Abstract
Objectives
We sought to conduct a detailed study on the risk factors of gestational diabetes mellitus (GDM) in Omani women to determine the actual and applicable risk factors and glucose profile in this population.
Methods
We conducted a cross-sectional case-control study using pregnant women diagnosed with GDM. Pregnant women without GDM were used as a control group. We collected information related to age, family history, prior history of pregnancy complications, age of marriage, age of first pregnancy, fasting glucose level, and oral glucose tolerance test (OGTT) results from three hospitals in Oman through face-to-face interviews and hospital records.
Results
The median age of women with GDM was 33 years old (p < 0.050). A significant risk was noted in women with a history of diabetes (p < 0.001), and those with mothers’ with a history of GDM. A significant (p < 0.010) relationship with a likelihood ratio of 43.9 was observed between the incidence of GDM in women with five or six pregnancies, a history of > 3 deliveries, height < 155 cm, and pregnancy or marriage at age < 18 years (p < 0.010). The mean difference in random plasma glucose, one-hour OGTT, and two-hour OGTT was significantly higher in GDM cases compared to control.
Conclusions
Glucose profile, family history, anthropometric profile, and age of first pregnancy and marriage should be considered while screening for GDM and determining the care needs of Omani women with GDM.
Keywords: Diabetes Mellitus, Gestational; Risk Factors; Women.; Oman
Introduction
Gestational diabetes mellitus (GDM) is a typical transient adaptive condition in which women previously undiagnosed with diabetes show higher levels of glucose intolerance during the third trimester of pregnancy. It is known to cause almost 7% of pregnancy-related complications.1 Patients with GDM have an almost 25% higher financial burden and 44–49% higher cost of inpatient care compared to a normal pregnancy.2 Pathogenesis of GDM is related to impaired β-cell functioning and insufficient insulin secretion to meet the demands made by the pregnancy, fetus, and placenta.3
A study conducted among Saudi women reported that patients with GDM had a significantly higher incidence of pre-eclampsia, preterm delivery, induction of labor, cesarean section, newborn mean birth weight, large for gestational age infants, macrosomia, and infant admission to the neonatal intensive care unit compared to control group women.4 A study carried out in Oman by the Obstetrics and Gynecology Departments of Sultan Qaboos University Hospital, Royal Hospital, Khoula Hospital, and Nizwa Regional Hospital reported GDM as one of the major reason for the gradual increase in cesarean section rate from 9.7% in 2000 to 15.72% in 2009.5 In another study, a higher number of preterm deliveries and premature rupture of membranes were reported compared to the control group.6 Pregnancies complicated by pregestational and gestational diabetes were found to be associated with higher rates of fetal macrosomia and increased neonatal shoulder-to-head size difference, significantly higher body fat, thicker upper extremity skinfolds, and a two- to six-fold increase in the likelihood of shoulder dystocia.7
We used the National Institute for Health and Care Excellence (NICE) definition to classify patients with GDM. Patients who had a fasting plasma glucose (FPG) ≥ 5.6 mmol/L and/or two-hour-oral glucose tolerance test (OGTT) ≥ 7.8 mmol/L were considered to have GDM.8
The increase in the prevalence of type 2 diabetes mellitus (T2DM) in Oman is attributed to various risk factors9 one of them being gestational diabetes.1 Retrospective studies carried out in Oman indicated that patients with gestational diabetes have a higher risk of developing hypertension and macrosomia.10 The Ministry of Health, Oman, reported a consistent rise in the incidence of GDM in Oman despite screening for glucose intolerance.11 A study conducted in Muscat showed that 17.2% of patients screened for GDM had a slightly higher risk of developing T2DM within 10 years.12 A recently published article reported that 5% of all pregnant women are diagnosed with GDM in Oman, and 14.3% of fetal deaths are attributed to GDM.13
Given the consistent rise in the burden of diabetes and cardiovascular diseases in Oman and the GCC, it is important to identify the causes and risk factors of GDM to provide a sufficient cushion to health care professionals to develop strategies for its primary prevention.14 A study carried out in Iran identified a positive family history of diabetes and GDM, multiparity, older age, obesity, infertility, chronic hypertension, and stillbirth and abortions as major risk factors.11 A retrospective study carried out in the United Arab Emirates concluded that women with advanced age, multiparity, and obesity had a greater risk for GDM.15 A study from Sudan reported a higher risk of GDM due to proteinuria, preeclampsia, higher blood pressure, and glycosuria in its study population.16 A study conducted in Bahrain suggested a consistent increase in the prevalence of GDM from 7.2% to 12.5%. Maternal age and weight were the major risk factors identified.17
GDM is linked to various risk factors and has differing rates of incidence due to diverse socioeconomic status, healthcare access, lifestyle, heterogenic populations, increased obesity, and geopolitical changes.18 Development of GDM is due to the transient progressive insulin resistance and causes adverse maternal, fetal, and neonatal complications.19 Despite glycemic regulation by dietary interventions, and the use of glibenclamide, metformin, and insulin therapies,1 the mother and fetus/neonate are not totally free from consequences.20,21 Many recent studies have stressed the need for early gestational screening to avoid the development of GDM, but not its prevention.22 However, the lack of detailed studies on risk factors in Omani women makes it difficult to plan and implement primary and secondary preventive measures.
