Abstract
Clonal haematopoiesis is thought to be a rare condition that increases in frequency with age and predisposes individuals to haematological malignancy. Recent studies, utilizing next-generation sequencing (NGS), observed haematopoietic clones in 10% of 70-year olds and rarely in younger individuals. However, these studies could only detect common haematopoietic clones—>0.02 variant allele fraction (VAF)—due to the error rate of NGS. To identify and characterize clonal mutations below this threshold, here we develop methods for targeted error-corrected sequencing, which enable the accurate detection of clonal mutations as rare as 0.0003 VAF. We apply these methods to study serially banked peripheral blood samples from healthy 50–60-year-old participants in the Nurses' Health Study. We observe clonal haematopoiesis, frequently harbouring mutations in DNMT3A and TET2, in 95% of individuals studied. These clonal mutations are often stable longitudinally and present in multiple haematopoietic compartments, suggesting a long-lived haematopoietic stem and progenitor cell of origin.
 Clonal haematopoiesis has been thought to occur in less than 10% of individuals younger than 70 years old. Here, the authors use an error corrected next-generation sequencing method to find clonal haematopoiesis in the peripheral blood of 19 of 20 healthy 50–70 year old individuals.
Clonal haematopoiesis has been thought to occur in less than 10% of individuals younger than 70 years old. Here, the authors use an error corrected next-generation sequencing method to find clonal haematopoiesis in the peripheral blood of 19 of 20 healthy 50–70 year old individuals.
The advent of cost-effective, next-generation sequencing (NGS) has permitted in-depth analysis of the spectrum of somatic mutations driving clonal evolution in malignancy1,2,3. Subsequently, benign clonal haematopoiesis was identified in healthy individuals4,5,6,7. Recent studies revealed that malignant and benign haematopoietic clones frequently harbour mutations in the epigenetic modifiers DNMT3A and TET2 (refs 1, 8, 9, 10, 11). Benign clones were rarely detected before 60 years old, but were detected in 10–20% of individuals older than 70 years old8,9,10,11. While compelling, these previous studies could only detect common clonal mutations—greater than 0.02 variant allele fraction (VAF)—due to the NGS error rate. Haematopoietic clones detected above this 0.02 VAF threshold have been termed clonal haematopoiesis of indeterminate potential (CHIP) and are associated with an increased risk of the developing haematological malignancy12.
Recently, the development of error-corrected sequencing (ECS) using single molecule tagging with unique molecular identifiers has permitted the detection of rare variants below the error rate of NGS13,14,15,16,17,18. Here we combined ECS with targeted capture for 54 genes, recurrently mutated in acute myeloid leukaemia (AML) to enable the detection of clonal mutations at VAFs two orders of magnitude lower than the detection limit of NGS. Using these methods, we sought to thoroughly describe the prevalence and mutation profile of rare haematopoietic clones in healthy individuals, determine if these clones are stable longitudinally, and determine if clonal mutations arise in long-lived haematopoietic stem and progenitor cells (HSPCs) or in more committed progenitors. We studied clonal haematopoiesis in longitudinally banked blood samples from middle-aged healthy participants in the Nurses' Health Study (NHS). We found clonal haematopoiesis, predominantly harbouring mutations in DNMT3A and TET2, in 95% of individuals studied. Many clonal mutations were stable longitudinally and detected in both myeloid and lymphoid lineages, suggesting they arose in long-lived HSPCs.
Results
Samples
We obtained paired buffy coat blood samples, banked ∼10 years apart, from 20 healthy participants in the NHS (Methods; Table 1)—a cohort of 121,701 female registered nurses longitudinally studied since 1976 (refs 19, 20, 21). The median ages at sample collection were 56.6 and 68.1 years old. This facilitated the investigation of benign clonal haematopoiesis in younger individuals, previously thought to only rarely harbour haematopoietic clones8,9,10,11,12. To identify haematopoietic clones, we combined ECS with targeted capture for 568 amplicons in 54 genes frequently mutated in AML (Methods)14,15,16,17. This enabled us to sequence a tractable subset of the genome, while still querying loci associated with clonal haematopoiesis and AML. Samples were prepared and sequenced in duplicate. We generated an average of 47.7 million paired-end sequencing reads, which yielded an average of 3.4 million error-corrected consensus sequences (ECCSs), per library (Supplementary Table 1).
