Abstract
Incidence of natural light stress renders it important to enhance our understanding of the mechanisms by which plants protect themselves from harmful effects of UV-B irradiation, as this is critical for fitness of land plant species. Here we describe natural variation of a class of phenylacylated-flavonols (saiginols), which accumulate to high levels in floral tissues of Arabidopsis. They were identified in a subset of accessions, especially those deriving from latitudes between 16° and 43° North. Investigation of introgression line populations using metabolic and transcript profiling, combined with genomic sequence analysis, allowed the identification of flavonol-phenylacyltransferase 2 (FPT2) that is responsible for the production of saiginols and conferring greater UV light tolerance in planta. Furthermore, analysis of polymorphism within the FPT duplicated region provides an evolutionary framework of the natural history of this locus in the Brassicaceae.
 Protection from UV-B is critical for land plant survival. Here Tohge et al. show that saiginols, a novel class of flavonols that efficiently absorb UV-B, accumulate in Arabidopsis accessions collected from high irradiance regions and identify a flavonol phenylacyltransferase gene required for saiginol production.
Protection from UV-B is critical for land plant survival. Here Tohge et al. show that saiginols, a novel class of flavonols that efficiently absorb UV-B, accumulate in Arabidopsis accessions collected from high irradiance regions and identify a flavonol phenylacyltransferase gene required for saiginol production.
The dependency of plants on sunlight inevitably brings them into exposure to ultraviolet (UV) light, including that in the wavelength range 280–320 nm (UV-B)1,2. This wavelength range is potentially damaging to DNA, RNA and proteins, and furthermore leads to increased production of free radicals that can activate transposons and cause further mutations3,4. Thus, although only 0.5% of energy reaching the earth is in this wavelength range5, the risk of UV-B damage is profound. For this reason plants have been under considerable natural selective pressure to generate elegant mechanisms to both sense and to respond to the presence UV-B irradiation1,2,6,7. The zonal average UV irradiance (flux UV, FUV) reaching the Earth's surface has continuously increased since 1979 at all latitudes except the equatorial zone8 even though the total area of the ozone hole has slightly decreased since 2006, most likely due to efforts restricting usage of chlorofluorocarbons. In addition to its potential impact on natural plant populations, this could greatly impair crop yields since the detrimental effects described above combine to considerably constrain plant seed yields and growth rates. Our understanding of the response to UV-B in land plants has been greatly enhanced by the study of the UV-B resistance 8 (UVR8) UV-B photoreceptor and the downstream cascades under its control9. One aspect of the downstream response is the production of additional antioxidant sunscreens including various phenylpropanoids like flavonoids and hydroxycinnamates, and other antioxidants such as ascorbate1. The reprogramming of these pathways after exposure to UV-B has been demonstrated to involve independent metabolic responses, and indicates that in Arabidopsis seedlings the role of flavonoids is probably of greater quantitative importance than that of hydroxycinnamates, despite the fact that hydroxycinnamates display better absorption of light quanta in the UV-B wavelength range2.
Flavonoids and hydoxycinnamates are phenylpropanoids, an important class of plant secondary metabolites that exhibits a range of diverse functions, including roles in protection against biotic and abiotic stresses and developmental processes10,11,12. They are synthesized from phenylalanine via a core pathway that is conserved among land plants, and additional side pathways that start from various intermediates of this core pathway10. Whilst some of these, such as the hydroxycinnamate biosynthetic pathways of angiosperms evolved early and are consequently widespread in major plant lineages, others such as the polyphenolic tannins, phenylamines and coumarin derivatives represent relatively recent adaptations and as such are more phylogenetically restricted in their occurrence10. Whilst natural variance screening has been carried out in many plant species including Arabidopsis with regard to the accumulation of specific primary and secondary metabolites13,14,15,16,17 relatively few studies have focused on phenylpropanoid metabolism18. Given that considerable evidence has accumulated concerning the UV-B protective function of various phenylpropanoids during the processes of flower development, pollination and seed production19, we therefore assayed their levels in flowers in a set of 64 Arabidopsis ecotypes demonstrated to provide good coverage of the overall natural variability of the species20. Here we studied the natural variation of floral secondary metabolites among Arabidopsis accessions, characterizing a novel class of phenylacylated-flavonols (saiginols) and a flavonol-phenylacyltransferase 2 (FPT2) gene corresponding to altered in planta UV-B light tolerance. Furthermore, genomic sequence and gene syntenic analysis of the FPT gene duplication region across Brassicaceae species allowed us to develop a picture of the evolutionary framework of the natural history of this locus with respect to natural light selection.
Results
Arabidopsis accession-specific phenylacylated-flavonols
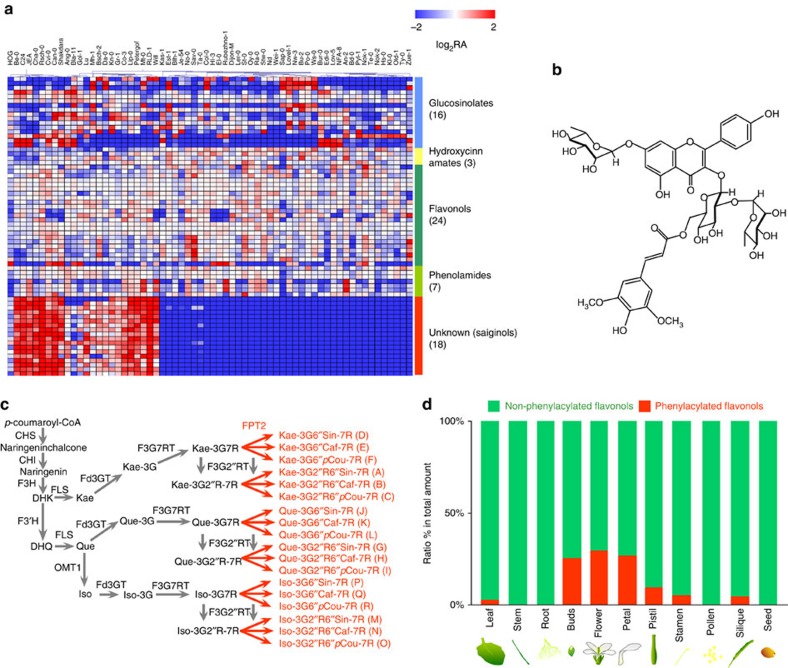
Application of a previously described liquid chromatography/mass spectrometry (LC/MS) protocol21, resulted in secondary metabolite profiles for flowers in a set of 64 Arabidopsis accessions and in the detection of a total of 68 peaks consisting of 16 glucosinolates, 3 hydroxycinnamates, 24 flavonoids, 7 putative phenolamides and 18 peaks of unknown chemical structure (Fig. 1a and Supplementary Data 1). Whilst there was considerable difference in the abundance of the peaks corresponding to chemicals of known chemical structure, most striking was the pattern for 18 peaks of unknown chemical structure, which were only present in a subset of the accessions (Supplementary Fig. 1). The abundance of these unknown peaks was highly correlated among unknown peaks (r2=0.6630–0.9997 Pearson's correlation co-efficient, Supplementary Fig. 2), but not with other secondary metabolites such as flavonol glycosides, glucosinolates and hydroxycinnamates with exception of a negative correlation between sinapoyl-glucose (r2=−0.6236 to −0.7117). Among the 64 Arabidopsis accessions, 31 accumulated considerable amounts of these compounds whilst 33, including the common laboratory ecotype Col-0, lacked these peaks (Supplementary Fig. 1). MS/MS fragmentation studies suggested that these unknown peaks are novel flavonol derivatives (Supplementary Data 1).
Figure 1. Novel phenylacylated-flavonol glycosides found in A. thaliana accessions.
(a) LC/MS profiling of secondary metabolites in Arabidopsis accession flowers. A total of 64 accessions were profiled in biological triplicate (n=3). Heatmap shows values displayed on a log2 relative peak area (RA) scaled by the average of all values. (b) Chemical structure of saiginol A isolated and characterized in this study. (c) Constructed novel biosynthetic branches for 18 saiginol productions in producer Arabidopsis accessions. (d) Tissue specificity of the accumulation of saiginols and relative ratio of phenylacylated(red)/non-phenylacylated(green) flavonols in producer C24 accession. Total phenylacylated- and non-phenylacylated-flavonol contents were compared using their abundance against total amount of all flavonols measured by LC/MS in each tissue type. Caf, caffeoyl; CHI, chalcone isomerase; CHIF3H, flavanone 3-hydroxylase; CHS, chalcone synthase; DHK, dihydrokaempferol; DHQ, dihydroquercetin; F3′H, flavonoid 3′-hydroxylase; Fd3GT, flavonoid 3-O-glycosyltransferase; F3G7RT, flavonol-7-O-rhamnosyltransferase; F3G2″RT, anthocyanin-3-O-glucoside-2″-O-rhamnosyltransferase; FLS, flavonol synthase; FPT, flavonol-phenylacyltransferase; G, glucose; Iso, isorhamnetin; Kae, kaempferol; OMT1, O-methyltransferase 1; pCou, p-coumaroyl; Que, quercetin; R, rhamnose; Sin, sinapoyl.
