Abstract
We report the uncommon case of an acute cavernous sinus syndrome in a patient who was consequently discovered to have both a cavernous internal carotid artery aneurysm and bacterial meningitis. Which came first, the chicken or the egg? Which of the two, the aneurysm or the meningitis, gave rise to the patient’s symptoms? We briefly reviewed the literature of similar cases and tried to analyze the possible pathophysiological relationship between these findings. Moreover, this case highlights the importance of a multidisciplinary management of these patients to better decide between a medical and a surgical and/or endovascular treatment.
Keywords: Cavernous sinus syndrome, Cavernous ICA aneurysm, Bacterial meningitis, MRI, DSA
Introduction
The cavernous sinus (CS) is a complex venous space surrounded by the dural folds, which contains important neurovascular structures (III, IV, VI nerves; first and second trigeminal branches; internal carotid artery [ICA], sympathetic plexus) and has a complex valveless venous communication system which involve directly or indirectly almost every important venous structure of the head and neck. Very different pathologic conditions, which include infective, inflammatory, vascular, and neoplastic diseases, can involve the CS and give rise to a CS syndrome (CSS) [1], [2], [3], [4].
We present the case of a patient with a facial Herpes Zoster (HZ) recurrence and an acute onset of a CSS, who was consequently discovered to have a cavernous ICA aneurysm at imaging examinations and a bacterial meningitis at a cerebral spinal fluid (CSF) examination. All these factors can be a cause of CSS; moreover, the cavernous ICA aneurysm can have an infective origin.
To decide between the most appropriate medical and surgical and/or endovascular treatment, we briefly reviewed the literature of similar cases and tried to analyze the possible pathophysiological relationship between these findings.
Case report
A 55-year-old woman was admitted to the emergency department with a 2-day story of worsening left headache and fronto-orbital pain, slightly responsive to drugs. She reported recurrent episodes of herpes labialis with concurrent migraine pain usually responsive to NSAIDs and a recent episode of HZ recurrence of the left side of the face, still under treatment with acyclovir.
Physical examination reported normal vital signs, oxygen saturation (98% in ambient air), body temperature (36.7°C), blood pressure, and pulse rate; at first, neurologic examination showed no cranial nerve deficit, but after a few hours, she suddenly developed a mild left eyelid ptosis. Pain was not responsive to intravenous ketoprofen or acetaminophen.
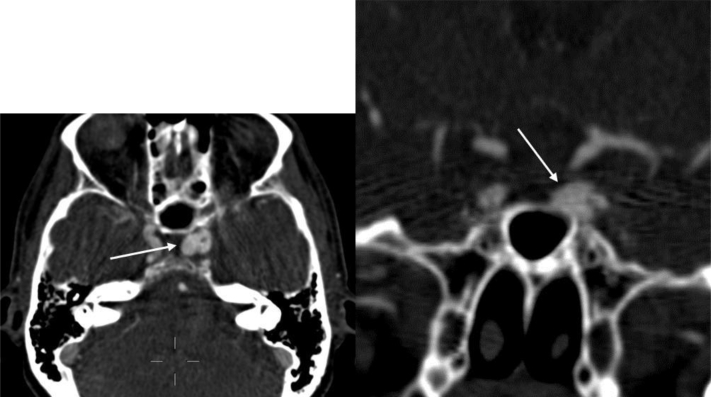
Despite a negative preliminary brain computed tomography (CT) examination, the headache was worsening therefore a CT angiography (CTA) study was performed, revealing an ICA aneurysm with intrasellar extension, ipsilateral to the facial pain side, also confirmed by a following digital subtraction angiography (Figs. 1 and 2).