Table 1. Age at sample collection for each NHS participant.
| Participant ID | Collection 1 age (years) | Collection 2 age (years) |
|---|---|---|
| 1 | 53.5 | 64.6 |
| 2 | 51.2 | 63.0 |
| 3 | 52.3 | 64.4 |
| 4 | 53.4 | 64.2 |
| 5 | 52.2 | 64.4 |
| 6 | 57.9 | 69.2 |
| 7 | 60.1 | 71.4 |
| 8 | 56.5 | 68.5 |
| 9 | 58.0 | 69.0 |
| 10 | 54.7 | 66.9 |
| 11 | 63.5 | 74.5 |
| 12 | 56.4 | 67.3 |
| 13 | 56.6 | 68.5 |
| 14 | 60.1 | 71.8 |
| 15 | 57.6 | 67.7 |
| 16 | 54.1 | 65.4 |
| 17 | 51.7 | 63.1 |
| 18 | 65.1 | 76.2 |
| 19 | 64.0 | 75.1 |
| 20 | 62.8 | 74.6 |
Error-corrected NGS
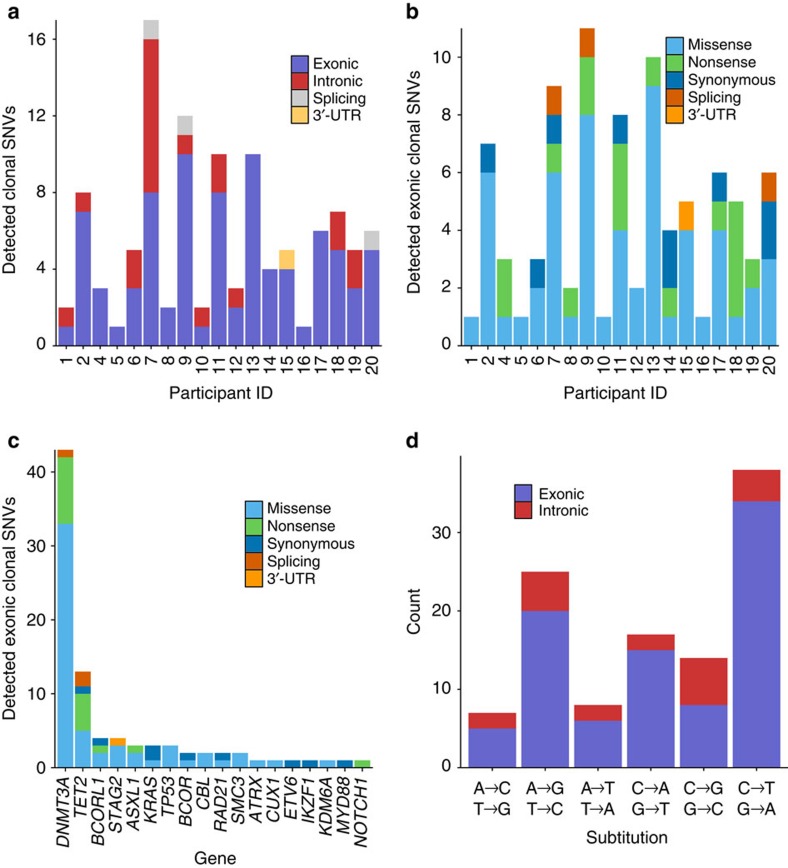
We modelled position-specific errors in the ECCSs using binomial statistics to identify clonal mutations (Methods). We identified 109 clonal single nucleotide variants (SNVs) in at least one time point <0.2 VAF in 95% (19/20) of individuals. We detected 1–17, mostly exonic, SNVs per individual at 0.0003–0.1451 (median 0.0024) VAF (Fig. 1a; Supplementary Table 2). Of note, the median VAF we observed was 10-fold less than the detection limit governing previous studies of clonal haematopoiesis8,9,10. Separately, we identified nine clonal insertion/deletion variants (indels) in six individuals (Supplementary Table 3). Indels were identified by ECCS coverage alone, as indel errors were not appropriately modelled by the same statistical framework implemented to identify SNVs.
