7.4 % to the class. Additional designer stimulants were less common and are included in the miscellaneous category.
Anticonvulsants and mood stabilizers are presented in Table 17, along with lithium, which was considered as a separate agent class. Valproic acid and lamotrigine were again the top reported agents accounting for 27.4 and 26.3 % of the agent class. Reported phenytoin exposures decreased from last year, making up 7.9 % of the agent class (N = 29), down from 14.0 % (N = 59) [4]. Topiramate, phenytoin, and oxcarbazepine were recorded in similar frequency and together made up 4.4 % of the class. There were 143 reported lithium exposures, making up 1.3 % of all agent encounters.
Psychoactives are summarized in Table 18. This category represents a diverse group of agents, including hallucinogens, NMDA antagonists, cannabinoid receptor agonists, and tryptamines. In 2015, synthetic cannabinoids were the largest recorded agent in the class, outnumbering marijuana. Synthetic cannabinoids made up 51.3 % of the psychoactive agent class, while marijuana contributed 23.9 %. There were an additional four entries of the specific synthetic cannabinoid, AB FUBINACA, adding another 1.1 % to this.
Past Registry years have shown the synthetic cannabinoid numbers steadily increasing as marijuana has decreased [4, 8–11]. This year, synthetic cannabinoids, including AB FUBINACA, were responsible for 1.7 % of Registry agent entries. Tables 19 shows the trend of reported synthetic cannabinoid cases in the Registry over time. The largest increases occurred between 2010 and 2011 when cases jumped from 13 to 66, and between 2014 and 2015, when synthetic cannabinoid encounters more than doubled. In 2014, they made up 27.4 % of the psychoactive agent class, but in 2015 represented 51.3 %. Marijuana encounters in the Registry have decreased since 2012 [4, 8, 10]. There were 101 cases in 2014 (32.4 % of the class) and 85 cases (23.9 %) in 2015 [4]. The average ages of those with synthetic cannabinoid or marijuana exposures were similar. Synthetic cannabinoid cases had an average age of 29.1, with a median of 26, and a range of 2 to 62. Marijuana encounters had an average age of 27.0, a median of 23.5, and an age range of 1 to 68. Those presenting with marijuana exposures were predominantly men (60.0 %), and 51.8 % of cases involved additional exposures. Cases of synthetic cannabinoid exposures were 87.6 % males with 81.2 % of cases being single agent exposures.
Table 20 summarizes envenomations and marine poisonings. The largest contributor to this class was Crotalus spp. (rattlesnake) envenomations (43.5 %), followed by Agkistrodon spp. (copperhead) (25.8 %). Total snake envenomations made up 80.6 % of the agent class. Spider species envenomations accounted for 10.5 %, and scorpion species envenomations for 4.0 %.
Table 21 includes antidiabetic medications reported in 2015. Metformin, insulin, and glipizide together were responsible for about 80 % of the agent class. Glimepiride and glyburide each contributed more than 5 % of the agent class as well.
Toxic alcohols are reported in Table 22. Ethanol is considered its own separate class and is also summarized in Table 22. Ethylene glycol (41 %) and isopropanol (31.1 %) were the leading toxic alcohols reported, as has been the case in past years [4, 8–11]. Ethanol was second only to acetaminophen as the most common single agent reported, constituting 6.2 % of the total agents entered (N = 669).
Table 23 presents cases involving plants and fungi. The plants and fungi category includes numerous, diverse species, but the most commonly reported were unspecified mold (37.3 %) and unspecified mushroom (17.9 %). The next most common agents in the class this year were Mitragyna speciosa (kratom) and psilocybin mushrooms (6 % each).
Table 24 summarizes metal agents. Lead, iron, and mercury composed the majority of the metal agent class, totaling 57.4 %. Fourteen other metals and a metal unspecified classification created a large miscellaneous category, which made up 20.9 % of the metal agent class.
Table 25 includes gases/irritants/vapors/dusts. As in past years, carbon monoxide was the most common agent in the category (45.7 %). Hydrogen sulfide and chlorine were present in equal numbers (9.5 %), followed by cyanide (6.0 %).
The household agent class is presented in Table 26. Sodium hypochlorite <6 % (household bleach) and laundry detergent pods were tied as the most common agents, together making up 53.8 % of the class. Nonspecific cleaning solutions and disinfectants were third, at 17.9 %.
The cough and cold agent class, presented in Table S1, was comprised almost exclusively of dextromethorphan entries (93.1 %). The group as a whole was responsible for less than 1 % of the 2015 Registry agent entries.
The antimicrobial class, presented in Table S2, shows a diverse group of agents contributing this group. The entire agent class made up less than 1 % of database agent entries. Most (68.3 %) were antibiotics, with dapsone and isoniazid occurring with equal frequency.
Anticoagulants are presented in Table S3. These were uncommonly reported (N = 30), with 17 (56.7 %) being warfarin. Rivaroxaban was the second most frequent agent at 16.7 % of the class.
Endocrine agents in Table S4 had a large miscellaneous group with 12 different agents making up 50.0 % of the class. Levothyroxine was the most commonly reported agent. Table S5 presents the “other pharmaceutical class,” in which memantine and rivastigmine were reported in equal numbers (18.5 %).
The most common agents in additional smaller agent classes were lidocaine (anesthetics), ondansetron (gastrointestinal), ropinirole (anti-Parkinsonism), theophylline (pulmonary), and methotrexate (chemotherapeutic and immunological) (Tables S6, S7, S8, S9, and S10).
Table S11 presents the herbal products and dietary supplements and demonstrates a predominance of caffeine entries in this class (39.0 %). Melatonin was the next most common at 14.9 %. There was a diverse assortment of infrequently reported agents in this class, with the miscellaneous category containing 34 different agents.
Table S12 describes hydrocarbon exposures. Unspecified hydrocarbons were responsible for 27.7 % of the class with multiple, assorted chemicals making up the remainder. The most common specified hydrocarbons were toluene (8.5 %) and benzene (6.4 %).
Table S13 includes caustic exposures. Unspecified caustics, cleaning agents, and formaldehyde were the most common agents, making up a total of 34.2 % of the class.
Additional smaller agent classes are presented in the Supplemental Materials.
Clinical Signs and Symptoms
Almost one-third of cases entered into the Registry in 2015 involved a diagnosed toxidrome, N = 2659 (32.8 %) (Table 27). A sedative-hypnotic toxidrome was the most commonly reported (11.4 %), followed by anticholinergic (7.1 %), and opioid (4.7 %).
Table 27.
Toxidromes
| N (%)a | |
|---|---|
| Sedative-hypnotic | 925 (11.4) |
| Anticholinergic | 579 (7.1) |
| Opioid | 374 (4.7) |
| Sympathomimetic | 332 (4.1) |
| Serotonin syndrome | 251 (3.1) |
| Alcoholic ketoacidosis | 65 (0.8) |
| Sympatholytic | 52 (0.6) |
| Washout syndrome | 32 (0.4) |
| NMS | 23 (0.3) |
| Overlap syndromes | 11 (0.1) |
| Cholinergic | 10 (0.1) |
| Anticonvulsant hypersensitivity | 5 (0.06) |
| Class total | 2659 (32.8) |
NMS neuroleptic malignant syndrome
aPercentage equals the number of cases reporting specific toxidrome relative to the total number of Registry cases in 2015 (N = 8115)
In Table 28, major abnormalities of vital signs are reported, affecting 2045 cases (25.2 %). Tachycardia, defined as a heart rate greater than 140 beats per minute, was present in 880 entries (10.8 %). Hypotension, defined as a systolic blood pressure less than 80 mmHg, was present in 508 entries (6.3 %). Hyperthermia greater than 105 °F was less common, with only 36 reported cases (0.4 %).