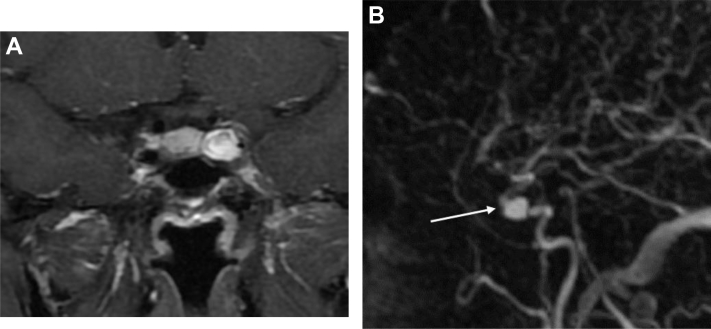
Fig. 1.
Axial and coronal CTA view showing the cavernous left ICA aneurysm (arrows), which is medially oriented and partially intrasellar.
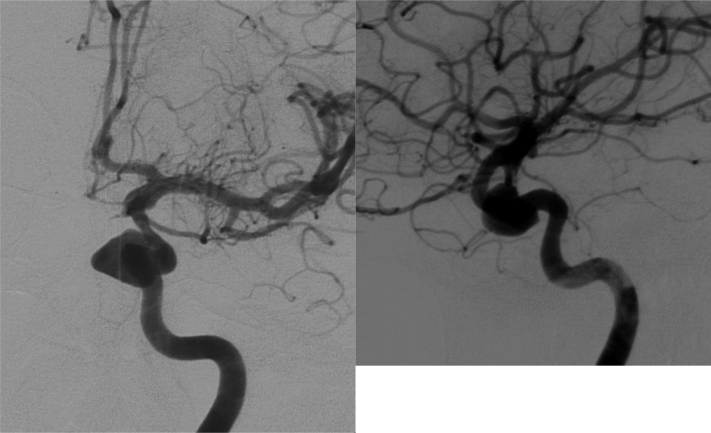
Fig. 2.
Digital subtraction angiography study (antero-posterior and latero-lateral projections) confirming the medial wall ICA aneurysm; the sac is irregular, medially oriented, with a maximum diameter of 12 mm.
An endovascular approach was planned but was immediately delayed because of a sudden clinical worsening associated with signs of sepsis: high fever (39°C), tachycardia (110 beats-per-minute), tachypnea (30 breaths-per-minute), decreased blood pressure (up to 90/60 mm Hg), and neck stiffness. CSF was turbid with hyperproteinorrhachia (171 mg/dL), hypoglycorrhachia (35 mg/dL), and neutrophil pleocytosis.
A brain magnetic resonance imaging (MRI) scan showed a partial aneurysmal sac thrombosis, an ipsilateral CS heterogenous enhancement, and a surrounding meningeal enhancement. A concomitant mild sphenoid sinus mucosal thickening was also noticed (Fig. 3).