Figure 1. Number and characteristics of clonal SNVs detected by ECS in the peripheral blood of healthy adult nurses.
(a) Clonal SNVs detected in each individual, colour-coded by annotation. (b) Exonic clonal SNVs detected in each individual, colour-coded by predicted effect. (c) Detected exonic clonal SNVs organized by gene, colour-coded by predicted effect. (d) Distribution of substitution types observed in clonal SNVs.
We were initially concerned that most of the identified rare variants were artefacts introduced during library preparation or sequencing. We first determined that SNV calls were not biased by coverage per amplicon (Supplementary Fig. 1) or by the number of bases captured per gene (Supplementary Fig. 2). Next, we validated these findings using droplet digital PCR (ddPCR)—an orthogonal non-sequencing-based technique for VAF quantification. We designed ddPCR assays for 21 SNVs that had been previously observed in malignancy22 and for one indel (Methods; Supplementary Fig. 3). The VAFs measured by ECS and ddPCR were highly correlated (R2=0.98; Supplementary Fig. 4; Supplementary Table 4), consistent with the previously observed accuracy of ECS17.
We next compared the mutation profile observed in these rare haematopoietic clones to previous findings in CHIP and AML. We detected 88 exonic clonal SNVs with 58 missense SNVs, 17 nonsense SNVs, 9 synonymous SNVs, 3 splicing SNVs and 1 SNV in a 3′-UTR (Fig. 1b). While exonic variants were detected in only 18 of the 54 genes in the panel, 64% (56/88) occurred in the epigenetic regulators DNMT3A and TET2 (Fig. 1c). We frequently detected multiple DNMT3A and TET2 mutations in the same individual, which were not necessarily in the same clone (Supplementary Fig. 5). The DNMT3A SNVs were predominantly nonsense mutations in the 5′ end of the gene or missense mutations in the three functional domains (Supplementary Fig. 6). For comparison, TET2 SNVs were primarily missense mutations in the functional domains or nonsense mutations throughout the gene (Supplementary Fig. 7), consistent with previous observations of AML23. While less prevalent, intronic clonal SNVs were observed in 12 genes with 29% (6/21) detected in DNMT3A and 5% (1/21) detected in TET2 (Supplementary Figs 8,9). The most common type of exonic substitution was the cytosine to thymine (C to T) transition (Fig. 1d), as previously observed in CHIP8,9,10. Conversely, intronic SNVs were evenly distributed across substitution types.
Longitudinal analyses
We characterized the temporal stability of these clones by tracking clonal mutations longitudinally within our 20 study participants. Variants were called independently from paired samples banked ∼10 years apart (Fig. 2). Of the 109 clonal SNVs identified, 27.5% (30/109) were detected at both time points, 13.8% (15/109) were detected at only the first time point and 58.7% (64/109) were detected at only the second time point (Supplementary Table 2). The stability of VAFs observed here was consistent with the previous observations at higher VAFs in a few instances of CHIP8. The presence of the same clonal mutations longitudinally suggested that these mutations arose in long-lived HSPCs or committed progenitors.
Figure 2. Longitudinal detection of clonal SNVs in NHS participants.
Clonal SNVs were detected by ECS in both time points for 16/20 NHS participants. For each participant ID (PID), the VAF measured by ECS was plotted relative to the age at sample collection. Variants detected in both time points were connected with a line.
To further elucidate the cell of origin for clonal haematopoiesis, we sorted 26 samples from 13 individuals into B lymphocyte (CD45+CD33–CD19+), T lymphocyte (CD45+CD33–CD3+) and myeloid (CD45+CD33+) compartments using flow cytometry (Methods; Supplementary Fig. 10). While cell recovery was variable per sample, we observed the same clonal SNVs in both myeloid and lymphoid compartments in 10/13 individuals (Fig. 3; Supplementary Table 5). Frequently, the VAF measured in the bulk sample was approximately equal to the VAF measured in each compartment. These observations were unlikely to have arisen due to contamination, given that variants were often detected at similar VAFs in different sorted compartments.