While studies on GDM have been conducted, hardly any studies in Oman measured the risk factors and glucose profile related to this disorder as a whole. This is despite the fact that GDM is associated with serious birth outcomes for both the mother and fetus. The literature confirms the association and correlation between GDM and certain risk factors; however, GDM may also occur in the absence of identifiable risk factors. Therefore, there is a need to study the risk profile of women who are likely to develop GDM, which will help physicians recognize GDM and advise women to modify their lifestyle appropriately early enough to minimize consequences to them and their fetus.
Our study was based on the hypothesis that the risk factors for GDM in Omani patients vary from other populations. Hence, we sought to identify the risk factors of GDM and the extent of their association with the incidence of GDM compared to the normal control group.
Methods
Our study was carried out from February 2015 to July 2015 at Nizwa Polyclinic, Sohar Hospital, and Rustaq Hospital after obtaining approval from Research and Ethics Committee, Ministry of Health, Oman. Primary data were collected by face-to-face interview assisted by staff nurses at each hospital, and secondary data was collected through retrospective chart review of GDM cases and controls. The control group contained pregnant women having a normal pregnancy without glucose intolerance.
Considering a 5% margin error, 95% confidence interval (CI), a population size of 61,000,23 and a response distribution of 50%, a sample size of 382 was calculated. We enrolled a larger population size of 591; 291 were diagnosed with GDM and made up the case group and 300 women without GDM made up the control group. The inclusion and exclusion criteria for both the case and control groups were those who agreed to take part in the study and had no association of glucose level fluctuation due to pancreatitis, pancreatectomy, no pre-natal check-up, and history of not attending follow-up appointments regarding their gestational glycemia.
A literature-based questionnaire24,25 was used to collect the women’s demographic data, obstetric history, gestational outcomes, glucose profile, diabetic history, and other information.
The identity of the participants and findings of this study were treated with the highest degree of confidentiality. The objective of the study was clearly explained to all participants and their written consent was sought before their inclusion. Participation in this study was voluntary and participants had the right to withdraw at any time. Team members and nurses working at the respective hospitals carried out the data collection. The study was approved by Research and Ethics Committee, Ministry of Health, Oman (Approval Number MH/DGP/R&S/PROPOSAL_APPROVED/10/2015 Dated 16/02/2015).
Each woman was given a case number, and the information was entered directly into SPSS Statistics (SPSS Statistics Inc., Chicago, US) version 19.0. Continuous numerical data were described as mean± SD, and categorical data by percentage-frequency distribution. Any difference in continuous and categorical variables of the control and case groups were described by descriptive analysis of frequencies, the chi-square test for categorical variables, Student’s t-test for independent samples, and Spearman’s analysis with the level of significance set at 5%.
Results
We conducted a multicenter systematic randomized case-control study involving 98, 94, and 99 women with GDM and 100 control women each from Nizwa polyclinic, Sohar Hospital, and Rustaq Hospital, respectively. Over half (57%) of the women were from Al Batinah governorate, which may be because two hospitals were located in this region. Thirty-two percent of patients were from Nizwa hospital in Al Dakhliya. However, 7.3% of the samples were originally from Muscat, and the remaining 4% were from Musandam, Al Suwaiq, Al Wusta and Al Sharqia [Figure 1].
Figure 1.