To characterize the chemical structure of these peaks, we re-grew large amounts of the C24 accession (which accumulates these compounds) and purified the corresponding compounds from extractions of ∼30 g FW (fresh weight) of entire flower samples. Following this procedure, we were able to isolate almost 3.5 mg of the major peak, which was then subjected to a suite of analytical chemical procedures, including one- and two-dimensional nuclear magnetic resonance spectroscopy, high-resolution electrospray ionization Orbitrap (ESI-Orbitrap)-MS (in both positive and negative modes) and UV–visible (VIS) spectroscopy (Supplementary Note 1). The combined results of these studies allowed us to identify the compound, which we termed saiginol A, as a novel phenylacylated-flavonol glycoside (flavonol-3-O-(2″-O-rhamnosyl-6″-O-sinapoyl)glucoside-7-O-rhamnoside)(Fig. 1b) and to demonstrate that it displayed superior UV-B absorbent properties to phenylpropanoids that contain the flavan ring and one additional phenolic ring structure. On the basis of the core chemical structure and decoration pattern of saiginol A, their retention times and the MS/MS experiments described above we were able to impute the chemical structure (Supplementary Fig. 3) and thus putative biosynthetic paths for the other unknown peaks, which we subsequently collectively refer to as saiginols (Fig. 1c). These peaks, we named as saiginols B-R, are putatively characterized by the presence or absence of three possible aglycones (kaempferol, quercetin and isorhamnetin) and by having one of three types of phenylacylation (sinapoyl, caffeoyl and p-coumaroyl moieties) based on annotation by fragmentation of MS/MS and elution time profile (Supplementary Data 1). Evaluation of SciFinder and the Handbook of Natural Flavonoids edited by Harborne and Baxter22 reveals that the presence of phenylacylated-flavonols is not without precedence with, in addition to the example given above, a total of 29 putative flavonol-phenylacylglycosides being documented in Brassica vegetables23. However the Brassica flavonol-phenylacylglycosides found and annotated to date are 2″- or 4″-O-phenylacylated-flavonols. Thus, the position to which the phenylacyl and sugar moieties is attached to the saiginols is structurally distinct from all phenylacylated-flavonols reported to date.
Having elucidated the structure of these compounds, we next evaluated their differential accumulation in various tissues. Saiginols were predominantly present in floral tissues but also to a lesser extent in the stem, silique, rosette leaf and the cauline leaf but are essentially, or even totally, absent in senescent leaf and the root (Fig. 1d and Supplementary Fig. 4). Further detailed spatial analysis of flavonol profiles revealed that saiginols predominantly accumulate in the floral petal and to a lesser extent in the stamen and pistil, but are absent in pollen.
FPT2 is a key gene for saiginol production
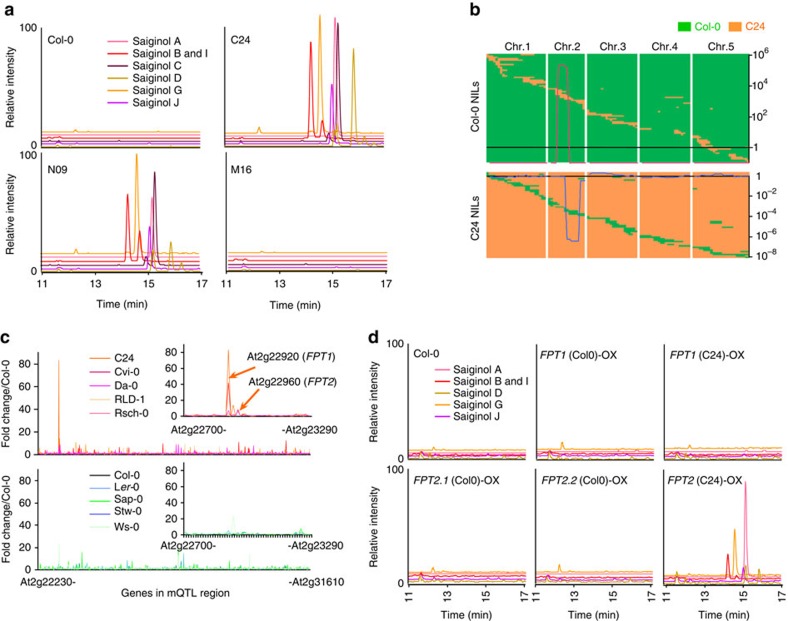
To identify the genes encoding the protein(s) responsible for the synthesis of these saiginols, we next re-grew previously generated reciprocal introgression lines (near-isogenic line population, NIL) harbouring chromosome segmental substitutions of Col-0 (saiginol non-producer) in C24 (saiginol producer), or substitutions of C24 in Col-0 (ref. 24) and assessed the presence or absence of the saiginols by LC/MS (Fig. 2a). Intriguingly, we found a single loss-of-function line in the C24 population, which showed the overlapping genome region as four gain-of-function lines in the Col-0 population (Fig. 2b and Supplementary Data 2). Given that this genomic region harbours a total of 829 genes (Fig. 2b; At2g22230–At2g31610), we next performed transcript profiling to compare gene expression in the gain-of-function donor line (C24; as well as the producer accessions Cvi-0, Da-0, Rsch-0 and RLD-1) and its recurrent parent (Col-0; as well as the non-producer accessions Ler-0, Ws-0, Sap-0 and Stw-0; Fig. 2c). Expression levels were generally very similar between the genotypes although a number of genes were significantly different between producing and non-producing accessions including several transposable elements (which comprise 9 of the top 50 most variable genes; Supplementary Table 1). However, when the 829 genes that localized to the chromosomal segment substitution were inspected, only two genes were markedly altered between producing and non-producing accessions. These were annotated in TAIR10 as a serine carboxypeptidase protein like 12 (SCPL12, At2g22920) and a pseudoSCPL gene (pSCPL, At2g22960). We subsequently renamed these genes as putative flavonol-phenylacyltransferases 1 and 2 (AtFPT1 and AtFPT2), respectively. Transcripts for these two genes were more than 17.7 and 6.7 times higher, respectively, in C24 than in Col-0. The fact that they are expressed at exceedingly low levels in Col-0 means that there is unfortunately a paucity of publically available data on their expression. To analyse the flavonol-phenylacyltransferases genes, we cloned the C24 and Col-0 alleles of both genes and evaluated their transcript production. Since the transcript of FPT2-Col-0 could not be observed in Col-0 floral material, we cloned FPT2-Col-0 from 10-day-old seedlings. Intriguingly, whilst the length of FPT1 transcript was invariant between Col-0 and C24, the FPT2-Col-0 allele encoded four different transcripts FPT2-Col-01–4 (743, 657, 637 and 566 bp in length, respectively) and FPT2-C24 encoded a considerably longer transcript (1,305 bp).
Figure 2. Functional identification of the FPT genes based on integrative approach.
(a) LC/MS chromatogram and (b) chromosomal mapping of gain-of-function and loss-of-function NILs obtained from metabolic quantitative trait locus (mQTL) analysis of reciprocal crosses between Col-0 and C24. N and M lines are Col-0 and C24 background introgression lines, respectively. Green and orange boxes indicate Col-0 and C24 DNA segments, respectively. Metabolic QTL was presented by log2-transformed relative amount of saiginol A. (c) Microarray analysis of gene expression of the genes encoded in the genomic region of chromosome 2 encoding the mQTL in five producing accessions (C24, Cvi, Da, Rsch and RLD) and five non-producing accessions (Col-0, Ler-0, Ws, Sap and Stw). Intensity indicates fold change estimated by average of expression level in Col-0. (d) LC/MS chromatogram showing in vivo functional characterization of FPT1 and 2 by metabolite profiling of 35S-driven overexpressing transgenic plants. All transgenic lines are Col-0 background. FPT1(Col-0), FPT1(C24), FPT2.1(Col-0), FPT2.2(Col-0) and FPT2(C24) indicate FPT1 cloned from Col-0 and C24, FPT2 cloned from Col-0 (FPT2.1 is shortest transcript and FPT2.2 is longest transcript) and C24, respectively. Chromatogram showed ion extracted chromatogram for major saiginol A (945 m/z), B and I (901 m/z), C (885 m/z), D (799 m/z), G (961 m/z) and J (815 m/z), respectively.