Figure 3. Haematopoietic compartment-specific detection of clonal SNVs in NHS participants.
Paired buffy coat samples from 13 individuals were sorted into B lymphocyte (pink), T lymphocyte (purple) and myeloid (blue) compartments using flow cytometry. For each NHS participant ID (PID), a single SNV, detected by ECS, was selected for compartment-specific quantification by ddPCR. Variants detected in both time points were connected with a line. The VAF measured by ddPCR in the bulk sample (green) was included for comparison.
Discussion
These findings suggest that clonal haematopoiesis-harbouring mutations in AML-associated genes is nearly ubiquitous (95%) in 50–70-year olds—an age group in which previous studies identified haematopoietic clones in only 5% of individuals8,9,10,11. Clonal mutations were detected in both the myeloid and lymphoid compartments in samples banked a decade apart in these healthy individuals, clearly demonstrating that these mutations arose in long-lived HSPCs. However, these clonal mutations conferred only a modest proliferation advantage, as most clonal mutations were rare (median 0.0024 VAF) and stable longitudinally. One possible explanation for these observations was that these mutations, often in epigenetic regulators, augmented self-renewal capacity without a concomitant increase in proliferation. This hypothesis may also explain why HSPC number increases and quiescence decreases as a function of age24,25. As HSPCs gradually senesce throughout life, the acquisition of these mutations may allow benign clonal haematopoiesis to maintain ostensibly normal blood production years after it would otherwise decline26. This hypothesis is supported by work in mice demonstrating that DNMT3A loss-of-function mutations in haematopoietic stem cells (HSCs) are associated with an increase in HSC self-renewal without increasing proliferation27. Comparably, TET2 loss-of-function mutations in mice increase HSC self-renewal and proliferation28.
While DNMT3A mutations are frequently observed in CHIP and AML1,8,9,10,11, we observed a different distribution of DNMT3A mutations, specifically at the arginine 882 (R882) residue. Previous studies showed that mutations in DNMT3A R882 comprised approximately two-thirds of total DNMT3A mutations in AML29 and one-third of DNMT3A mutations in CHIP8,9. However, we observed only a single DNMT3A R882H variant. These findings suggest that DNMT3A R882 mutations potently drive clonal expansion, explaining their prior detection in common CHIP clones (median 0.11 VAF)8 and their rarity in these lower frequency clones.
The detection limit of ECS was ∼1:10,000 cells. Thus, given an estimated 11,000 HSCs in adults, of which only a fraction actively contribute to haematopoiesis at any given time30, we expected to observe unique somatic mutations marking each active HSC (a random distribution of synonymous and nonsynonymous mutations throughout the 54 AML-associated genes captured). Instead, over half of the detected mutations were in DNMT3A or TET2. This observation alone could have occurred if DNMT3A and TET2 were hotspots of somatic mutation. However, 89% (78/88) of the detected exonic mutations were nonsynonymous, truncating or splicing mutations. Given this skew towards presumed functional mutations, it was more likely that these haematopoietic clones were enriched by selection.
Due to technical limitations of our methods, we likely underreported the number of clonal mutations present in each individual. Specifically, we likely underreported the number of C to T (G to A) substitutions present in these rare haematopoietic clones due to the stringency of the binomial variant calling strategy and the background rate of cytosine deamination, which is a predominant artefact of ECS14,16,31. Here 38/109 substitutions identified were C to T (G to A) substitutions. Conversely, in previous studies of CHIP and AML, C to T (G to A) substitutions comprised ∼50–60% of identified substitutions8,9,32. In addition, the binomial statistical framework underreported hotspot mutations occurring in multiple individuals. However, in our raw data we only observed a single likely instance of an uncalled hotspot mutation—a DNMT3A R882H variant in individual 5 observed at a lower VAF than the variant reported in individual 13. In addition, we could not routinely co-localize mutations within the same haematopoietic clone. However, we co-localized mutations in three instances where they co-occurred in the same sequenced reads (participant ID (PID) 2, TET2 R1216G/A1217A; PID 13, DNMT3A G498V/C494F; PID 14, KRAS A66A/S65S). Future adaptations of this technology could address these limitations by targeting a larger capture panel and implementing single-cell sequencing approaches.
