Abstract
There has been a significant increase in the number of patients receiving cardiovascular implantable electronic devices (CIED) over the last two decades. CIED infection represents a serious complication after CIED implantation and is associated with significant morbidity and mortality. Recently, newly advanced technologies have offered attractive and suitable therapeutic alternatives. Notably, the leadless pacemaker and anti-bacterial envelope decrease the potential risk of CIED infection and the resulting mortality, when it does occur. A completely subcutaneous implantable cardioverter defibrillator is also an alternative to the transvenous implantable cardioverter defibrillator (ICD), as it does not require implantation of any transvenous or epicardial leads. Among the patients who require ICD removal and subsequent antibiotics secondary to infection, the wearable cardioverter defibrillator represents an alternative approach to inpatient monitoring for the prevention of sudden cardiac death. In this review paper, we aimed to introduce the advanced technologies and devices for prevention of CIED infection.
Keywords: Cardiac implantable electronic devices infection (CIED) infection, Wearable cardioverter defibrillator (WCD), Anti-bacterial envelope, Subcutaneous implantable cardioverter defibrillator (S-ICD), Leadless pacemaker
1. Introduction
There has been a significant increase in the number of patients receiving cardiovascular implantable electronic devices (CIED) over the last two decades [1], [2]. This is largely owing to the expanding indications of CIED based on technological improvements and new evidence demonstrating improved survival and quality of life among certain groups of patients having structural heart diseases [3], [4]. However, the advantage of these devices is limited by associated adverse events and complications. CIED infection represents a serious complication of cardiac device therapy and is associated with significant morbidity and mortality. Despite appropriate care, in-hospital mortality among patients admitted because of CIED infection ranges from 4% to 10% and one-year mortality from 15% to 20% [5], [6], [7], [8], [9], [10], [11].
The majority of patients with CIED infection have pocket and/or endovascular lesions (Fig. 1). If aggressive antibiotic therapy fails to control CIED infection, then complete removal of the device is recommended in many instances [2], [6]. The timing of re-implantation is another critical issue. An early re-implantation should be performed in patients who are solely dependent on the CIED; however, at least one week is required to control local or systemic bacterial infections [12]. Currently, the advanced technologies may contribute to a decrease in infection risk and mortality and may bridge the critical period between device removal and re-implantation.
Fig. 1.
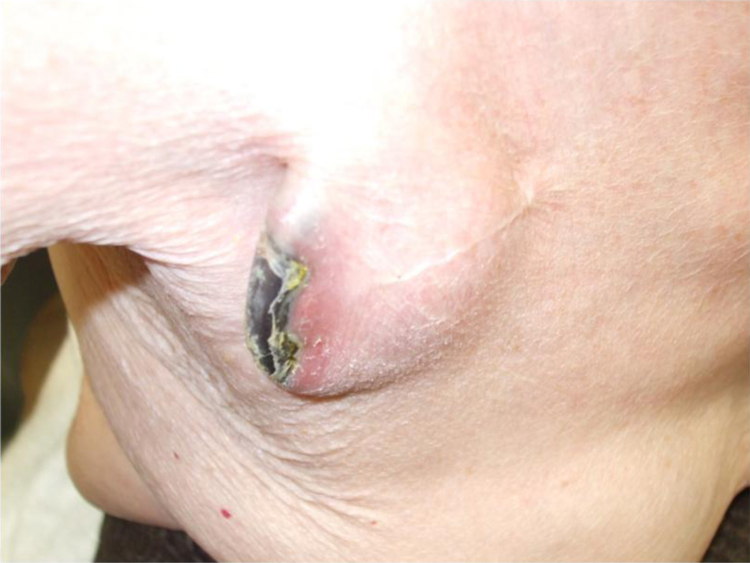
A case of cardiac implantable electronic device infection.
2. New technologies to reduce the risk of CIED infection
In the USA and Europe, some new alternatives to prevent CIED infection are available. The leadless pacemaker and antibacterial envelope represent attractive and suitable therapeutic options to minimize the risk of CIED infection.
2.1. Leadless pacemaker
To reduce the complications associated with the standard transvenous electrode lead of the pacemaker, a leadless pacemaker has been invented. The concept of a completely self-contained VVIR intracardiac pacemaker, first explored about 45 years ago by Spickler JW et al., has finally become a reality with the development of the Nanostim™ Leadless Pacemaker (St Jude Medical, Inc., St. Paul, MN, USA) and the Micra™ Transcatheter Pacing System (Medtronic plc) for use in humans [13], [14], [15], [16]. Technological advances in electronics miniaturization and battery chemistries have enabled creation of a device small enough to be implanted within the heart via a percutaneous, transvenous approach, while still providing similar battery longevity without leads. The leadless pacemaker has been expected to reduce CIED infections, because this system has no physical connection between the endocardium and the subcutaneous pocket, which are the most likely source and channel of bacterial infection, respectively. Furthermore the leadless stand-alone system never produces subclavian or supra vena-cava occlusions. Both systems have received the CE Mark in Europe, but are not approved in the USA.