With the purpose of testing the function of various FPT proteins experimentally, we performed complementation assays in Col-0 and knockout fpt1 and fpt2 mutants. CaMV 35S driven complementation with the C24 allele of FPT2 resulted in the production of all 18 saiginols in flowers of the transgenics. However, neither expression of the Col-0 allele of FPT2 in the Col-0 fpt2 knockout line nor expression of the Col-0 or the C24 allele of FPT1 in the Col-0 fpt1 mutants yielded flowers, which produce saiginols (Fig. 2d and Supplementary Fig. 5). Complementation experiments also revealed that FPT2 expression corresponds to the production of the flavonol-phenylacylglucosides, but FPT1 expression did not. The saiginol levels in FPT2-C24-overexpressing and Col-0/C24 NIL lines revealed differences in floral tissues (The NIL lines showed 10 times higher accumulation than the 35S-driven transgenic plants).
Saiginols accumulation correlates with UV light tolerance
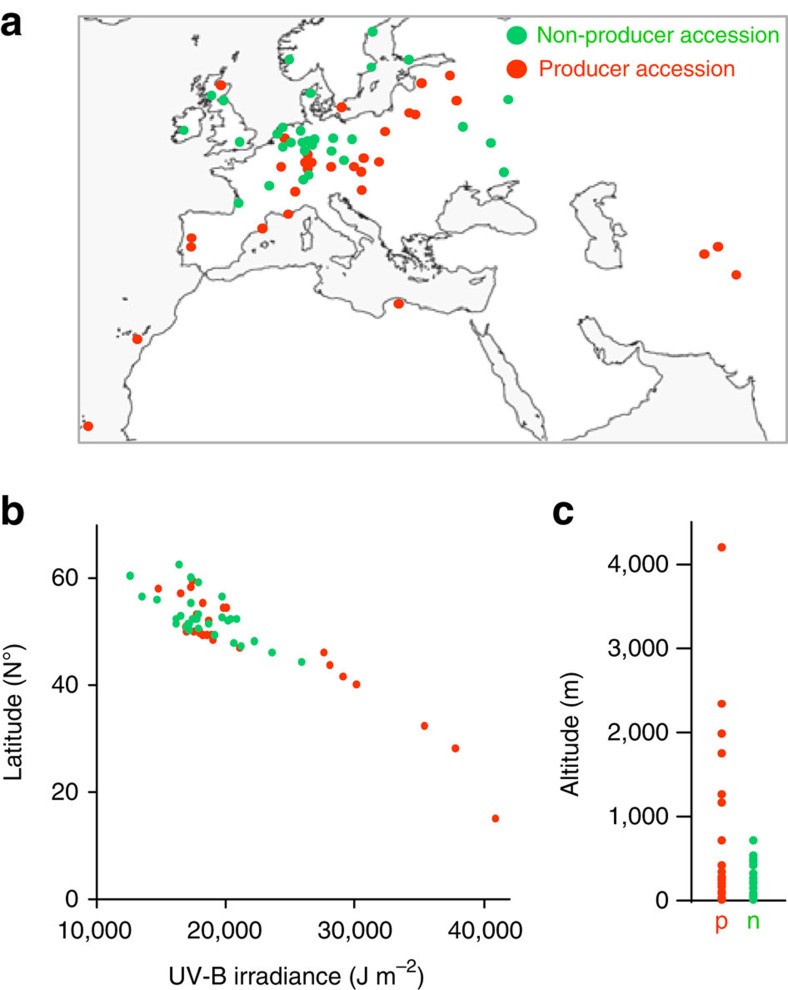
To place the occurrence of these novel compounds in an eco-physiological context, the producers and non-producers were mapped on a world map according to their site of origin (Supplementary Table 2). Intriguingly, although the sample number is admittedly relatively small, all of the accessions deriving from high irradiance habitats produce these metabolites. By contrast, in low irradiance areas such as mid and north Europe habits and lower altitude (Fig. 3a), both producing accessions and non-producing accessions can be found. Thus, the presence of the phenylacylated-flavonols may well be selected for in populations exposed to higher UV-B irradiance only in high light irradiance regions. The average of daily UV-B irradiance during 1985 and 2005 was assessed for the region of origin of the accession from satellite data (obtained from Soda (http://www.soda-is.com/eng/index.html). These data alongside latitude data were plotted in Fig. 3b which shows that there is clear relationship between at UV-B irradiance above 26,000 J m−2 where only producers are found. In addition, plots of the altitude calculated by Shuttle Radar Topography Mission (http://www2.jpl.nasa.gov/srtm/) showed that the accessions isolated from above 1,000 m altitude are exclusively saiginol producers (Fig. 3c and Supplementary Fig. 6).
Figure 3. Geographical distribution of A. thaliana accessions used in this study.
(a) Geographical distribution of producers (orange) and non-producers (green) in the world scale map. A total of 63 A. thaliana ecotypes are mapped. (b) Plots of producers and non-producers (by latitude (N°) and UV-B irradiation (J m−2) averaged of daily UV-B irradiance during 1985 and 2005 estimated from satellite data (Soda (http://www.soda-is.com/eng/index.html)). r2=0.8624 (producers) and 0.4476 (non-producers). (c) Plots of producers and non-producers by altitude (m) estimated by Shuttle Radar Topography Mission (http://www2.jpl.nasa.gov/srtm/). Map by FreeVectorMaps.com. Green and orange indicate non-producing and producing accessions, respectively.
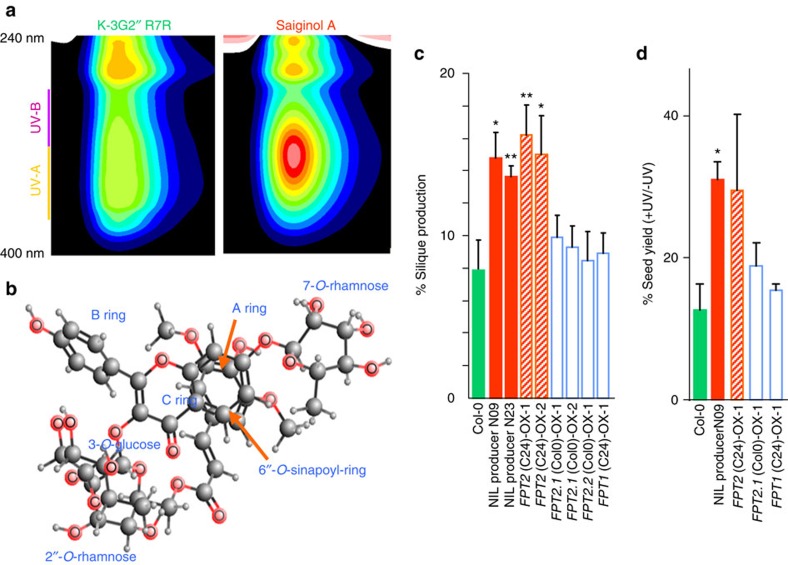
The UV–VIS spectra of saiginol A (kaempferol-3-O-(2″-O-Rha-6″-O-sinapoyl)Glc-7-O-Rha), revealed λmax at 206, 223, 267 and 332 nm (Supplementary Note 1), indicating that the phenylacylated-flavonol is much more efficient in UV-A/B absorption than its precursor, kaempferol-3-O-Glc-2″-O-Rha-7-O-Rha (Fig. 4a) (λmax at 202, 249 and 266 nm)25, which is most abundant flavonol in Arabidopsis thaliana. Such an absorption addition by one more phenolic ring (for example, from sinapoyl-donor, sinapoyl-Glc, λmax at 203, 241 and 329 nm)25 in flavonol decoration has previously been reported for 3″,6″-di-O-(p-coumaroyl)isoquercitrin in Scots pine26 which is a similar, although structurally distinct, molecule to those described here. This thus presents the possibility that the synthesis of saiginols in Scots pine and Arabidopsis may have occurred as a result of convergent evolution. Furthermore, computational estimation of the most stable stereochemical structure of saiginol A using MMFF94 resulted in a bridge piled structure between the aromatic rings A and sinapoyl-ring (Fig. 4b) similar to that of the intramolecular co-pigmentation of phenylacylated-anthocyanins reported in Scots pine27. Previous research of absorption shift by intermolecular co-pigmentation of p-coumaroyl-isoquercitrins in Scots pine suggested that phenylacylation into 6″-O- position indicates a further enhancement of its absorption at UV-B range. Importantly, neither of these compounds absorb in the photosynthetically active radiation range meaning that their presence does not result in a trade-off against photosynthetic efficiency.
Figure 4. Functional characterization of the role of saiginols and FPT2 in protection against UV-B irradiation.