In summary, we demonstrate that clonal haematopoiesis, originating in long-lived HSPCs, is far more common than previously thought in healthy middle-aged adults. Despite its prevalence, clonal haematopoiesis shares many mutations with AML, raising additional questions regarding the sequence of mutation acquisition and cooperating events necessary for malignant transformation. Furthermore, in previous studies of CHIP the detection of a haematopoietic clone (at any age) was associated with an 11–13-fold increased risk of developing a haematological malignancy8,9. These earlier findings suggested that CHIP was comparable to monoclonal gammopathy of undetermined significance and monoclonal B-cell lymphocytosis, which are benign clonal proliferative conditions that occasionally progress to haematological malignancy6,7,12. Conversely, our findings suggest that clinically silent clonal haematopoiesis is present in almost all individuals by middle age, and that progression to haematological malignancy is exceptionally rare. Given the current public interest in precision medicine33, these findings have practical implications for sequencing-based screening of nascent malignancy or recurrence. Future research must focus on reliably distinguishing benign clonal haematopoiesis, however rare, from malignant clonal haematopoiesis that could drive transformation and relapse. This imperative extends to sequencing-based non-invasive screening34, which will require even finer discrimination between nascent malignancy and benign clonal expansion.
Methods
Study population
The NHS began in 1976 with 121,701 female United States registered nurses age 30–55 years old who returned an enrolment questionnaire, which queried medical history, anthropometric measures and lifestyle/environmental risk factors19. Since enrolment, the participants have returned biennial follow-up questionnaires to update information on potential exposures and diagnoses of chronic disease. To date, follow-up rates have been consistently high (>90%). In 1989–1990, 32,826 women provided a heparinized whole blood sample by methods described previously20. In 2000–2001, 18,743 of the women who had provided a sample in 1989–1990 provided a second whole blood sample using the same protocol21. In brief, participants willing to provide blood samples received kits that included all supplies necessary for their collection and overnight return (including a chill pack), and a brief questionnaire. Upon receipt, specimens were separated into plasma, buffy coat and red blood cell fractions, and frozen in liquid nitrogen. Informed consent to participate in the NHS was implied by return of the enrolment and follow-up questionnaires; written informed consent was obtained for biomarker studies at time of blood collection.
Among women who provided blood samples in 1989–1990 and 2000–2001, we identified 20 with no history of cancer or other major chronic disease. De-identified aliquots from those 40 buffy coat samples were prepared and shipped overnight to Washington University for the detection of persistent rare haematopoietic clones harbouring AML-associated somatic mutations as described below. Since each sample was de-identified and the capture space for targeted genomic DNA sequencing was not enough to enable the individual identification (141 kb per person), the Washington University Human Research Protection Office deemed this study as non-human research.