The Nanostim system is delivered to the implant site at the lower septum of the right ventricle (RV) via a transfemoral route and allows for bradycardia pacing via a miniature pulse generator with a built-in battery and electrodes that can be entirely and permanently implanted (Fig. 2). The first successful Nanostim implantation in humans took place in December 2012 in Prague, Czech Republic. Recently, a nonrandomized first-in-human study demonstrated this system to be safe and feasible over a 90-day period [15]. This preclinical study expanded on the previous study by demonstrating that the pacing and sensing properties remain adequate for up to 18 months. In addition, the histological analyses at the 90-day mark revealed a limited local response to the implanted device at the RV apex. Furthermore, there were no significant adhesions between the device and the RV walls. These pathological features may have important implications related to the long-term efficacy and safety of this system, as well as for designing approaches to extract the device.
Fig. 2.
The Nanostim™ Leadless Pacemaker (St Jude Medical, Inc., St. Paul, MN, USA) Reprinted with permission from St Jude Medical, Inc.
The Micra system, similar to the Nanostim system, is an investigational device and is being assessed in a pivotal global clinical trial. The miniaturized device is only one-tenth the size of a conventional pacemaker (Fig. 3). The Micra system is also delivered directly into the heart through a catheter inserted in the femoral vein. Once positioned, the pacemaker is securely attached to the heart wall in the RV and can be repositioned or retrieved during implantation if needed (Fig. 4). The device does not require the use of leads and is attached via small tines securing it to the heart wall. The first successful in-human Micra implantation occurred in December 2013 in Linz, Austria. It is currently being evaluated in the Medtronic Micra Transcatheter Pacing System (TPS) Global Clinical Trial, which is a single-arm, multicenter study that will enroll up to 780 patients at approximately 50 centers [16].
Fig. 3.

The Micra™ Transcatheter Pacing System (Medtronic plc). Reprinted with permission from Medtronic plc.
Fig. 4.
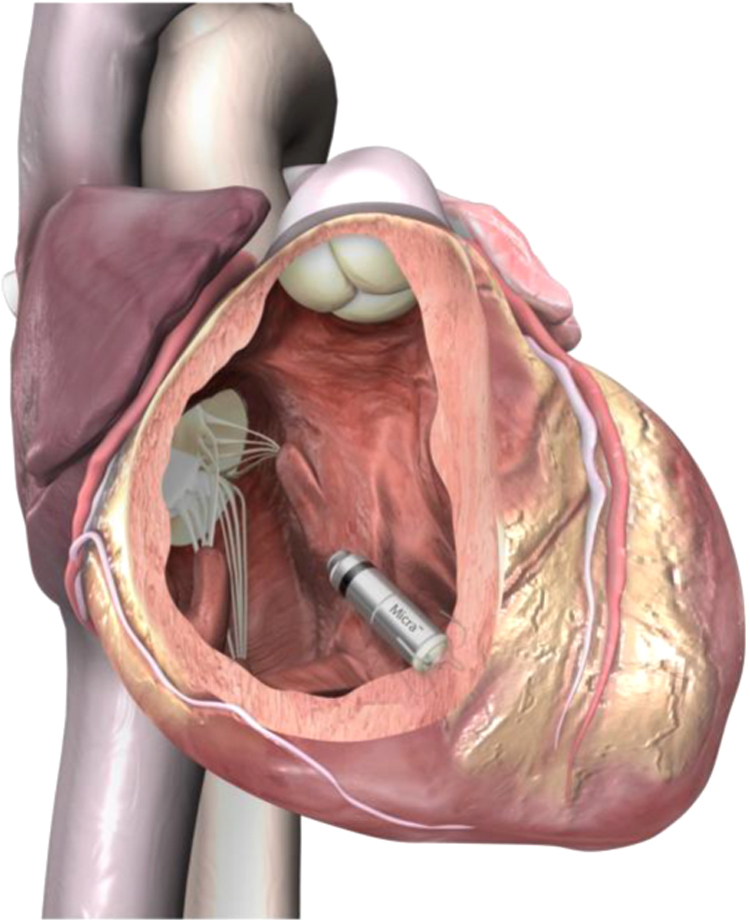
Micra inside the right ventricle. Reprinted with permission from Medtronic plc.
Both systems allow for retrievability, if needed; however, there are significant differences in the designs that are worth noting: (i) the Micra device has an active fixation mechanism consisting of four electrically-inactive extendable and retractable tines to anchor it to the cardiac tissue, whereas the Nanostim device uses an electrically active fixed helix, (ii) the Micra device is wider (20 Fr) and shorter (25.9 mm) than the Nanostim pacemaker (18 Fr and 41.4 mm), (iii) the Micra pacemaker׳s communication between the device and programmer is established using a standard programming head, whereas the Nanostim pacemaker communicates with the St. Jude Medical Merlin™ Patient Care System using a programmer link and surface electrocardiographic electrodes, and (iv) the Micra device uses a three-axis accelerometer for rate response, whereas the Nanostim pacemaker utilizes a blood temperature sensor.
