Abstract
Background
We conducted a survey of the infection burden associated with the implantation of cardiac implantable electronic devices (CIEDs) in Japan.
Methods
The institutes were selected using annual device implantation data provided by Medtronic Japan Co., Ltd. The data sampling period was from January 1 to December 31, 2013. Institutes were classified into Group P, at which only pacemakers were implanted, and Group A, at which other CIEDs were implanted. Group P was further classified into three sub-groups by implantation number. The infection rate was compared between groups using logistic regression analysis.
Results
A total of 129 of 138 institutes responded. The annual infection rate was 1.12% for overall CIEDs. The institute at which 15–29 pacemakers were implanted had a high infection rate (2.11%). No statistically significant difference was observed (adjusted p=0.1131). The overall migration rate was 0.50%. Complete removal of the CIED system was performed in 55.8% of patients who underwent implantation.
Conclusions
This survey was the first on CIED infection and migration in Japan. The CIED infection rate (1.12%) was similar to that previously reported. A high infection rate (2.77%) was observed in the infection experienced institutes.
Keywords: Cardiac implantable electronic device, Infection, Migration, Lead extraction, Implantable cardioverter defibrillator
1. Introduction
Cardiovascular implantable electronic device (CIED) treatment is indispensable as cardiac treatment, and the implantation number of CIEDs has significantly increased over the past several years [1], [2], [3]. Increase in implantable cardioverter defibrillator (ICD) implantation is responsible for the increasing number of CIED implantations [4]. With this trend, augmenting CIED infection has become concerning. CIED infection has detrimental effects on mortality and increases financial burden [3], [4], [5]. Therefore, guidelines for the management of CIED infection were recently updated [5], [6]. When a patient is diagnosed with CIED infection, even if the infection is restricted locally to the pocket, complete removal of the CIED system is required by guidelines [5], [6]. However, a nationwide study on CIED infection has not been conducted in Japan. This survey is the first to investigate the current status of CIED infection and migration in Japan.
2. Methods
From October 27, 2014 to December 12, 2014, a survey on device infection and migration was conducted in Japan. The number of cases of device infection or migration after CIED implantation was assessed retrospectively. Respondents did not necessarily undergo CIED implantation. The institutes at which pacemakers, ICDs, cardiac resynchronization therapy pacemakers (CRT-Ps), or cardiac resynchronization therapy defibrillators (CRT-Ds) were implanted from January 1 to December 31, 2013 were selected using annual device implantation data provided by Medtronic Japan Co., Ltd. Patients who reported infection(s) during that period, and the infection or migration incidents that occurred from January 1, 2013 and December 12, 2014 were included in this study. The implant was not categorized based on whether it was a new or replacement device. All applicable events were counted regardless of manufacturer. The sample size was calculated based on the following assumptions. An 80% power at a significance level of 0.05 was the goal. The estimated annual infection rates are 0.6% for pacemakers [9], and for all other CIEDs, the estimated annual infection rate is 2.8%, which is derived from the infection rate of each device type and annual implant number. Estimation accuracy of the 95% confidence interval was set at ±0.6% for the pacemaker group and ±2.8% for all other CIEDs group. After applying an attrition rate of 20%, 2052 and 432 subjects were found to be required to meet the above criteria. With regard to data collection, referred cases were excluded. Specific information collected was treatment for infection and complications due to migration. The questionnaire is shown in Table 1, and the original language was Japanese.
Table 1.
Survey questionnaire.
| Question |
| The number of the CIED implantations in 2013 |
| The number of CIED infection and migration. |
| The time infection occurred, within 1 year or more than 1 year |
| Treatment for infection |
| Complication due to migration |
| Treatment for migration |
CIEDs: ICD, CRT-D, CRT-P.
Statistical analyses were performed with SAS Ver. 9.3. A p-value less than 0.05 was used to assess statistical significance.
3. Results
3.1. Institutes
A total of 138 institutes from each geographical area in Japan were randomly chosen. One hundred twenty-nine institutes responded to the survey. The response rate was 93.5%. A total of 84.5% (109 sites) of institutes at which only pacemakers were implanted and 15.5% (20 sites) at which other CIEDs were implanted responded to the survey. The total number of implantation cases was 3840: 3331 pacemaker cases (86.7%) and 509 other CIED cases (13.3%).
