Abstract
Schwannomas are benign tumors arising from the peripheral nerve sheath, commonly occurring in the head, neck, and extensor surfaces of the extremities. They can be associated with neurofibromatosis type II. Our case describes a 48-year-old woman with a 2-week history of a left-sided palpable breast mass. She was referred to radiology, where additional imaging revealed a 1.1-cm mass. A biopsy was performed; histology revealed an intramammary schwannoma. Mammography findings include a well-defined mass without calcification. Ultrasound images have shown hypoechoic, encapsulated, and well-defined lesions without calcification. Histologically, schwannomas reveal alternating Antoni A and Antoni B cellular areas. Schwannomas are also S100-positive on immunohistochemistry. This case is best categorized as a BI-RADS 4A lesions. This case report highlights the importance of both imaging and pathology in the diagnosis of breast neoplasms. Although breast schwannomas are not a common entity, they are an important consideration when evaluating a breast mass.
Keywords: Breast, Intramammary, Schwannoma, Palpable breast mass, Mammography, Ultrasound
Introduction
Schwannomas are benign lesions that arise from schwann cells of the peripheral nerve sheath [1]. They can arise from any nerve in the body and are commonly found in the extremities, head, and neck region [2]. These slow-growing neoplasms commonly affect young adults and present during the third decade of life [3]. Although schwannomas are common, palpable breast lumps that manifest as intramammary schwannomas are extremely rare with a limited amount of cases reported in literature [3]. With this in mind, we present a unique case of a patient who presented with a palpable breast mass and underwent an ultrasound-guided biopsy, which demonstrated atypical findings consistent with a breast schwannoma.