The most relevant limitation is that the current Nanostim and Micra devices are indicated for patients requiring a single-chamber pacemaker only, limiting their use to a relatively small percentage of patients. Current indications focus on patients with chronic atrial fibrillation and second- or third-degree atrioventricular block, patients with sinus rhythm with second- or third-degree atrioventricular block and a low level of physical activity or short expected lifespan, and patients with sinus bradycardia with infrequent pauses or unexplained syncope.
2.2. Anti-bacterial envelope
Previously published randomized controlled studies indicate that perioperative intravenous administration of a cephalosporin antibiotic can help to reduce CIED infections [2], [18]. The European Society of Cardiology, American Heart Association, and Heart Rhythm Society recommendations for prophylaxis at the time of CIED placement consist of an antibiotic that has in-vitro activity against staphylococci. In recent large studies, the vast majority of patients received antimicrobial prophylaxis with CIED placement [19], [20]. Despite widespread use of antimicrobial prophylaxis, CIED infection rates are increasing faster than implantation rates [21]. Effective antimicrobial prophylaxis could help reduce CIED infections and improve clinical outcomes.
The TYRX™ Antibacterial Envelope (Medtronic plc) consists of a Food and Drug Administration (FDA)-approved surgical mesh envelope that releases minocycline and rifampicin in the generator pocket after implantation with a CIED (Fig. 5) [17]. The biocompatible mesh is coated with antibiotics that elute (dissolve) within an approximately 7-day period. The TYRX™ is of two types, with substrate meshes that are 100% absorbable or non-absorbable. A recent report suggests that the use of the TYRX™ absorbable envelope was associated with a very low prevalence (0%) of CIED-related infections that was comparable to that seen with the non-absorbable envelope. However, data from a randomized clinical trial are needed to support increased use of the antibacterial envelope [22]. Both TYRX™ Antibacterial Envelopes are sterile devices constructed of an open-pore weave, knitted filaments of a lightweight mesh, and an absorbable polymer coating impregnated with antimicrobial agents. The antibacterial envelope is indicated for holding CIEDs, thereby creating a stable environment surrounding the device and leads after surgical placement.
Fig. 5.
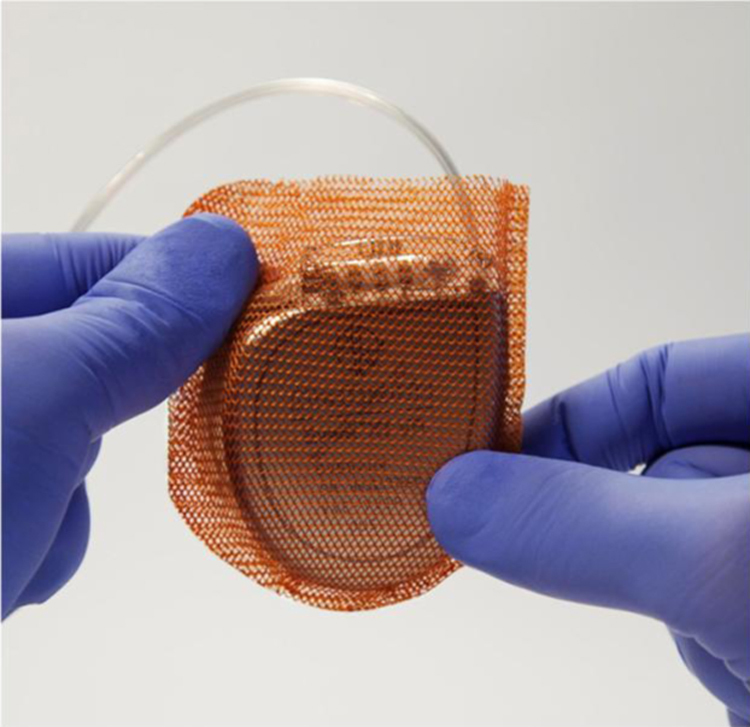
The TYRX™ Antibacterial Envelope (Medtronic plc). Reprinted with permission from Medtronic plc.
At least one-half to two-thirds of CIED infections are caused by Staphylococcus aureus (S. aureus) and coagulase-negative staphylococcus species (CoNS) [18], [20], [23], [24], [25]. In vitro, methicillin-resistant strains of S. aureus and many strains of CoNS are susceptible to a combination of two antibiotics with distinct mechanisms of actions: minocycline and rifampicin [26], [27], [28]. Rifampicin is bacteriostatic and inhibits DNA-dependent RNA polymerase. Minocycline is bacteriostatic and inhibits protein synthesis. Minocycline has an antimicrobial spectrum against a wide range of gram-positive and gram-negative organisms. Rifampin is a semi-synthetic compound derived from Amycolatopsis rifamcinica and has antimicrobial activity against select gram-positive and gram-negative organisms. The substrate mesh varies between the two types of envelopes and consists of either polypropylene or Glycoprene II. Polypropylene has been utilized in surgically implanted medical devices for decades. The most common use is in hernia repairs.