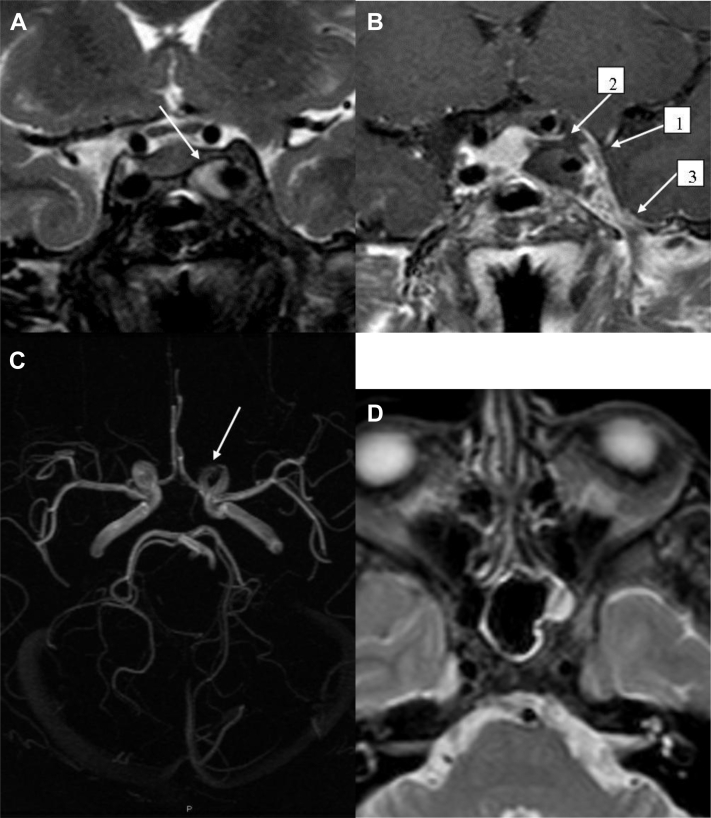
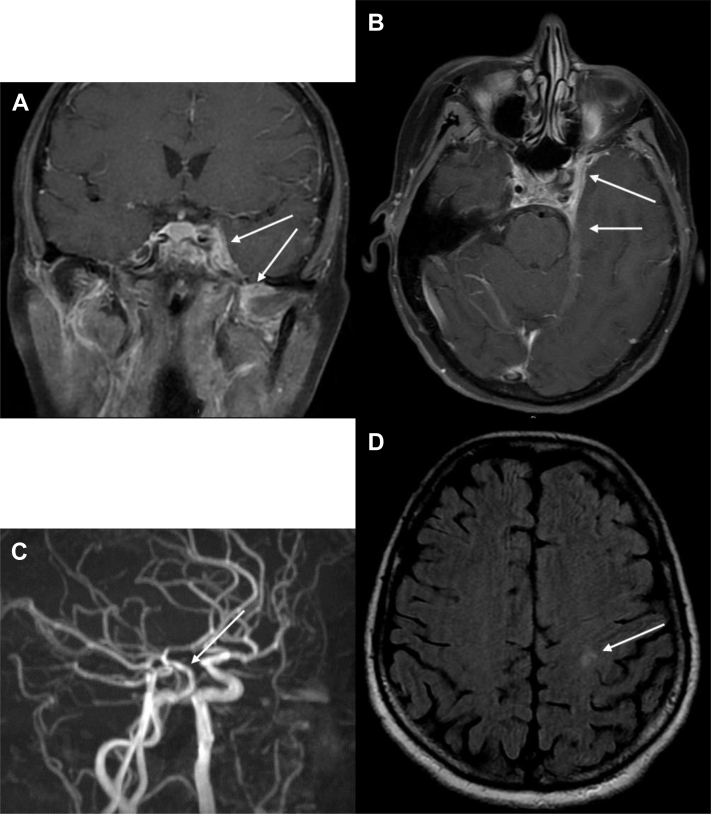
Fig. 3.
MRI coronal T2-w (A) showing homogeneous hyperintensity into the aneurysmal sac (arrow) without flow void signs, due to reduced blood flow or partial thrombosis; coronal T1-w after gadolinium with fat suppression (B) revealing an enlargement of the left CS with a convex lateral wall (arrow 1), a filling defect of its superior portion (arrow 2) and an enhancement of the surrounding meninges (arrow 3); 3-dimensional time-of-flight images (C) showing an irregular and narrow appearance of the cavernous and ophthalmic tract of the ICA (arrow); axial T2-w (D) revealing mild signs of left sphenoid sinus mucosal thickening.
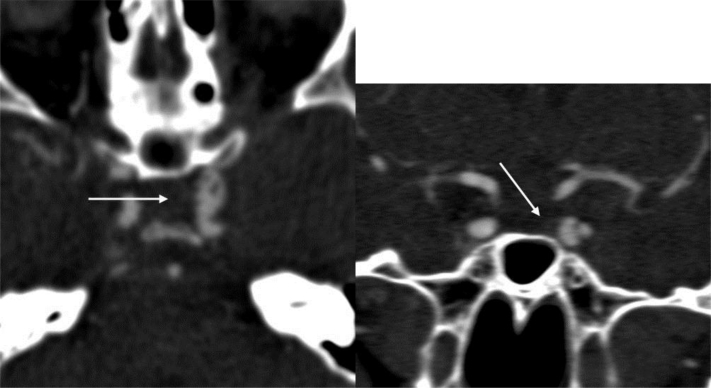
A second CTA scan confirmed the ongoing worsening, by revealing a reduced contrast opacification of the aneurysm due to thrombosis, and an evident irregular appearance of the ICA wall (Fig. 4).