3.2. Infection burden
The duration that the survey covered was 1 year from January 1, 2013 to December 31, 2013. New device implantation and replacement were not distinguished in the analysis. We identified 43 cases of device infection. The overall infection rate was 1.12% (95% CI: 0.812–1.505); however, the rate was 2.77% at the sites with more experience with implantation.
The infection rates according to the device type were 1.08% in pacemakers and 1.38% in other CIEDs (Table 2). The infection rates according to institute type were 1.18% in the institutes at which only pacemakers were implanted and 1.05% in the institutes at which other CIEDs were implanted (Table 3.)
Table 2.
Comparison of the infection rate between pacemakers and other CIEDs.
| Devices | Operation numbers | Infection numbers | Infection rate (%) | Exact-95% CI (min-MAX ) | RR | p-Valuea |
|---|---|---|---|---|---|---|
| Pacemakers | 3331 | 36 | 1.081 | 0.758–1.493 | 1.27 | 0.5005 |
| Other CIEDsb | 509 | 7 | 1.375 | 0.555–2.813 | ||
| Total | 3840 | 43 | 1.120 | 0.812–1.505 |
CI, Confidence interval; RR, relative risk.
Fisher exact test.
other CIEDs: ICD, CRT-D, CRT-P.
Table 3.
Comparison of the infection rate between institutes: pacemakers only and other CIEDs.
| Institute | Institution numbers | Operation numbers | Average implantation | Infection numbers | Infection rate (%) | Exact-95% CI (min-MAX) | RR | p-Valuea |
|---|---|---|---|---|---|---|---|---|
| Pb | 109 | 2037 | 18.7 | 24 | 1.178 | 0.756–1.748 | 0.89 | 0.7603 |
| Ac | 20 | 1803 | 90.2 | 19 | 1.054 | 0.636–1.641 | ||
| Total | 129 | 3840 | 29.8 | 43 | 1.120 | 0.812–1.505 |
CI, Confidence interval; RR, relative risk.
Fisher exact test.
P: The institutes at which only pacemakers were implanted.
A: The institutes at which all other CIEDs were implanted.
Infection occurred within 1 year of implantation in 35 cases (81.4%) and more than 1 year in 8 cases (18.6%). Regarding treatments for infection, complete CIED system removal was performed in 24 cases (55.8%), generator removal in 11 cases (25.6%), administration or prolongation of antibacterial drugs or both in 7 cases (16.3%), and movement of the pocket to the opposite side in 1 case (2.33%) (Fig. 1).
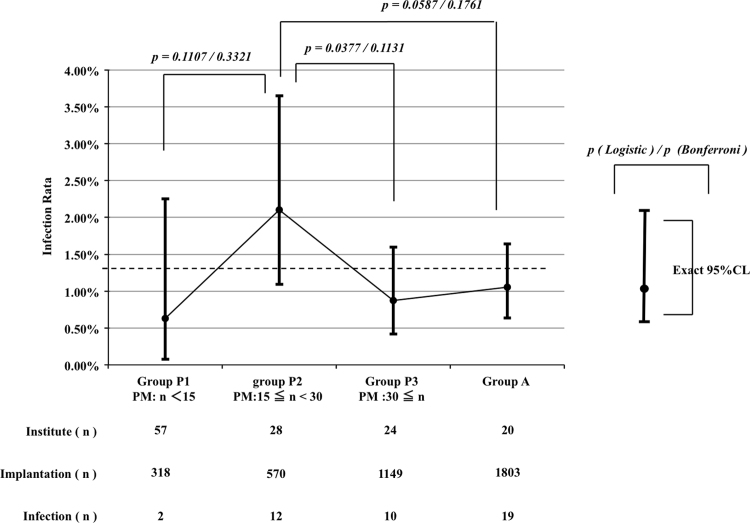
Fig. 1.
Comparison of the Group P subgroups with Group A. Group P was classified into three sub-groups based on the number of implantations. Group P1 included the institutes in which less than 14 devices per year were implanted. Institutes at which 15–29 implantations were performed were classified as Group P2. The institutes at which more than 30 implantations were performed were classified as Group P3. The infection rate of Group P2 was 2.11%, and it was higher than that of the other groups and the infection rate in the US in 1996 [4]; dotted line. The infection rate in Group P2 was compared with that of the other groups using logistic regression analysis. The results of the analysis showed the inferiority of Group P2 to Group P3 with respect to the infection rate. However, no statistically significant difference was observed after Bonferroni correction.