The absorbable tyrosine-based polymer coating is designed to degrade to well-characterized natural metabolites. It has been demonstrated to resorb benignly, in the same manner as absorbable surgical sutures, while eliciting a minimal inflammatory response. It also has a long history of use with other FDA-approved implantable medical devices. Randomized controlled trials demonstrated that coating or impregnating catheters with the combination of rifampin and minocycline significantly reduces device-associated infections of central venous, hemodialysis, and cerebrospinal fluid drain catheters, especially infections with S. aureus and CoNS [29], [30], [31], [32], [33], [34], [35].
Preclinical studies demonstrated that the antibacterial envelope helped reduce the risk for infection by several pathogens, including Staphylococcus epidermidis, within CIED implant pockets [36]. A previous large clinical study indicated that the envelope is associated with a high rate of successful CIED implantation and a low risk of infection in a population at significant risk for CIED infection. Furthermore, standard use of an antibacterial envelope was associated with a significantly lower rate of CIED infection and appeared to be economically viable [37].
3. New technology for prevention of fatal CIED infection
It is widely accepted that complication rates are higher with re-implantations, particularly if a lead implantation or revision is involved [38], [39]. In addition, morbidity and mortality is particularly high in patients with an infected transvenous implantable cardioverter defibrillator (transvenous-ICD) system, especially when a systemic infection or endocarditis is present.
The risk of reinfection following system re-implantation is also a concern [40], [41].
A completely subcutaneous ICD was developed as an alternative to the transvenous-ICD system, as it is implanted without any transvenous or epicardial leads. The rate of infections resulting in explantation or revision of this new device was not lower than that reported in previous ICD registries. However, it should be emphasized that none of the documented device infections were systemic [42].
3.1. Complete subcutaneous implantable cardioverter defibrillator
The completely subcutaneous ICD system (S-ICD™ System, Boston Scientific Corp., Marlborough, MA, USA) was developed to provide life-saving defibrillation therapy while leaving the heart and vasculature untouched [43]. The S-ICD system is preferred over transvenous-ICD for patients having no vascular access, a history of recurrent transvenous lead infections, or primary electrical disease with ventricular fibrillation as the major life-threatening rhythm. The first pilot-phase human studies of the S-ICD commenced in 2008, followed by subsequent regulatory and post-marketing studies. Approved by the FDA in September 2012, to provide defibrillation therapy for the treatment of ventricular tachyarrhythmias, the S-ICD system was developed after 10 years of defibrillation and sensing research, acute human feasibility studies, and long-term clinical studies [43], [44], [45], [46], [47], [48], [49]. This system demonstrated a very high shock efficacy for spontaneous ventricular arrhythmias and a decreased incidence of inappropriate shocks [48].
The S-ICD system is comprised of a pulse generator, subcutaneous electrode, electrode-insertion tool, and device programmer. The pulse generator has an estimated longevity of 5 years and is slightly larger with a weight (145 g) approximately double that of a modern transvenous ICD generator [49]. It provides high-energy defibrillation shock (80 J) therapy through the use of a constant-tilt biphasic form. In addition, the new generation S-ICD System (EMBLEM™ [Fig. 6]), which is 20% thinner and is projected to last 40% longer than the previous S-ICD system, is available in a small number of centers in Europe and USA. This system is also enabled for remote patient management for increased patient convenience. The generator is placed subcutaneously in a left lateral position over the 6th rib between the midaxillary and anterior axillary lines. Via two parasternal incisions, a 3 mm tripolar parasternal electrode (polycarbonate urethane) is positioned parallel to and 1 to 2 cm to the left of the sternal midline with the distal sensing electrode localized adjacent to the manubriosternal junction and the proximal sensing electrode positioned adjacent to the xiphoid process (Fig. 7).
Fig. 6.
The entirely subcutaneous ICD system (EMBLEM™ S-ICD System, Boston Scientific Corp., Marlborough, MA, USA). Image provided courtesy of Boston Scientific. © 2015 Boston Scientific Corporation or its affiliates. All rights reserved.
Fig. 7.