Case report
A 48-year-old woman with no significant medical history, including a negative history of neurofibromatosis type II (NF II), presented to her primary care physician with a 2-week history of a palpable left-sided breast mass that was incidentally found on self breast examination. At the time of the evaluation, a palpable mass was appreciated, and she was referred to radiology for further diagnostic imaging.
The radiologist performed the diagnostic evaluation. The mammogram revealed a 1.1-cm oval-shaped focal asymmetry with an indistinct margin in the left breast at the 6-o'clock posterior depth (Figs. 1 and 2). Because the etiology of the mass was inconclusive, further imaging was recommended. An ultrasound of the left breast was performed and revealed 4 distinct masses. A 1.6 cm mass was found at the 7 o'clock position, for which ultrasound guided core biopsy was recommended as this lesion fell under the category of Breast Imaging-Reporting and Data System (BI-RADS) 4: a lesion suspicious for malignancy (Fig. 3). Additional findings included 1-cm, 9-mm, and 6-mm masses, which were considered to be benign, and therefore, no follow-up for these lesions was required.
Fig. 1.
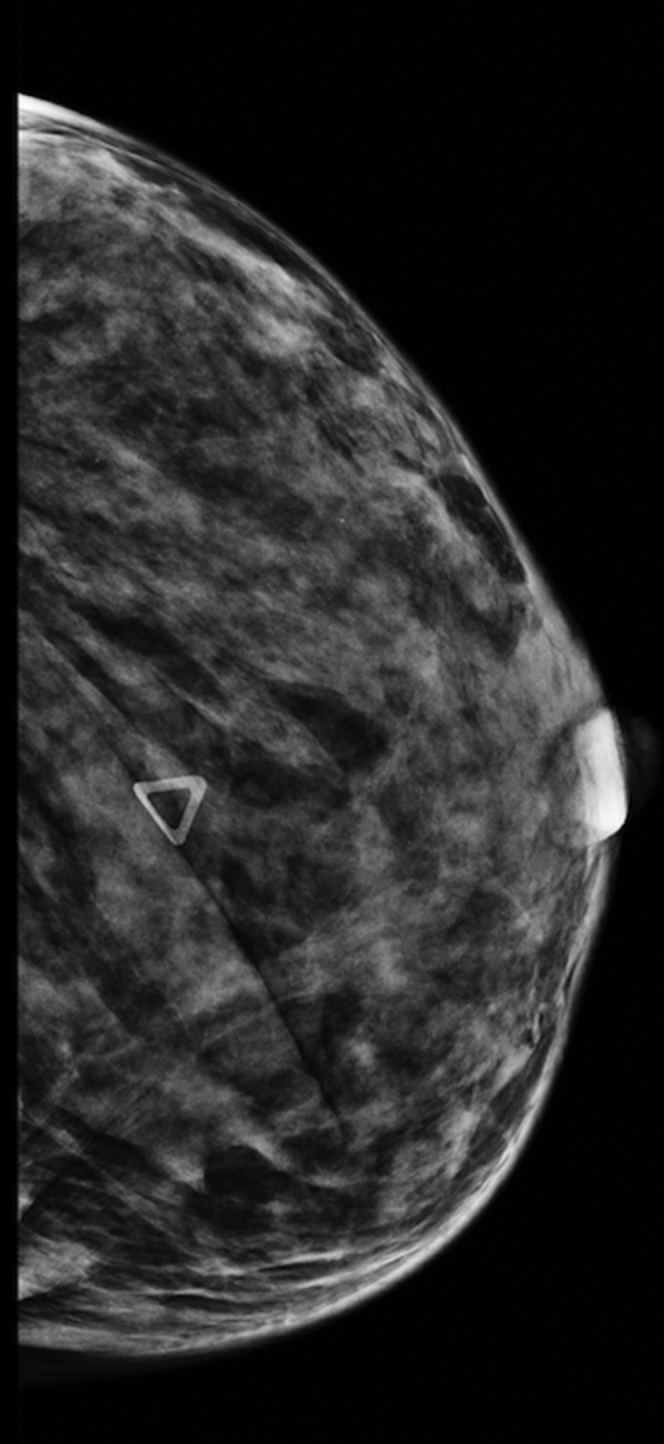
Mammogram: Mammogram (craniocaudal) showing an under-skin marker at the site of palpable abnormality. There is an ill-defined soft-tissue asymmetry, which blends with the rest of the breast tissue.
Fig. 2.
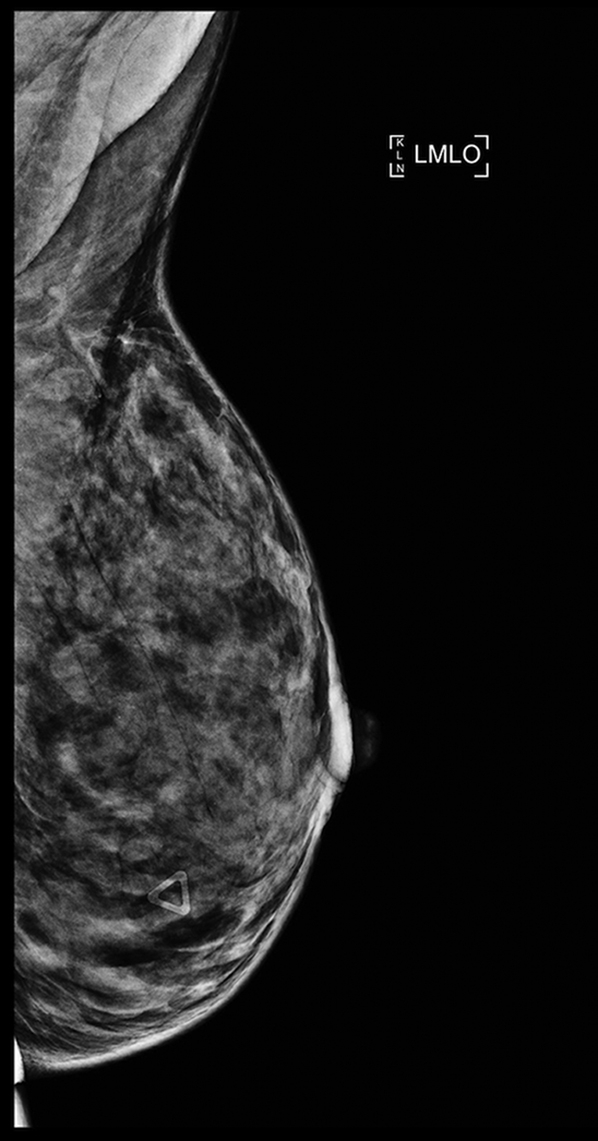
Mammogram: Mammogram in mediolateral-oblique view.
Fig. 3.
