Abstract
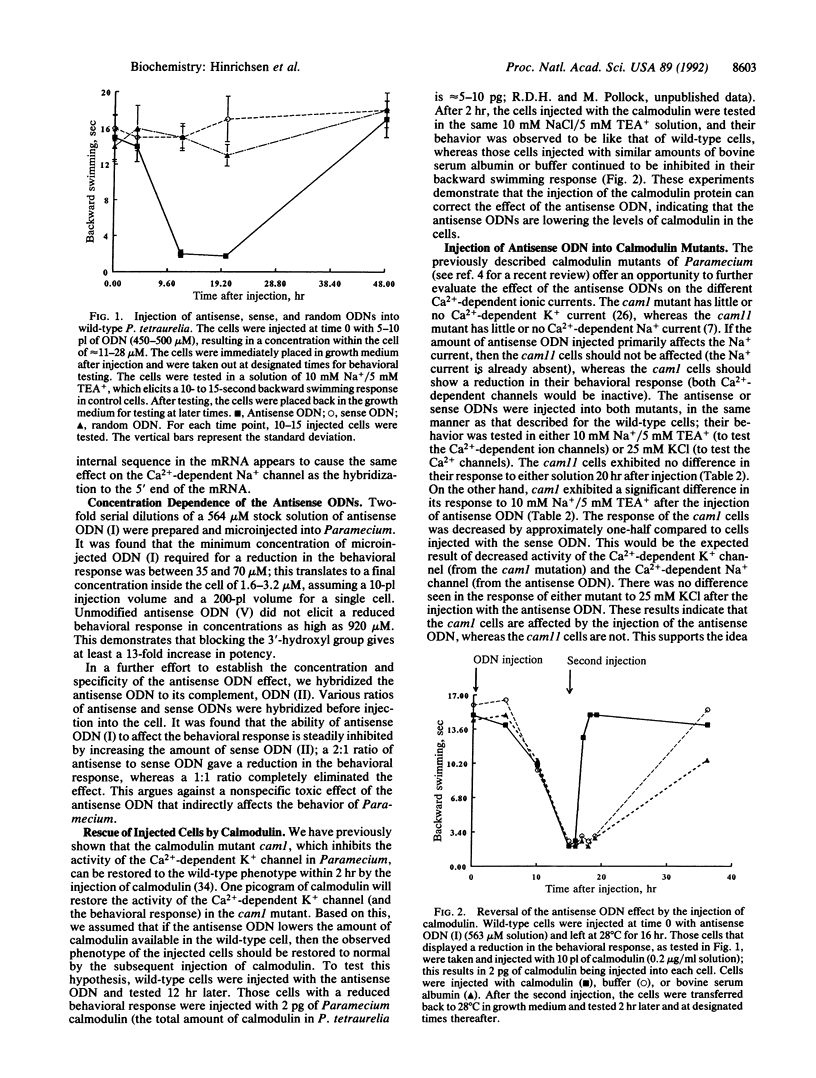
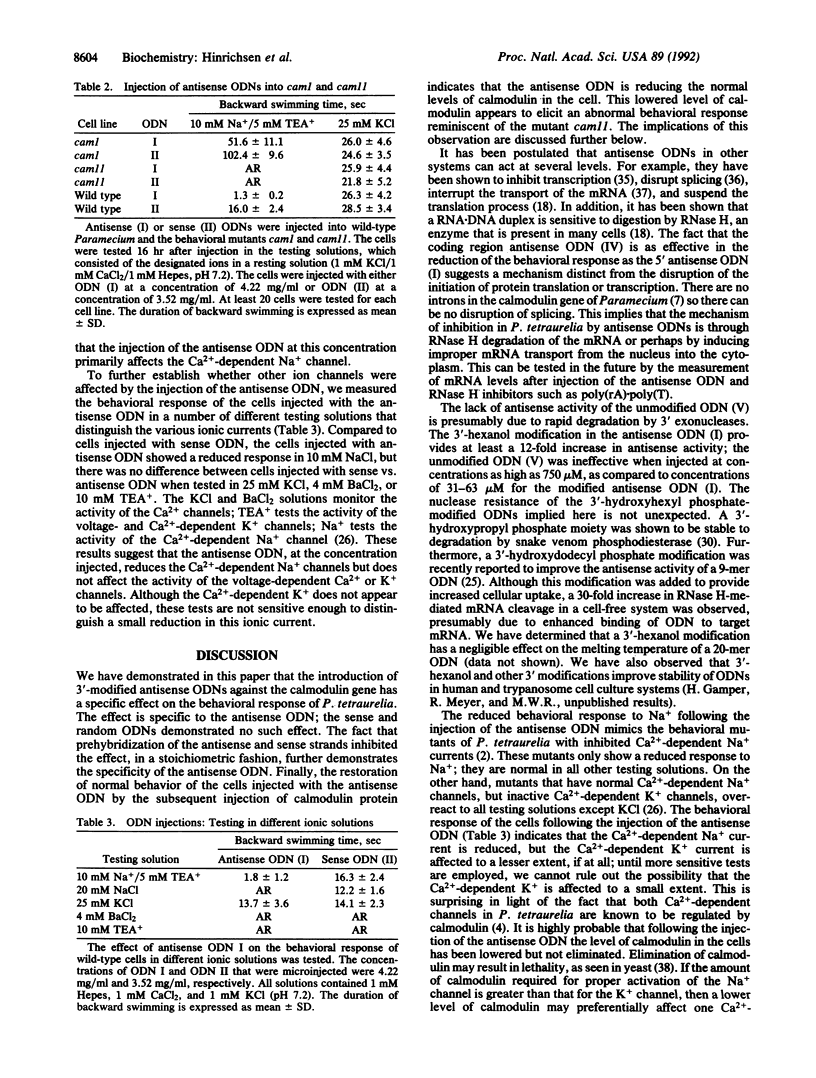
The calcium-binding protein calmodulin has been shown to modulate the Ca(2+)-dependent ion channels of Paramecium tetraurelia. Mutations in the calmodulin gene of Paramecium result in an altered pattern of behavioral responses. Antisense oligodeoxyribonucleotides (ODNs), complementary to calmodulin mRNA in Paramecium, were synthesized from a modified solid support that introduced a 3'-hydroxyhexyl phosphate. These 3'-modified ODNs were tested for their ability to alter the behavioral response of Paramecium. The microinjection of antisense ODNs temporarily reduced the backward swimming behavior of the cells in test solutions containing Na+. The injection of sense and random 3'-modified ODNs, or unmodified antisense ODNs, had no effect. The antisense ODN-induced effect was reversed by the injection of calmodulin protein. The pattern of response of the injected cells in various behavioral test solutions indicated that the calmodulin antisense ODNs reduce the Ca(2+)-dependent Na+ current. Antisense ODNs, complementary either to the 5' start site or to an internal sequence of the calmodulin mRNA, were similarly effective in altering behavior. These results show that antisense ODNs may be utilized in ciliated protozoa as a tool for reducing the expression of specific gene products. In addition, Paramecium represents a powerful model system with which to study and develop antisense ODN technology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor C. R., Warshaw M. M., Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9(9):1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- Chiang M. Y., Chan H., Zounes M. A., Freier S. M., Lima W. F., Bennett C. F. Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J Biol Chem. 1991 Sep 25;266(27):18162–18171. [PubMed] [Google Scholar]
- Colman A. Antisense strategies in cell and developmental biology. J Cell Sci. 1990 Nov;97(Pt 3):399–409. doi: 10.1242/jcs.97.3.399. [DOI] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Weeks D. L., Walder J. A. Pathways of degradation and mechanism of action of antisense oligonucleotides in Xenopus laevis embryos. Antisense Res Dev. 1991 Spring;1(1):11–20. doi: 10.1089/ard.1991.1.11. [DOI] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Eid J. E., Sollner-Webb B. Homologous recombination in the tandem calmodulin genes of Trypanosoma brucei yields multiple products: compensation for deleterious deletions by gene amplification. Genes Dev. 1991 Nov;5(11):2024–2032. doi: 10.1101/gad.5.11.2024. