Abstract
Background
The efficacy and safety of non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in Asian octogenarian atrial fibrillation (AF) patients have not been established in a real-world setting. We aimed to evaluate the efficacy and safety of NOACs and warfarin in Korean octogenarian patients.
Methods
A total of 293 consecutive patients aged ≥ 80 years with non-valvular AF who had taken either NOACs (148 cases, 50.5%) or warfarin (145 cases, 49.5%) were retrospectively reviewed. The efficacy outcome was the composite of stroke or systemic embolism. The safety outcome was major bleeding.
Results
The follow-up duration was 375 patient-years (172 patient-years with NOACs and 203 patient-years with warfarin). Patients on NOACs were slightly older (P = 0.006) and had slightly higher HAS-BLED scores (P = 0.034). The efficacy of both anticoagulants was high (1.16% for NOACs vs. 2.98% for warfarin per 100 patient-years, P = 0.46). The safety outcome was relatively high in both NOACs and warfarin groups (8.96% vs. 12.46%, P = 0.29). The efficacy and safety outcomes tended to decrease non-significantly in low dose NOACs than in common dose NOACs or warfarin (0.85% vs. 1.84% vs. 2.98% in efficacy outcome, P = 0.69; and 6.97% vs. 13.29% vs. 12.46% in safety outcome, P = 0.34).
Conclusions
NOACs were highly effective for prevention of stroke or systemic embolism in Asian octogenarian AF patients. However, major bleeding occurred excessively high in both anticoagulant groups. Further study is required on the optimal anticoagulant regimen in octogenarian population.
Keywords: Anticoagulants, Atrial fibrillation, Efficacy, Octogenarian, Safety
1. Introduction
The incidence of atrial fibrillation (AF) and AF-related strokes increase with age.[1]–[3] Warfarin has been clearly shown to effectively prevent thromboembolic events in patients with AF, especially in elderly patients.[4]–[6] However, warfarin therapy is often underutilized in elderly patients due to a higher risk of bleeding and poor tolerability.[7]
Recently, non-vitamin K antagonist oral anticoagulants (NOACs) such as dabigatran and rivaroxaban have been shown to be non-inferior or even superior to warfarin for long-term stroke prevention in patients with non-valvular AF.[8],[9] A recent meta-analysis reported that NOACs did not cause excess bleeding and were associated with equal or greater efficacy than warfarin therapy in elderly patients (≥ 75 years).[10] Moreover, subgroup analysis of major clinical trials in Asian populations showed even better results for NOACs in terms of bleeding complications.[11],[12] Therefore, it is expected that NOACs would be an effective alternative to warfarin, or even a better option, in patients with a higher risk of bleeding. As societies worldwide have aged, the efficacy and safety of NOACs in extremely elderly patients or octogenarians have emerged as an important area of research. As renal function decreases with age and creatinine levels poorly reflect renal function in elderly patients, the bleeding risk of NOACs could be substantial in this extreme age group. Our present study aimed to evaluate the efficacy (prevention of stroke or systemic embolism) and safety (risk of major bleeding) of NOACs and warfarin in Korean octogenarian patients in a real-world setting.