3.3. Device migration
Nineteen device migration cases were identified. The overall migration rate was 0.50% (95% CI: 0.298–0.772). There were 19 complications due to device migration. These complications included lead dislodgment in 14 cases (73.7%), and perforation, lead fracture, and infection in 1 case each (15.8%), and an unknown complication in 2 cases (10.5%). Migration was treated by lead repositioning in 10 cases (52.6%), whole CIED system extraction and re-implantation in 2 cases (10.5%), and observation alone in 3 cases (15.8%). Lead extraction with an additional procedure was performed in 2 cases (10.5%).
4. Discussion
4.1. Quality and quantity of the survey
To review implant status as a whole, private annual device implantation data provided by Medtronic Japan Co., Ltd., were used in this survey. The private data may introduce bias; however, the data showed good correlation in the annual implantation number with the data from the Japan Arrhythmia Device Industry Association. The geographical area coverage rate of this survey was 93.6% (44 out of 47 prefectures). The variance of the annual implantation numbers among the institutes was 1–221. Out of randomly selected sites sampled from the above data, 93.5% of the institutes replied to the questionnaire. Therefore, the findings of this survey are well representative of the entire Japanese CIED infection rate, and this study is the first of its kind in Japan.
The data did not include personal data such as patients’ demographics, physicians’ experience, and details on the time of infection occurrence.
4.2. Infection burden
The overall infection rate was 1.12% and it was similar or lower than that previously reported in the USA and Europe [1], [4], [8], [10], [11]. In the European survey [7], the number of infection-free institutes was 27.1% (13/48). In this survey, 76.7% of the enrolled institutes did not experience device infection, and this rate is higher than that observed by the European survey. Nevertheless, the data from this survey cannot be compared with that from previous studies in the USA and Europe, because the background of the enrolled institutes may be different. One difference was that this survey included institutes with a low implant volume. The limitation of the European survey [7] was the low number of small volume institutes. The same survey showed that at 62.5% of the enrolled institutes, more than 200 devices were implanted per year. In contrast with this finding, less than 50 devices were implanted at 82.2% of the institutes in this survey and at 9.30% of the institutes, only 1 device per year was implanted. These findings reflect the number of small volume institutes in Japan. In a small volume institute, only 1 case of infection may reflect a high infection rate, e.g., the infection rate would be 100% at an institute at which only 1 implantation per year is performed if the patient contracts infection.
Another difference was the smaller number of ICDs implanted than that reported by previous studies in the USA and Europe. Greenspon et al. studied the trend of device infection from 1993 to 2008 in the USA using the Nationwide Inpatient Sample (NIS) discharge records [4]. The infection rate was 1.53% in 2004 and 2.41% in 2008. The infection rate in this survey (1.12%) was lower than that in Greenspon et al.’s study. While ICD implantation represented 35% of all CIED implantations in Greenspon et al.’s study, the implantation rate of ICDs and CRTs was only 13.3% in this survey. Therefore the two values cannot be compared. For this reason, we intended to normalize the results to the data in the literature [4] with the same ICD implantation rate. In the literature, the trend graph of the annual number of CIED implantations from 1993 to 2008 is shown in Fig. 1 of the article. According to the figure, the ICD implantation rate in 1996 was the same as that in this survey (approximately 13%). The infection rate was able to read from it and the rate was approximately 1.3%. This value is similar to the infection rate in this survey.
To reduce the rate of CIED infection, the importance of preoperative antibiotic prophylaxis has been discussed [8], [12], [13]. de Oliveira et al. [14] studied the efficacy of perioperative antibiotic prophylaxis in a prospective, randomized, double-blinded, placebo-controlled trial. The study was terminated by the safety committee due to a significant difference in favor of the antibiotic arm. The infection rate of the control arm, which administrated placebo, was 3.28%, whereas that in the prophylactic antibiotic administration arm was 0.63%. Almost 100% of institutes in Japan administer antibiotics pre- and perhaps postoperatively. This prophylaxis is thought to contribute to the reduction in the infection rate in Japan. Assuming that all the institutes in this survey administered prophylactic antibiotics, the annual infection rate, which is1.12% in this survey, is much higher than that in the prophylaxis group in de Oliveira et al.’s study (0.63%.)
In Japan, ICDs and CRTs are implanted only in institutes certified by the Japanese Heart Rhythm Society. On the other hand, no such regulations apply for implantation of pacemakers. Accordingly, the institutes were classified as all CIED-approved institutes (Group A) and pacemaker institutes (Group P), and the infection rate was compared between these groups (Table 3). There was no statistical significance between the two groups in the annual infection rate (p=0.7603.)