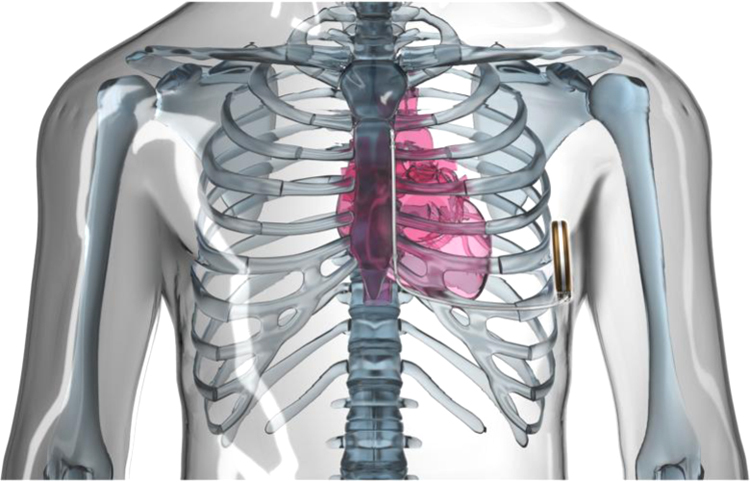
The entirely subcutaneous ICD system with the pulse generator implanted subcutaneously in a left lateral position and the parasternal lead-electrode positioned parallel to and 1 to 2 cm to the left of the sternal midline. The lead-electrode contains 2 sensing electrodes separated by an 8 cm shocking coil. Image provided courtesy of Boston Scientific. © 2015 Boston Scientific Corporation or its affiliates. All rights reserved.
A population-based decrease in mortality with a new device is paramount, but can be negated if the implant is associated with a higher risk of removal due to pocket infection. Infection without any bacteremia remained the most common complication requiring invasive action in the early experience with the S-ICD [44], [45], [46]. Many steps were taken to mitigate this risk and prevent device removal, including better operative preparation training and techniques and aggressive management of skin infections [45]. Advances in implantation techniques were introduced in the literature by Knops et al. in an effort to reduce the incisional surface area and resulting infection risk [50]. Advances in operator experience, preparation, and implantation techniques appear to have positively affected the rates of infection, as use of the S-ICD system has expanded worldwide. In recent studies, the simplicity of implantation which avoids vascular access was reflected in the very low rate (2%) of acute major complications such as device system infection [48]. The S-ICD could be a new alternative to the conventional transvenous-ICD system to minimize device system infections.
The limitations of the current S-ICD include its inability to provide anti-tachycardia pacing for ventricular tachycardia, limited bradycardia pacing support, and absence of endovascular monitoring capabilities for collateral data gathering such as impedance monitoring for chronic heart failure. One estimate of potential candidates for the S-ICD includes every patient indicated for primary SCD prevention without a pacing indication. In addition, the use of a subcutaneous sensing electrode with the S-ICD may theoretically increase the risk of over-sensing noise or myopotential signals and under-sensing low-amplitude cardiac signals during ventricular fibrillation. The previous trial compared the arrhythmia detection of 3 commercially available transvenous ICD lead systems with the S-ICD electrode [51]. All devices excelled in detecting ventricular tachyarrhythmia (100%); however, the S-ICD demonstrated greater specificity in discriminating supraventricular from ventricular tachycardia (98% S-ICD vs. 76.7% single-chamber transvenous-ICD vs. 68% dual-chamber transvenous-ICD). Ideally, greater user programming experience and improvements in S-ICD technology may reduce the rate of inappropriate shocks.
4. New technology to reduce the risk of sudden cardiac death after the removal of ICD: a wearable cardioverter defibrillator
ICD therapy has been established as a cornerstone of cardiology practice for reducing the incidence of SCD [52], [53], [54], [55]. Unfortunately, ICD system infection represents a complication that occurs even in experienced centers. Among patients who require ICD removal and subsequent antibiotic therapy, a wearable cardioverter defibrillator (WCD; LifeVest WCD4000, ZOLL, Pittsburgh, PA, USA) represents an alternative approach to prevention of SCD. Removal of the ICD deprives the patient of the protection against potential life-threatening ventricular tachyarrhythmias, particularly in patients with ICD implantation for secondary prevention of SCD. The Heart Rhythm Society recommends the use of WCD as a bridge to ICD re-implantation when the ongoing infection is a concern [56].
The WCD was introduced into clinical practice in 2002, and indications for its use are expanding currently. It has been in use worldwide, especially in the USA and Germany [57], [58].
This device consists of an external defibrillator vest that automatically detects and treats ventricular tachyarrhythmias without bystander assistance [59], [60]. A WCD is composed of a garment containing two defibrillation patch electrodes on the back, an elastic belt with a front-defibrillation patch electrode, and four non-adhesive ECG electrodes connected to a monitoring and defibrillation unit (Fig. 8). Recent trials demonstrated the efficacy of the WCD in the detection and treatment of lethal ventricular arrhythmias [57], [61]. The WCD therapy can prevent SCD until ICD re-implantation is feasible in patients who underwent device removals for device system infections [62].
Fig. 8.