2. Methods
2.1. Study subjects
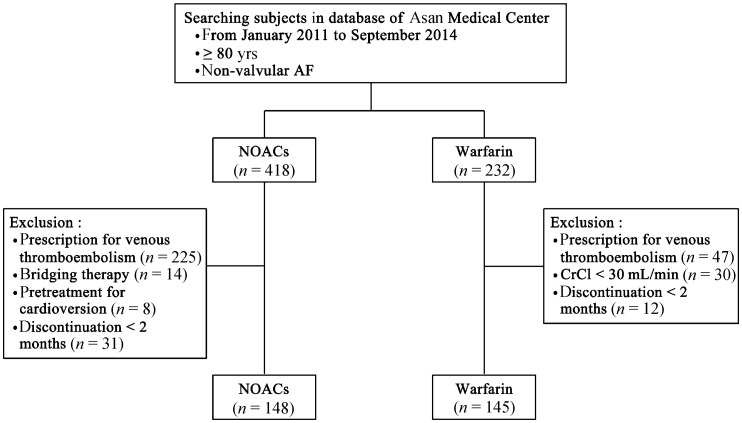
We performed a systematic computerized search of our hospital's database (Asan Biomedical Research Environment), and selected patients who were ≥ 80 years old with non-valvular AF (418 patients on NOACs and 232 patients on warfarin) at Asan Medical Center between January 2011 and September 2014. Patients receiving anticoagulant therapy for the purpose of primary or secondary prevention of ischemic stroke or systemic embolism were solely selected. Non-valvular AF was defined as rhythm disturbance occurring in the absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair.[13] Patients were excluded if they were taking anticoagulants for other purposes including prevention or treatment of pulmonary thromboembolism or deep vein thrombosis; if they had bridging therapy with NOAC or pretreatment before cardioversion; if they did not have at least two months of follow-up after medication; if they had a creatinine clearance (CrCl) of < 30 mL/min. CrCl was calculated with the use of the Cockcroft–Gault formula.
Finally, 148 patients were enrolled into the NOACs group and 145 patients were enrolled into the warfarin group, respectively (Figure 1). All patients were treated with NOACs (148 patients, 50.5%) or warfarin [target international normalized ratio (INR): 2.0–3.0; 145 patients, 49.5%] according to the patient's or physician's preference. Of the 148 patients that received NOACs, 52 (35.1%) received dabigatran [48 on a low-dose (110 mg twice a day) and 4 on a common dose (150 mg twice a day)] and 96 (64.9%) received rivaroxaban [32 on a low dose (15 mg once daily) and 64 on a common dose (20 mg once daily)]. Apixaban was not included in our analysis because it was not available in our hospital during the study period. Types (dabigatran or rivaroxaban) and doses (low dose or common dose) of NOACs were prescribed at the discretion of the attending physicians. The study was approved by the institutional review board of Asan Medical Center.
Figure 1. Recruitment of study subject.
CrCl: creatinine clearance; NOACs: non-vitamin K antagonist oral anticoagulants.
2.2. Efficacy and safety outcomes
The efficacy outcome was the composite of stroke or systemic embolism. Stroke was defined as the sudden onset of a focal neurological deficit lasting at least 24 h. Systemic embolism was defined as acute vascular occlusion of an extremity or major organ documented at the time of autopsy, angiography, or vascular imaging.
The safety outcome was the incidence of major bleeding. Major bleeding was defined as (1) fatal bleeding, (2) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, or pericardial, or intramuscular with compartment syndrome, and/or (3) bleeding causing a fall in hemoglobin level of ≥ 2 g/dL or leading to the transfusion of ≥ 2 units of whole blood or red cells.[14]
Two investigators (Kwon CH and Kim MS) reviewed all medical records of the study patients during follow-up and adjudicated all stroke, systemic embolism, and bleeding events that contributed to the pre-specified outcomes. The efficacy and safety outcomes for the warfarin and NOACs groups were compared. Event rates are presented as event numbers per 100 patient-years of follow-up.