(a) UV absorbance shift of flavonol by phenylacylation measured by HPLC-PDA. Purified compounds, a precursor (K-3G2″R7R) and saiginol A were used for the profile. (b) Computational estimation of the most stable stereochemical structure performed by MMFF94 of Marvin (http://www.chemaxon.com/). (c) Rate of silique production under UV-B irradiation after long-term (2 h per day) UV-B treatment (1 W m−2) using the detached immature inflorescences of first bolting. Error bars indicate the s.e. of six biological replicates. (d) Seed yield after long-term (2 h per day for 28 days, 2 W m−2) UV-B treatment using 3-week-old intact plants. Seeds were collected on a single plant basis but three individual plants per genotype were measured. Data are presented as mean±s.e.m. *P<0.05, **P<0.05.
To assess the role of FPT2 and by implication the role of saiginols, in conferring additional protection against UV-B, we grew the overexpressing lines for both alleles of FPT1 and FPT2 alongside Col-0 the gain-of-function NIL and subjected them to a range of UV-B treatments. A difference between these genotypes most likely directly reflects the role of saiginols as we do not see major changes in the accumulation of other flavonols in either natural accessions or overexpressors. Nevertheless we cannot formally rule out that differences in FPT2 expression may be correlated with other phenotypic effects that influence UV-B sensitivity independently of saiginols. Since saiginols accumulate in floral tissue especially in buds and petal but not in pollen, although pollen is extremely sensitive to UV-B irradiation, we hypothesize that saiginols may protect pollen or its haploid state from UV-B light before flower opening and pollination. We, therefore, ensured using a detached flower experiment28 that the flower organs were equidistant from the UV-B source. As documented in Fig. 4c and Supplementary Fig. 7, both NIL producers (N09 and N23) and FPT2-C24-overexpressing lines showed significantly higher silique production following UV-B treatment, whilst no significant higher silique production in normal condition in saiginol-producing lines. We next assessed the total seed yield of intact plants at maturity. Although, as is typical of such experiments, there was a high variability between replicates, the experiments revealed a consistently significantly higher seed yield of the gain-of-function NIL. In addition, FPT2-C24-overexpressing line that exhibit relatively lower accumulation of saiginols in floral tissues (Supplementary Fig. 5) was characterized as exhibiting higher seed yield to the control plants under UV-B irradiation. Consistent with their lack of saiginols, lines overexpressing the FPT1 alleles and the FPT2-Col-0 allele displayed similar seed yield following UV-B treatment to those in control lines (Fig. 4d).
Deletion of FPT2 gene in SCPL duplication region
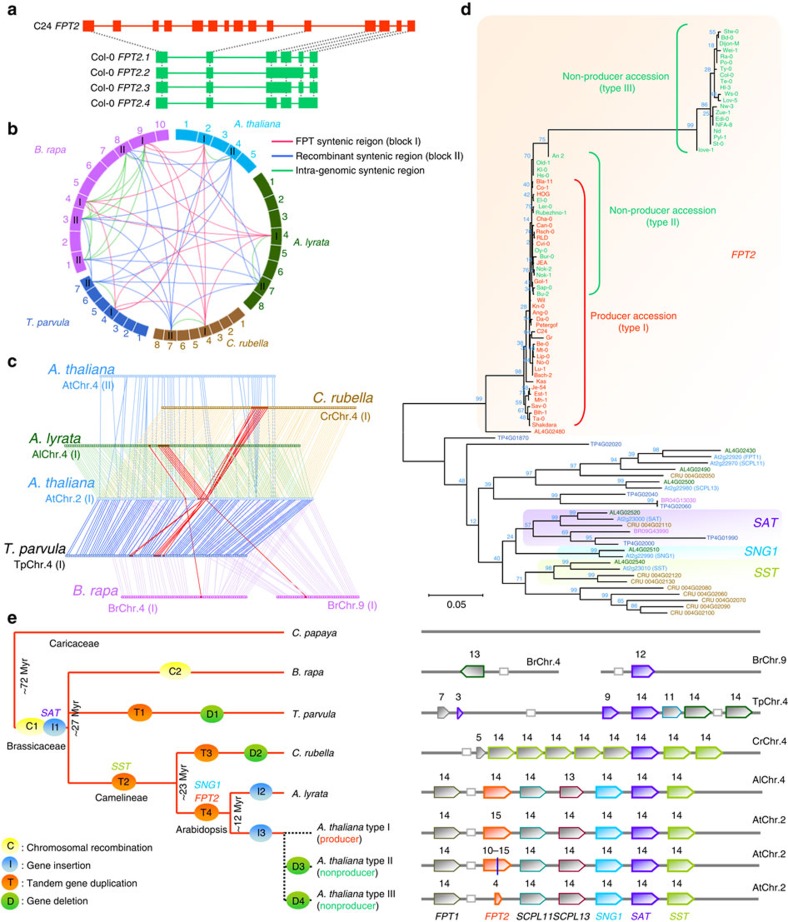
Having established that full-length FPT2 from C24 functions to enhance UV-B protection, we next sought to clarify the molecular reason for the natural variance for the presence of the saiginols. We first performed genomic sequencing of FPT1-C24 and FPT2-C24 and mapped their full-length cDNA sequences obtained from Col-0 and C24. Analyses of FPT1 revealed a deletion (∼600 bp) in the FPT1-C24 promoter region and an insertion (∼10 and 6 bp) within the first and second introns of FPT1-C24 (Supplementary Fig. 8). The protein-coding sequences, however, were highly similar and comprised genome sequence with complete match of all 14 exons. On the other hand, genomic sequence analyses of FPT2 (Col-0, 2,039 bp; C24, 4,262 bp) revealed a large gene deletion (∼2,279 bp) in Col-0, spanning the region corresponding to the second to eleventh exons of FPT2-C24 (Supplementary Fig. 9). This analysis indicated that the four transcripts of FPT2-Col-0 described above are alternative splicing variants (Fig. 5a). One of the splice variants (At2g22960) was previously identified as a pseudoSCPL gene since the ATPase-like sequences upstream of the first exon suggest that this SCPL gene lacks a promoter29, however, importantly the predicted translation products of all four splice variants terminate shortly after the putative catalytic Ser (ref. 30), and thus lack the active site Asp and His residues. Thus, it is highly likely that FPT2-Col-0 does not a function as a phenylacyltransferase in vivo. Furthermore, the result of BLAST search of this deleted sequence (2,279 bp) revealed that the deleted sequence has similarity to SCPL10 (At2g23000, SAT, anthocyanin sinapoyltransferase) in the coding sequence, as well as one fragment of copia-like retrotransposon in its untranslated regions. The FPT genomic region in chromosome II (AtChr.2) is very close to the SCPL cluster region generally discussed as containing five SCPL genes (SCPL11, At2g22970; SCPL13, At2g22980; SCPL08, SNG1, At2g22990; SCPL10, SAT, At2g23000; and SCPL09, SST, At2g23010) of which three to date have been assigned specific enzymatic activities, namely sinapoyl-glucose: malate sinapoyltransferase 1 (SNG1); sinapoyl-glucose: anthocyanin sinapoyltransferase (SAT); and sinapoyl-glucose: sinapoyl-glucose sinapoyltransferase (SST)30. Interestingly, all SCPL genes have 14 exons except FPT2-C24, which has 15 exons due to a short insertion in second exon, but FPT2-Col-0 has just 4–6 exons (Supplementary Fig. 10). This result revealed that the difference between FPT2-Col-0 and FPT2-C24 is the result of transpositional gene deletion as opposed to an insertional gain of function. The shared number of exons in this FPT duplication cluster, as well as the similarity of the gene sequence between FPT genes and SCPL later in this cluster, suggest that this tandem gene duplication in FPT region occurred before the transpositional gene deletion of FPT2 in non-producing accessions.
Figure 5. Genomic structure and sequence analysis of FPT gene cluster region in plant species.
(a) Gene deletion identified in the FPT2-Col-0 genomic sequence. Coding sequence including splicing variance found in Col-0 and C24 was mapped with their genomic sequence. (b) FPT gene syntenic regions found in plant species. I, red, syntenic block I (containing FPT gene cluster); II, blue, syntenic block II (recombinant syntenic region); green, linkage of intra-genomic syntenic region. (c) Syntenic gene linkage between plant species. Red box and line indicate FPT/SCPL genes. Similarity between AtSCPL and SCPL genes obtained from Brassicaceae species is evaluated by TAIR-BLAST (https://www.arabidopsis.org/). (d) Phylogenetic analysis using CDS of SCPL genes in syntenic region of Brassicaceae species. A short gene TP4G01860 was excluded from this analysis. The phylogenetic tree was created using MEGA5.2 (ref. 60). Scale implies nucleotide substitutions per site. (e) Hypothetical scheme of evolutional events in FPT gene cluster region among Brassicaceae species. The taxonomical tree of Brassicaceae species was constructed via reference to PLAZA (http://bioinformatics.psb.ugent.be/plaza/) based on NCBI taxonomic analysis (http://www.ncbi.nlm.nih.gov/taxonomy). FPT tandem duplication cluster of SCPL genes including FPT1 and FPT2. Numbers above genes indicate number of exons. Red, yellow and blue indicate sub-clades found by phylogenetic trees analysis in d. C1 and C2, chromosomal recombination in Brassicaceae and B. rapa, respectively; I1, hypothetical time of SAT insertion into FPT region; I2, gene insertion into fourth and eleventh exons of AL4G02540; I3, gene insertion into second intron of FPT2; T1–4, tandem gene duplication in T. parvula, Camelineae (C. rubella, A. lyrata and A. thaliana), C. rubella and Arabidopsis (A. lyrata and A. thaliana), respectively; D1–4, gene deletion in T. parvula, C. rubella, A. thaliana non-producer type II and type III.