Table 28.
Major vital sign abnormalities
| N (%)a | |
|---|---|
| Tachycardia (HR > 140) | 880 (10.8) |
| Hypotension (systolic BP < 80 mmHg) | 508 (6.3) |
| Bradycardia (HR < 50) | 323 (4.0) |
| Bradypnea (RR < 10) | 153 (1.9) |
| Hypertension (systolic BP > 200 mmHg and/or diastolic BP > 120 mmHg) | 145 (1.8) |
| Hyperthermia (temp > 105 °F) | 36 (0.4) |
| Total | 2045 (25.2)b |
HR heart rate, BP blood pressure, RR respiratory rate
aPercentage equals the number of cases relative to the total number of Registry cases in 2015 (N = 8115)
bTotal reflects cases reporting at least one major vital sign abnormality. Cases may be associated with more than one major vital sign abnormality
There were 4646 (57.3 %) total reported neurological signs or symptoms (Table 29). Some cases may have reported more than one finding. The majority of reported neurological signs or symptoms were coma or central nervous system depression (N = 2639, 32.5 %). Agitation (15.2 %) and delirium (13.4 %) and hyperreflexia/myoclonus/tremor (9.8 %) followed. Seizures occurred in 467 cases (5.8 %). Hallucinations affected 392 cases (4.8 %).
Table 29.
Clinical signs and symptoms—neurological
| N (%)a | |
|---|---|
| Coma/CNS depression | 2639 (32.5) |
| Agitation | 1233 (15.2) |
| Delirium | 1087 (13.4) |
| Hyperreflexia/myoclonus/tremor | 792 (9.8) |
| Seizures | 467 (5.8) |
| Hallucinations | 392 (4.8) |
| Weakness/paralysis | 132 (1.6) |
| Dystonia/rigidity/extrapyramidal symptoms | 127 (1.6) |
| Numbness/paresthesia | 97 (1.2) |
| Peripheral neuropathy | 29 (0.4) |
| Total | 4646 (57.3)a, b |
CNS central nervous system
aPercentage equals the number of cases relative to the total number of Registry cases in 2015 (N = 8115)
bTotal reflects cases reporting at least one neurological symptom. Cases may be associated with more than one neurological symptom
Respiratory depression was the most commonly reported pulmonary sign, present in 682 cases (8.4 % of total registry cases). Other cardiovascular and pulmonary signs were relatively uncommon, each involving less than 5 % of Registry cases. Table 30 presents collected data on cardiovascular and pulmonary signs.
Table 30.
Clinical signs—cardiovascular and pulmonary
| N (%)a | |
|---|---|
| Cardiovascular | |
| Prolonged QTc (≥500 ms) | 339 (4.2) |
| Prolonged QRS (≥120 ms) | 119 (1.5) |
| Ventricular dysrhythmia | 80 (1.0) |
| AV block (>1st degree) | 35 (0.4) |
| Total | 494 (6.1)b |
| Pulmonary | |
| Respiratory depression | 682 (8.4) |
| Aspiration pneumonitis | 150 (1.8) |
| Acute lung injury/ARDS | 89 (1.1) |
| Asthma/Reactive airway disease | 67 (0.8) |
| Total | 893 (11.0)b |
ARDS acute respiratory distress syndrome
aPercentage equals the number of cases reporting signs of symptoms relative to the total number of Registry cases in 2015 (N = 8115)
b Total reflects cases reporting at least one cardiovascular or pulmonary symptom. Cases may be associated with more than one symptom
Table 31 demonstrates additional clinical signs affecting multiple various organ systems, with each sign individually making up less than 5 % of the total Registry. Among subgroups, metabolic dysfunction was the most common, predominantly due to hypoglycemia (4.7 %), and an anion gap greater than 20 (4.0 %). Renal and musculoskeletal abnormalities, consisting of rhabdomyolysis and acute kidney injury, were present in 8.4 % of database entries. Gastrointestinal/hepatic dysfunction was reported in 4.8 % of Registry cases. Hepatotoxicity, defined as an aspartate aminotransferase (AST) over 1000 IU/L, was the most commonly reported gastrointestinal/hepatic abnormality (3.2 %). Hematologic dysfunction, manifested as coagulopathy, occurred in 2.3 % of cases, followed by thrombocytopenia (1.2 %), and leukocytosis (1.1 %). The smallest subgroup was dermatological signs. Rash was the top reported dermatologic sign (1.5 %) followed by blisters/bullae (0.8 %).
Table 31.
Clinical signs—other organ systems
| N (%)a | |
|---|---|
| Metabolic | |
| Hypoglycemia (glucose < 50 mg/dL) | 382 (4.7) |
| Elevated anion gap (>20) | 321 (4.0) |
| Metabolic acidosis (pH < 7.2) | 140 (1.7) |
| Elevated osmole gap (>20) | 78 (1.0) |
| Total | 921 (11.3)b |
| Gastrointestinal/hepatic | |
| Hepatotoxicity (AST ≥ 1000 IU/L) | 261 (3.2) |
| Pancreatitis | 55 (0.7) |
| Gastrointestinal bleeding | 45 (0.6) |
| Corrosive injury | 28 (0.3) |
| Intestinal ischemia | 3 (0.0) |
| Total | 392 (4.8)b |
| Hematological | |
| Coagulopathy (PT > 15 s) | 187 (2.3) |
| Thrombocytopenia (platelets < 100 K/μL) | 96 (1.2) |
| Leukocytosis (WBC > 20 K/μL) | 88 (1.1) |
| Hemolysis (Hgb < 10 g/dL) | 40 (0.5) |
| Methemoglobinemia (MetHgb ≥ 2 %) | 23 (0.3) |
| Pancytopenia | 12 (0.1) |
| Total | 446 (5.5)b |
| Renal/musculoskeletal | |
| Rhabdomyolysis (CPK > 1000 IU/L) | 398 (4.9) |
| Acute kidney injury (creatinine > 2.0 mg/dL) | 283 (3.5) |
| Total | 681 (8.4)b |
| Dermatological | |
| Rash | 122 (1.5) |
| Blister/bullae | 68 (0.8) |
| Necrosis | 25 (0.3) |
| Angioedema | 18 (0.2) |
| Total | 233 (2.9)b |
AST aspartate aminotransferase, PT prothrombin time, WBC white blood cells, Hgb hemoglobin, CPK creatine phosphokinase
aPercentage equals the number of cases reporting specific clinical signs compared to the total number of Registry cases in 2015 (N = 8115)
bTotal reflects cases reporting at least one sign in the category. Cases may be associated with more than one symptom
Fatalities
Fatality data with a known toxicological exposure are summarized in Tables 32 and 33. There were 98 fatalities reported in the Registry in 2015, constituting 1.2 % of all database cases. Of these, 49 cases (50.0 %) were single agent exposures, and 26 (26.5 %) were exposures to multiple toxicological agents. An additional 23 fatality cases did not have a clear toxicological exposure as determined by the examining medical toxicologist. Information on fatalities reported in the Registry without a known toxicological exposure are presented in Supplemental Table S17.