Fig. 4.
Axial and coronal CTA view showing a partial thrombosis of the ICA aneurysm and irregularities of the ICA walls (arrows).
The patient was then hospitalized in the Infectious Disease Unit and started a treatment with ceftriaxone, levofloxacin, and dexamethasone according to our protocol for purulent meningitis. Blood investigations revealed peripheral leukocytosis (12,090/mmc with 85% of granulocytes) and an elevated C-reactive protein (13 mg/dL), without any other significant alterations.
During the following days, clinical conditions got better (the patient was afebrile, hemodynamic conditions restored, neck stiffness disappeared, headache improved with persisting mild eyelid ptosis). Microbiological tests performed resulted negative (blood cultures, CSF cultures, polymerase chain reaction for Neisseria meningitidis, Streptococcus pneumoniae, Lysteria monocytogenes, Haemophilus influenzae, and Mycobacterium tuberculosis, urinary pneumococcal antigen). However, antibiotic therapy was continued, whereas dexamethasone de-escalation was started.
An MRI scan performed a week later (Fig. 5) showed a reduction of the pathologic tissue surrounding the cavernous ICA aneurysm but revealed the appearance of small subclinic ischemic frontoparietal areas, a reduced contrast opacification of the aneurysm due to thrombosis, and increasing irregularities of the ICA walls. Because of the residual aneurysmal thrombosis, an anticoagulant therapy with LMWH was started; according to MR signs of cerebral arteritis and embolism, dexamethasone was readministered at high doses, ceftriaxone was replaced by linezolid because of the better CSF pharmacokinetics, and antiplatelet therapy with acetylsalicylic acid (100 mg/die) was started.
Fig. 5.
MRI coronal T1-w fat sat after gadolinium (A) showing a pathologic hyperintense tissue surrounding the cavernous ICA aneurysm and extending toward Meckel’s cave (superior arrow) and, inferior, to the infratemporal fossa (inferior arrow); axial T1-w fat saturation after gadolinium (B) showing a pathologic hyperenhancement of the ipsilateral pachimeninges (anterior arrow) and tentorium (posterior arrow); 3-dimensional time-of-flight (C) showing a further worsening of the irregular and narrow appearance of the intracranial siphon (arrow); fluid attenuation inversion recovery T2-w (D) images revealing the occurrence of left hemispheric small foci of subacute ischemia (arrow).
In the following days, blood examinations (white blood cell and c-reactive protein) and CSF examination returned completely normal. Antibiotic therapy was stopped, and the patient was discharged with cortisonic therapy.
A follow-up with serial MRI scans showed an ongoing improvement of the neuroradiologic signs (Fig. 6) with the persistence of the aneurysm and of the carotid artery siphon narrowing.
Fig. 6.
MRI coronal T1-w fat sat after gadolinium (A) showing the disappearance of the inflammatory enhancement of CS, meninges and infratemporal fossa, and the reperfusion of the aneurysm (maximum diameter of 9 mm); angiographic sequences time-resolved imaging of contrast kinetics (TRICKS) (B) confirming the aneurysmal blood flow and the persistence of intracranial carotid siphon narrowing (arrow).
Discussion
Patients with a CSS may have a combination of periorbital or hemicranial pain, opthalmoplegia and ptosis due to oculomotor (III), trochlear (IV) or abducens (VI) nerves involvement, or periarterial sympathetic plexus palsies (Horner syndrome), facial sensory loss in the territory of distribution of opthalmic (V1) and maxillary (V2) division of trigeminal nerve. A possible involvement of the venous drainage may give rise to chemosis, proptosis, and papilledema [1], [2], [3], [4].