We next extended the coding region sequencing across further all 64 accessions and were able to identify a strict relationship between this polymorphism and saiginol production among the accessions. For this purpose sequences were obtained using two primer sets one designed for the full-length coding region and the other to include 71 bp forward upstream and 55 bp reverse downstream of deleted region for a total of 44 ecotypes. Further, sequences were evaluated and constructed with publically available sequence (Arabidopsis 1,001 genome (http://signal.salk.edu/atg1001/3.0/gebrowser.php). A total of three classes of genotype were observed: type I, producer, similar to C24 (31 accessions); type II, non-producer, similar to C24 but with a 2 bp deletion in the eleventh exon (Ler-0, Sap-0, Bur-0 and Rubezhno-1) with 14 bp deletion in third exon (Nok-1 and Nok-2), deletion of seventh exon (El-0, Oy-0 and Bu-2) and deletion in the seventh to tenth exons (Hs-0, Kl-0, Old-1 and An-2); and type III, non-producer, similar to Col-0 (deletion in the second to tenth exons, 18 accessions) and similar to Col-0 but with a 9 or 72 bp deletion in the tenth exon (Stw-0 and Bd-0) (Supplementary Fig. 11). The inactivation pattern does not reflect geographic demography of accessions, for example, accessions Ler-0, Sap-0, Bur-0 and Rubezhno-1, which have 2 bp deletion in the eleventh exon from Poland, Czech Republic, Ireland and Ukraine, respectively. The fact that a large or short deletion in the coding region of FPT2, was always and exclusively observed in saiginol non-producing genotypes (Supplementary Fig. 11), suggests that this deletion invokes the observed natural diversity of saiginol production. That said the presence of such a large variety of genomic polymorphism across Arabidopsis accessions suggests that the gene deletion within the FPT–SCPL region is a relatively recent event.
Genomic signatures and evolutionary framework in FPT region
To assess the evolutionary context of the FPT2 gene deletion within the SCPL cluster region, we first performed cross-species genome-wide analysis to identify gene conservation and syntenic regions of FPT2. Results of a PLAZA (http://bioinformatics.psb.ugent.be/plaza/) search and further genomic sequence analysis suggest that the sequence of FPT2 is not found in other plant species with the exception of Arabidopsis lyrata AL4G02480, although several orthologues of other SCPL genes have been found in several plants especially those of the Brassicaceae. We next carried out a syntenic cross species orthologue analysis of the AtFTP region. Syntenic blocks (defined as block I) were found in AlChr.4 (seven SCPL genes in A. lyrata), CrChr.4 (nine SCPL genes in Capsella rubella), TpChr.4 (seven SCPL genes in Thellungiella parvula), BrChr.4 (one SCPL gene in Brassica rapa) and BrChr.9 (one SCPL gene in B. rapa)(Fig. 5b, Supplementary Fig. 12 and Supplementary Data 3 and 4). Furthermore, intrasyntenic regions (defined as block II regions see Supplementary Figs 12 and 13, and Supplementary Data 4) possibly caused by chromosomal recombination were found in AtChr.4, AlChr.7, CrChr.7, TpChr.7, BrChr.1, BrChr.3 and BrChr.8 (Fig. 5b and Supplementary Fig. 12), but do not contain SCPL genes. However, papaya does not have such a syntenic region in its genome but rather has two short similar blocks, which share genes with both AtChr.2 and AtChr.4, but do not harbour FPT. The identified syntenic blocks in other Brassicaceae species, however, fit to previously reported segmental collinearity between A. thaliana and those species31,32,33,34,35. This observation suggests that FPT genes, like all members of the SCPL family, evolved after chromosomal recombination split this block across two chromosomes. Further detailed syntenic block analysis revealed that numbers of total SCPL genes in this syntenic region are different between species except A. thaliana and A. lyrata (Fig. 5c and Supplementary Fig. 14). This suggests that SCPL genes were not duplicated in same manner and that SCPL gene duplication occurred after speciation. To test metabolic conservation of FPT2 in Arabidopsis species including A. thaliana and A. lyrata, we performed LC/MS profiling of saiginols in A. lyrata and C. rubella. Saiginol A was clearly present in A. lyrata but not in C. rubella suggesting that FPT2 evolved after speciation between Arabidopsis and C. rubella (Supplementary Fig. 15). Phylogenetic analysis of the SCPL genes of the block I syntenic region in Brassicaceae species and FPT2 in all A. thaliana accessions studied here revealed that anthocyanin-sinapoyltransferase (SAT) appears to be the first FPT inserted into this region because only this gene is well conserved across the Brassicaceae (Fig. 5d). By contrast, FPT1 and SCPL11 genes appear to have evolved relatively recently since only A. lyrata harbours an orthologue of these genes. Further phylogenetic analysis using predicted protein sequences also supports similarity between them (Supplementary Figs 16 and 17). In addition it appears that the C. rubella SCPL was duplicated from sinapoyl-glucose: SST gene subsequent to its speciation from Arabidopsis.
Discussion
Our hypothesized evolutionary framework for genes in this gene cluster is summarized in Fig. 5e with speciation tree based on NCBI taxonomic analysis. The presence/absence of SCPL within the intrasyntenic region demonstrates that the origin of SCPL likely occurred after speciation of the Caricaceae and Brassicaceae within the Brassicales that is, around 72 million years ago (Myr ago)36. Between 27 and 72 Myr ago37, inter-chromosomal recombination (between C1 as described in Fig. 5e) occurred independently within Brassicaceae species. Subsequent to chromosomal recombination, the first SCPL appeared in syntenic block I (I1) ultimately evolving into SAT (anthocyanin sinapoyltransferase) gene. Furthermore, 23–27 Myr ago after speciation to Camelineae/Capsella37, in B. rapa a second chromosomal recombination occurred (C2), whereas FPT was tandemly duplicated in T. parvula (T1). On the other hand, SST gene was created by tandem gene duplicated in this cluster (T2). During the relative recent period 12–23 Myr ago, FPT1 and FPT2 genes occurred by tandem gene duplication (T4) only on the origin of the Arabidopsis species, since tandem gene duplication in C. rubella occurred in different manner. The observed variation of genotypes among non-producing A. thaliana accessions suggests that FPT2 gene has been deleted or differentially mutated. However, it is important to note that although the mechanisms resulting in the non-production of saiginols differ the phenotypes, they confer that is, robustness in the face of high UV irradiance is the same most likely indicating exposure to similar selective pressure. Interestingly, classification of A. thaliana accessions based on function/non-function of FPT2 genes was not similar to any other classification of accessions based on criteria such as global single-nucleotide polymorphisms, cold response, salt tolerance, pathogen response or glucosinolate content38,39,40,41,42. This observation suggested that the evolutionary events have occurred individually and have been differentially filtered by natural selection. This fact suggests that the pressure of natural light selection was independent of that occurring for the other environmental natural selection factors listed above.