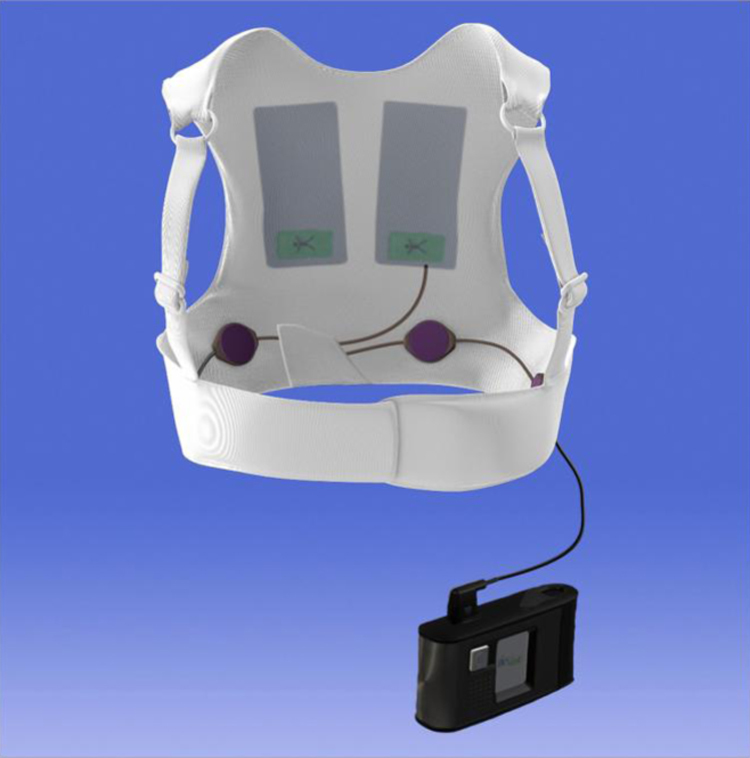
The wearable cardioverter defibrillator (LifeVest WCD4000, ZOLL, Pittsburgh, PA, USA). Adopted from ZOLL homepage (http://lifevest.zoll.com/).
The efficacy of the WCD in the prevention of arrhythmic SCD would seem to be highly dependent on patient compliance. Proper instruction on the use of WCD is also important to avoid inappropriate shocks. In previous studies, inappropriate shock is a rare event that occurs in 0–3% of patients using WCD [63], [64]. Shocks may be inappropriately delivered due to noise, device malfunction, or the rate criteria. A WCD is a unique tool designed to avoid unnecessary shock therapy. If a persistent arrhythmia is detected, the WCD notifies the patient via a “responsiveness test,” allowing a conscious patient to prevent treatment. A conscious patient can hold the “response buttons” during the “responsiveness test” to prevent an unnecessary treatment. Therefore, much attention is being paid to the provision of medical education and information to patients in order to optimize their understanding and acceptance of the WCD therapy [59].
The strategy for re-implantation after removal of an ICD must be individualized to each patient and clinical situation. For many patients, continuous inpatient/outpatient monitoring may be impossible or at least highly undesirable. The WCD is a cost-effective alternative to protect patients against SCD following the removal of an infected ICD while waiting for ICD re-implantation, as compared to keeping patients in the hospital or discharging them to go home or to a skilled nursing facility [12].
5. Conclusion
CIED system infection represents a relevant complication after CIED implantation and is associated with a significant risk of morbidity and mortality. However, newly developed technologies and devices represent attractive and suitable therapeutic options to reduce the incidence of this increasing problem.
Conflict of interest
Dr. Kondo has received a research grant from St. Jude Medical, Biotronic. Dr. Ueda has received an unrestricted educational donation from St. Jude Medical. Dr. Kobayashi has received a research grant from Medtronic, St. Jude Medical, Boston Scientific, and Biotronik. Dr. Schwab has received speaking honoraria and research support from Medtronic, Biotronik, St. Jude Medical, Boston Scientific, and Sorin.