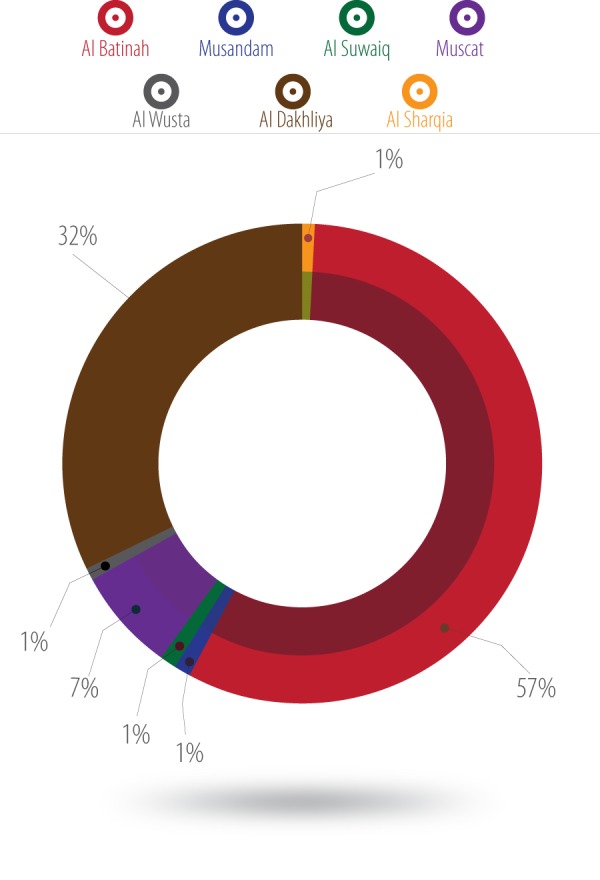
Distribution of the study population.
Pearson’s r was statistically significant (p < 0.050) when comparing the mean age women with GDM (32.3±5.5 years) and the age of the normal control group (28.6±4.7 years). The mean age difference between GDM and normal cases was significantly (p < 0.001) higher (3.7 years, CI 95% 2.9–4.5). Most GDM cases were seen in the 32–36 year age group. The oldest woman was 44 years old in the GDM group and 39 years old in the control group. Simple boxplot analysis of the data showed a significant (p < 0.050) shift in higher age of pregnancy for women with GDM compared to women without GDM [Figure 2].
Figure 2.
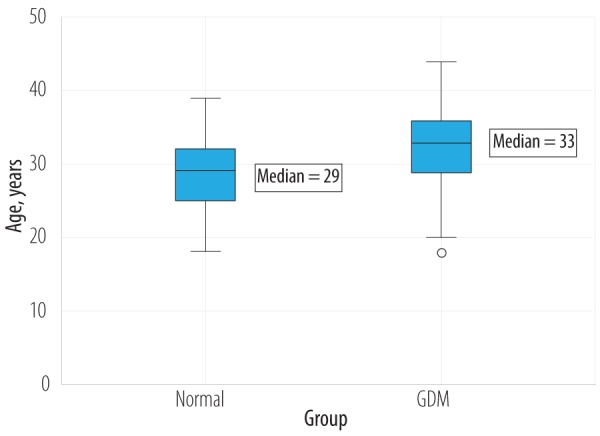
Simple boxplot comparative analysis of age in normal and GDM population.
Spearman correlation analysis indicated a significant relationship between GDM and a family history of type I or type II diabetes (p < 0.001). There was a significant (p < 0.001) risk and a likelihood ratio of 37.3 to developing GDM in a patient with a personal history of DM type I or II. The odds ratio of a patient having a mother with diabetes was 1.2 (CI 0.6–1.9), and a father was 1.0 (CI 0.3–1.7). The odds ratio for patients having a history of diabetes in other family members including grandfather, grandmother, cousins, family relatives, and distant relatives was 1.9 (CI 0.4–9.6) [Figure 3 and Table 1]. Almost 84% of patients with GDM in this study had a family history of GDM compared to 16% of normal cases. The total number of GDM cases with a history of GDM were 157, and 83 without a family history. Some of the patients were not aware of a family history of GDM [Figure 4].
Figure 3.