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Amberger E., Saimi Y., Burgess-Cassler A., Kung C. Genetic analysis of mutants with a reduced Ca2+-dependent K+ current in Paramecium tetraurelia. Genetics. 1985 Nov;111(3):433–445. doi: 10.1093/genetics/111.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Burgess-Cassler A., Soltvedt B. C., Hennessey T., Kung C. Restoration by calmodulin of a Ca2+-dependent K+ current missing in a mutant of Paramecium. Science. 1986 Apr 25;232(4749):503–506. doi: 10.1126/science.2421410. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Pollock M., Hennessey T., Russell C. An intragenic suppressor of a calmodulin mutation in Paramecium: genetic and biochemical characterization. Genetics. 1991 Nov;129(3):717–725. doi: 10.1093/genetics/129.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Saimi Y., Kung C. Mutants with altered Ca2+-channel properties in Paramecium tetraurelia: isolation, characterization and genetic analysis. Genetics. 1984 Nov;108(3):545–558. doi: 10.1093/genetics/108.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Schultz J. E. Paramecium: a model system for the study of excitable cells. Trends Neurosci. 1988 Jan;11(1):27–32. doi: 10.1016/0166-2236(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M. A. An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Maley M. E., Preston R. R., Ling K. Y., Wallen-Friedman M. A., Saimi Y., Kung C. Mutations in paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell. 1990 Jul 13;62(1):165–174. doi: 10.1016/0092-8674(90)90250-i. [DOI] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Rudman B. M., Barnett A. J. Deviation from the universal code shown by the gene for surface protein 51A in Paramecium. Nature. 1985 Mar 14;314(6007):188–190. doi: 10.1038/314188a0. [DOI] [PubMed] [Google Scholar]
- Preston R. R., Kink J. A., Hinrichsen R. D., Saimi Y., Kung C. Calmodulin mutants and Ca2(+)-dependent channels in Paramecium. Annu Rev Physiol. 1991;53:309–319. doi: 10.1146/annurev.ph.53.030191.001521. [DOI] [PubMed] [Google Scholar]