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 21 (SPSS Inc., Chicago, IL), and data are expressed as the mean ± SD (continuous variables) or as frequency (categorical variables). When comparing baseline variables between the NOACs and warfarin groups, continuous variables were compared using the Student's t test and Mann-Whitney U test, and categorical variables were compared using the chi-square test or Fisher's exact test. Hazard ratios (HRs) and P values were calculated by the use of multivariable Cox's regression analysis that included sex, anticoagulant type, concomitant antiplatelet use, and HAS-BLED score. The Kaplan-Meier method was used to estimate the survival-free rate of major bleeding between the treatment groups. Additionally, the survival rates were compared using the log-rank test. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Study subjects
The baseline characteristics of the study patients are listed in Table 1. Patients in the NOACs group were significantly older than those in the warfarin group. In addition, the HAS-BLED scores—as determined by previous bleeding history, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, and drug or alcohol use— were significantly higher in the patients in the NOACs group than in those in the warfarin group. The overall patient follow-up duration was 375 patient-years at risk. The median follow-up duration was 10.9 months (interquartile range, 5.2–21.2 months) for the NOACs group and 12.8 months (interquartile range, 5.3–24.9 months) for the warfarin group. The mean CHA2DS2 VASc score was 4.7 for both the NOACs and warfarin groups. A total of 29 patients (9.9%) had concomitant antiplatelet agent with none on dual antiplatelet therapy. There was no significant difference in number of patients on concomitant antiplatelet agents between NOACs group (n = 15, 10.1%) and warfarin group (n = 14, 9.7%). Mean INR of patients in the warfarin group was measured 2.1 ± 1.5 at the last follow-up.
Table 1. Baseline characteristics of patients treated with either NOACs or warfarin.
| Total (n = 293) | NOACs (n = 148) | Warfarin (n = 145) | P value | |
| Follow-up duration, months | 12.3 (5.3–23.0) | 10.9 (5.2–21.2) | 12.8 (5.3–24.9) | 0.047 |
| Age, yrs | 83.7 ± 3.4 | 84.2 ± 3.5 | 83.2 ± 3.1 | 0.006 |
| Female | 175 (59.7%) | 89 (60.1%) | 86 (59.3%) | 0.886 |
| Body mass index, kg/m2 | 24.1 ± 3.6 | 24.4 ± 3.6 | 23.7 ± 3.6 | 0.082 |
| Type of arrhythmia | 0.296 | |||
| Paroxysmal atrial fibrillation | 117 (39.9%) | 59 (39.9%) | 58 (30.0%) | |
| Persistent atrial fibrillation | 170 (58.0%) | 84 (56.8%) | 86 (59.3%) | |
| Atrial flutter | 6 (2.0%) | 5 (3.4%) | 1 (0.7%) | |
| Concomitant antiplatelet agent | 29 (9.9%) | 15 (10.1%) | 14 (9.7%) | 0.891 |
| Congestive heart failure | 56 (19.1%) | 27 (18.2%) | 29 (20.0%) | 0.702 |
| Hypertension | 216 (73.7%) | 107 (72.3%) | 109 (75.2%) | 0.576 |
| Diabetes mellitus | 81 (27.6%) | 38 (25.7%) | 43 (49.5%) | 0.446 |
| Previous stroke/SE | 123 (42.0%) | 68 (45.9%) | 55 (37.9%) | 0.165 |
| Previous bleeding | 52 (17.7%) | 41 (27.7%) | 11 (7.6%) | < 0.0001 |
| Previous MI | 16 (5.5%) | 6 (4.1%) | 10 (6.9%) | 0.284 |
| Previous CAD | 61 (20.8%) | 32 (52.5%) | 29 (47.5%) | 0.732 |
| CHA2DS2 VASc score | 4.7 ± 1.4 | 4.7 ± 1.4 | 4.7 ± 1.4 | 0.745 |
| HAS-BLED score | 2.5 ± 0.9 | 2.6 ± 1.0 | 2.4 ± 0.9 | 0.034 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.239 |
| Creatinine clearance, mL/min | 52.1 ± 15.8 | 51.0 ± 13.9 | 53.1 ± 17.4 | 0.259 |
Values are expressed as the mean ± SD, median (interquartile range), or n (%). CAD: coronary artery disease; CHA2DS2 VASc score: cumulative score of congestive heart failure, hypertension, age (≥ 75 years or ≥ 65 years), diabetes, stroke, vascular disease, and sex (female); eGFR: estimated glomerular filtration rate; HAS-BLED score: cumulative score of hypertension, abnormal renal and liver function, stroke, bleeding, labile INRs, elderly (e.g., age > 65 years), and drugs or alcohol; MI: myocardial infarction; NOACs: non-vitamin K antagonist oral anticoagulants; SE: systemic embolism.
