Abstract
Penile metastasis from prostate cancer represents a rare condition, associated with poor prognosis. In the literature, authors have reported less than 500 cases of secondary penile cancers, and among these cases of metastases, only 33% are from prostate cancer. Overall reported rate of survival is about 1-24 months. Here, we present an uncommon case of penile metastasis from prostatic adenocarcinoma, with particular focus on the role of computed tomography and magnetic resonance imaging in diagnosis and follow-up.
Keywords: metastasis, penis, prostate cancer, diagnostic imaging
Case report
In December 2006, a 77-year-old male patient was treated with radical prostatectomy and dissection of lymph nodes, total adrenergic block (BAT), and cycles of radiation therapy for prostate adenocarcinoma (Gleason score 5 + 4 = 9; T3b N0 Mx).
During routine oncologic follow-up, his levels of serum prostate-specific antigen (PSA) had remained below 1 ng/mL for 6 years (normal values for PSA at our institution: 0-4.4 ng/mL). In March 2012, the patient's value of PSA was 0.98 ng/mL. In June 2012, laboratory tests revealed hematic level of PSA of 3.24 ng/mL. His value of PSA had tripled in only 3 months, about 5 years after radical surgery and therapies.
After this unexpected increase, complete clinical examination of the patient happened to be negative, without any skin alteration on the penile glans or any evidence of palpable inguinal lymph nodes.
In October 2012, the value of PSA had reached 6.16 ng/mL, almost twice compared to June 2012.
Follow-up computed tomography (CT) scan did not reveal any metastatic localization in the abdomen, but scans showed osteolytic areas: one area with osteolytic features was visualized on the vertebral body of L2 and one on the left femur.
Bone scintigraphy confirmed the positivity of these 2 lesions. Thus, oral therapy with estramustine phosphate was started.
During further follow-up, the patient did not complain any local complications or penile pain, but a continuous increase of his values of PSA was observed.
Nevertheless, the disease remained stable until January 2013 (PSA values of 8.07 ng/mL), when another CT examination showed an oval subcutaneous nodule (maximum diameter 21 mm) that was located on the left margin of corpus cavernosum. After injection of iodinated contrast agent, this mass had homogenous and early contrast enhancement, indicating high angiogenesis. Furthermore, left scrotal hydrocele was observed (Fig. 1).
Fig. 1.
CT scan on axial (A, B) and multiplanar reconstruction in the sagittal and coronal planes (C, D) detects a nodule on the left side of corpus cavernosum (indicated by white arrow in all figures) with an early contrast enhancement after iodinated contrast agent injection. In addition, CT reveals an ipsilateral hydrocele (D). CT, computed tomography.
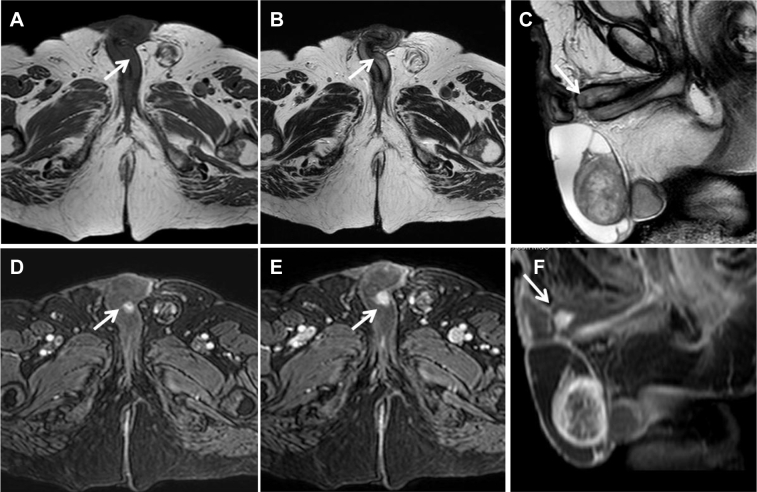
Magnetic resonance imaging (MRI) of the penis was performed on a 1.5-T scanner (GyroscanIntera; Philips Medical Systems, Best, The Netherlands) equipped with 8-channel dedicated coil. MRI images were acquired on axial, sagittal, and coronal planes using T1-weighted and T1 spectral presaturation with inversion recovery–weighted sequences and T2-weighted and T2 spectral presaturation with inversion recovery–weighted sequences; T1-weighted dynamic study after injection of gadolinium was also performed. MRI confirmed the previous findings, showing nodular area of altered signal intensity, hypointense on T1-weighted and T2-weighted sequences, characterized by an early and persistent contrast enhancement on dynamic sequences after injection of gadolinium (Fig. 2).