- Reed M. W., Adams A. D., Nelson J. S., Meyer R. B., Jr Acridine- and cholesterol-derivatized solid supports for improved synthesis of 3'-modified oligonucleotides. Bioconjug Chem. 1991 Jul-Aug;2(4):217–225. doi: 10.1021/bc00010a005. [DOI] [PubMed] [Google Scholar]
- Saimi Y., Kung C. Behavioral genetics of Paramecium. Annu Rev Genet. 1987;21:47–65. doi: 10.1146/annurev.ge.21.120187.000403. [DOI] [PubMed] [Google Scholar]
- Saimi Y., Ling K. Y. Calmodulin activation of calcium-dependent sodium channels in excised membrane patches of Paramecium. Science. 1990 Sep 21;249(4975):1441–1444. doi: 10.1126/science.2169650. [DOI] [PubMed] [Google Scholar]
- Saison-Behmoaras T., Tocqué B., Rey I., Chassignol M., Thuong N. T., Hélène C. Short modified antisense oligonucleotides directed against Ha-ras point mutation induce selective cleavage of the mRNA and inhibit T24 cells proliferation. EMBO J. 1991 May;10(5):1111–1118. doi: 10.1002/j.1460-2075.1991.tb08051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. H., Hinrichsen R. D., Burgess-Cassler A., Kung C., Blair I. A., Watterson D. M. A mutant Paramecium with a defective calcium-dependent potassium conductance has an altered calmodulin: a nonlethal selective alteration in calmodulin regulation. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3931–3935. doi: 10.1073/pnas.84.11.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Kaiser K. Oligodeoxyribonucleotides containing 1,3-propanediol as nucleoside substitute. Nucleic Acids Res. 1987 Apr 10;15(7):3113–3129. doi: 10.1093/nar/15.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker M. O., Lau W., Shattuck R. L., Kwiatkowski A. P., Matrisian P. E., Guerra-Santos L., Wilson E., Lukas T. J., Van Eldik L. J., Watterson D. M. Use of DNA sequence and mutant analyses and antisense oligodeoxynucleotides to examine the molecular basis of nonmuscle myosin light chain kinase autoinhibition, calmodulin recognition, and activity. J Cell Biol. 1990 Sep;111(3):1107–1125. doi: 10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers T., Baker B. F., Cook P. D., Zounes M., Buckheit R. W., Jr, Germany J., Ecker D. J. Inhibition of HIV-LTR gene expression by oligonucleotides targeted to the TAR element. Nucleic Acids Res. 1991 Jun 25;19(12):3359–3368. doi: 10.1093/nar/19.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. M. Antisense RNA and DNA. Sci Am. 1990 Jan;262(1):40–46. doi: 10.1038/scientificamerican0190-40. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]