Sample preparation for ECS
Genomic DNA was extracted from 50 μl of purified buffy coat from each sample using the QIAmp DNeasy Blood and Tissue Kit (Qiagen) with MinElute columns (Qiagen) instead of standard columns to facilitate elution in a lower volume (three 30 μl elutions). The concentration of the extracted genomic DNA was measured using the Qubit dsDNA HS Assay Kit (Life Technologies). Enrichment of 568 amplicons in 54 genes (141 kb) commonly mutated in AML was performed using 250 ng of genomic DNA via the Illumina TruSight Myeloid Panel (Illumina). Technical replicates were prepared for each sample (80 libraries total). Following extension–ligation, the amplified fragments were eluted in 50 mM NaOH. Recovered fragments were amplified using the Q5 High-Fidelity 2x Master Mix (New England Biolabs) in a 75 μl reaction (37.5 μl 2x master mix, 20 μl DNA in 50 mM NaOH, 2 μl Tris-HCl pH 7.5 and 0.4 μM i5/i7 primers). Illumina's standard i7 primers were used to enable sample multiplexing. The i5 primer was redesigned to contain a random 16 nucleotide index to facilitate ECS (Supplementary Table 6). The following conditions were used for amplification: 98C for 30 s; six cycles of 98 °C for 10 s, 66 °C for 30 s, 72 °C for 30 s; 72 °C for 2 min; hold 10 °C. The PCR reaction was purified using a modified Ampure bead (Beckman Coulter) clean up to purify the amplified fragments (>400 bp). A modified poly-ethylene glycol (PEG) solution was made containing 382.5 μl 50% wt/vol PEG (Sigma), and 562.5 μl 5 M NaCl and 555 μl ddH2O. One-hundred microlitres of beads were washed twice with ddH2O to remove the stock PEG solution. One-hundred-fifty microlitres of the modified PEG solution was added to the washed Ampure beads with the 75 μl PCR reaction and otherwise purified using the standard Ampure protocol. The fragments were eluted in 20 μl ddH2O and the concentration of each library was quantified with Qubit (Life Technologies).
Quantification by ddPCR
Our goal was to generate each ECS library from 4M uniquely tagged molecules. We quantified each library's concentration using the QX200 ddPCR platform (Bio-Rad). A 2 μl aliquot of each library was diluted 1,000-fold and quantified in duplicate wells. Each well contained the following reaction mixture: 10 μl 2 × EvaGreen 2 × ddPCR master mix (Bio-Rad), 5 μl 1:1,000 diluted ECS library, 100 nM P5/P7 primers (Supplementary Table 6) and ddH2O added to 20 μl total. Droplets were generated using the standard Bio-Rad protocol. Amplification was completed using the following conditions: 95 °C for 5 min; 40 cycles of 95 °C for 30 s, 66 °C for 1 min; 4 °C for 5 min; 90 °C for 5 min; 4 °C hold. With the calculated concentration, we aliquotted the appropriate volume of each library to introduce 4M molecules into the subsequent amplification step.
Amplification and normalization
Following ddPCR quantification, 4M molecules for each library were amplified using Q5 High-Fidelity 2 × Master Mix (New England Biolabs) using 1 μM P5/P7 primers (Supplementary Table 6) in a 50 μl reaction under the following conditions: 98 °C for 30 s; 16 cycles of 98 °C for 10 s, 66 °C for 30 s, 72 °C for 30 s; 72 °C for 2 min; 4 °C hold. The PCR reaction was purified using the modified Ampure bead clean up. One-hundred microlitres of beads were washed twice with ddH2O and replaced with 100 μl of the modified PEG solution described above. The PCR reaction was then added to the mixture and purified using the standard protocol. The fragments were eluted in 20 μl ddH2O. A 2 μl aliquot of each library was diluted 10-fold and quantified on the Agilent 2200 Tape Station. Libraries were then pooled in equimolar groups of eight. Once pooled, each library was again quantified on the Tape Station and submitted for sequencing.
Sequencing
Each library was sequenced on the Illumina NextSeq platform using a 300 cycle high output kit as specified by the manufacturer. Approximately 5–10% of PhiX control DNA was spiked into each sequencing experiment. Each sequencing run contained roughly 400 M paired-end 144 bp reads with corresponding 16 bp unique molecular index and 8 bp sample-specific index sequences. Sequenced reads were demultiplexed by sample-specific index allowing for at most one mismatch in the index sequence (Supplementary Table 1). Raw sequence reads were aligned to the PhiX genome using Bowtie 2 (ref. 35). Sequence reads that did not align to PhiX were retained for the subsequent analysis (below).