Fig. 2.
MRI T1-weighted images on the axial plane (A) and T2-weighted images on the axial and sagittal planes (B, C) showing an oval-shaped area, hypointense on both sequences (white arrow) that is localized on the left side of corpus cavernosum. On dynamic sequences, after paramagnetic contrast agent injection, the lesion shows early contrast enhancement in the arterial phase (D), and persistent enhancement in the delayed phases (E, F). MRI, magnetic resonance imaging.
In February 2013, penile biopsy was performed. Pathology report from biopsy specimens was fibromuscular adipose tissue with infiltration of poorly differentiated carcinoma, all positive for PSA at the immunohistochemistry (Fig. 3). Levels of PSA at this time were 8.81 ng/mL.
Fig. 3.
Histologic findings of surgical biopsy. Fibromuscular tissue characterized by an infiltration from a poorly differentiated carcinoma (panel A, hematoxylin-eosin, 10×), showing a positive reaction with the anti-prostate-specific antigen antibody at the immunohistochemistry (panel B, 10×).
In August 2013, values of PSA reached 13.57 ng/mL and another bone scintigraphy revealed new sites of metastatic localization.
Twelve months after the diagnosis of penile metastasis, due to cardiologic toxicity and bad general conditions of the patient, multidisciplinary group of our institution decided to treat him with best supportive care. The patient died about 30 months after the diagnosis of penile metastasis because of his bad general conditions.
Discussion
Penile metastases are relatively uncommon, event though the penis is provided by rich and complex vascular supply, well connected to the pelvic organs. In 1870, Eberth [1] first described a case of penile metastasis. In 1997, a Japanese Scientific Review reported only 110 described cases (Table 1).
Table 1.
Primary sites of cancers metastasized to penis (Japanese Scientific Review).
| Primary sites | N. patients (%) |
|---|---|
| Genitourinary tract | 76 (69) |
| Bladder | 32 (29) |
| Prostate | 25 (23) |
| Kidney | 11 (10) |
| Ureter | 4 (3.6) |
| Testis | 2 (1.8) |
| Urethra | 2 (1.8) |
| Gastrointestinal systems | 21 (19) |
| Rectum | 12 (11) |
| Stomach | 5 (4.5) |
| Esophagus | 3 (2.7) |
| Cecum | 1 (0.9) |
| Respiratory system | 8 (7.2) |
| Lung | 6 (5.4) |
| Other | 2 (1.8) |
| Others | 5 (4.5) |
| Total | 110 (100) |
About 75% of all cases originate from the genital tract [2].
Clinical signs and symptoms associated with penile metastases are priapism, urinary retention, penile palpable masses or skin alterations, pelvic and perineum pain, dysuria, and hematuria [3].
Although prostatic carcinoma is the most common cancer in males and the second leading cause of cancer-related deaths, penile metastasis from prostatic cancer is a relatively rare condition [4].
From a review of the latest literature, we could find 451 published cases of secondary penile metastasis, and among these, only 33% had originated from prostate adenocarcinoma. Primary tumor generally involved the genitourinary tract (69%) followed by gastrointestinal cancer (19%). All described cases were associated with very poor prognosis—variable survival rates from 1 to 24 months—and in all these cases, autopsy has identified multiple coexistent sites of metastasis in the patients [5], [6], [7]. Only 2 articles from the recent literature have described a penile metastatic localization from osteosarcoma and cholangiocarcinoma [8], [9].