Table 32.
2015 fatalities reported in ToxIC Registry with known toxicological exposure (based on the response from a medical toxicologist “Did the patient have a toxicological exposure?” equals Yes with known agent(s)): single agent
| Age/gendera | Agents involved | Clinical findingsb | Life support withdrawn | Brain death confirmed | Treatmentc |
|---|---|---|---|---|---|
| 17F | Methanol | HT, TC, QRS, CNS, SZ, HGY, MA, AG | Yes | Yes | NaHCO3, vasopressors, glucose, intubation |
| 76F | Acetaminophen | HT, TC, AP, ALI, CNS, MA, CPT | No | Unknown | Calcium, fomepizole, NAC, NaHCO3, vasopressors, benzodiazepines, steroids, continuous renal replacement, intubation, IV fluids |
| 55F | Acetaminophen | HT, TC, QRS, ALI, RD, AGT, CNS, HGY, MA, HPT, CPT, AKI | Unknown | Unknown | NAC, intubation, IV fluids |
| 45M | Diphenhydramine | TC, VD, AGT, DLM, SZ, RBM | No | Unknown | Benzodiazepines, IV fluids |
| 50F | Acetaminophen | GIB | Yes | No | NAC |
| 89F | Digoxin | None listed | No | Unknown | None listed |
| 54F | Cocaine | VD, CNS | Yes | Unknown | None listed |
| 63M | Ethanol | None listed | No | Unknown | None listed |
| 43M | Acetaminophen | HT, VD, ALI, RD, CNS, MA, AG, HPT, AKI | Yes | Yes | NAC, vasopressors, antiarrhythmics, benzodiazepines, activated charcoal, hemodialysis, continuous renal replacement, CPR, ECMO, intubation, IV fluids |
| 28M | Methamphetamine | HT, TC, HYT, RD, CNS, RFX, HGY, MA, AG, HPT, GIB, INT, CPT, AKI, RBM | Yes | Yes | Vasopressors, benzodiazepines, hemodialysis, IV fluids |
| 70F | Morphine | TC, AP, CNS, MA | Yes | Unknown | Vasopressors |
| 16F | Acetaminophen | None listed | Unknown | Unknown | NAC |
| >89F | Amlodipine | HT, BC, QTC, QRS, CNS, MA, AKI | Yes | Unknown | Vasopressors, bronchodilators, intubation, IV fluids, pacemaker |
| 45M | Acetaminophen | HT, RD, CNS, SZ, MA, HPT, INT, PLT, AKI, RBM, OTH1 | Yes | Yes | Fomepizole, NAC, benzodiazepines, IV fluids |
| 54F | Verapamil | HT, BC, BP, QTc, RD, CNS, MA, AG, HPT, AKI | No | Unknown | Insulin-euglycemic therapy, vasopressors, whole bowel irrigation, CPR, intubation, IV fluids |
| 60F | Acetaminophen | HT, TC, VD, QRS, RD, CNS, HGY, MA, AG, HPT, CPT | No | Unknown | NAC, vasopressors, glucose, hemodialysis, CPR, cardioversion, intubation, IV fluids |
| 80M | Drain cleaner (caustic) | CRV | Yes | No | Steroids, IV fluids |
| 19M | Mannitol | RAD, CNS | No | Unknown | Vasopressors, bronchodilators, CPR, intubation |
| 58F | Amphetamine | TC, VD, CNS | Yes | Yes | Anithypertensives, neuromuscular blockers, intubation |
| 65F | Digoxin | None listed | Yes | Unknown | None listed |
| 12 mo M | Oxycodone | HT, VD, ALI, CNS, CPT | Yes | Yes | Naloxone, vasopressors, intubation |
| 42F | Acetaminophen | HT, CNS, RFX, MA, AG, HPT, CPT, WBC, AKI | Unknown | Unknown | Fomepizole, NAC, NaHCO3, vasopressors, continuous renal replacement, intubation, IV fluids |
| 17F | MDMA | HT, TC, HYT, RFX, MA, AG, HPT, HYS, CPT, PLT, AKI, RBM | Yes | Yes | Vitamin K, vasopressors, benzodiazepines, neuromuscular blockers, opioids, continuous renal replacement, intubation, IV fluids |
| 31F | Acetaminophen | CNS, HGY, HPT, CPT | Yes | Unknown | Glucagon, NAC, vitamin K, continuous renal replacement, intubation |
| 36F | Heroin | HT, ALI, RD, CNS, HPT | Yes | Yes | Insulin-euglycemic therapy, naloxone, NaHCO3, vasopressors, CPR, intubation |
| 33F | Acetaminophen | TC, QTc, AP, CNS, MA, AG, HPT, HYS, CPT | Yes | Yes | NAC, vasopressors, anticonvulsants, IV fluids |
| 49M | Sympathomimetic unspecified | TC, HYT, VD, QRS, RD,CNS, MA, CPT, WBC, AKI, RBM | No | Unknown | Vasopressors, antiarrhythmics, benzodiazepines, CPR, intubation, IV fluids |
| 19M | Acetaminophen | HT, QTc, ALI, SZ, MA, AG, HPT, HYS, CPT, PLT, AKI | Yes | No | NAC, vasopressors, benzodiazepines, neuromuscular blockers, opioids, steroids, continuous renal replacement, ECMO, intubation, IV fluids |
| 31F | Acetaminophen | TC, CNS, SZ, MA, AG, OG, PNC, HYS, CPT, AKI | Yes | Yes | NAC, NaHCO3, vitamin K, anticonvulsants, continuous renal replacement, intubation, transfusion |
| 45F | Cocaine | RD, CNS, AG, HYS, CPT | Unknown | Unknown | Calcium, flumazenil, NaHCO3, thiamine, vasopressors, benzodiazepines, vasopressors, intubation, IV fluids |
| 72M | Ethanol | AGT, RFX, CPT, PLT | Yes | No | Benzodiazepines, beta blockers, intubation |
| 4M | Smoke | HT, MA | Yes | Yes | Hydroxocobalamin, vasopressors, CPR, intubation, IV fluids |
| 40F | Acetaminophen | HT, BC, AVB | Yes | Unknown | NAC, intubation |
| 67M | Digoxin | HT, BC, AVB | Yes | Unknown | Digoxin Fab, vasopressors |
| 55M | Cocaine | HT, BP, RD, CNS, MA, AG, RBM | Yes | Yes | Naloxone, vasopressors, opioids, IV fluids |
| 32M | Methadone | HT, AP, ALI, RD, CNS, MA, AG, HPT, CPT, PLT, AKI, RBM, OTH2 | Yes | Yes | Naloxone, vasopressors, CPR, intubation, IV fluids, therapeutic hypothermia |
| 17F | Diltiazem | None listed | Yes | No | Continuous renal replacement, IV fluids |
| Unknown F | Acetaminophen | HT, TC, RD, CNS, HGY, MA, AG, HPT, CPT, AKI | No | Unknown | NAC, vitamin K, vasopressors, glucose, continuous renal replacement, intubation, IV fluids, transfusion |
| 19 mo F | Oxycodone | HT, TC, AP, ALI, CNS | Yes | Yes | Naloxone, vasopressors, intubation, IV fluids |
| 55F | Acetaminophen | HT, HGY, AG, HPT, CPT, PLT, AKI | No | Unknown | NAC, vasopressors, glucose, continuous renal replacement, intubation, IV fluids, therapeutic hypothermia, transfusion |
| 35F | Acetaminophen | HT, ALI, RD, CNS, DLM, MA, AG, HPT, CPT, PLT, WBC, AKI | Yes | Yes | NAC, NaHCO3, vasopressors, continuous renal replacement, intubation, IV fluids |
| 34M | Cocaine | HT, TC, VD, QRS, ALI, RD, CNS, MA, AG, AKI, RBM | Yes | Unknown | Calcium, NaHCO3, vasopressors, CPR, intubation, IV fluids, therapeutic hypothermia |
| 29F | Laundry detergent pod | HT, AP, ALI, RD, CNS | No | Unknown | Vasopressors, CPR, intubation, IV fluids |
| 26M | Diesel fuel | HT, TC, BP, VD, AP, ALI, CNS, MA, AG, HYS, CPT, PLT, RBM | No | Unknown | None listed |
| 30M | Heroin | HT, BC, ALI, CNS, MA, AG, PLT, AKI, RBM | Yes | Yes | Calcium, vasopressors, continuous renal replacement, CPR, ECMO, intubation, IV fluids, transfusion |
| 2M | Amphotericin | None listed | Yes | Yes | IV fluids, peritoneal dialysis |
| 65M | Vitamin A | PLT, PCT | No | Unknown | None listed |
| 17F | Metoprolol | HT, BC, RD, CNS | No | Unknown | Lipid resuscitation therapy |
| 22F | Amphetamine | CNS, RFX, WKN | Yes | Unknown | Dantrolene, intubation, IV fluids |
aAge in years unless otherwise stated. wk weeks, mo months
b AG anion gap, AGT agitation, AKI acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis
cPharmcological and nonpharmacological support as reported by a medical toxicologist; BAL dimercaprol, CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation, NAC N-acetyl cysteine, NaHCO 3 sodium bicarbonate
Table 33.
