Abstract
Acanthamoeba spp. are pathogenic protozoa that are uncommonly encountered. They tend to infect immunocompromised patients, most often causing cutaneous lesions and in some instances granulomatous amebic encephalitis, as well as rare instances of dissemination to other organs. We present a case of amebic osteomyelitis of the fibula in a patient with rejection of a transplanted kidney who was chronically immune-suppressed.
Keywords: Ameba, Amoeba, Acanthamoeba, Osteomyelitis, Infection, MRI
Case report
A 36-year-old diabetic woman with a remote history of renal transplant being maintained on immunosuppressant therapy for chronic transplant rejection was initially diagnosed with cutaneous infection by an Acanthamoeba sp. She presented to an outside facility emergency department approximately one month after initial diagnosis with fever, mild dyspnea, nausea, vomiting, and severe pain in the left knee that started several days before presentation. Physical examination revealed a small, warm, tender, and erythematous area along the lateral aspect of the inferior left knee. Initial radiographs of the left knee revealed a permeative pattern with cortical destruction in the proximal fibula and swelling of the overlying soft tissues, concerning for osteomyelitis (Fig. 1). Magnetic resonance imaging (MRI) without contrast of the left knee was performed, revealing cortical destruction and exuberant increased T2 signal intensity (SI) with corresponding decreased T1 SI in the proximal fibula consistent with osteomyelitis. A large abscess with surrounding soft-tissue edema was present lateral and posterior to the fibula (Fig. 2). The patient was admitted and treated in an intensive care unit at the outside institution. She underwent 2 surgical procedures several days apart for drainage of the abscess and excisional debridement of the proximal fibula. Tissue samples from surgery were sent to the Centers of Disease Control and Prevention in Atlanta, which confirmed the presence of Acanthamoeba. The patient underwent complex antimicrobial therapy and improved sufficiently for discharge to a long-term acute care facility, where she continued to recover and eventually returned home.
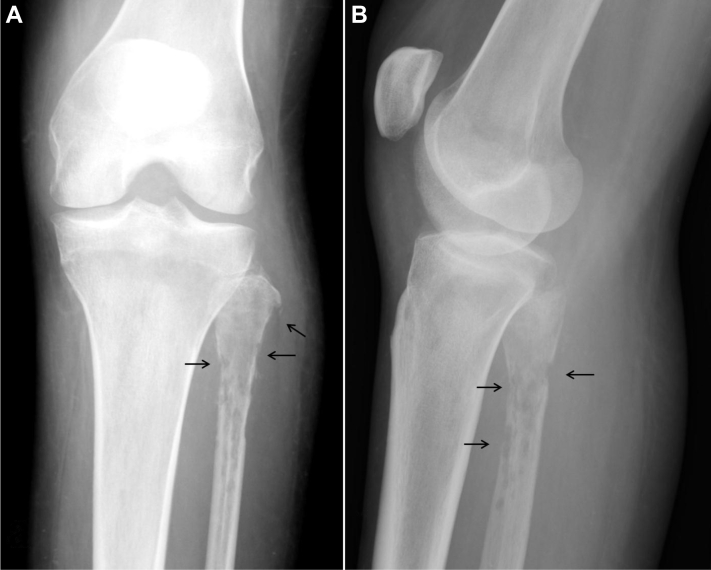
Fig. 1.
A 36-year-old woman with Acanthamoeba spp. osteomyelitis of the left fibula. Frontal (A) and lateral (B) radiographs demonstrate a permeative pattern of lucency within the proximal fibula with interruptions in the cortex (arrows) and soft-tissue swelling.