Typically, prostatic carcinoma metastasizes to local regional lymph nodes and to the bone, but it can spread also to lungs, bladder, liver, and adrenal glands. Classification for prostatic cancer is made using the Gleason score, based on the degree of glandular differentiation and the pattern of tumor growth (stromal component), as identified at relatively low magnification [10]. Despite the proximity and his rich vascularization, prostate carcinoma reaches the penis infrequently: in 219 cases of penis metastases reported in the literature, only 26 cases were secondary to prostatic cancer [5], [11].
In the natural history of disseminated prostate cancer, penile metastasis may be a late presentation, and it is generally associated with very poor prognosis: the literature reports that 41% of patients died within 6 months after the diagnosis [5], [12].
Abeshouse et al [13] tried to explain the possible mechanism of metastasis from prostate to penis as follows: (1) direct invasion, (2) implantation, (3) dissemination through the blood stream, and (IV) dissemination through lymphatic system. The most likely route of spread is retrograde venous transport because there are high chances of communication between the pelvic venous plexuses and the penile dorsal venous system [13]. This way of spreading could explain why penile erectile tissues, mainly corpora cavernosa are preferentially involved.
Typically, metastases occur in the central portion of the corpus cavernosum or spongiosum, and this distinguishes them from primary penile lesions; in particular, squamous cell carcinoma, which represents 95% of primary tumors of the penis, is mainly localized at glans. When diagnosed, most of these cases are associated with disseminated disease, and patients usually present with priapism, painful nodule (Peyronie's disease), and urinary obstruction. The typical occurrence of priapism is due to metastatic replacement of corpora cavernosa by the tumor, with blockage of the venous blood flow and lasting painful erection. Differential diagnosis should include primary penile tumors, chancre, tuberculosis, and nonspecific inflammatory lesions.
In prostatic cancer, the clinical management and follow-up is aided by laboratory testing of values of PSA and radiologic examinations.
Serum levels of PSA enable not only the early detection of prostatic adenocarcinoma but also the identification of eventual metastases during follow-up [14]. Despite this, we could find in the literature, a reported case of penile metastases without any increase of PSA levels [15], [16].
Radiologic diagnosis of penile metastasis involves several steps: CT scan has an important role for the detection of secondary lesions at diagnosis and during the follow-up. MRI represents the most reliable technique in the differentiation of penile lesions and for staging: due to its soft-tissue contrast high capability, it can evaluate the invasion of tunica albuginea, corpora, and urethra. Typical MRI features of penile metastases are hypointensity on both T1- and T2-weighted sequences and nonspecific enhancement after gadolinium injection [17].
Currently the use of ultrasound has not been standardized yet.
The final diagnosis is made by needle core biopsy.
Our case is extremely rare: a presentation of penile metastasis 6 years after radical prostatectomy has not been previously reported in the literature. The average interval of presentation of penile metastatic localizations is reported to be about 38-50 months after the first diagnosis of prostate carcinoma [18].
In conclusion, metastatic prostatic cancer may present different localizations, including penile structures. Despite the rarity of this disease, considering its poor prognosis and its capability to metastasize after several years, it emerges the crucial role of radiologic examinations during oncologic follow-up, especially after significant increases of PSA levels.