2015 fatalities reported in ToxIC Registry with known toxicological exposure (based on the response from a medical toxicologist “Did the patient have a toxicological exposure?” equals Yes with known agent(s)): multiple agents
| Age/gendera | Agents involved | Clinical findingsb | Life support withdrawn | Brain death confirmed | Treatmentc |
|---|---|---|---|---|---|
| 42M | Metoprolol, methamphetamine | HT, ALI, CNS, MA, HPT | No | Unknown | Insulin-euglycemic therapy, vasopressors, continuous renal replacement, IV fluids |
| 27F | Acetaminophen, oxycodone | HT, BP, RD, CNS, HPT | Yes | Yes | NAC, vasopressors, intubation |
| 39M | Acetaminophen, diphenhydramine | RD, AKI | Yes | No | NAC, intubation |
| 23M | Cannabinoid synthetic, LSD, cathinone | RD, AGT, DLM, AKI | Yes | Yes | Hemodialysis, intubation, IV fluids |
| 71F | Alprazolam, hydrocodone | HT, BC, VD, RD, CNS, HPT, AKI | Yes | No | None listed |
| 16M | Bupropion, guanfacine, lamotrigine, hydroxyzine | HT, TC, VS, QRS, RD, AGT, CNS, DLM, HCN, PST, WKN, AG, GIB | Yes | No | Lipid resuscitation therapy, vasopressors, antiarrhythmics, anticonvulsants, benzodiazepines, neuromuscular blockers, CPR, cardioversion, ECMO, intubation, IV fluids, transfusion |
| 26M | Antihypertensive unspecified, acetaminophen | HT, BC, VD, QRS, RD, CNS, MA, AG, CPT, WBC, AKI | No | Unknown | Insulin-euglycemic therapy, lipid resuscitation therapy, methylene blue, NAC, vasopressors, neuromuscular blockers, opioids, steroids, CPR, ECMO, intubation, IV fluids, pacemaker, therapeutic hypothermia, transfusion |
| 50M | Olanzapine, lorazepam, diphenhydramine | QTc, RD, AGT, CNS, DLM, HCN, AG, RBM | Yes | No | Antipsychotics, benzodiazepines, CPR, intubation |
| 60M | Pyrethroid unspecified, petroleum vapors | HT, QTc, ALI, CNS, RFX | Unknown | Unknown | 2-PAM, fomepizole, lipid resuscitation therapy, vasopressors, intubation, IV fluids, therapeutic hypothermia |
| 47F | Trazodone, heroin | QTc, RD, CNS, GIB, HYS, PLT | Yes | No | Calcium, naloxone, NaHCO3, vasopressors, CPR, intubation, IV fluids, transfusion |
| 3 mo F | Phenobarbital, fosphenytoin, lorazepam | TC, RD, CNS, SZ, MA, HYS | Yes | Yes | Vasopressors, anticonvulsants, benzodiazepines, intubation |
| 14F | Carbon monoxide, cyanide | HT, BP, VD, AP, CNS, MA | Yes | Yes | Hydroxocobalamin, vasopressors, hemodialysis, intubation, IV fluids |
| 39M | Carbon monoxide, cyanide | HT, TC, VD, RD, CNS, MA, WBC | Yes | Yes | Hydroxocobalamin, vasopressors, intubation, IV fluids |
| 19F | Olanzapine, escitalopram | None listed | Yes | Unknown | None listed |
| 73F | Digitoxin, torsemide, diltiazem, metformin | HT, BC, QRS, AG, AKI | No | Unknown | Calcium, digoxin Fab, NaHCO3, vasopressors, continuous renal replacement, |
| 27F | Tramadol, hydromorphone, lorazepam, gabapentin, quetiapine | RD, CNS, SZ | No | Unknown | Flumazenil, naloxone, anticonvulsants, benzodiazepines, opioids, CPR, intubation, IV fluids |
| 53F | Verapamil, oxycodone, fluoxetine, duloxetine, quetiapine | HT, QTc, RD, CNS, MA, RBM | No | Unknown | Calcium, insulin-euglycemic therapy, NaHCO3, vasopressors, glucose, CPR, intubation, IV fluids |
| 19F | Methamphetamine, amphetamine, phentermine | HT, TC, HYT, AP, ALI, CNS, DLM, MA, CPT, PLT, WBC, AKI, RBM | No | Unknown | Vasopressors, neuromuscular blockers, CPR, cardioversion, ECMO, intubation, IV fluids |
| 30M | Heroin, cocaine, clonzepam, methedrone | HT, AP, CNS, RBM | No | Unknown | Naloxone, vasopressors, anticonvulsants, benzodiazepines, neuromuscular blockers, opioids, intubation, IV fluids, therapeutic hypothermia |
| Unknown F | Tramadol, cyclobenzaprine, meloxicam | HT, TC, ALI, CNS, HGY, MA, AG, HPT, CPT, AKI, RBM | No | Unknown | None listed |
| 83M | Memantine, donepezil, dabigatran, levodopa/carbidopa | None listed | Unknown | Unknown | None listed |
| 45M | Oxycodone, cyclobenzaprine | HT, BC, QRS, ALI, CNS, MA, AG, GIB, CPT, WBC, AKI RBM | Yes | No | Atropine, glucagon, lipid resuscitation therapy, NaHCO3, vasopressors, benzodiazepines, neuromuscular blockers, opioids, CPR, intubation, IV fluids, transfusion |
| 46M | Nifedipine, metoprolol, gabapentin | HT, BC, ALI, CNS, MA, HPT, PNC, AKI | Yes | Unknown | Atropine, insulin-euglycemic therapy, lipid resuscitation therapy, NAC, naloxone, vasopressors, intubation, IV fluids |
| 43F | Pregabalin, hydrocodone, amitriptyline, metoprolol | HT, BC, RD, CNS, RFX, SZ, MA | Yes | No | NaHCO3, vasopressors, hemodialysis, CPR, intubation, IVF |
| 29M | Clonazepam, methocarbamol, clonidine, cyclobenzaprine, tizanidine | HT, CNS | No | Unknown | Flumazenil, naloxone |
| 65M | Carbon monoxide, cyanide | HTN, ALI, RD, AGT, CNS, MA, AG, WBC, AKI, skin blisters | Yes | No | hydroxocobalamin, antihypertensives, antipsychotics, benzodiazepines, steroids, intubation, IV fluids |
aAge in years unless otherwise stated. wk weeks, mo months
b AG anion gap, AGT agitation, AKI acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis
cPharmcological and nonpharmacological support as reported by a medical toxicologist; BAL Dimercaprol, CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation, NAC N-acetyl cysteine, NaHCO 3 sodium bicarbonate