Some studies found that infection rate of ICDs was higher than that of peacemakers [9], [11], and CRT is a risk factor for CIED infection [14]. In this survey, the annual infection rate of ICDs and CRT was 1.05% and that of pacemakers was 1.18% in the certified institutes, with no statistically significant difference in the infection rate between pacemakers and ICDs.
Al-Khatib et al. [15] analyzed the relationship between physician experience and ICD infection rate, and found an association between a higher volume of ICD implantations and a lower rate of infections. Unfortunately, the infection rate in the groups was not reported. However, the total number of implantations and the infection numbers are shown in a table in the literature. The calculated infection rate for physicians who performed more than 29 implantations was 0.9%. On the other hand, 3 of 6 institutes in which more than 18 CEIDs were implanted without pacemakers experienced infections with an infection rate of 0.70% in Japan.
Implantation numbers were 15–29 in Group P, and the proportion of institutes with more experience with infections was 42.9%. The number is similar to that of other CIEDs in Group A. However, the average implantation number in Group P was significantly smaller than that in Group A. The infection rate in Group P was 1.18%, which is almost equal to the infection rate in the US, in which 35% of implantations were those for ICDs in 2008 [4]. Group P was classified into 3 sub-groups based on the number of implantations. Group P1 included institutes at which less than 14 devices per year were implanted, and Group P2 included institutes at which 15–29 devices per year were implanted, and Group P3 included institutes at which 30 CIEDs were implanted. According to Al-Khatib’s study [15], less experienced physicians had less in patient mortality and more mechanical complications, and this group showed better infection rate than the relatively experienced physicians group. The same trend was observed in this survey. The infection rate in Group P1, which included the fewest CIED implantations, was not inferior to that of Group P2. The CIED infection rate does not seem to be related to either skill or implantation number.
The infection rate in the three P sub-groups and Group A was compared using logistic regression analysis (Fig. 2). The results of the analysis showed the inferiority of Group P2 to P3 (p=0.0377). However, no statistical significance was observed after p value adjustment using Bonferroni correction (p=0.1131.) This analysis was validated comprehensively using the Cochran–Mantel–Haenszel general association test. Statistical significance was not observed in all groups (p=0.0934).
Fig. 2.
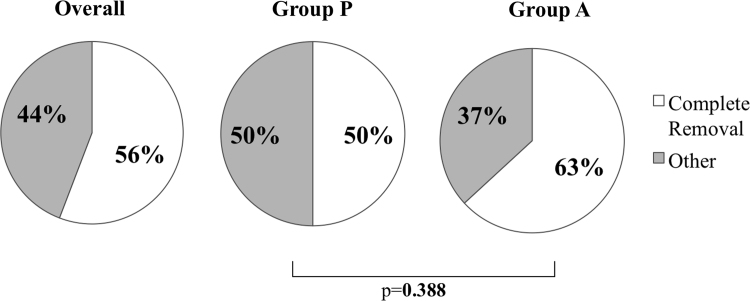
Treatment of infection: Comparison of Group P with A. 50% of infections in Group P and 63% in Group A were treated with complete removal of the CIED system. There was no statistically significant difference between groups in the total removal rate. Overall complete removal rate was 56%.
In this survey, 76.7% of the participating institutes did not experience the infection, which was a high rate. However in the remaining institutes (23.3%) that experienced infection, most had high infection rates (2.77%) despite prophylactic antibiotic use as the standard of care. To reduce the rate of CIED infection in Japan, the root cause of the infection should be identified. However, in this survey few parameters were assessed and hence risk stratification was not performed. In this survey, the operations were not categorized as new device implantation, device replacement, up-grade operation, or reoperation due to system failure. However, many studies have emphasized that device replacement is a risk factor for infection [7], [15], [16], [17].
4.3. Device migration
Device migration is a serious complication of CIED implantation. The causes of device migration are thought to be the device size and procedure. Device migration sometimes causes lead complications and most require reoperation. Reoperation is regarded as a risk factor for CIED infection [8], [16], [17], [18]. No survey or study has focused on device migration; therefore this survey is unique.
It is notable that the overall migration rate was 0.50%, and the rate of ICD and CRT (0.20%) infection is lower than that of pacemakers (0.54%). Migrations induced a high rate of lead dislodgement (73.7%) that require reoperation, which is an additional risk factor for infection. Therefore, this survey showed that device migration was an important factor for device infection.