ECS analysis
The first 30 nucleotides of each sequenced read were hard clipped to remove the primer sequences from the TruSight Myeloid panel. Next, the sequenced read pairs tagged with the same random index sequence (originating from the same uniquely tagged DNA molecule) were aligned to each other to generate read families in a manner similar to the previously published methods14,15,16,17. Read families were required to have five or more reads sharing the same index sequence. Paired-end reads within a read family were error corrected to generate an ECCS in a stepwise manner. First, at every position, the nucleotides called by each sequence read were compared and a consensus nucleotide was called if there was at least 90% agreement between the reads. If there was <90% agreement, an N was called in the consensus sequence at that position. Errors that occurred during the library preparation and sequencing were corrected or removed because they were not shared between different reads within a read family. Second, an ECCS was discarded if >10% of the 228 nucleotides comprising the paired-end read were reported as N nucleotides. ECCSs were then locally aligned to UCSC hg19/GRCh37 using Bowtie2 and realigned with GATK's Indel Realigner36. Next, the aligned ECCSs were processed with Mpileup using the parameters -BQ0 -d 10,000,000,000,000. This removed the coverage thresholds to ensure that all of the pile up output was returned regardless of VAF or coverage. The parsed pile up output was further filtered to ignore positions with <1,000x ECCS coverage or outside of the Illumina TruSight Myeloid target space. In addition, germline variants identified by the 1,000 Genomes Project above a 0.01 minor allele fraction were excluded from the analysis.
We implemented a position-specific binomial error model to improve rare clonal SNVs calling as described previously17. For each sample, we generated a nucleotide position-specific error profile using all sequenced libraries that were not from the same individual. A variant was called if: (a) the binomial P value was <0.05 after Bonferroni correction; (b) the variant was observed in at least five ECCSs; (c) the VAF was >0.0001; and (d) the variant was identified with criteria a–c in at least two replicates from one of the two time points. Likely, clonal SNVs (<0.2 VAF) were reported and annotated using Annovar37, with the COSMIC 68 (ref. 22) and 1,000 Genomes (October 2014 release)38 databases. The amino-acid substitutions were predicted based on the canonical transcript reported in the GENCODE (v22)39 as retrieved from the UCSC Table Browser40.
We identified potential insertion/deletion (indel) events using VarScan 2 (ref. 41), from the filtered Mpileup output (described above), with the following parameters: min-coverage 1,000; min-reads2 5; min-var-freq 0.001; strand-filter 0; and output-vcf 1. We filtered out single-nucleotide indels in homopolymer runs at least four nucleotides long and indels that were observed in multiple samples to remove technical artefacts in the variant calling. We reported likely clonal indels (<0.2 VAF) that were detected in at least two replicates from one of the collection time points. Reported indels were annotated with Annovar37 as described previously.
ddPCR validation
We validated 21 SNVs and 1 indel using the ddPCR probe assay (Bio-Rad)42. Probes were designed by Bio-Rad based on MIQE guidelines for the quantitative digital PCR43. All reagents were purchased from Bio-Rad. For each sample and control, 45 ng of the genomic DNA was aliquoted per well of generated droplets. We generated between 8 and 32 wells of droplets for each validation experiment, depending on the expected VAF for the assayed mutation. Each control sample was assayed with the same number of wells as the corresponding sample. Droplets were generated on the QX200 Droplet Generator (Bio-Rad) and assayed on the QX200 droplet reader (Bio-Rad) using the standard protocol42. The VAF was estimated from droplets lacking the reference allele and the Poisson-estimated number of singleton droplets as described previously17.
Flow cytometry
Cells were sorted from the buffy coat samples using a Sony iCyt Synergy SY3200 BSC 17-colour, 5-laser cell sorter (Sony Biotechnology Inc.) and analysed with FlowJo (Treestar) using standard protocols (Supplementary Fig. 10). Cells were stained with the following antibodies (BioLegend): CD45 (BV-421), CD33 (APC), CD19 (FITC) and CD3 (PE-CY7) per the manufacturer's instructions. Variants were detected in purified cell populations using the ddPCR assay described previously.
Data availability
The sequencing data have been deposited into the NCBI Sequence Read Archive under accession number SRP078948. All other relevant data are included in the article or supplementary files, or available from the authors upon request.