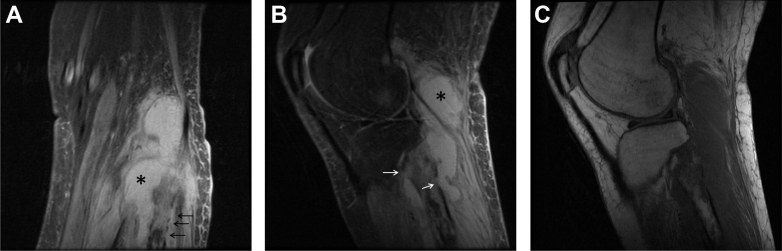
Fig. 2.
A 36-year-old woman with Acanthamoeba spp. osteomyelitis of the left fibula. Coronal (A) and sagittal (B) proton density fat saturation images show high SI within the medullary canal of the proximal fibula. Cortical disruption (arrows) and subcutaneous edema correlate with findings seen radiographically (Fig. 1), and the cortical interruptions are shown to communicate with a homogeneous, high SI collection (*) confirmed at surgery to be an abscess cavity. Sagittal T1WI (C) shows well-circumscribed regions of low internal T1 SI in the proximal fibula.
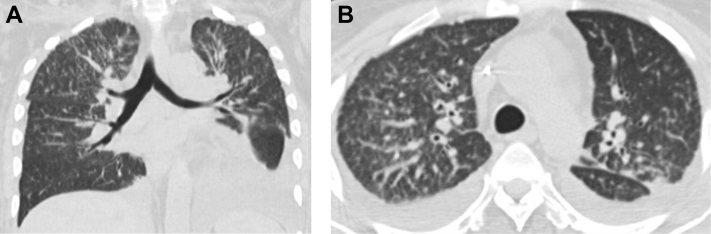
The patient was followed closely on an outpatient basis and maintained on both prednisone for transplant rejection and a multi-agent regimen for disseminated amebiasis. Several weeks after discharge from the long-term care facility, she complained of an increasing number of painful cutaneous nodules on her right elbow, right lower back, and left thigh and was subsequently admitted to our facility. New radiographic and MRI examinations of the left tibia and fibula (Figs. 3 and 4, respectively) showed recurrence of a fluid collection consistent with abscess around the remainder of the proximal fibula. The patient underwent another excisional debridement with resection of the proximal fibula. Pathologic examination of tissue samples again showed organisms consistent with amoebae. Of note, the patient also had tree-in-bud opacities with a right upper lobe predominance and small bilateral pleural effusions on noncontrast thoracic computed tomography which, although nonspecific, was felt could represent pulmonary involvement of acanthamebiasis (Fig. 5).
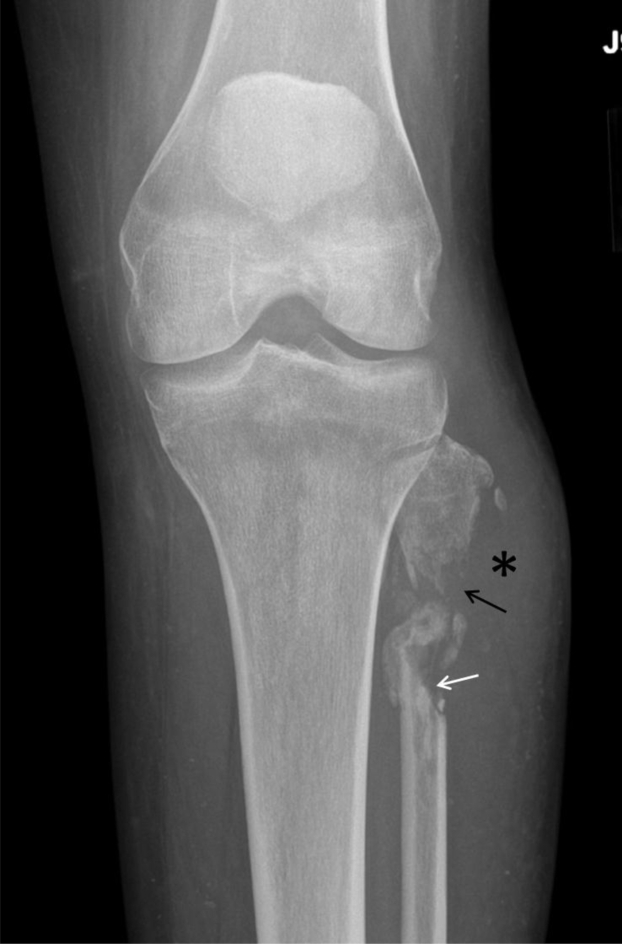
Fig. 3.