Acknowledgments
Conception and design of the study was done by VF, VL, GL and GC. Acquisition of data was carried out by VL, GC, VF, VF, and AM. Revision of the article was performed by VF, VF, GL, MR, and RF. Final approval was done by VF, MR, AM, RF.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Eberth C.J. Krebsmetastasen des corpus cavernosum penis. (Cancer metastases of the corpus cavernosum of penis) Virchows Arch. 1870;51:145. [Google Scholar]
- 2.Osther P.F., Løntoft E. Metastasis to the penis. Case reports and review of the literature. Int Urol Nephrol. 1991;23:161–167. doi: 10.1007/BF02549714. [DOI] [PubMed] [Google Scholar]
- 3.Hizly F., Berkmed F. Penile metastases from other malignancies. A study of ten cases and review of the literary. Urol Int. 2006;76(2):118–121. doi: 10.1159/000090872. [DOI] [PubMed] [Google Scholar]
- 4.Mugharbil Z.H., Childs C., Tannenbaum M., Schapira H. Carcinoma prostate metastatic to penis. Urology. 1985;25(3):314–315. doi: 10.1016/0090-4295(85)90339-5. [DOI] [PubMed] [Google Scholar]
- 5.Ansari H., Prashant R., Franks A. Prostatic carcinoma metastasis to the penis–an uncommon site. Lancet Oncol. 2003;4(11):705–706. doi: 10.1016/s1470-2045(03)01251-8. [DOI] [PubMed] [Google Scholar]
- 6.Sönmez N.C., Coşkun B., Arisan S., Güney S., Dalkiliç A. Early penile metastasis from primary bladder cancer as the first systemic manifestation: a case report. Cases J. 2009;2:7281. doi: 10.4076/1757-1626-2-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamoto T., Azuma H., Iwamoto Y., Sakamoto T., Katsuoka Y. A rare case of penile metastasis of testicular cancer presented with priapism. Hinyokika Kiyo. 2005;51(9):639–642. [PubMed] [Google Scholar]
- 8.Aparna C., Renuka I.V., SailaBala G., Annapurna P. Osteosarcoma metastases in penis. South Asian J Cancer. 2013;2(3):136. doi: 10.4103/2278-330X.114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastore A.L., Palleschi G., Manfredonia G., Maceroni P., Alvaro D., De Santis D. Penile metastasis from primary cholangiocarcinoma: the first case report. BMC Gastroenterol. 2013;13:149. doi: 10.1186/1471-230X-13-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotran R.S., Kumar V., Robbins S.L. WB Saunders Company; Philadelphia: 1994. Pathologic basis of disease; p. 1029. [Google Scholar]
- 11.Krpina K., Markić D., Spanjol J., Valencić M., Fuckar Z. Penile metastases of prostate cancer. Acta Clin Croat. 2011;50:431–443. [PubMed] [Google Scholar]
- 12.Kotake Y., Gohji K., Suzuki T., Watsuji T., Kusaka M., Takahara K. Metastases of penis from carcinoma of the prostate. Int J Urol. 2001;8(2):83–86. doi: 10.1046/j.1442-2042.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Abeshouse B.S., Abeshouse G.A. Metastatic tumors of the penis: a review of the literature and report of two cases. J Urol. 1961;86:99–112. doi: 10.1016/S0022-5347(17)65117-6. [DOI] [PubMed] [Google Scholar]
- 14.Rohan V., Hanji A., Patel J., Goswami J., Tankshali R. Penile metastases from prostate cancer. Urol J. 2009;6:217–219. [PubMed] [Google Scholar]
- 15.Senkul T., Karademir K., Silit E., Işeri C., Erden D., Baloğlu H. Penile metastasis of prostatic adenocarcinoma. Int J Urol. 2002;9:597–598. doi: 10.1046/j.1442-2042.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 16.Pierro A., Cilla S., Digesù C., Morganti A.G. Penile metastases of recurrent prostatic adenocarcinoma without PSA level increase: a case report. J Clin Imaging. 2012;2:44. doi: 10.4103/2156-7514.99178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocher L., Glas L., Cluzel G., Ifergan J., Bellin M.F. Imaging tumors of the penis. Diagn Interv Imaging. 2012;93:319–328. doi: 10.1016/j.diii.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Tu S.M., Reyes A., Maa A., Bhowmick D., Pisters L.L., Pettaway C.A. Prostate carcinoma with testicular or penile metastases. Clinical, pathologic, and immunohistochemical features. Cancer. 2002;94(10):2610–2617. doi: 10.1002/cncr.10546. [DOI] [PubMed] [Google Scholar]