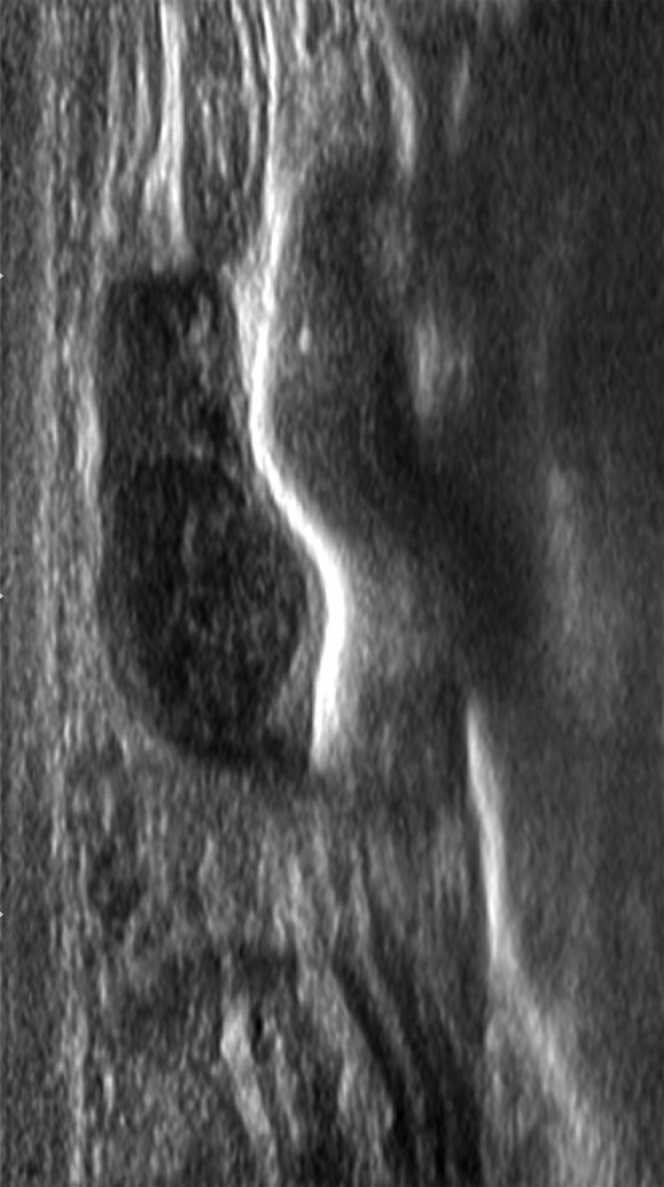
Breast ultrasound. At the site of palpable abnormality in the left breast, there is a bilobed lesion 9.6-mm hypoechoic, well defined, and encapsulated against the chest wall. There is no evidence of calcifications.
An ultrasound-guided core needle biopsy was done using a 14-gauge needle (Fig. 4). Four specimens were obtained using an Achieve automated firing device and were sent to the laboratory for pathologic analysis. Postmammogram imaging was done, but the biopsy clip was placed too far posterior as reported in the report by the radiologist.
Fig. 4.
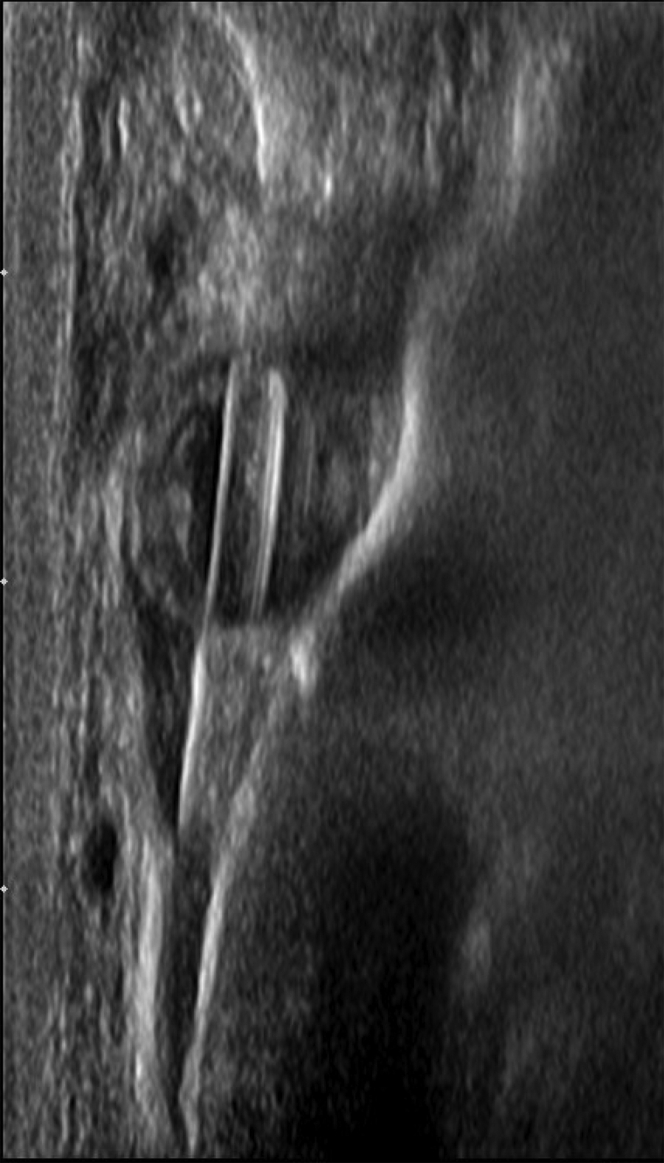
Ultrasound-guided core biopsy. Percutaneous ultrasound-guided biopsy of the left breast palpable lesion with a needle in the mass. There is a 14-gauge Achieve core needle in the mass.