Studies focusing on the clinical mechanism of migration and identification of the appropriate surgical procedure to prevent device migration are important.
4.4. Treatment of CIED infection
Even if CIED infection is only observed locally in the pocket, complete removal of the CIED system, i.e., device and lead(s), should be performed [5], [6], [7]. Annual system removal rate in this survey was 55.8%. The removal rate was 50.0% in Group P and 63.2% in Group A. There was no statistical significance between the two groups (Fig. 2). This rate is higher than that in the European survey, which was 43.5%. Device removal is a conventional method to treat CIED infection and is recommended in the guidelines. The recurrence rate of CIED infection is higher if device removal and debridement are performed for device infection [18]. In this survey, device removal was performed in 11 cases, but details of the treatment were not available.
4.5. Study limitations
Even though the data were obtained from a geographically wide area and are from various institutions, a retrospective questionnaire survey was used for collection. Hence, individual patient data, physician experience, and treatment detail were not collected.
5. Conclusions
This survey was the first on CIED infection and migration in Japan. The rate of CIED infection was 1.12%, which was similar to that previously reported in the USA and Europe. The burden resulted from the high rate of infection-free institutes. However the high infection rate (2.77%) was observed in institutes with more experience with infection. Device migration occurred more frequently than expected, and 73.7% of migrations were caused by lead insufficiency. In more than 50% of cases, the CIED system was completely removed.
Conflict of interest
The survey was conducted by Medtronic Japan Co. Ltd., and H. Nakajima, MD is a consultant of the company.
Contributor Information
Hiroshi Nakajima, Email: hnakajima-cvs@umin.ac.jp.
Masami Taki, Email: masami.taki@medtronic.com.
References
- 1.Kurtz S.M., Ochoa J.A., Lau E. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol. 2010;33:705–711. doi: 10.1111/j.1540-8159.2009.02670.x. [DOI] [PubMed] [Google Scholar]
- 2.Mond H.G., Irwin M., Ector H. The world survey of cardiac pacing and cardioverter defibrillators: calendar year 2005: an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202–1212. doi: 10.1111/j.1540-8159.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 3.Uslan D.Z., Tleyjeh I.M., Baddour L.M. Temporal trends in permanent pacemaker implantation: a population study. Am Heart J. 2008;155:896–903. doi: 10.1016/j.ahj.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenspon A.J., Patel J.D., Lau E. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Baddour L.M., Epstein A.E., Erickson C.C. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 6.Wilkoff B.L., Love C.J., Byrd C.L. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–1104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Mittal Suneet, Shaw Richard E., Michel Kimberly. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11:595–601. doi: 10.1016/j.hrthm.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Bongiorni M.G., Marinskis G., Lip G.Y.H. Blomström-Lundqvist C. How European centres diagnose, treat, and prevent CIED infections: results of a European Heart Rhythm Association survey. Europace. 2012;14:1666–1669. doi: 10.1093/europace/eus350. [DOI] [PubMed] [Google Scholar]
- 9.Uslan D.Z., Sohail M.R., St, Sauver J.L. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–675. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]
- 10.Cabell C.H., Heidenreich P.A., Chu V.H. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J. 2004;147:582–586. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Voigt A., Shalaby A., Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:590–591. doi: 10.1016/j.jacc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa A., Kirkorian G., Cucherat M. Antibiotic prophylaxis for permanent pacemaker implantation : a meta-analysis. Circulation. 1998;97:1796–1801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira J.C., Martinelli M., Nishioka S.A.D. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. doi: 10.1161/CIRCEP.108.795906. [DOI] [PubMed] [Google Scholar]
- 14.Romeyer-Bouchard C., Da Costa A., Dauphinot V. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J. 2010;31:203–210. doi: 10.1093/eurheartj/ehp421. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib S.M., Lucas F.L., Jollis J.G. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating medicare beneficiaries. J Am Coll Cardiol. 2005;46:1536–1540. doi: 10.1016/j.jacc.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Klug D., Balde M., Pavin D. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 17.Lekkerkerker J.C., van Nieuwkoop C., Trines S.A. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart Cardiac Soc) 2009;95:715–720. doi: 10.1136/hrt.2008.151985. [DOI] [PubMed] [Google Scholar]
- 18.Sohail M.R., Uslan D.Z., Khan A.H. Management and outcome of permanent and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]