A 36-year-old woman with Acanthamoeba spp. osteomyelitis of the left fibula. Repeat frontal radiograph shows progressive destruction of the fibula. The sharp superior margin of the distal piece of the fibula is consistent with prior surgical debridement (white arrow), but the irregular margin along with poor cortication and focal demineralization of the proximal fibula suggests persistent or recurrent infection (black arrow). Homogeneous soft-tissue density in the adjacent soft tissues represents recurrence of abscess (*).
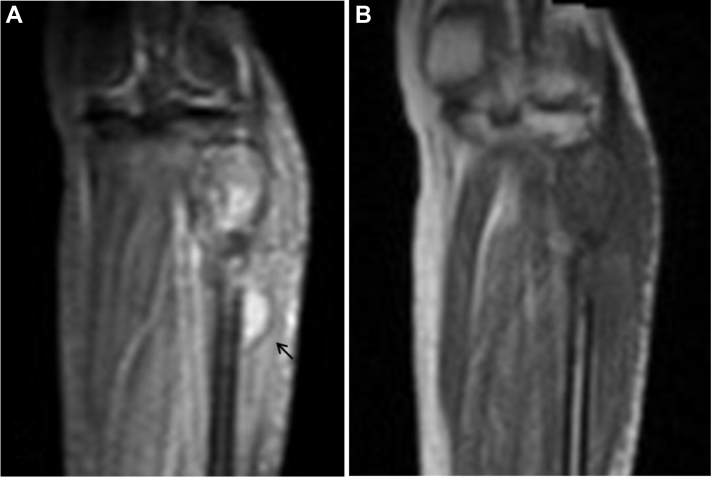
Fig. 4.
A 36-year-old woman with Acanthamoeba spp. osteomyelitis of the left fibula. MRI of the proximal left tibia performed the same day as the radiograph of Fig. 3. Coronal short-tau inversion recovery (A) and T1WI (B) images show abnormally high short-tau inversion recovery SI and low T1 SI within the proximal fibula with contiguous fluid collection (arrow), which was a recurrent abscess. Bone loss resulted from a combination of destruction by the infectious process and prior surgical debridement.
Fig. 5.
A 36-year-old woman with Acanthamoeba spp. osteomyelitis of the left fibula. Coronal (A) and axial (B) images from unenhanced computed tomography of the chest show tree-in-bud opacities with a right upper lobe predominance and small bilateral pleural effusions, nonspecific findings most frequently associated with atypical infections.
At last follow-up, the patient was undergoing treatment for disseminated amebiasis, roughly 6 months after her initial diagnosis with cutaneous Acanthamoeba spp. infection.
Discussion
Amoebae are widespread in nature. They are free-living, meaning they are able to survive outside a host, and only a few of the many species are known to opportunistically infect humans as parasites. These include Naegleria fowleri, several members of the genus Acanthamoeba, and Balamuthia mandrillaris, any of which can inhabit the central nervous system. Specifically, in addition to infections of the lungs and skin, Acanthamoeba and B. mandrillaris, which are closely related taxonomoically, are known to cause granulomatous amebic encephalitis (GAE), a chronic and almost uniformly fatal disease that favors immunocompromised hosts, children, and the elderly. Acanthamoeba spp. also rarely cause a highly morbid keratitis that usually strikes wearers of contact lenses [1], [2], [3]. True incidence of amebiasis is unknown due to multiple factors. Many cases likely go undiagnosed in areas where the tools for formal diagnosis are lacking. In the developed world, lack of awareness and low suspicion are thought to contribute to underrecognition [2].