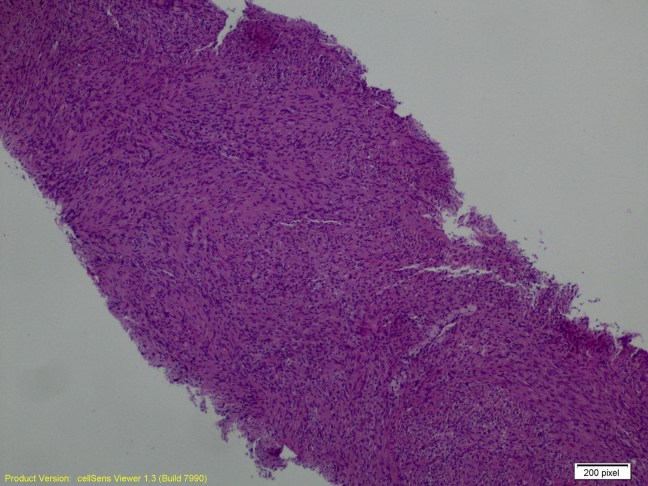
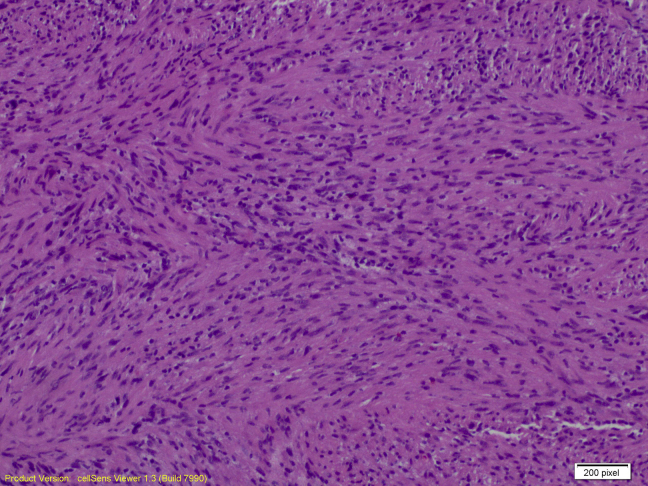
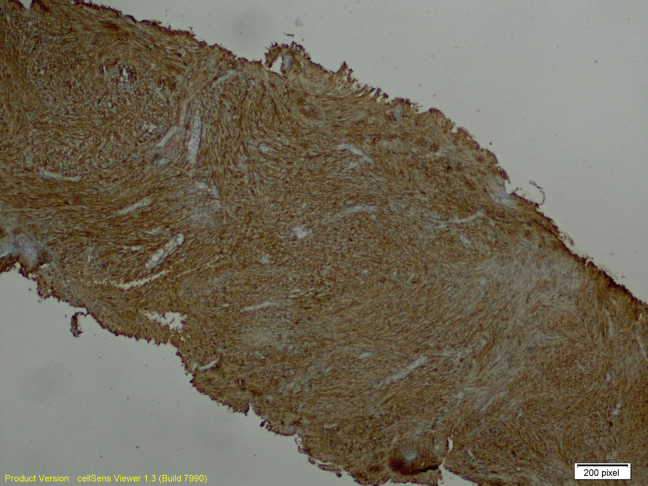
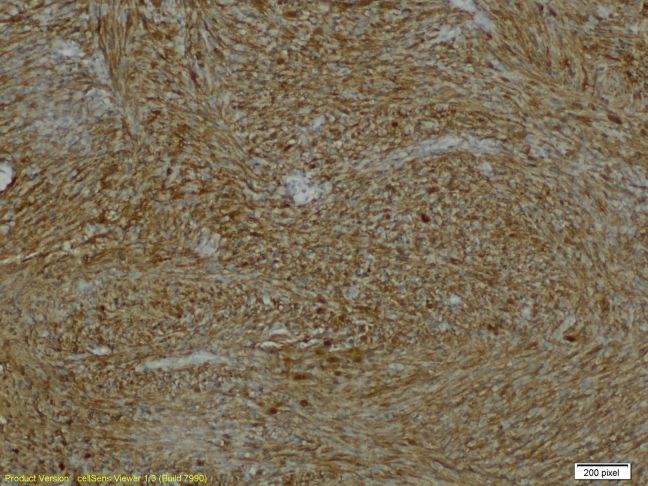
The ultrasound-guided core biopsy sampled benign breast tissue and a lesion characterized by intersecting fascicles of spindled cells with bland oval nuclei. There is no significant pleomorphism, necrosis, or mitotic activity (Figs. 5 and 6). Spindle cell lesions of the breast have a broad differential diagnosis, including superficial nodular fasciitis, desmoid fibromatosis, myofibroblastic lesions, stromal components of a phyllodes tumor, spindle cell carcinoma, and schwannomas. These cells in the core biopsy are diffusely and strongly positive for S100 protein but negative for cytokeratin AE1/AE3, P63, desmin, actin, CD10, and CD117 on immunohistochemical studies (Figs. 7 and 8). These support neural differentiation, which when combined with the morphology is characteristic of a breast schwannoma.
Fig. 5.
Breast schwannoma at 40×, hematoxylin and/or eosin stain.
Fig. 6.
Breast schwannoma at 100×, hematoxylin and/or eosin stain.
Fig. 7.
Positive S100 immunohistochemical study at 40×.
Fig. 8.
Positive S100 immunohistochemical study at 100×.
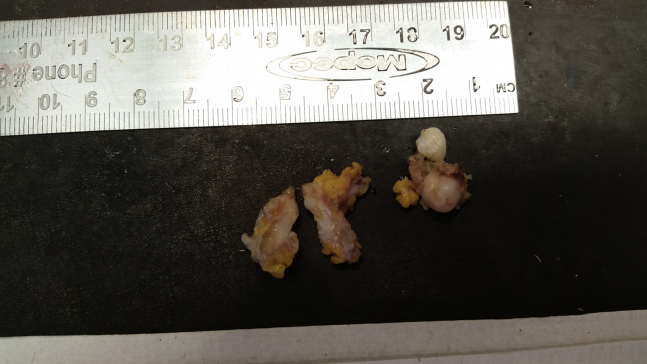
The gross specimen obtained after surgical excision is noted in Figure 9. No further pathology was done after excision.
Fig. 9.
Gross specimen of breast schwannoma after surgical excision.
Discussion
Schwannomas are the most common tumors arising from the Schwann cells comprising the nerve sheath [4]. As a whole, there have been a handful of reported breast schwannoma cases that have been generally benign, although there have been a few cases demonstrating malignant transformation [5]. In addition, they are known to have an association with NF II, although only 3% of schwannomas occur as a part of NF II, while 90% occur sporadically, as it did in this patient [6]. When they arise in the chest, they most commonly arise in the posterior aspect of the mediastinum. In this patient, it was clear that instead of presenting in the mediastinal area of the chest, the palpable mass proved to be originating in the breast tissue itself [6].