Patients involved in fatalities with a toxicological exposure had an average age of 40.7 years, with a range from 3 months old to greater than 89 years. Median age was 40. There were 5 fatalities in children under the age of 5 years. Four of these were single agent exposures, and one involved multiple agents. Seven fatalities occurred in the 13–18-year age range. The most commonly reported single exposure agent was acetaminophen (N = 16). Three additional multiple toxicological exposure cases involved acetaminophen. Sympathomimetics (N = 9), cardiovascular agents (N = 7), and opioids (N = 6) were the next most commonly reported single agent exposures. This year, there was a fatality reported in a 29-year-old female with a known toxicological exposure to a laundry detergent pod. The multiple toxicological exposure group showed a similar trend of agents as the single agent exposures with 12 cardiovascular, 10 opioid, and 7 sympathomimetics. There were a relatively large number of sedative/hypnotic agents (N = 15) contributing in the multiple agent exposure group, compared to none in the single agent group. Five deaths in the multiple agent category involved a combination of an opioid and a sedative/hypnotic agent. In 22 cases (22.2 %), there was no agent reported. Forty-six (46.5 %) of the fatality cases reported that life supportive measures were withdrawn, and among these, 22 (22.2 %) were formally declared brain dead.
Adverse Drug Reactions
Adverse drug reactions (ADRs), as defined in the Registry, describes any unintended response to a medication as it is used in standard therapeutic dosing. A total of 352 ADR cases were reported in the Registry in 2015, making up 4.3 % of case entries. A total of 536 agents were entered into cases recorded as ADRs, with the possibility of multiple agents reported for each case, as in a drug-drug interaction. There were 177 unique agents entered at least once; 118 cases had multiple agents listed. There were 14 cases without an agent listed (4.0 % of ADR cases).
As reported in Table 34, the top 3 agents were lithium (N = 25), lorazepam (N = 20), and quetiapine (N = 19). Only the top 10 agents are reported in the table. Percentages were calculated out of the number of ADR case entries, so the total percentage is more than 100, although only the most frequent agents are shown. Similar to last year, the most common agent classes involved in ADRs were distinct from the most common agent exposures overall [4]. Cardiovascular agents were involved in 20.2 % of ADRs, followed by antipsychotics (19.3 %) and antidepressants (19.0 %). This year, sedative/hypnotic medications were much more commonly reported to be involved with ADRs (15.3 %), with lorazepam being the second most common single agent reported.
Table 34.
Most common drugs associated with ADRs
| N (%)a | |
|---|---|
| Lithium | 25 (7.1) |
| Lorazepam | 20 (5.7) |
| Quetiapine | 19 (5.4) |
| Digoxin | 16 (4.5) |
| Haloperidol | 15 (4.3) |
| Valproic acid | 15 (4.3) |
| Fentanyl | 11 (3.1) |
| Bupropion | 10 (2.8) |
| Diphenhydramine | 10 (2.8) |
| Metoprolol | 10 (2.8) |
ADRs adverse drug reactions
aPercentages are calculated out of the total number of cases reporting an ADR (N = 352)
Treatment
Table 35 presents all data for antidotal therapy. In 2015, there were 5298 cases (65.3 %) that received a toxicological treatment. There were 3044 instances of specific antidotal therapy given in 2681 Registry cases (33.0 %). Some individuals received more than one antidote. By far, the most frequent antidotal therapy was N-acetylcysteine, making up 29.0 % of the total antidotes given (N = 883). Naloxone/nalmefene was the next most common antidote (17.6 %), followed by sodium bicarbonate (11.6 %) and physostigmine (6.6 %). These were also the top 4 antidotes in 2014 with a similar distribution. Methylene blue was given more frequently compared to last year (N = 16, 0.5 % vs. N = 3, 0.1 %) [4].
Table 35.
Antidotal therapy
| N (%)a | |
|---|---|
| N-acetylcysteine | 883 (29.0) |
| Naloxone/nalmefene | 535 (17.6) |
| Sodium bicarbonate | 352 (11.6) |
| Physostigmine | 201 (6.6) |
| Thiamine | 180 (5.9) |
| Folate | 144 (4.7) |
| Flumazenil | 105 (3.4) |
| Fomepizole | 91 (3.0) |
| Calcium | 76 (2.5) |
| Glucagon | 73 (2.4) |
| Atropine | 57 (1.9) |
| Octreotide | 45 (1.5) |
| Cyproheptadine | 44 (1.4) |
| l-Carnitine | 38 (1.2) |
| Fab for digoxin | 36 (1.2) |
| Vitamin K | 31 (1.0) |
| Insulin-euglycemic therapy | 30 (1.0) |
| Pyridoxine | 27 (0.9) |
| Lipid resuscitation | 25 (0.8) |
| Methylene blue | 16 (0.5) |
| Hydroxocobalamin | 15 (0.5) |
| Dantrolene | 11 (0.4) |
| Bromocriptine | 10 (0.3) |
| 2-PAM | 7 (0.2) |
| Anticoagulation reversal | 3 (0.1) |
| Ethanol | 3 (0.1) |
| Coagulation factor replacement | 3 (0.1) |
| Sylimarin | 2 (0.1) |
| Thiosulfate | 1 (0.0) |
| Total | 3044 (100) |
aPercentages are out of the total number of antidotes administered (3044); 2681 registry cases (33.0 %) received at least one antidote. Cases may have involved the use of multiple antidotes
Specific antivenom therapy (Table 36) was delivered in 187 cases, representing 2.3 % of total Registry cases. The overwhelming majority of antivenom was crotalidae polyvalent immune fab (ovine) (N = 172). No cases reported the use of spider antivenom.
Table 36.