Acanthamoeba spp. are ubiquitous; they are found worldwide and have been isolated from air, soil, and a wide variety of water sources including bottled water, plumbing fixtures, air conditioning units, and swimming pools, among others. Indeed, most healthy individuals have been found to carry anti-Acanthamoeba antibodies, suggesting there are subclinical states of infection which are cleared by immunocompetent hosts. Their life cycle includes 2 stages: cyst and trophozoite, either of which may be infectious. They enter the body via inhalation, through breaks in the skin, or through the nasopharynx, and then spread hematogenously. In contrast to primary amebic meningoencephalitis, which is caused by N. fowleri, for a patient to become infected with Acanthamoeba there need not be a history of exposure to fresh water. Risk factors include any cause of immunocompromise, including HIV and/or AIDS, diabetes mellitus, malnutrition, and organ transplantation [1], [2], [3].
Patients present with pustules or firm, usually nontender nodules on the extremities, abdomen, thorax, or face that may ulcerate or develop abscesses, as in the presented case. Alternatively, patients with GAE may complain of headache, nuchal rigidity, nausea, vomiting, and/or focal neurologic deficits progressing over days to weeks or even months. The syndrome is nonspecific, overlapping with signs and symptoms of central nervous system (CNS) infections caused by bacteria, viruses, and fungi, confounding diagnosis. Cutaneous lesions and GAE may be found singly or in combination. Diagnosis is made by examination of tissue from brain or skin biopsy, frequently postmortem. A high degree of suspicion is required, as amebic cysts and trophozoites are easily missed using standard analytical approaches. PCR and real-time PCR methods also have been created. Alternatively, detection of a host immune response to the presence of Acanthamoeba spp. via indirect immunofluorescence may be suggestive [1], [2], [3].
Imaging findings of amebic infection are nonspecific, both in the CNS and in the skeleton [1], [2], [3]. The findings are similar to those of bacterial and fungal osteomyelitis, which can themselves present a broad spectrum of imaging findings that overlap significantly with imaging characteristics of many tumors. Although the lesions are generally lytic on radiographs, they may or may not be expansile, have associated periosteal reaction, or have sclerotic borders. There may be obliteration of adjacent fat planes and/or soft-tissue fluid collections. When osteomyelitis occurs at a joint, cartilage loss and joint effusion are expected but again are nonspecific [4], [5]. MRI findings typically include decreased T1 SI and increased T2 or short-tau inversion recovery SI in the involved bone marrow [4], [5], [6].
Cases of amebic osteomyelitis have been very rarely reported. The first 2 case reports involved AIDS patients and were published in the mid-1990s [7], [8]. These were followed by reported postmortem diagnosis of disseminated Acanthamoeba infection with osteomyelitis of a finger in a renal transplant patient [9]. In another case, a patient developed fungal rhinosinusitis several months after lung transplantation and was found to have Acanthamoeba cysts in tissue samples taken during sinus debridement surgery. This patient was successfully treated with a combination of amphotericin, voriconazole, and caspofungin [10].
Treatment for cutaneous amebiasis in the absence of GAE or other organ involvement consists of topical regimens including chlorhexidine gluconate and ketoconazole or itraconazole. In cases of CNS disease or other dissemination systemic therapy multidrug regimens of pentamidine, sulfadiazine, flucytosine, and fluconazole or itraconazole are used. Rifampin and azithromycin also have been prescribed [1], [2], [3], [7], [8], [9], [10]. Miltefosine (Impavido; Knight Therapeutics, Inc., Montreal, Quebec, Canada) is an agent that has been previously used for treatment of leishmaniasis. It has recently been used with some success for amebic infections and at the time of this writing is under investigation by the United States’ Centers of Disease Control and Prevention for treatment of infections with free-living amebae. Reportedly, there are preliminary indications that miltefosine offers survival advantage over other regimens [9]. Regardless of treatment approach, severe medication side effects are often a limiting factor [2], [3]. Even when the diagnosis is made antemortem, prognosis in cases of GAE or disseminated infection is poor, with mortality of over 90% [11]. The patient in our case has been treated with multidrug regimens both with and without miltefosine. Outpatient treatment with miltefosine failed due to intolerable gastrointestinal side effects; however, as an inpatient, she was able to receive miltefosine as well as flucytosine, amphotericin, and posaconazole in addition to topical ketoconazole and chlorhexidine gluconate, with routine antiemetic premedication before doses of miltefosine.