Histologically, schwannomas are usually comprised of 2 areas—Antoni A and Antoni B areas. Antoni A areas are orderly areas made up of compact spindle cells, indistinct cytoplastmic borders, and possibly intranuclear vacuoles. Antoni B areas, on the other hand, are less orderly and less cellular than Antoni A areas. Our findings from the fine needle biopsy demonstrate the presence of these patterns on analysis. This was further confirmed by the S-100 positivity demonstrating the presence of a schwannoma [6].
A handful of other cases of breast schwannomas have been reported, with many using both mammography and follow-up ultrasound as the used imaging techniques [7]. Ultrasound imaging of schwannomas are commonly defined as hypoechoic and well defined, as in our patient [2], [7]. As with our case report, other case reports also defined the lesion as encapsulated and without calcifications [3], [8], [9]. Mammography findings are not as descriptive as ultrasound findings in the literature, although commonly used terms also include well defined without microcalcifications [7], [9].
Management for intramammary schwannomas follows the general principles of benign breast tumor management. According to Kopans' Breast Imaging, lesions that appear to be benign may be followed up every 6 months for 2 years. If the lesion remains stable over the 2 years, then the patient may return to routine annual screening procedures. Kopan's also notes hat any non-cyst mass above 8 mm should have a needle or excisional biopsy to determine its histology, as done in this patient [3], [10]. According to Shin et al. [4], excisional biopsy is the only required therapy.