3.2. Efficacy and safety outcomes
The efficacy and safety outcomes according to the treatment group are presented in Table 2 and Figure 2. None of the study patients experienced transient ischemic events. The incidence rates of stroke or systemic embolic events were low in both anticoagulant groups and there were no significant differences in stroke or systemic embolic events between the treatment groups. However, major bleeding events occurred in a significant numbers of patients treated with both NOACs and warfarin. Major bleeding events tended to be lower in the low dose NOACs group than the common dose NOACs or warfarin groups, but the difference was not statistically significant (Figure 2B).
Table 2. Efficacy and safety outcomes according to treatment groups.
| Low dose NOACs (n = 97) |
Common dose NOACs (n = 51) |
Warfarin (n = 145) |
P value | ||||
| No. of events | Event rate (No./100 patient-years) | No. of events | Event rate (No./100 patient-years) | No. of events | Event rate (No./100 patient-years) | ||
| Efficacy | |||||||
| Stroke or systemic embolism | 1 | 0.85 | 1 | 1.84 | 6 | 2.98 | 0.69 |
| Stroke | 1 | 0.85 | 1 | 1.84 | 5 | 2.47 | 0.83 |
| Systemic embolism | 0 | – | 0 | – | 1 | 0.49 | 0.88 |
| Safety | |||||||
| Major bleeding | 8 | 6.97 | 7 | 13.29 | 24 | 12.46 | 0.34 |
| Decrease in Hb ≥ 2 g/dL | 7 | 6.10 | 7 | 13.29 | 21 | 10.89 | 0.33 |