In summary, Acanthamebiasis is being increasingly recognized in patients with HIV/AIDS and other immunocompromised individuals. Prognosis in cases of disseminated disease with or without CNS involvement is poor. Signs and symptoms of Acanthamoeba infection, including imaging findings, overlap with those of more common infectious agents, necessitating a high degree of suspicion for diagnosis. When the disease has been recognized before development of GAE, occasional cases of successful treatment have been reported. Miltefosine is a therapeutic agent currently under investigation that has shown particular promise.
Footnotes
Disclosures: None.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Khan N.A. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 2.Visvesvara G.S. Infections with free-living amebae. In: Garcia H.H., Tanowitz H.B., Del Brutto O.H., editors. 114 (3rd series) Elsevier B.V.; New York, NY: 2009. pp. 153–168. (Neuroparasitology and Tropical Neurology. Handbook of Clinical Neurology). [DOI] [PubMed] [Google Scholar]
- 3.Visvesvara G.S., Moura H., Schuster F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Gold R.H., Hawkins R.A., Katz R.D. Bacterial osteomyelitis: findings on plain radiography, CT, MRI, and scintigraphy. AJR Am J Roentgenol. 1991;157:365–370. doi: 10.2214/ajr.157.2.1853823. [DOI] [PubMed] [Google Scholar]
- 5.Pineda C., Vargas A., Rodriguez A.V. Imaging of osteomyelitis: current concepts. Infect Dis Clin N Am. 2006;20:789–825. doi: 10.1016/j.idc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Unger E., Moldofsky P., Gatenby R., Hartz W., Broder G. Diagnosis of osteomyelitis by MR imaging. AJR Am J Roentgenol. 1988;150:605–610. doi: 10.2214/ajr.150.3.605. [DOI] [PubMed] [Google Scholar]
- 7.Murakawa G.J., McCalmont T., Altman J., Telang G.H., Hoffman M.D., Kantor G.R. Disseminated acanthamebiasis in patients with AIDS. A report of five cases and a review of the literature. Arch Dermatol. 1995;131(11):1291–1296. [PubMed] [Google Scholar]
- 8.Selby D.M., Chandra R.S., Rakusan T.A., Loechelt B., Markle B.M., Visvesvara G.S. Amebic osteomyelitis in a child with acquired immunodeficiency syndrome: a case report. Pediatr Pathol Lab Med. 1998;18:89–95. [PubMed] [Google Scholar]
- 9.Steinberg J.P., Galindo R.L., Kraus E.S., Ghanem K.G. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin Infect Dis. 2002;35(5):e43–e49. doi: 10.1086/341973. [DOI] [PubMed] [Google Scholar]
- 10.Vernon S.E., Acar B.C., Pham S.M., Fertel D. Acanthamoeba infection in lung transplantation: report of a case and review of the literature. Transpl Infect Dis. 2005;7:154–157. doi: 10.1111/j.1399-3062.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 11.Cope J.R. Vol. 666. 2013. Investigational drug available directly from CDC for the treatment of infections with free-living amebae.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6233a4.htm MMWR. Morbidity and Mortality Weekly Report. [accessed 03.03.16] [PMC free article] [PubMed] [Google Scholar]