Antivenom therapy
| N (%)a | |
|---|---|
| Crotalidae polyvalent immune fab (ovine) | 172 (92.0) |
| Other snake antivenom | 11 (5.9) |
| Scorpion antivenom | 4 (2.1) |
| Total | 187 (100) |
aPercentages are out of the total number of antivenom treatments administered (N = 187)
The trends of pharmacological supportive care were stable compared to last year with agents and the frequency with which they were given (Table 37). Benzodiazepines were by far the most common, comprising 55.5 % of supportive pharmacological interventions out of 3287 total cases requiring supportive treatment, followed by opioids (10.2 %), vasopressors (8.9 %), and antipsychotic agents (7.1 %).
Table 37.
Supportive care—pharmacological
| N (%)a | |
|---|---|
| Benzodiazepines | 1825 (55.5) |
| Opioids | 336 (10.2) |
| Vasopressors | 293 (8.9) |
| Antipsychotics | 233 (7.1) |
| Anticonvulsants | 134 (4.1) |
| Glucose (concentration > 5 %) | 123 (3.7) |
| Antihypertensives | 102 (3.1) |
| Neuromuscular blockers | 94 (2.9) |
| Albuterol (or other bronchodilator) | 46 (1.4) |
| Corticosteroids | 44 (1.3) |
| Antiarrhythmics | 34 (1.0) |
| Beta blockers | 20 (0.6) |
| Vasodilators | 3 (0.1) |
| Total | 3287 (100) |
aPercentages are out of the total number of treatments administered (3287); 2423 registry cases (29.9 %) received at least one form of pharmacological treatment. Cases may have involved the use of multiple forms of treatment
Nonpharmacological supportive care was given in 3371 cases with intravenous fluid resuscitation making up 69.6 % of these (Table 38). Intubation or ventilatory management was required in 837 cases. There were 19 cases reporting the use of extracorporeal membrane oxygenation (ECMO). Fifteen cases underwent hyperbaric oxygen therapy.
Table 38.
Supportive care—nonpharmacological
| N (%)a | |
|---|---|
| IV fluid resuscitation | 2345 (69.6) |
| Intubation/ventilatory management | 837 (24.8) |
| CPR | 66 (2.0) |
| Transfusion | 29 (0.9) |
| Therapeutic hypothermia | 26 (0.8) |
| ECMO | 19 (0.6) |
| Cardioversion | 18 (0.5) |
| Hyperbaric oxygen | 15 (0.4) |
| Pacemaker | 13 (0.4) |
| Aortic balloon pump | 2 (0.1) |
| Organ transplantation | 1 (0.02) |
| Total | 3371 (100) |
CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation
aPercentages are out of the total number of treatments administered (3371); 2657 registry cases (32.7 %) received at least one form of nonpharmacological treatment. Cases may have involved the use of multiple forms of treatment
The use of chelation therapy is described in Table 39. There were 25 total cases in which chelation was performed, most of these with dimercaptosuccinic acid (DMSA) (N = 16, 64 %).
Table 39.
Chelation therapy
| N (%)a | |
|---|---|
| DMSA | 16 (64.0) |
| Dimercaprol (BAL) | 3 (12.0) |
| Deferoxamine | 3 (12.0) |
| EDTA | 2 (8.0) |
| Pencillamine | 1 (4.0) |
| Total | 25 (100) |
DMSA dimercaptosuccinic acid, EDTA ethylenediamine-tetraacetic acid
aPercentages are out of the total number of chelation treatments administered (25)
Decontamination procedures were performed in 340 cases (4.2 %) (Table 40). Some cases involved more than one decontamination measure, so that a total of 361 decontamination treatments were administered; 82.3 % of decontamination was activated charcoal. Gastric lavage was only performed in nine cases (2.5 %), compared with 23 cases last year [4].
Table 40.
Decontamination
| N (%)a | |
|---|---|
| Activated charcoal | 297 (82.3) |
| Whole bowel irrigation | 38 (10.5) |
| External irrigation | 17 (4.7) |
| Gastric lavage | 9 (2.5) |
| Total | 361 (100) |
aPercentages are out of the total number of treatments administered (361); 340 registry cases (4.2) received at least one form of decontamination
Enhanced elimination techniques were used in 244 cases (3.0 %), with some receiving multiple modalities, for a total of 292 reported treatments (Table 41). Hemodialysis was the most common modality, performed in 87 cases (29.8 %) for an indication of toxin removal, and in an additional 59 cases (20.2 %) for other indications. These numbers as well as those for the additional modalities reported in Table 41 were consistent with last year’s findings.
Table 41.
Enhanced elimination
| N (%)a | |
|---|---|
| Hemodialysis (toxin removal) | 87 (29.8) |
| Urinary alkalinization | 75 (25.7) |
| Hemodialysis (other indication) | 59 (20.2) |
| Continuous renal replacement therapy | 53 (18.2) |
| Multiple-dose activation charcoal | 14 (4.8) |
| Exchange transfusion | 4 (1.4) |
| Total | 292 (100) |
aPercentages are out of the total number of treatments administered (292); 244 registry cases (3.0 %) received at least one form of enhanced elimination
Discussion
This report is the summary for the sixth year of data collected in the ACMT ToxIC Registry. The most common agent classes, location of encounter, and reason for consultation were similar to prior years. Some broad trends or differences in this year’s results from past years are discussed.
Synthetic cannabinoids have steadily increased in cases each year since the Registry began in 2010 (Table 19). The most notable increases were from 2010 to 2011 and 2014 to 2015. The first increase may have been, at least in part, related to an increased awareness of this class of psychotropic drugs. The recent increase in 2015, with cases more than doubling, occurs as marijuana cases continue to decrease in the Registry. In 2015, synthetic cannabinoid encounters were more frequently reported than marijuana making up 51.3 % of the class (Table 18). It is unclear if the two trends are related, or if synthetic cannabinoids are being used as a substitute product for marijuana. As the number of states allowing medical marijuana or decriminalization of marijuana continues to grow, one might expect an increased frequency of marijuana exposures presenting for care. Those presenting with exposures to synthetic cannabinoids and marijuana appear to be different users, despite a similar age group. Those presenting after synthetic cannabinoid exposure are more commonly males and more likely to have a single agent exposure. Marijuana exposures by comparison still had a male predominance, but less marked. They were also approximately equally likely to present with more than one substance or with marijuana as a single agent. These characteristics describe those who presented for care and may not be generalizable to all individuals using these substances. According to 2010–2014 National Poison Data System (NPDS) reports, the years the Registry has been active, the number of marijuana exposures has been approximately stable, with an uptick in 2014 [12–16]. In the same time period, synthetic cannabinoids, referred to as THC homologs, peaked in 2011, then decreased significantly, although also showing an increase from 2013 to 2014. NPDS data for 2015 is not yet available to compare trends since 2014. Differences between NPDS and ToxIC Registry data may reflect the differences between overall exposures and those which are referred for consultation by a medical toxicologist. Alternatively, this may be a partial reflection of geographic distribution patterns of drugs of abuse, with the possibility that certain substances will be seen more or less often depending upon what drugs are common in the practice areas of participating medical toxicologists.