These neurovascular structures are all contained inside the CS, a loculated venous space on either side of the sella turcica, surrounded by the meningeal and periosteal dural folds. As it can be found in literature, the CS can be affected by very different pathologic conditions, which include infective, inflammatory, vascular, and neoplastic diseases.
In our case, the first relevant imaging finding at CTA and digital subtraction angiography (Figs. 1 and 2) was the presence of an aneurysm of the cavernous segment of the left ICA. Therefore, as described before, a relationship between the aneurysm and the patient's signs and symptoms was presumed, and an endovascular treatment was planned.
Literature reports that cavernous aneurysms of the ICA are the most common lesions that may occur in the CS, with an incidence of 2%-5% of all intracranial aneurysm. They tend to be asymptomatic, but they may produce a CSS due to mass effect, inflammation, or rupture with subsequent carotid-cavernous fistula. Other symptoms include headaches, transient ischemic attacks, and thromboembolic strokes. Most of them are idiopathic, but they may have a traumatic or mycotic origin [1], [2], [4], [5], [6].
Mycotic aneurysms account for a minority of all intracranial aneurysms and only a small part of these occurs into the cavernous ICA. In general, mycotic intracranial aneurysms have a fusiform morphology, whereas those involving the cavernous segment of the ICA are often saccular, facilitating endovascular treatments. However, cavernous ICA aneurysms have multiple appearances on CT and MR images due to variable size, morphology, stage of thrombosis, calcifications, and flow velocity [2], [7]. Our patient’s aneurysmal sac was irregular shaped, medially oriented, with a maximum diameter of 12 mm, with a contrast medium stasis in the venous phase; therefore, it is quite impossible to establish with certainty its etiology only on morphology basis.
The patient’s unexpected clinical aggravation (left ptosis, fever, and blood flogosis markers) revealed an underlying or concomitant disease, which led to the suspension of the endovascular treatment, and to the execution of an MRI and a CSF examination.
MR is useful in detecting and defining the extent of lesions of the CS. Three-dimensional heavily T2-weighted and thin (3 mm) T1-weighted contrast-enhanced sequences are needed for accurate definition. MR angiography helps in visualizing ICA anomalies (eg, aneurysm, stenosis). Combining clinical and MRI findings helps in making a proper diagnosis in most cases [1], [2], [3]. In fact, MRI examination revealed the appearance of a partial thrombosis of the aneurysmal sac[8], well-showed in T2-weighted images and an enlargement of the left CS with a heterogeneous enhancement in T1-weighted images after contrast medium administration. Postcontrast MRI also showed enhancement of the surrounding pachimeninges. A thin mucosal thickening of the left sphenoidal sinus was also noticed (Fig 3A, B, and D). These findings supported the hypothesis of a CS involvement and thrombosis. Furthermore, the subsequent CTA confirmed the onset of the aneurysmal thrombosis and also showed an irregular and stenotic carotid siphon, suggesting an ICA wall inflammation (Fig. 4).
CS thrombosis is a rare but potentially lethal disease. It can be due to aseptic causes such as trauma, surgery, tumor growth, hematologic disease, hypercoagulability states, or vascular conditions like aneurysm or carotid-cavernous fistula. A frequent early complication of CS thrombosis is reported to be a cavernous carotid arterial wall inflammation with narrowing and subsequent arterial ischemia or venous drainage thrombosis [9], [10]. In our case, there were neither radiological nor clinical signs of carotid-cavernous fistula; moreover, the CSF examination revealed a purulent meningitis.