Additional information
How to cite this article: Young, A. L. et al. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7:12484 doi: 10.1038/ncomms12484 (2016).
Supplementary Material
Supplementary Figures 1-10 and Supplementary Tables 1-6.
Acknowledgments
We thank the participants and staff of the Nurses' Health Study for their valuable contributions, D. Link for discussions and feedback on the manuscript and A. Kothari for assistance with the cell-sorting experiments. The authors assume full responsibility for analyses and interpretation of these data. Funding for this project was provided by the National Institutes of Health (UM1 CA186107, R01 CA49449 and R01 CA149445), the Children's Discovery Institute of Washington University and St Louis Children's Hospital (MC-II-2015-461), and Hyundai Hope on Wheels (2015Q3-3).
Footnotes
Author contributions A.L.Y. designed and performed the research, analysed the data and wrote the manuscript. G.A.C. contributed to the flow cytometry assay. B.M.B. provided samples from the Nurses' Health Study and contributed to the study design. T.E.D. supervised all of the research and edited the manuscript, which was approved by all co-authors.
References
- Cancer Genome Research Atlas Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976). [DOI] [PubMed] [Google Scholar]
- Busque L. et al. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 88, 59–65 (1996). [PubMed] [Google Scholar]
- Busque L. et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 44, 1179–1181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren O. et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113, 5412–5417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawstron A. C. et al. Monoclonal B-Cell lymphocytosis and chronic lymphocytic leukemia. N. Engl. J. Med. 359, 575–583 (2008). [DOI] [PubMed] [Google Scholar]
- Jaiswal S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrell T. et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell. Rep. 10, 1239–1245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. W. et al. Sequencing small genomic targets with high efficiency and extreme accuracy. Nat. Methods 12, 423–426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. W. et al. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl Acad. Sci. USA 109, 14508–14513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde I., Wu J., Papadopoulos N., Kinzler K. W. & Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl Acad. Sci. USA 108, 9530–9535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. L. et al. Quantifying ultra-rare pre-leukemic clones via targeted error-corrected sequencing. Leukemia. 29, 1608–1611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. N. et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518, 552–555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. R. et al. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat. Protoc. 9, 2586–2606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz G. A. & Hankinson S. E. The Nurses' Health Study: lifestyle and health among women. Nat. Rev. Cancer 5, 388–396 (2005). [DOI] [PubMed] [Google Scholar]
- Hankinson S. E. et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J. Natl Cancer Inst. 87, 1297–1302 (1995). [DOI] [PubMed] [Google Scholar]
- Zhang X., Tworoger S. S., Eliassen A. H. & Hankinson S. E. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res. Treat. 137, 883–892 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S. A. et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 44, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann S. et al. Landscape of TET2 mutations in acute myeloid leukemia. Leukemia. 26, 934–942 (2012). [DOI] [PubMed] [Google Scholar]
- Pang W. W. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda K. et al. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell 10, 542–546 (2011). [DOI] [PubMed] [Google Scholar]
- Holstege H. et al. Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 24, 733–742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G. A. et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 44, 23–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J. et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 363, 2424–2433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin S. N., Busque L., Gale R. E., Guttorp P. & Abkowitz J. L. The replication rate of human hematopoietic stem cells in vivo. Blood 117, 4460–4466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou D. I. et al. High-throughput DNA sequencing errors are reduced by orders of magnitude using circle sequencing. Proc. Natl Acad. Sci. USA. 110, 19872–19877 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J. S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S. & Varmus H. A new initiative on precision medicine. N. Engl. J. Med. 372, 793–795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L. A. & Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M. & Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164–e164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVean G. A. et al. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J. et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 22, 1760–1774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolchik D. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 32, 493D–496D (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett J. F. et al. The digital MIQE guidelines: minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 59, 892–902 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-10 and Supplementary Tables 1-6.
Data Availability Statement
The sequencing data have been deposited into the NCBI Sequence Read Archive under accession number SRP078948. All other relevant data are included in the article or supplementary files, or available from the authors upon request.