Sedative hypnotics and muscle relaxants were the second most commonly reported agent class, and the sedative hypnotic toxidrome was the most common toxidrome. This is consistent with NPDS data which found the sedative/hypnotics/antipsychotics agent class to have the greatest increase in serious exposures in 2014 [16]. Clonazepam and alprazolam were the top reported sedative hypnotic drugs in the ToxIC Registry in 2015. Lorazepam was the third most common, making up 10.2 % of the class; however, it was the second most frequent agent reported in ADRs. Sedative hypnotic agents overall were commonly reported as ADRs this year (15.3 %). In seven ADR cases, lorazepam was listed as the only agent. An additional four cases report an ADR for lorazepam and ethanol and another case for lorazepam and oxycodone. Other ADRs involving lorazepam with more than one other agent tended to involve other sedating drugs such as diazepam, oxycodone, methadone, haloperidol, trazodone, or quetiapine.
Among fatalities, there were a relatively large number of sedative/hypnotic agents (N = 15) contributing in the multiple agent exposure group, compared to none in the single agent group. There were five fatalities from opioids as a single agent in 2015. Nine fatality cases involved opioids in combination with other agents; of these, five deaths in the multiple agent category involved a combination with sedative hypnotic agents. These findings are in line with what is currently known regarding the increased risk of respiratory depression and mortality when both benzodiazepines and opioids are used in combination, either with or without ethanol.
In 2015, heroin remained the most common agent in the opioid class, being implicated in 243 case entries. Stimulants, benzodiazepines, and other opioids were the most commonly coingested substances. As might be predicted, heroin present in combination with additional agents was associated with a higher rate of intubation. Heroin users who present for medical care and subsequently receive a medical toxicology consult may represent more severe cases, or may have unique features and clinical course, so these cases may not be generalizable to the average heroin overdose seen in the community.
Limitations of the Registry exist and continue to be addressed. The central inclusion criterion for entry into the Registry is consultation by a medical toxicologist. This very likely creates a reporting bias toward more severe presentations or more unique cases both at the level of the decision to consult, and by the reporting medical toxicologist since all cases are entered by the examining medical toxicologist. This is partly controlled by an agreement with all participating sites that every case must be included, so that the database is a representation of all cases seen by the practicing medical toxicologist. All case entries represent those who have presented for clinical care rather than follow symptoms at home. Therefore, one would predict a bias toward more severe or unusual exposures within the Registry as compared to sources such as Poison Control Centers.
There exist a limited number of medical toxicologists in practice, and the Registry includes cases only from those who agree to the participation rules and guidelines set forth. This limits the entry of cases to only those geographic areas with actively practicing and participating medical toxicologists. Thus, the Registry is not a population-based database. Despite this, the Registry has grown to include sites from 24 states across the country, as well as sites in Canada, Israel, and Saudi Arabia, and therefore is expected to include a diverse collection of cases and practice patterns. The distribution of ToxIC sites around the country is sufficiently diverse that the Registry probably is a reasonable representation of trends around the country as a whole. However, some areas, such as the southeast part of the USA, are relatively unrepresented and trends unique to that area may not be fully reflected in the database.
The qualification of having a medical toxicologist at the bedside to examine each case included into the Registry also ensures that the toxicological data collected is of the highest quality. Determinations of relationships between signs or symptoms and exposure are as reliable as possible in this database.
A centralized quality assurance review was initiated in 2014 and has been ongoing. Efforts have focused on improving data entry and correcting inappropriately coded responses or absent data fields. 2015 is the first complete year of data collection since the conversion of several of the data fields into mandatory responses in 2014. This included fields such as reason for encounter, presence of suicidal intent, signs and symptoms, treatments, other interventions, and fatalities. Prior to this, it was unknown if items left blank were not applicable or simply not recorded. This improvement has been successful in nearly eliminating blank fields, and future research will benefit from the improved clarity of responses.
One particular limitation in this report is the limited data reported for race and ethnicity. These fields were added in 2014 as mandatory responses. Although reporting has increased since last year, approximately one-quarter of cases still have “unknown/uncertain” race and ethnicity listed. This may be in part due to its recent addition to the database. These data may not routinely be reflected in patient charts and may require the examiner to specifically recall this information. Participating members may not have made this a routine part of their information gathering. Going forward with the database, this will need to be addressed to improve the quality of demographic data.
Significant physical examination findings, vital sign abnormalities, supportive interventions, and some laboratory data are collected; however, the severity of illness is not directly reported in Registry cases. The completeness of this data has improved since creating the mandatory entry fields, although there remain some cases, such as fatalities, where multiple interventions might be expected, but are not recorded. A study is ongoing with a goal to develop a severity score for the Registry. Fatality data are also somewhat limited in the Registry. The timeline of events and cause of death cannot be described. Multiple agents may be reported, but unless free text fields are used, it may be unclear to what degree each agent contributed.
Additional limitations include those inherent in any database. Every case is unique and some details may be too complicated or nuanced to be adequately described in a series of data fields. There are free text fields available to enter additional information on each case at the medical toxicologist’s discretion, but information in these areas is not easily searchable and must be reviewed manually.
Since its inception in 2010, the Registry has continued to grow, consisting of 43,099 total cases as of December 31, 2015. It is the only database of its kind with a log of all cases encountered by participating medical toxicologists. While there may be variation in agent frequencies from year to year, and new trends may emerge, overall the most common exposures, toxidromes, clinical abnormalities, and antidotes as recorded in the Registry represent the routine practice of the specialty of medical toxicology. The Registry forms a representation of our specialty and the expertise that a medical toxicologist brings to the bedside. This database has potential to be used not only for toxico-surveillance and research but also as an informational and instructional tool for fellows, residents, and medical students in medical toxicology.
Conclusions
Intentional pharmaceutical product exposures were the most common reason for a medical toxicology encounter in 2015. The majority of intentional pharmaceutical exposures involved an attempt at self-harm. Nonopioid analgesics, sedative-hypnotic/muscle relaxants, antidepressants, and opioids were the most commonly reported agent classes. Treatment was required in almost two-thirds of all cases; however, fatalities were uncommon.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOCX 28 kb)
(DOCX 13 kb)
(DOCX 29 kb)
(DOCX 12 kb)
(DOCX 36 kb)
(DOCX 12 kb)
(DOCX 37 kb)
(DOCX 28 kb)
(DOCX 28 kb)
(DOCX 35 kb)
(DOCX 50 kb)
(DOCX 43 kb)
(DOCX 43 kb)
(DOCX 37 kb)
(DOCX 42 kb)
(DOCX 29 kb)
(DOCX 15 kb)
Acknowledgments
The authors express their sincere gratitude to the staff at the American College of Medical Toxicology for supporting the ToxIC Registry project. We very much appreciate the contribution to the Registry from each of the ToxIC sites. The following is a list of the principle coordinators from each site contributing cases in 2015.