| Critical organ bleeding | 1 | 0.85 | 0 | – | 3 | 1.51 | 0.84 |
| Fatal bleeding | 1 | 0.85 | 0 | – | 2 | 0.99 | 0.97 |
P value between treatment groups was achieved for the overall follow-up duration by univariable Cox regression model. Hb: hemoglobin; NOACs: non-vitamin K antagonist oral anticoagulants.
Figure 2. The efficacy (A) and safety (B) outcomes according to low dose NOACs, common dose NOACs, and warfarin treatment.
NOACs: non-vitamin K antagonist oral anticoagulants.
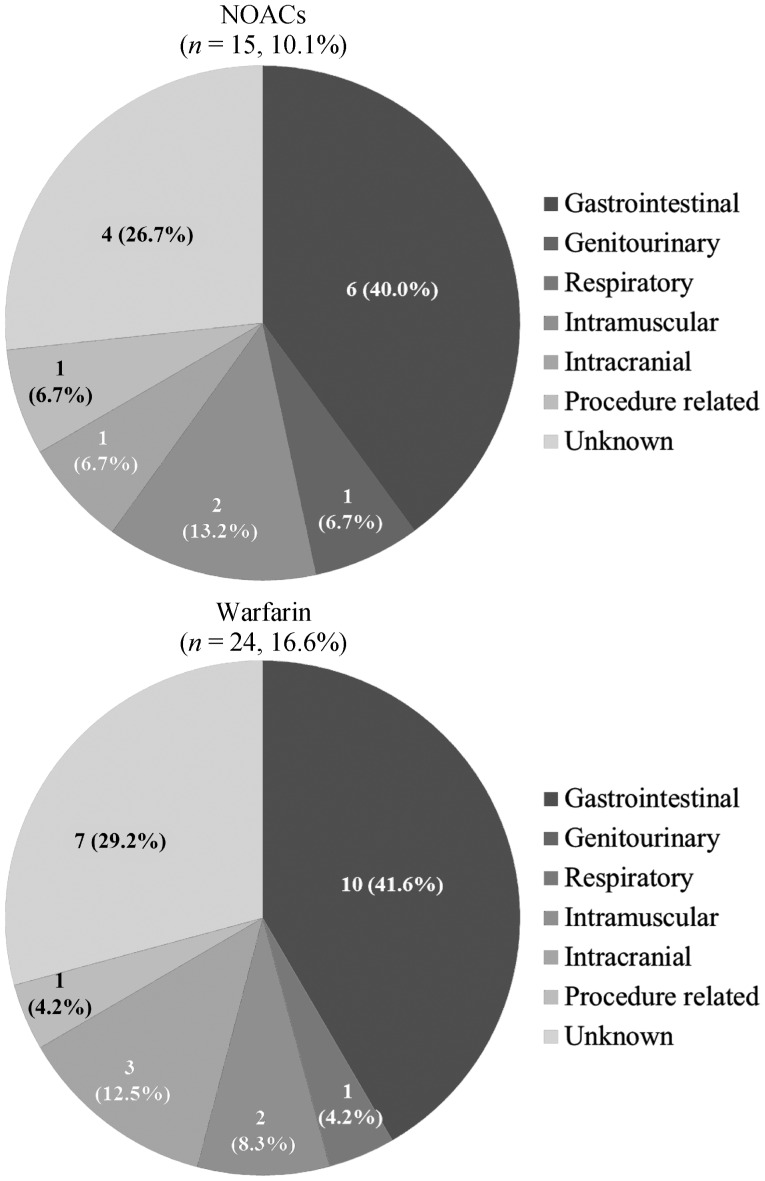
Major bleeding occurred in 15 patients (10.1%) in the NOACs group and 24 patients (16.4%) in the warfarin group (Figure 3). Gastrointestinal bleeding was the most common cause of major bleeding in both groups [6 patients (4.1%) in the NOAC group vs. 10 patients (6.8%) in the warfarin group]. Intracranial bleeding occurred in one patient (0.7%) in the NOAC group and three patients (2.1%) in the warfarin group. These results were not tested for statistical significance.
Figure 3. Sites of major bleeding.
NOACs: non-vitamin K antagonist oral anticoagulants.
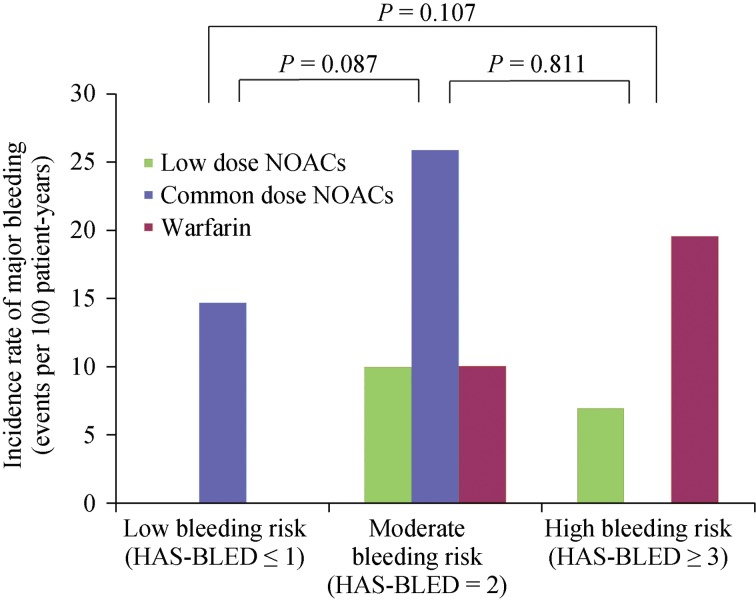
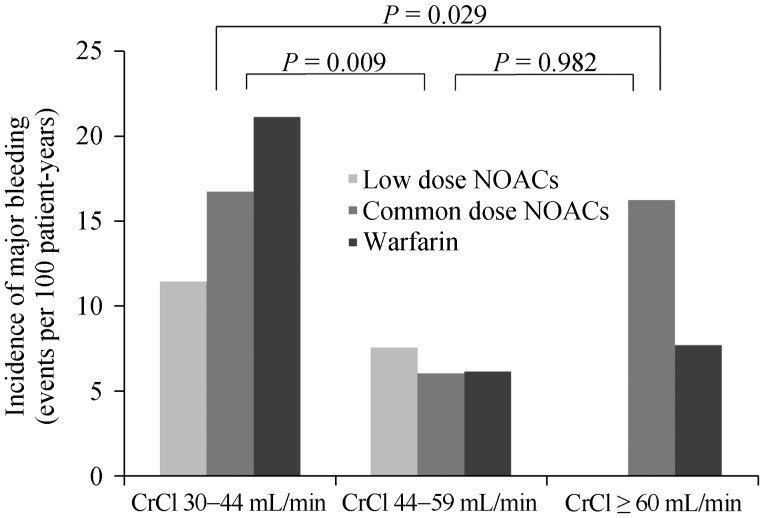
Major bleeding events tended to increase according to HAS-BLED scores but no statistically significant differences were shown (Online data Figure S1). Likewise, no significant difference was found between the patients with vs.. without concomitant