We demonstrate here that the FPT2 protein corresponds to one step of a multi-step pathway for the production of the 18 newly identified and annotated saiginols, which have enhanced UV-B-absorbing properties to non-phenylacylated-flavonol-glycosides. As such this is another example, similar to that of the recently described alpha-pyrones43, of a rapidly evolved biochemical pathway, which exploits the pre-existing metabolic infrastructure. In this instance, 18 novel phenylacylated-flavonols are produced following neofunctionalization of the FPT2 gene after gene duplication, possibly through divergence of substrate specificity. Phenylacyltransferases are not uncommon in plant natural product biosynthesis. Members of the BEAT-AHCT-HCBT-DAT (BAHD) family are collectively responsible for the acylation of a wide range of compounds such as flavonoids, hydroxycinnamates, terpenoids and alkaloids being very well characterized. Three BAHD anthocyanin phenylacyltransferases from Gentiana44, Arabidopsis45 and Tomato46 have been characterized, but no flavonol-phenylacyltransferase have been reported previously. The serine carboxypeptidase-like (SCPL) acyltransferase family was more recently identified and characterized in both Arabidopsis and oat, and is involved in the acylation of anthocyanins, sinapoyl-derivatives, glucosinolates and the important defence compound and triterpene saponin, avenacin47,48. While BAHD enzymes use CoA-thioesteters as the acyl donor, SPCL enzymes use O-glucose esters48. To date, four SCPL genes have been characterized as encoding specific sinapoyltransferases of malate (SNG1), choline (SNG2), sinapoyl-glucose (SST) and anthocyanin (SAT) in Arabidopsis. Intriguingly, FPT2 is proposed to catalyse a highly similar (in terms of position and acyl-donor) phenylacylation, to that carried out by the Arabidopsis anthocyanin phenylacyltransferase, A3G6″pCouT, of the BAHD family45,49. However, the precise biological function of the phenylacylated-anthocyanins are as yet unknown.
Characterization of FPT2 in this study suggests it is capable of catalysing several single steps of a multi-step reaction with substrate flexibility for both acceptors and donors of the phenylacyl moiety. Such an enzyme potentially involved in such single steps of a multi-step reaction supports the suggestion that a competitive advantage is conferred to genotypes, which produce saiginols and that flavonol decoration may represent a powerful tool for enhancing seed yield in crops. In addition, analysis of the origin of producing and non-producing accessions revealed that the presence of a functional FPT2 seemingly confers a selective advantage in high light growth habits. Furthermore, analysis of polymorphism within the FPT duplicated genomic region provides an evolutionary framework of the natural history and current status of this locus in the Brassicaceae.
Methods
Plant materials
A. thaliana accessions used in this article were described in Supplementary Table 2. Plants were cultured on agar plate in a growth chamber under standard long day light conditions (16 h day, 140–160 μmol m−2 s−1, 20 °C; 8 h night, 16 °C) for 14 days and transferred to soil (type GS-90 Einheitserde; Gebrueder Patzer). Materials were collected from individual plants, immediately frozen in liquid nitrogen, and stored at −80 °C until further use. A. lyrata (MN47) and Capella rubella (MTE) used in this article were grown in a greenhouse under control conditions, and were immediately frozen with liquid nitrogen after harvest.
Secondary metabolite profiling using LC/MS
Metabolite profiling of secondary metabolites were performed by the method described by Tohge et al.21. Ground frozen flowers were aliquoted and homogenized in 20 μl of extraction buffer (80% MeOH, 5 μg ml−1 isovitexin as an internal standard) per milligram of fresh weight of tissue in a mixer mill for 3 min at 25 Hz with zirconia ball. After centrifugation at 12,000g, the supernatants were immediately used for secondary metabolite profiling. Secondary metabolite analysis was performed on HPLC system Surveyor (Thermo Finnigan, USA) coupled to Finnigan LTQ-XP system (Thermo Finnigan, USA). All data were processed using Xcalibur 2.1 software (Thermo Fisher Scientific, Waltham, USA). Peak identification and annotation were performed with a combination approach using standard chemical confirmation25, MS/MS profiling, retention time profiling, mutant analysis50,51 and literature survey21,49,50,52,53,54,55. To carry out mutant analysis for flavonoid derivatives, 14 mutants; ugt78d2 mutant (flavonoid-3-O-glucoside-less)49, tt7 mutant (quercetin and isorhamnetin derivative-less)50, ugt78d1 mutant (flavonol-3-O-rhamnoside-less)52, ugt78d3 mutant (flavonol-3-O-arabinoside-less)56, omt1 mutant (isorhamnetin-derivative-less)50, ugt89c1 mutant (flavonol-7-O-rhamnoside-less)57, tt4 mutant (all flavonoid-less)58 and pap1-D mutant (anthocyanin-overaccumulator)49,59, and La-er background tt mutant series obtained from NASC (tt3,N84; tt4, N85; tt5, N86; and tt6, N87) were used.
Procedure of purification and characterization of saiginol A
Column chromatography was carried out over ODS (Nacalai Tesque, Cosmosil 75C18-OPN). HPLC analysis was carried out on an Atlantis (φ 4.6 × 150 mm, Waters) at a flow rate of 0.5 ml min−1. Preparative HPLC was performed on a LC 10A system (Shimadzu) using an Inertsil ODS-EP 5 μm (φ 6.0 × 150 mm) at 30 °C and monitoring was accomplished by photodiode array detector (PDA) (200–600 nm). HPLCPDA/ESIMS was performed on a Finnigan LCQ-DECA mass spectrometer (ThermoQuest, San Jose, CA, USA) and an Agilent HPLC 1100 series (Agilent Technologies, Palo Alto, CA, USA) (Tohge et al.49). HR-ESI-MS was performed on an Exactive mass spectrometer (ThermoQuest, San Jose, CA, USA). Optical rotations were determined on a JASCO P-1020. UV spectra were recorded on a JASCO V-560. Nuclear magnetic resonance data were recorded on JEOL JNM ECP-600. The deuterated solvent CD3OD was used for peak 4. Coupling constants are expressed in Hz.
Plant samples (FW, 26.72 g) were collected and immediately frozen in liquid nitrogen, the whole of which was immediately extracted with methanol. After concentration, MeOH liquid extraction was extracted with n-hexane, CHCl3 to remove low-polarity metabolites. After the liquid–liquid partition and concentration, MeOH soluble fraction was obtained and was dissolved with H2O. After liquid–liquid partition with n-BuOH, n-BuOH fraction was obtained (256.9 mg). This fraction was applied to ODS column (φ 3.5 × 7 cm), and roughly separated by eluting with a gradient of H2O as solvent A and CH3CN as solvent B and the following elution profile (fraction 1: 0% CH3CN; fraction 2: 10% CH3CN; fraction3: 20% CH3CN; fraction 4: 30% CH3CN; fraction 5: 40% CH3CN; fraction 6: 50% CH3CN; fraction 7: 60% CH3CN; fraction 8: 70% CH3CN; fraction 9 80% CH3CN; and fraction 10: 100% CH3CN (elution solvent: 70 ml per fraction)) to give 10 fractions. After LC/MS analysis for trace of peak 4, fraction 2–4 was assembled (fraction A). Fraction A was applied to ODS column (φ 3.5 × 7 cm) again, and separated by eluting with a gradient of H2O as solvent A and CH3CN as solvent B and the following elution profile (fractionA-1 and A-2: 0% CH3CN; fraction A-3 and A-4: 10% CH3CN; fraction A-5 to A-7: 20% CH3CN; fraction A-8 to A-11: 30% CH3CN; and fraction A-12: 100% CH3CN (elution solvent: 30 ml (fraction A-1 to A-5) and 15 ml (fraction A-6 to A-12))) to give 12 fractions. After LC/MS analysis for trace of peak 4, fraction A-10 and A-11 were assembled (fraction B). Fraction B (12.0 mg) was applied to preparative HPLC using an isocratic elution (20% CH3CN in H2O) at a flow rate of 4 ml min−1 to give peak 4 (3.5 mg).
Profiling UV–VIS spectrum of saiginol A and K-3G2″ R7R
Saiginol A and K-3G2″R7R (kaempferol-3-O-(-2″-O-rhamnosyl)glucoside-7-O-rhamoside, which were purified in this study and previous work25, were subjected to HPLC-PDA analysis. HPLC analysis was performed on a Summit HPLC system (Dionex, Idstein, Germany) using an Luna C18(2) (φ 2.0 × 150 mm, Waters) at 25 °C and monitoring was accomplished by PDA-100 photodiode array detector (Dionex, Idstein, Germany) (190–600 nm). Peaks were separated by eluting with a gradient described previously21.
Metabolite QTL analysis for saiginols
Introgression line of NILs was obtained as described previously24. Inflorescences from 5-week-old Arabidopsis plants were collected from three individual plants of 45 M lines (C24 background) and 69 N lines (Col-0 background) for metabolite profiling of secondary metabolites.
Microarray analysis
Transcriptome analysis was carried out using ATH1 microarrays as described previously49 with five producing accessions (C24, Cvi, Da, Rsch and RLD) and five non-producing accessions (Col-0, Ler-0, Ws, Sap and Stw). Duplicate hybridizations were carried out for Col-0 and C24, and a single hybridization was performed for all other accessions. Intensity indicates fold change estimated by average of expression level in Col-0.