References
- 1.Goldberger Z., Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. J Am Med Assoc. 2006;295:809–818. doi: 10.1001/jama.295.7.809. [DOI] [PubMed] [Google Scholar]
- 2.Baddour L.M., Epstein A.E., Erickson C.C. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 3.Hayes D.L., Furman S. Cardiac pacing: how it started, where we are, where we are going. Pacing Clin Electrophysiol. 2004;27:693–704. doi: 10.1111/j.1540-8159.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 4.Epstein A.E., Dimarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Kolek M.J., Dresen W.F., Well Q.S. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. Pacing Clin Electrophysiol. 2013;36:354–361. doi: 10.1111/pace.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarakji K.G., Chan E.J., Cantillon D.J. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Uslan D.Z., Sohail M.R., St, Sauver J.L. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–675. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]
- 8.Baman T.S., Gupta S.K., Valle J.A. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009;2:129–134. doi: 10.1161/CIRCEP.108.816868. [DOI] [PubMed] [Google Scholar]
- 9.Athan E., Chu V.H., Tattevin P. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. J Am Med Assoc. 2012;307:1727–1735. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- 10.Le K.Y., Sohail M.R., Friedman P.A. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011;8:1678–1685. doi: 10.1016/j.hrthm.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Deharo J.C., Quatre A., Mancini J. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart. 2012;98:724–731. doi: 10.1136/heartjnl-2012-301627. [DOI] [PubMed] [Google Scholar]
- 12.Healy C.A., Carrillo R.G. Wearable cardioverter defibrillator for prevention of sudden cardiac death following infected implantable cardioverter defibrillator removal: a cost-effectiveness Evaluation. Heart Rhythm. 2015;12:1565–1573. doi: 10.1016/j.hrthm.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 13.Spickler J.W., Rasor N.S., Kezdi P. Totally self-contained intracardiac pacemaker. J Electrocardiol. 1970;3:325–331. doi: 10.1016/s0022-0736(70)80059-0. [DOI] [PubMed] [Google Scholar]
- 14.Koruth J.S., Rippy M.K., Khairkhahan A. Feasibility and efficacy of percutaneously delivered leadless cardiac pacing in an in vivo ovine model. J Cardiovasc Electrophysiol. 2015;26:322–328. doi: 10.1111/jce.12579. [DOI] [PubMed] [Google Scholar]
- 15.Reddy V.Y., Knops R.E., Sperzel J. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129:1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 16.Ritter P., Duray G.Z., Zhang S. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace. 2015;17:807–813. doi: 10.1093/europace/euv026. [DOI] [PubMed] [Google Scholar]
- 17.Shariff N., Eby E., Adelstein E. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015;26:783–789. doi: 10.1111/jce.12684. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira J.C., Martinelli M., Nishioka S.A.D.O. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter defibrillators: results of a large, prospective, randomized, double blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. doi: 10.1161/CIRCEP.108.795906. [DOI] [PubMed] [Google Scholar]
- 19.Gould P.A., Gula L.J., Champagne J. Outcome of advisory implantable cardioverter defibrillator replacement: one-year follow-up. Heart Rhythm. 2008;5:1675–1681. doi: 10.1016/j.hrthm.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Klug D., Balde M., Pavin D. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 21.Voigt A., Shalaby A., Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33:414–419. doi: 10.1111/j.1540-8159.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 22.Kolek M.J., Patel N.J., Clair W.K. Efficacy of a bio-absorbable antibacterial envelope to prevent cardiac implantable electronic device infections in high-risk subjects. J Cardiovasc Electrophysiol. 2015;26:1111–1116. doi: 10.1111/jce.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohail M.R., Uslan D.Z., Khan A.H. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 24.Catanchin A., Murdock C.J., Athan E. Pacemaker infections: a 10-year experience. Heart Lung Circ. 2007;16:434–439. doi: 10.1016/j.hlc.2007.02.097. [DOI] [PubMed] [Google Scholar]
- 25.Lekkerkerker J.C., van Nieuwkoop C., Trines S.A. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715–720. doi: 10.1136/hrt.2008.151985. [DOI] [PubMed] [Google Scholar]
- 26.Zinner S.H., Lagast H., Klastersky J. Antistaphylococcal activity of rifampin with other antibiotics. J Infect Dis. 1981;144:365–371. doi: 10.1093/infdis/144.4.365. [DOI] [PubMed] [Google Scholar]
- 27.Darouiche R.O., Raad, Bodey G.P., Musher D.M. Antibiotic susceptibility of staphylococcal isolates from patients with vascular catheter-related bacteremia: potential role of the combination of minocycline and rifampin. Int J Antimicrob Agents. 1995;6:31–36. doi: 10.1016/0924-8579(95)00017-3. [DOI] [PubMed] [Google Scholar]
- 28.Segreti J., Gvazdinskas L.C., Trenholme G.M. In vitro activity of minocycline and rifampin against staphylococci. Diagn Microbiol Infect Dis. 1989;12:253–255. doi: 10.1016/0732-8893(89)90022-9. [DOI] [PubMed] [Google Scholar]
- 29.Raad I., Darouiche R., Dupuis J. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections: a randomized, double-blind trial. Ann Intern Med. 1997;127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Marik P.E., Abraham G., Careau P. The ex vivo antimicrobial activity and colonization rate of two antimicrobial bonded central venous catheters. Crit Care Med. 1999;27:1128–1131. doi: 10.1097/00003246-199906000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Leon C., Ruiz-Santana S., Rello J. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30:1891–1899. doi: 10.1007/s00134-004-2378-2. [DOI] [PubMed] [Google Scholar]