antiplatelet agents (5/29, 17.2% vs. 34/264, 12.9%). Concomitant antiplatelet agent was not associated with an increased incidence of major bleeding in Cox regression analysis (HRs = 1.273, 95% CI: 0.497−3.263, P = 0.615). Among the 29 patients on antiplatelet therapy, major bleeding occurred in 2 out of 14 patients in the warfarin, 3 of 11 patients in the low dose NOACs, and no one (0/4 patients) in common dose NOACs group, respectively (Log rank, P = 0.572). On the other hand, major bleeding events significantly increased in patients with decreased renal function (CrCl: 30–44 mL/min) (Figure 4). Kaplan–Meier analysis revealed a significant difference in the incidence of major bleeding according to renal function (Figure 5).
Figure S1. The incidence rate of major bleeding according to HAS-BLED scores.
HAS-BLED: cumulative score of hypertension, abnormal renal and liver function, stroke, bleeding, labile INRs, elderly (e.g., age > 65 years), and drugs or alcohol; NOACs: non-vitamin K antagonist oral anticoagulants.
Figure 4. The incidence rate of major bleeding according to renal function.
CrCl: creatinine clearance; NOACs: non-vitamin K antagonist oral anticoagulants.
Figure 5. Kaplan-Meier curves showing the probability of survival free of major bleeding events during overall follow-up according to renal function.
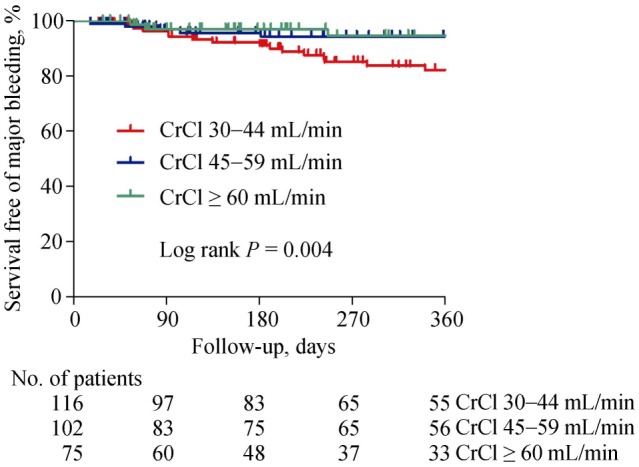
CrCl: creatinine clearance.