Overexpression of FPT1 and FPT2 genes
FPT1 and FPT2 overexpression constructs were created by cloning the full-length cDNAs of FPT1 and FPT2 genes from Col-0 and C24 Arabidopsis under the control of the CaMV 35S promoter in vector pK7GW2 (Invitrogen), a binary vector with a gateway cassette, using the In-fusion HD cloning kit (Takara). Binary plasmids were transferred to Agrobacterium tumefaciens GV3101 (pMP90) and transformed to Arabidopsis plants (Col-0) and T-DNA insertion lines (SALK_067799 and SALK_111019) according to the floral dip method. Transgenic plants were selected with 50 mg l−1 kanamycin sulfate for pK7GW2 and T4 progenies were used for the analysis. Primers used for the cloning are described in Supplementary Table 3. Full-length cDNA sequences of FPT1(Col0), FPT1(C24), FPT2(Col0) and FPT2(C24) are shown in Supplementary Figs 8, 9 and 11.
Full-length cDNA and genomic sequence of FPT2 genes
Full-length cDNA of FPT2 from 64 A. thaliana accessions and genomic sequences of FPT2 from 2 Arabidopsis accessions (Col-0 and C24) were sequenced. Primers used for amplification and sequencing of FPT2 are described in Supplementary Table 3. All primers were designed using NCBI blast primer (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Silique and seed productions under UV-B irradiation
For silique production experiment, inflorescences from the primary bolts of 5-week-old Arabidopsis plants were used. The detached inflorescences were irradiated with UV-B light (1 W m−2) for 2 h per day during midday time (5–7 h after the onset of normal light) for 14 days by placing their cut ends in wells of a 96-well microtitre plate containing water. For seed yield experiment, 3-week-old Arabidopsis plants were irradiated with UV-B light (1 W m−2) for 2 h per day during midday time (5–7 h after the onset of normal light) for 28 days.
Synteny analysis and gene duplication of FPT genes
To compare the genomic context of Arabidopsis FPT genes with that in other plant species, information on their patterns of synteny and their orthologues was retrieved from the database PLAZA (http://bioinformatics.psb.ugent.be/plaza/). Duplication analysis of Arabidopsis FPT genes was performed by whole genomic data of plant species obtained from PLAZA v3.0 platform.
Phylogenetic analysis of FPT genes in Brassicaceae species
A phylogenetic tree was constructed with the aligned sequences of FPT-related genes from Brassicaceae species by MEGA5.2 (ref. 60) using the Maximum Likelihood. Sequences of FPT2 were aligned by ClustalW implemented in MEGA 7.0. A phylogenetic tree of protein sequences was built by ClustalW in MEGA 7.0 with G-blocks using Gblocks 0.91b in Phylogeny.fr (http://phylogeny.lirmm.fr/phylo_cgi/one_task.cgi?task_type=gblocks)61.
Data availability
Microarray data generated as part of this study has been deposited into the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database with accession code GSE83291. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author on request.
Additional information
How to cite this article: Tohge, T. et al. Characterization of a recently evolved flavonol-phenylacyltransferase gene provides signatures of natural light selection in Brassicaceae. Nat. Commun. 7:12399 doi: 10.1038/ncomms12399 (2016).
Supplementary Material
Supplementary Figures 1 - 17, Supplementary Tables 1 - 3 and Supplementary Note 1
Secondary metabolite profiling of Arabidopsis thaliana accession flower
Saiginol profiling of near-isogenic line (NIL) Arabidopsis thaliana lines mQTL. Samples obtained from 45 M lines (C24 background) and 69 N lines (Col-0 background) were evaluated with three independent biological replications (n=3).
Identification of syntenic genes located in the Brassicaceae syntenic block of AtFPT/SCPL genomic region. Syntenic block (defined as Block I) of AtFPT/SCPL genomic region, 1) Arabidopsis thaliana chromosome 2 (AtChr.2), were found in 2) Arabidopsis lyrata chromosome 4 (AlChr.4), 3) Capsella rubella chromosome 4 (CrChr.4), 4) Thellungiella parvula chromosome 4 (TpChr.4), 5) Brassica rapa chromosome 4 (BrChr.4) and 6) B. rapa chromosome 9 (BrChr.9). Syntenic blocks were found with 116 genes in AtFPT genomic region (AT2G22500- AT2G23470) using Plaza (http://bioinformatics.psb.ugent.be/plaza/versions/plaza_v3_dicots/). Red indicates FPT/SCPL related genes.
Identification of syntenic genes located in the intrasyntenic genomic region of AtFPT/SCPL genomic region in the Brassicaceae species. Syntenic genes of intrasyntenic block, Arabidopsis thaliana chromosome 4 (AtChr.4)(defined as Block II) of AtFPT/SCPL genomic region, were found in 2) Arabidopsis lyrata chromosome 7 (AlChr.7), 3) Capsella rubella chromosome 7 (CrChr.7), 4) Thellungiella parvula chromosome 7 (TpChr.7), 5) Brassica rapa chromosome 1 (BrChr.1), 6) Brassica rapa chromosome 3 (BrChr.3) and 7) B. rapa chromosome 8 (BrChr.8). Syntenic blocks were found with 132 genes in intrasyntenic genomic region (AT4G36940- AT4G38080) of AtFPT/SCPL region using Plaza (http://bioinformatics.psb.ugent.be/plaza/versions/plaza_v3_dicots/). Red indicates FPT/SCPL related genes.
Acknowledgments
This work was financially supported by the Max Planck Society and an Alexander von Humboldt grant (to T.T.), as well as the European Commission's Directorate-General for Research within the seventh Framework Program (FP7/2007–2013) under grant agreements 270089 (MULTIBIOPRO). We thank Dr Ales Pecinka (Max Planck Institute for Plant Breeding Research), Dr Ute Kraemer (Ruhr-Universität Bochum) and Dr Jamshaid Ali (Ruhr-Universität Bochum) for providing A. lyrata materials; Dr Sebastian Proost (Max Planck Institute of Molecular Plant Physiology) for discussion regarding to plaza cross species comparison and taxonomical phylogenetic tree.
Footnotes
T.T., R.W., H.I., R.S., M.S. and A.R.F. have filed patent applications on saiginol derivatives and FPT gene function. The remaining authors declare no competing financial interests.
Author contributions T.T. and A.R.F. managed the project; T.T., R.W., M.W. and A.R.F. designed the analysis; T.T. and R.W. performed metabolite profiling; T.T., R.W., R.N., H.T. and K.S. contributed chemical purification and characterization of structures; H.I., R.S. and M.S. conducted DNA sequencing; R.W., M.W. and R.H. conducted cDNA sequencing; T.T., R.W. and M.W. identified and evaluated candidate genes; T.T. contributed in silico sequence analysis; T.T., M.S. and A.R.F. wrote the manuscript with help from all authors.