However, despite its decreasing incidence and more benign course since antibiotic availability, one of the most frequent cause of CS thrombosis is septic involvement due to locoregional infections, mainly spreading from sinonasal cavities, followed by orbit and facial cutaneous infections, with Staphylococcus aureus as the most common organism found [4], [5], [9], [11], [12], [13]. The CS, in fact, has a complex valveless venous communication system which involve directly or indirectly almost every important venous structure of the head and neck. These connections play an important role in symptomatology and provide pathways for infections from extracranial to intracranial sites: a septic thrombophlebitis may reach the CS by direct retrograde extension or venous embolism [4], [9], [13].
In this case, we can exclude a bacterial sinusal origin of the CS thrombosis because the patient’s paranasal sinus and nasal cavities were almost normal, except for a thin mucosal thickening of the left sphenoidal sinus. The presence of a HZ facial recurrence is instead more suspicious [14]. Unfortunately, all microbiologic tests performed resulted negative.
Literature suggests that mycotic aneurysms formation may have 2 possible mechanisms: intravascular, which usually implies septic embolism and focal arteritis with a rapid destruction of the internal elastic membrane; extravascular, which is mainly due direct spread from contiguous foci of infection of low-virulence organisms that have enough time to reach the vessel intima and start the microvascular changes that lead to the aneurysm formation without significant clinical manifestation [15].
As for this case, we may think that the most likely hypothesis is that of a pre-existing cavernous ICA aneurysm with an intercurrent infection (A locoregional HZ virus infection? or A bacterial infection due to an idiopathic temporary immunosuppression? or A less probable sinonasal infection?) resulting in a CS thrombosis, carotid inflammation, and spreading of the infection toward the intracranial compartment and the development of a meningitis. On the other hand, we may have the hypothesis of the direct formation of a cavernous ICA aneurysm due to a CS infection, with a related ICA wall inflammation and vasculitis, and intracranial spread of the pathogen resulting in meningitis. Unfortunately, we are unable to date the aneurysm formation with confidence without previous imaging studies. Therefore, we can not define with certainty the aneurysm as mycotic.
Furthermore, it is not possible to say if the symptoms at the onset were related either to the presence of the aneurysm itself or if they were due to a mild CS inflammation that subsequently involved the siphon and the pre-existing aneurysm. In our opinion, the second hypothesis is more probable because of the bacterial meningitis demonstration and because of the prompt response to the antibiotic therapy with a reduction of the pathologic tissue surrounding the cavernous ICA aneurysm as shown in Fig. 5.
However, we learned that in the context of a CSS, the finding of a carotid aneurysm should be carefully evaluated as the only possible cause for the symptoms.
Eventually, according to literature, if an infectious vasculitis is suspected the surgical and/or endovascular treatment of a cavernous ICA aneurysm should be delayed until the antibiotic therapy eradicates the infection, to decrease the risk of iatrogenic rupture [15]. In our case, the medical treatment consisted of a prompt antibiotic therapy, directed at the primary infection source according to the infectious disease unit protocol, and an early anticoagulation with low-molecular-weight heparin, which have been reported to decrease mortality and morbidity [16]. Moreover, anticoagulation with low-molecular-weight heparin is widely considered the standard initial therapy for patients with cerebral venous thrombosis [17].
Conclusions
The CS is a complex anatomic landmark in the brain. Despite the wide range of different disorders that can affect it, the clinical presentation is almost unique, named CSS. Imaging, in conjunction with clinical history, is an invaluable instrument in diagnosing the underlying cause of a CSS. Yet, the imaging finding of a possible cause of CSS should not lower the attention to the presence of other concomitant disorders. As our experience shows, sometimes it is not really possible to define an exact and unique cause for a CSS, in particular when multiple possible etiologic factors are involved. However, due to the multiple structures and pathologies involved in this complex venous space, a multidisciplinary management of these patients is mandatory to better define the medical or the surgical and/or endovascular therapy.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Federico Sacchetti and Silvia Stagni contributed equally.