Toxicology Investigators Consortium
Albuquerque, NM
Steven Seifert, MD
Atlanta, GA
Adam Pomerleau, MD
Ziad Kazzi, MD
Boston, MA (Beth Israel Deaconess Medical Center)
Michael Ganetsky, MD
Boston, MA (Children’s Hospital of Boston)
Michele Burns, MD
Charlotte, NC
Michael Beuhler, MD
Charlottesville, VA
Joshua D. King, MD
Chicago, IL
Steven Aks, DO
Dallas, TX
Jakub Furmaga, MD
Paul Wax, MD
Denver, CO
Jeffrey Brent, MD
Christopher Hoyte, MD
Evanston, IL
Jerrold Leikin, MD
Fresno, CA
Rais Vohra, MD
Grand Rapids, MI
Bryan Judge, MD
Brad Riley, MD
Greenville, SC
William J Meggs, MD
Haifa, Israel
Didi Bentur, MD
Harrisburg, PA
Phil Moore, MD
Hartford, CT
Lynn Farrugia, MD
Houston, TX
Spencer Greene, MD
Indianapolis, IN
Daniel Rusyniak, MD
Kansas City, MO
Jennifer Lowry, MD
Adam Algren, MD
Loma Linda, CA
Brian Wolk, MD
Los Angeles, CA
Michael Levine, MD
Manhasset, NY
Josh Nogar, MD
Milwaukee, WI
Mark Kostic, MD
David Gummin, MD
Morristown, NJ
Diane Calello, MD
New Brunswick, NJ
Ann-Jeannette Geib, MD
New York, NY (Mount Sinai Hospital)
Stephanie Hernandez, MD
New York, NY (NYU Langone Medical Center)
Silas Smith, MD
New York, NY (Bellevue Medical Center)
Lewis Nelson, MD
Newark, NJ
Steven Marcus, MD
Omaha, NE
Ronald Kirschner, MD
Philadelphia (Einstein Medical Center)
Adam Rowden, MD
Philadelphia, PA (Hahnemann University Hospital)
David Vearrier, MD
Rita McKeever, MD
Phoenix, AZ
Michelle Ruha, MD
Pittsburgh, PA
Anthony Pizon, MD
Portland, OR
Robert Hendrickson, MD
Nate McKeown, MD
Richmond, VA
Brandon Wills, DO
Kirk Cumpston, DO
Riyadh, Saudi Arabia
Mohammed Alhelail, MD
Rochester, NY
Timothy Wiegand, MD
Salt Lake City, UT
E. Martin Caravati, MD
San Antonio, TX
Daniel Sessions, MD
Joseph Maddry, MD
San Diego, CA
Alicia Minns, MD
San Francisco, CA
Derrick Lung MD
Craig Smollin MD
St. Louis, MO
Thomas Kibby, MD
Evan Schwarz MD
St. Paul, MN
Samuel Stellpflug, MD
Kristin Engebretsen, MD
Staten Island, NY
Nima Majlesi, MD
Syracuse, NY
Ross Sullivan, MD
Tampa, FL
Tamas Peredy, MD
Toronto, Canada
Yaron Finkelstiein, MD
Worcester, MA
Jennifer Carey, MD
Compliance with Ethical Standards
Conflict of Interest
None.
Funding Sources
This study received funding from the NIH National Institute of Neurological Disorders and Stroke 3U01NS083452-01 (Bird, SB), NIH National Institute on Drug Abuse 1R56DA38366 (Boyer, EW/ Carlson, RG) and 1R01DA037317-01 (Manini, A), and BTG International Inc.
Contributor Information
Lynn A. Farrugia, Phone: 860-972-0001, Email: Lynn.Farrugia@hhchealth.org
On behalf of the Toxicology Investigators Consortium:
Steven Seifert, Adam Pomerleau, Ziad Kazzi, Michael Ganetsky, Michele Burns, Michael Beuhler, Joshua D. King, Steven Aks, Jakub Furmaga, Paul Wax, Jeffrey Brent, Christopher Hoyte, Jerrold Leikin, Rais Vohra, Bryan Judge, Brad Riley, William J. Meggs, Didi Bentur, Phil Moore, Lynn Farrugia, Spencer Greene, Daniel Rusyniak, Jennifer Lowry, Adam Algren, Brian Wolk, Michael Levine, Josh Nogar, Mark Kostic, David Gummin, Diane Calello, Ann-Jeannette Geib, Stephanie Hernandez, Silas Smith, Lewis Nelson, Steven Marcus, Ronald Kirschner, Adam Rowden, David Vearrier, Rita McKeever, Michelle Ruha, Anthony Pizon, Robert Hendrickson, Nate McKeown, Brandon Wills, Kirk Cumpston, Mohammed Alhelail, Timothy Wiegand, E. Martin Caravati, Daniel Sessions, Joseph Maddry, Alicia Minns, Derrick Lung, Craig Smollin, Thomas Kibby, Evan Schwarz, Samuel Stellpflug, Kristin Engebretsen, Nima Majlesi, Ross Sullivan, Tamas Peredy, Yaron Finkelstiein, and Jennifer Carey
References
- 1.Wax PM, Kleinschmidt KC, Brent J. ACMT ToxIC Case Registry Investigators. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein Y, Goel G, Hutson JR, Armstrong J, Baum CR, et al. Drug misuse in adolescents presenting to the emergency department. Pediatr Emerg Care. 2015 doi: 10.1097/PEC.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 3.Froberg BA, Levine M, Beuhler MC, Judge BS, et al. Acute methylenedioxypyrovalerone toxicity. J Med Toxicol. 2015;11(2):185–94. doi: 10.1007/s13181-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhyee SH, Farrugia L, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2014 experience. J Med Toxicol. 2015;11(4):388–409. doi: 10.1007/s13181-015-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SW, Farmer BM. Toxicology in the service of patient and medication safety: a selected glance at past and present innovations. J Med Toxicol. 2015;11(2):245–252. doi: 10.1007/s13181-015-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins JW, Schwarz ES, Arroyo-Plasencia AM, Mullins ME. The use of physostigmine by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11(2):179–84. doi: 10.1007/s13181-014-0452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelner I, Matlow J, Hutson JR, Wax P, et al. Acute poisoning during pregnancy: observations from the Toxicology Investigators Consortium. J Med Toxicol. 2015;11(3):301–8. doi: 10.1007/s13181-015-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhyee SH, Farrugia L, Wiegand T, et al. The Toxicology Investigators Consortium Case Registry—the 2013 experience. J Med Toxicol. 2014;10(4):342–359. doi: 10.1007/s13181-014-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiegand TJ, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol. 2012;8(4):360–377. doi: 10.1007/s13181-012-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand T, Wax P, Smith E, et al. The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol. 2013;9(4):380–404. doi: 10.1007/s13181-013-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brent J, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2010 experience. J Med Toxicol. 2011;7(4):266–276. doi: 10.1007/s13181-011-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronstein AC, Spyker DA, Cantilena LR, et al. 2010 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol. 2011;49(10):910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- 13.Bronstein AC, Spyker DA, Cantilena LR, et al. 2011 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol. 2012;50(10):911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 14.Mowry JB, Spyker DA, Cantilena LR, et al. 2012 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 15.Mowry JB, Spyker DA, Cantilena LR, et al. 2013 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st annual report. Clin Toxicol. 2014;52(10):1032–1283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mowry JB, Spyker DA, Brooks DE, et al. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol. 2015;53(10):962–1147. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28 kb)
(DOCX 13 kb)
(DOCX 29 kb)
(DOCX 12 kb)
(DOCX 36 kb)
(DOCX 12 kb)
(DOCX 37 kb)
(DOCX 28 kb)
(DOCX 28 kb)
(DOCX 35 kb)
(DOCX 50 kb)
(DOCX 43 kb)
(DOCX 43 kb)
(DOCX 37 kb)
(DOCX 42 kb)
(DOCX 29 kb)
(DOCX 15 kb)