- 32.Hanna H., Benjamin R., Chatzinikolaou I. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol. 2004;22:3163–3171. doi: 10.1200/JCO.2004.04.124. [DOI] [PubMed] [Google Scholar]
- 33.Hockenhull J.C., Dwan K., Boland A. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1–154. doi: 10.3310/hta12120. [DOI] [PubMed] [Google Scholar]
- 34.Chatzinikolaou I., Finkel K., Hanna H. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115:352–357. doi: 10.1016/s0002-9343(03)00367-x. [DOI] [PubMed] [Google Scholar]
- 35.Zabramski J.M., Whiting D., Darouiche R.O. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725–730. doi: 10.3171/jns.2003.98.4.0725. [DOI] [PubMed] [Google Scholar]
- 36.Hansen L.K., Brown M., Johnson D. In vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing Clin Electrophysiol. 2009;32:898–907. doi: 10.1111/j.1540-8159.2009.02406.x. [DOI] [PubMed] [Google Scholar]
- 37.Bloom H.L., Constantin L., Dan D. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol. 2011;34:133–142. doi: 10.1111/j.1540-8159.2010.02931.x. [DOI] [PubMed] [Google Scholar]
- 38.Steckman D.A., Varosy P.D., Parzynski C.S. In-hospital complications associated with reoperations of implantable cardioverter defibrillators. Am J Cardiol. 2014;114:419–426. doi: 10.1016/j.amjcard.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Krahn A.D., Lee D.S., Birnie D. Ontario ICD Database Investigators. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;2:136–142. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 40.Sohail M.R., Henrikson C.A., Braid-Forbes J.M. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol. 2015;38:231–239. doi: 10.1111/pace.12518. [DOI] [PubMed] [Google Scholar]
- 41.Tarakji K.G., Chan E.J., Cantillon D.J. Cardiac implantable electronic device infections presentation, management and patient outcomes. Heart Rhythm. 2010;7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Boersma L., Burke M.C., Neuzil P. Infection and mortality after implantation of the subcutaneous ICD following transvenous ICD extraction. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.08.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Bardy G.H., Smith W.M., Hood M.A. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 44.Weiss R., Knight B.P., Gold M.R. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 45.Lambiase P.D., Barr C., Theuns D.A. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olde Nordkamp L.R., Dabiri Abkenari L., Boersma L.V. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939. doi: 10.1016/j.jacc.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen S.S., Lambiase P., Boersma L.V. Evaluation oF FactORs impacTing cLinical outcome and cost Effectiveness of the S-ICD: design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol. 2012;35:574–579. doi: 10.1111/j.1540-8159.2012.03337.x. [DOI] [PubMed] [Google Scholar]
- 48.Burke M.C., Gold M.R., Knight B.P. Safety and Efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 49.Akerström F., Arias M.A., Pachón M. Subcutaneous implantable defibrillator: state-of-the art 2013. World J Cardiol. 2013;5:347–354. doi: 10.4330/wjc.v5.i9.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knops R.E., Olde Nordkamp L.R., de Groot J.R. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2013;10:1240–1243. doi: 10.1016/j.hrthm.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Gold M.R., Theuns D.A., Knight B.P. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012;23:359–366. doi: 10.1111/j.1540-8167.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 52.The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 53.Bardy G.H., Lee K.L., Mark D.B. The sudden cardiac death in heart failure trial (SCD-HeFT) investigators: amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 54.Kurita T. Primary prevention of sudden cardiac death in patients with ischemic heart disease – possible role of the shock device in the Asia. J Arrhythm. 2007;23:264–268. [Google Scholar]
- 55.Shimizu A., Nitta T., Kurita T. Actual conditions of implantable defibrillation therapy over five years in Japan. J Arrhythm. 2012;28:263–272. [Google Scholar]
- 56.Wilkoff B.L., Love C.J., Byrd C.L. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–1104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 57.Feldman A.M., Klein H., Tchou P. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004;27:4–9. doi: 10.1111/j.1540-8159.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki S., Tomita H., Shibutani S. Usefulness of the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death: a single-center primary experience. Circ J. 2014;78:2987–2989. doi: 10.1253/circj.cj-14-1098. [DOI] [PubMed] [Google Scholar]
- 59.Kondo Y., Linhart M., Schwab J.O. Usefulness of the wearable cardioverter defibrillator in patients in the early post-myocardial infarction phase with high risk of sudden cardiac death: a single-center European experience. J Arrhythm. 2015;31:293–295. doi: 10.1016/j.joa.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein H.U., Goldenberg I., Moss A.J. Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J. 2013;34:2230–2242. doi: 10.1093/eurheartj/eht167. [DOI] [PubMed] [Google Scholar]
- 61.Chung M.K., Szymkiewicz S.J., Shao M. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol. 2010;56:194–203. doi: 10.1016/j.jacc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanawuttiwat T., Garisto J.D., Salow A. Protection from outpatient sudden cardiac death following ICD removal using a wearable cardioverter defibrillator. Pacing Clin Electrophysiol. 2014;37:562–568. doi: 10.1111/pace.12319. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu A. How should we use the wearable cardioverter-defibrillator in Japan? Circ J. 2014;78:2851–2853. doi: 10.1253/circj.cj-14-1188. [DOI] [PubMed] [Google Scholar]
- 64.Adler A., Halkin A., Viskin S. Wearable cardioverter-defibrillators. Circulation. 2013;127:854–860. doi: 10.1161/CIRCULATIONAHA.112.146530. [DOI] [PubMed] [Google Scholar]