References
- Tilbrook K. et al. The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11, e0164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano M. et al. Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. Plant J. 67, 354–369 (2011). [DOI] [PubMed] [Google Scholar]
- Foyer C. H. et al. Adaptations of photosynthetic electron-transport, carbon assimilation, and carbon partitioning in transgenic Nicotiana plumbaginifolia plants to changes in nitrate reductase-activity. Plant Physiol. 104, 171–178 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries G. et al. Elevated UV-B radiation reduces genome stability in plants. Nature 406, 98–101 (2000). [DOI] [PubMed] [Google Scholar]
- Blumthaler M. in UV-B Radiation and Ozone Depletion: Effects on Humans, Animals, Plants, Microorganisms, and Materials (1993). [Google Scholar]
- Sablowski R. W. M. et al. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 13, 128–137 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 60, 407–431 (2009). [DOI] [PubMed] [Google Scholar]
- Herman J. R. Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J. Geophys. Res. Atmos. 115, (2010). [Google Scholar]
- Rizzini L. et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106 (2011). [DOI] [PubMed] [Google Scholar]
- Tohge T., Watanabe M., Hoefgen R. & Fernie A. R. The evolution of phenylpropanoid metabolism in the green lineage. Crit. Rev. Biochem. Mol. Biol. 48, 123–152 (2013). [DOI] [PubMed] [Google Scholar]
- Taylor L. P. & Grotewold E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8, 317–323 (2005). [DOI] [PubMed] [Google Scholar]
- Nakabayashi R. et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 77, 367–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. & Fernie A. R. Plant metabolomics: towards biological function and mechanism. Trends Plant Sci. 11, 508–516 (2006). [DOI] [PubMed] [Google Scholar]
- Rowe H. C., Hansen B. G., Halkier B. A. & Kliebenstein D. J. Biochemical networks and epistasis shape the Arabidopsis thaliana metabolome. Plant Cell 20, 1199–1216 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller A. L. et al. Functionally divergent alleles and duplicated Loci encoding an acyltransferase contribute to acylsugar metabolite diversity in solanum trichomes. Plant Cell 27, 1002–1017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W. et al. Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat. Commun. 5, 3438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno-Quintero N., Bouwmeester H. J. & Keurentjes J. J. B. Genetic analysis of metabolome-phenotype interactions: from model to crop species. Trends Genet. 29, 41–50 (2013). [DOI] [PubMed] [Google Scholar]
- Ishihara H. et al. Natural variation in flavonol accumulation in Arabidopsis is determined by the flavonol glucosyltransferase BGLU6. J. Exp. Bot. 67, 1505–1517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozema J., Blokker P., Fuertes M. A. M. & Broekman R. UV-B absorbing compounds in present-day and fossil pollen, spores, cuticles, seed coats and wood: evaluation of a proxy for solar UV radiation. Photochem. Photobiol. Sci. 8, 1233–1243 (2009). [DOI] [PubMed] [Google Scholar]
- Sulpice R. et al. Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 30, 1163–1175 (2007). [DOI] [PubMed] [Google Scholar]
- Tohge T. & Fernie A. R. Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat Protoc. 5, 1210–1227 (2010). [DOI] [PubMed] [Google Scholar]
- Handa A. K. & Mattoo A. K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 48, 540–546 (2010). [DOI] [PubMed] [Google Scholar]
- Cartea M. E., Francisco M., Soengas P. & Velasco P. Phenolic compounds in brassica vegetables. Molecules 16, 251–280 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjek O. et al. Construction and analysis of 2 reciprocal Arabidopsis introgression line populations. J. Hered. 99, 396–406 (2008). [DOI] [PubMed] [Google Scholar]
- Nakabayashi R. et al. Metabolomics-oriented isolation and structure elucidation of 37 compounds including two anthocyanins from Arabidopsis thaliana. Phytochemistry 70, 1017–1029 (2009). [DOI] [PubMed] [Google Scholar]
- Jungblut T. P. et al. Structures of UV-B induced sunscreen pigments of the Scots Pine (Pinus sylvestris L.). Angew. Chem. Int. Ed. 34, 312–314 (1995). [Google Scholar]
- Bakowska-Barczak A. Acylated anthocyanins as stable, natural food colorants—a review. Pol. J. Food Nutr. Sci. 14, 107–115 (2005). [Google Scholar]
- Trivellini A. et al. Carbon deprivation-driven transcriptome reprogramming in detached developmentally arresting Arabidopsis inflorescences. Plant Physiol. 160, 1357–1372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehfeldt C. et al. Cloning of the SNG1 gene of arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12, 1295–1306 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle F., Brandt W., Stubbs M. T., Milkowski C. & Strack D. Sinapoyltransferases in the light of molecular evolution. Phytochemistry 70, 1652–1662 (2009). [DOI] [PubMed] [Google Scholar]
- Hu T. T. et al. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat. Genet. 43, 476–481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake M. et al. The genome of the extremophile crucifer Thellungiella parvula. Nat. Genet. 43, 913–U137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. et al. Identification and profiling of novel microRNAs in the Brassica rapa genome based on small RNA deep sequencing. BMC. Plant Biol. 12, 218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. et al. The reference genome of the halophytic plant Eutrema salsugineum. Front. Plant Sci. 4, 46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 5, 3930 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming R. et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452, 991–996 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat F. et al. Understanding Brassicaceae evolution through ancestral genome reconstruction. Genome Biol. 16, 262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwell S. et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah M. A. et al. Natural genetic variation of freezing tolerance in arabidopsis. Plant Physiol. 142, 98–112 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V., Garcia-Martinez S., Piqueras P., Ponce M. R. & Micol J. L. Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol. 130, 951–963 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva K. V. et al. Global analysis of Arabidopsis/downy mildew interactions reveals prevalence of incomplete resistance and rapid evolution of pathogen recognition. PLoS One 6, e28765 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R. et al. Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J.-K., Li Y., Mo H. & Chapple C. Assembly of an evolutionarily new pathway for alpha-pyrone biosynthesis in Arabidopsis. Science 337, 960–964 (2012). [DOI] [PubMed] [Google Scholar]
- Fujiwara H. et al. cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J. 16, 421–431 (1998). [DOI] [PubMed] [Google Scholar]
- Luo J. et al. Convergent evolution in the BAHD family of acyl transferases: identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J. 50, 678–695 (2007). [DOI] [PubMed] [Google Scholar]
- Tohge T. et al. Ectopic expression of snapdragon transcription factors facilitates the identification of genes encoding enzymes of anthocyanin decoration in tomato. Plant J. 83, 686–704 (2015). [DOI] [PubMed] [Google Scholar]
- Lee S. et al. Benzoylation and sinapoylation of glucosinolate R-groups in Arabidopsis. Plant J. 72, 411–422 (2012). [DOI] [PubMed] [Google Scholar]
- Mugford S. T. et al. A serine carboxypeptidase-like acyltransferase is required for synthesis of antimicrobial compounds and disease resistance in oats. Plant Cell 21, 2473–2484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T. et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 42, 218–235 (2005). [DOI] [PubMed] [Google Scholar]
- Tohge T., Yonekura-Sakakibara K., Niida R., Watanabe-Takahashi A. & Saito K. Phytochemical genomics in Arabidopsis thaliana: a case study for functional identification of flavonoid biosynthesis genes. Pure Appl. Chem. 79, 811–823 (2007). [Google Scholar]
- Tohge T., Scossa F. & Fernie A. R. Integrative approaches to enhance understanding of plant metabolic pathway structure and regulation. Plant Physiol. 169, 1499–1511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Messner B., Nakajima J. I., Schaffner A. R. & Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 278, 43910–43918 (2003). [DOI] [PubMed] [Google Scholar]
- Hirai M. Y. et al. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl Acad. Sci. USA 104, 6478–6483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno M. et al. Evolution of a novel phenolic pathway for pollen development. Science 325, 1688–1692 (2009). [DOI] [PubMed] [Google Scholar]
- Saito K. et al. The flavonoid biosynthetic pathway in Arabidopsis: structural and genetic diversity. Plant Physiol. Biochem. 72, 21–34 (2013). [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K. et al. Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlations in Arabidopsis. Plant Cell 20, 2160–2176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K., Tohge T., Niida R. & Saito K. Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. J. Biol. Chem. 282, 14932–14941 (2007). [DOI] [PubMed] [Google Scholar]
- Shikazono N., Tanaka A., Watanabe H. & Tano S. Rearrangements of the DNA in carbon ion-induced mutants of Arabidopsis thaliana. Genetics 157, 379–387 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz J. O., Xia Y. J., Blount J., Dixon R. A. & Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12, 2383–2393 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1 - 17, Supplementary Tables 1 - 3 and Supplementary Note 1
Secondary metabolite profiling of Arabidopsis thaliana accession flower
Saiginol profiling of near-isogenic line (NIL) Arabidopsis thaliana lines mQTL. Samples obtained from 45 M lines (C24 background) and 69 N lines (Col-0 background) were evaluated with three independent biological replications (n=3).
Identification of syntenic genes located in the Brassicaceae syntenic block of AtFPT/SCPL genomic region. Syntenic block (defined as Block I) of AtFPT/SCPL genomic region, 1) Arabidopsis thaliana chromosome 2 (AtChr.2), were found in 2) Arabidopsis lyrata chromosome 4 (AlChr.4), 3) Capsella rubella chromosome 4 (CrChr.4), 4) Thellungiella parvula chromosome 4 (TpChr.4), 5) Brassica rapa chromosome 4 (BrChr.4) and 6) B. rapa chromosome 9 (BrChr.9). Syntenic blocks were found with 116 genes in AtFPT genomic region (AT2G22500- AT2G23470) using Plaza (http://bioinformatics.psb.ugent.be/plaza/versions/plaza_v3_dicots/). Red indicates FPT/SCPL related genes.
Identification of syntenic genes located in the intrasyntenic genomic region of AtFPT/SCPL genomic region in the Brassicaceae species. Syntenic genes of intrasyntenic block, Arabidopsis thaliana chromosome 4 (AtChr.4)(defined as Block II) of AtFPT/SCPL genomic region, were found in 2) Arabidopsis lyrata chromosome 7 (AlChr.7), 3) Capsella rubella chromosome 7 (CrChr.7), 4) Thellungiella parvula chromosome 7 (TpChr.7), 5) Brassica rapa chromosome 1 (BrChr.1), 6) Brassica rapa chromosome 3 (BrChr.3) and 7) B. rapa chromosome 8 (BrChr.8). Syntenic blocks were found with 132 genes in intrasyntenic genomic region (AT4G36940- AT4G38080) of AtFPT/SCPL region using Plaza (http://bioinformatics.psb.ugent.be/plaza/versions/plaza_v3_dicots/). Red indicates FPT/SCPL related genes.
Data Availability Statement
Microarray data generated as part of this study has been deposited into the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database with accession code GSE83291. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author on request.