4. Discussion
Our present study is a retrospective analysis to compare NOACs with warfarin therapy for stroke prevention in Asian octogenarian patients with non-valvular AF. The major findings of our analyses are as follows: (1) the incidence rates of stroke or systemic embolism were low in octogenarian patients, and there was no significant difference in the efficacy outcomes (stroke or systemic embolism) between the NOACs and warfarin treatments (1.16% for NOACs vs. 2.98% for warfarin per 100 patient-years, P = 0.46); and (2) in contrast, major bleeding occurred in a significant number of patients with both NOACs (8.96 per 100 patient-years) and warfarin (12.46 per 100 patient-years) treatments, which was not significantly different between the two groups (P = 0.29).
4.1. Efficacy of NOACs and warfarin
In our present study series, stroke and systemic embolism event rates were low and were not significantly different between the NOACs and warfarin groups. Recent studies in Asian populations reported similar rates of stroke or systemic embolism, ranging from 1.26% to 2.6% per year with NOACs and from 2.61% to 3.4% per year with warfarin.[11],[12],[15] The mean age of the study population of the previous Asian studies was around 70 years, whereas the mean age of our study patients was 83.7 years. In addition, the patients in the NOACs group were significantly older than those in the warfarin group in this study. Taking these facts into consideration, NOACs appear to be not only non-inferior, but may also be more effective than warfarin at preventing embolic events in very old (≥ 80 years) AF patients. Moreover, a recent meta-analysis of elderly patients (≥ 75 years) that supported this assumption also reported that the incidence of stroke or systemic embolism was significantly lower with NOACs than warfarin therapy (3.3% vs. 4.7%; odds ratio = 0.65; 95% CI: 0.48–0.87).[10]
In this study, patients treated with low dose NOACs experienced numerically fewer stroke or systemic embolism events than those treated with common dose NOACs or warfarin. This result was consistent with those of previous studies. In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, the risks of stroke or systemic embolism were not statistically different between the two doses (110 mg and 150 mg) of dabigatran in elderly patients aged ≥ 75 years.[16] In a Japanese trial that used low-dose (15 mg once daily) rivaroxaban (mean age of study population, 71 years), there was a strong trend for a reduction in the rate of stroke/systemic embolism with low dose rivaroxaban vs. warfarin (HR = 0.49; P = 0.050).[15] A recent Chinese study suggested that low dose dabigatran achieved superior stroke risk reduction than warfarin in Chinese octogenarian patients with AF.[17] Therefore, low dose NOACs may be non-inferior than common dose NOACs or warfarin at preventing stroke or systemic embolism in East Asian octogenarians.
4.2. Safety of NOACs and warfarin
The risks of total bleeding and major bleeding were relatively high in our current study patients. Previous results of major bleeding risks in octogenarians treated with warfarin were inconsistent. One study found that the cumulative incidence of major hemorrhage for octogenarians was 13.1 per 100 patient-years, which was consistent with our present findings.[7] However, an Italian prospective observational study reported a low rate of major bleeding (1.87 per 100 patient-years) in octogenarians on warfarin.[18] In other recent sub-analyses of randomized trials, the risk of major bleeding was 4.37–4.40 per 100 patient-years on warfarin in patients aged ≥ 75 years.[16],[19] Bleeding risks in octogenarian patients taking NOACs have not been reported. Previous sub-analyses according to old age (≥ 75 years) reported that the incidence of major bleeding was 4.43–5.10 per 100 patient-years on NOACs.[16],[19] In Asian patients (mean age, about 70 years), the risk of major bleeding on NOACs was reported to be 2.99–3.59 per 100 patient-years.[11],[12],[15] Compared with these previous results, the risk of major bleeding on NOACs was about twice as high in our current study population. Given that our result was drawn from the real-world setting, the risk of major bleeding in octogenarian patients seems to be markedly high with both NOACs and warfarin.