In clinical practice, a breast schwannoma is considered a BI-RADS 4A lesion (a lesion that has low suspicion for malignany) as they have a very low risk of malignant transformation such that it may be ignored [5]. BI-RADS 4A lesions are normally followed up with biopsy, as was done in this patient. Management after biopsy is done on a case-by-case basis, but patients may choose to return to routine screening mammograms as the lesion has a low risk of malignant transformation.
Conclusions
Breast schwannomas are a relatively rare entity, comprising 2.6% of all schwannomas [4]. When evaluating these masses, this case report demonstrates the importance of including breast schwannoma as a part of the differential diagnosis for palpable breast masses during routine diagnostic mammography. It is important to recognize that imaging modalities can narrow the differential, but the diagnosis can only be established via confirmatory testing with biopsy.
Clinical practice points
-
●
Schwannomas are encapsulated tumors of the nerve sheath most commonly occurring in the head and neck regions as well as the extensor surfaces of the extremities.
-
●
The breast is mostly supplied by 2 nerves, the anterior and lateral cutaneous branches of the thoracic intercostal nerves, while also being innervated by the cervical plexus.
-
●
Intramammary schwannomas make up only 2.6% of all schwannomas.
-
●
Malignant transformation of any schwannoma is extremely rare; however, it has been identified in a number of cases in the literature.
-
●
When a schwannoma presents as an intramammary lesion, it is worked up as a breast mass using the usual breast imaging of mammography, ultrasound, and possible magnetic resonance imaging if considered necessary by the radiologist.
-
●
Biopsy is necessary to make the diagnosis, as the nature of the tumor cannot be confirmed by imaging alone.
-
●
On biopsy, a schwannoma is histologically characterized as alternating Antoni A and Antoni B areas as well as a strong presence of positive S-100 protein on immunohistochemistry.
-
●
Because schwannomas are generally benign entities with low malignant potential, they are regarded as BI-RADS 4A lesions. If a patient does not want to excise the tumor, it may be watched, and the patient may return to annual screening mammogram regulations.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Dialani V., Hines N., Wang Y., Slanetz P. Breast schwannoma. Case Rep Med. 2011;2011:930841. doi: 10.1155/2011/930841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho K.S., Choi H.Y., Lee S.W., Sung S.H. Sonographic findings in solitary schwannoma of the breast. J Clin Ultrasound. 2001;29(2):99–101. doi: 10.1002/1097-0096(200102)29:2<99::AID-JCU1005>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Tan Q.T., Chuwa E.W.L., Chew S.H., Hong G.S. Schwannoma: an unexpected diagnosis from a breast lump. J Surg Case Rep. 2014;2014(9):4. [Google Scholar]
- 4.Shin S., Rabban J. Elsevier; Philadelphia, PA: 2012. Breast Pathology: Mesenchymal Neoplasms of the Breast; p. 31. [Google Scholar]
- 5.Nayler S.J., Leiman G., Omar T., Cooper K. Malignant transformation in a schwannoma. Histopathology. 1996;29(2):189–192. doi: 10.1046/j.1365-2559.1996.d01-491.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldblum J.R., Folpe A.L., Weiss S.W., Enzinger F.M. 6th ed. Vol. xiv. Saunders/Elsevier; Philadelphia, PA: 2014. p. 1155. (Enzinger and Weiss's soft tissue tumors). [Google Scholar]
- 7.Fujii T., Yajima R., Morita H., Tsutsumi S., Asao T., Kuwano H. A rare case of anterior chest wall schwannoma masquerading as a breast tumor. Int Surg. 2014;99(3):196–199. doi: 10.9738/INTSURG-D-13-00145.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellezza G., Lombardi T., Panzarola P., Sidoni A., Cavaliere A., Giansanti M. Schwannoma of the breast: a case report and review of the literature. Tumori. 2007;93(3):308–311. [PubMed] [Google Scholar]
- 9.Uchida N., Yokoo H., Kuwano H. Schwannoma of the breast: report of a case. Surg Today. 2005;35(3):238–242. doi: 10.1007/s00595-004-2904-4. [DOI] [PubMed] [Google Scholar]
- 10.Kopans D.B. Breast imaging. 3rd ed. Lippincott Williams & Wilkins; Philadelphia, PA; London: 2007. [Google Scholar]