References
- 1.Tang Y., Booth T., Steward M., Solbach T., Wilhelm T. The imaging of conditions affecting the cavernous sinus. Clin Radiol. 2010;65(11):937–945. doi: 10.1016/j.crad.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Bakan A.A., Alkan A., Kurtcan S., Aralaşmak A., Tokdemir S., Mehdi E. Cavernous sinus: a comprehensive review of its anatomy, pathologic conditions, and imaging features. Clin Neuroradiol. 2015;25(2):109–125. doi: 10.1007/s00062-014-0360-0. [DOI] [PubMed] [Google Scholar]
- 3.Ruscalleda J. Imaging of parasellar lesions. Eur Radiol. 2005;15:549–559. doi: 10.1007/s00330-004-2628-2. [DOI] [PubMed] [Google Scholar]
- 4.Abdel Razek A.A.K., Castillo M. Imaging lesions of the cavernous sinus. AJNR Am J Neuroradiol. 2009;30:444–452. doi: 10.3174/ajnr.A1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korchi A.M., Cuvinciuc V., Caetano J., Becker M., Lovblad K.O., Vargas M.I. Imaging of the cavernous sinus lesions. Diagn Interv Imaging. 2014;95(9):849–859. doi: 10.1016/j.diii.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Pant B., Joshi H.C., Isser D.K. Atypical presentation of cavernous sinus infection with intracavernous ICA aneurysm. Indian J Otolaryngol Head Neck Surg. 2014;66(1):118–121. doi: 10.1007/s12070-012-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun J.Y., Smith W., Halbach V.V., Higashida R.T., Wilson C.B., Lawton M.T. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001;48:1203–1213. doi: 10.1097/00006123-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo M., Scomazzoni F., Cirillo L., Cadioli M., Simionato F., Iadanza A. Comparison of 3D TOF-MRA and 3D CE-MRA at 3T for imaging of intracranial aneurysms. Eur J Radiol. 2013;82(12):e853–e859. doi: 10.1016/j.ejrad.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 9.Schuknecht B., Simmen D., Yuksel C., Valavanis A. Tributary venosinus occlusion and septic cavernous sinus thrombosis: CT and MR findings. AJNR Am J Neuroradiol. 1998;19:617–626. [PMC free article] [PubMed] [Google Scholar]
- 10.Pinardi F., Stracciari A., Spinardi L., Guarino M. Postpneumococcal Moyamoya syndrome case report and review of the postinfective cases. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellie E., Houang B., Louail C., Legrain-Lifermann V., Laurent F., Drouillard J. CT and high-field MRI in septic thrombosis of the cavernous sinuses. Neuroradiology. 1992;34:22–24. doi: 10.1007/BF00588427. [DOI] [PubMed] [Google Scholar]
- 12.Sakaida H., Kobayashi M., Ito A., Takeuchi K. Cavernous sinus thrombosis: linking a swollen red eye and headache. Lancet. 2014;384(9946):928. doi: 10.1016/S0140-6736(14)61404-5. [DOI] [PubMed] [Google Scholar]
- 13.Ebright J.R., Pace M.T., Niazi A.F. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001;161(22):2671–2676. doi: 10.1001/archinte.161.22.2671. [DOI] [PubMed] [Google Scholar]
- 14.Khatri I.A., Wasay M. Septic cerebral venous sinus thrombosis. J Neurol Sci. 2016;362:221–227. doi: 10.1016/j.jns.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Ghali M.G., Ghali E.Z. Intracavernous internal carotid artery mycotic aneurysms: comprehensive review and evaluation of the role of endovascular treatment. Clin Neurol Neurosurg. 2013;115(10):1927–1942. doi: 10.1016/j.clineuro.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Lemos J., Eggenberger E. Neuro-ophthalmological emergencies. Neurohospitalist. 2015;5(4):223–233. doi: 10.1177/1941874415583117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutinho J.M., Ferro J.M., Canhão P., Barinagarrementeria F., Bousser M.G., Stam J. ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575–2580. doi: 10.1161/STROKEAHA.110.588822. [DOI] [PubMed] [Google Scholar]