The risks of major bleeding tended to be lower in patients treated with low dose NOACs than in those treated with common dose NOACs and warfarin therapy in our analysis. Moreover, the patients in our NOACs group were significantly older and had higher HAS-BLED scores and previous bleeding history than those in the warfarin group. Based on these results, low dose NOACs may not only be non-inferior, but may be superior to common dose NOACs or warfarin for preventing major bleeding in frail patients such as octogenarians. A simple laboratory test with a capability of indicating the effects of different dosages of NOACs or gauging the degree of anticoagulation would be of great help to adjust dosage, especially at the beginning of NOAC treatment.
In the sub-analysis, major bleeding risks tended to be higher in patients with moderate-to-high bleeding risk (HAS-BLED score ≥ 2) than in those with low bleeding risk (HAS-BLED score ≤ 1). However, major bleeding was significantly higher in patients with stage 3B chronic kidney disease (CrCl 30–44 mL/min) than in other less decreased renal function groups. Therefore, circumspection is needed when prescribing NOACs and warfarin to patients with decreased renal function.
Although warfarin is effective for the prevention of stroke and systemic embolism in AF, advanced age and Asian ethnicity are associated with a greater risk for warfarin-related bleeding, especially intracranial hemorrhage.[20]–[22] Our current analysis also showed an increased tendency for major bleeding with warfarin compared with NOACs. Therefore, NOACs would be an attractive alternative to warfarin for achieving fewer thromboembolic and bleeding events in Asian octogenarian patients.
4.3. Limitations of the study
Our present study had several limitations. First, our analysis was retrospective. Second, the number of patients analyzed was relatively small and the follow-up period was relatively short. Third, different kinds and doses of NOACs were prescribed for our study patients. Thus, we could not compare the efficacy and safety between different NOACs. Of note, apixaban was not included in our analysis. Considering previous results of apixaban on the superior safety outcomes, bleeding in our patient cohort might have been different if apixaban was available. However, as there has been no head-to-head comparison using different NOACs, it is difficult to extrapolate individual NOACs results to comparison of different NOACs. Fourthly, we could not test patient's adherence to prescribed anticoagulant regimen. Finally, we did not calculate time-in-therapeutic range for the warfarin group, and thus could not evaluate the appropriateness of warfarin therapy. However, our study largely reflects the current status of “real-world” anticoagulation practice, and deserves its significance. Despite these limitations, our findings are meaningful because they reveal the incidence of stroke or systemic embolic events and major bleeding risks in NOACs or warfarin groups in a real-world clinical setting for Asian octogenarian patients.
4.4. Conclusions
The incidence rates of stroke or systemic embolism are low in Asian octogenarian AF patients treated with warfarin or NOACs. However, major bleeding events can be excessively high in both anticoagulant groups, especially in patients with decreased renal function. The efficacy and safety outcomes tended to decrease non-significantly in patients with low dose NOACs than in those treated with common dose NOACs or warfarin therapy. In the future, a well-designed, prospective study on the optimal anticoagulant regimen (low dose regimen and new criteria for prescription of low dose NOACs) is required in elderly Asian population, especially in patients with impaired renal function.
Acknowledgments
The results of the present study were presented in the 8th Asia Pacific Heart Rhythm Society Scientific Sessions in Melbourne, Australia.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 6.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 10.Sardar P, Chatterjee S, Chaudhari S, et al. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 11.Wong KS, Hu DY, Oomman A, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 12.Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 16.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 17.Chan PH, Huang D, Hai JJ, et al. Stroke prevention using dabigatran in elderly Chinese patients with atrial fibrillation. Heart Rhythm. 2016;13:366–373. doi: 10.1016/j.hrthm.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Poli D, Antonucci E, Testa S, et al. Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation. 2011;124:824–829. doi: 10.1161/CIRCULATIONAHA.110.007864. [DOI] [PubMed] [Google Scholar]
- 19.Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) Circulation. 2014;130:138–146. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 20.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 21.Rosand J, Eckman MH, Knudsen KA, et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 22.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]