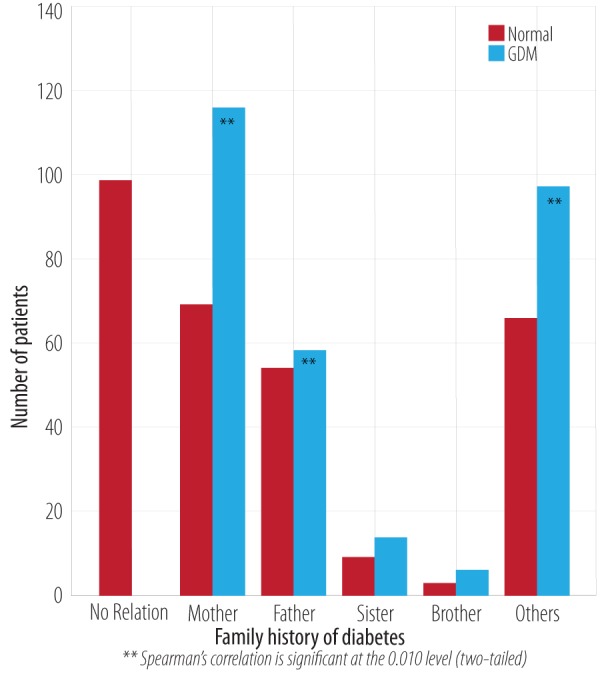
Comparative analysis of family history of diabetes in normal and GDM patients.
Table 1. Family history of diabetes in normal and GDM Cases.
| Diabetes type | Normal patients, n | GDM patients, n |
|---|---|---|
| Type I | 74 | 108** |
| Type II | 63 | 95** |
| Unknown | 161 | 88 |
**p < 0.010 with a likelihood ratio of 3.
**Spearman correlation significant at the 0.010 level (two-tailed).
Figure 4.
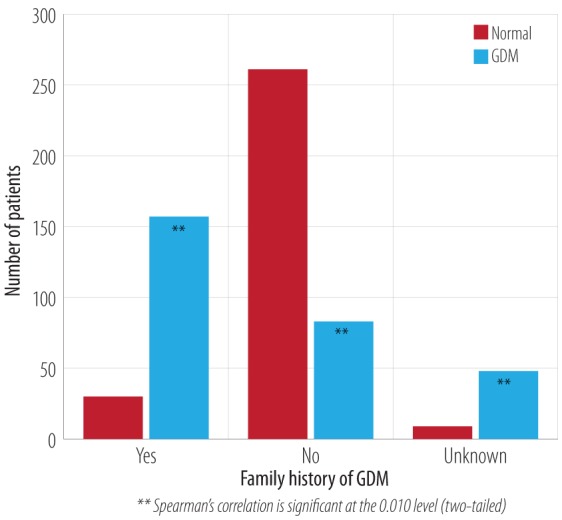
Frequency of family history of GDM and incidence of GDM.
Chi-square analysis showed a significant (p < 0.010) variation in the incidence of GDM with respect to patient height [Figure 5]. A shorter height was related to a higher rate of GDM cases. The mean height of normal patients was 156.3±6.5 cm and 155.6±5.9 cm in patients with GDM. The number of GDM cases was significantly higher in women ≤ 155 cm, but women > 156 cm had a significantly lower risk of GDM (p < 0.010).
Figure 5.
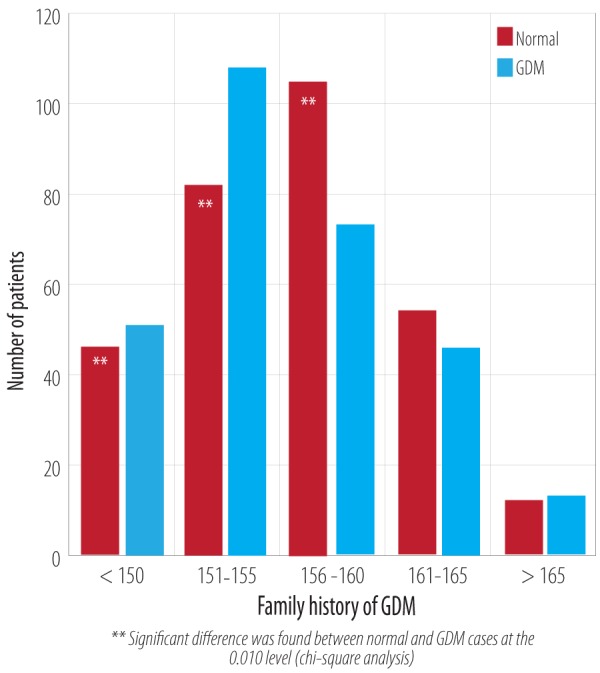
Comparison of height in patients with GDM and the normal population.
Spearman’s correlation analysis of the number of pregnancies and incidence of GDM compared to the control group revealed a significant relationship with a likelihood ratio of 43.9 in women with five and six pregnancies (p < 0.010) [Figure 6]. The average number of pregnancies in the normal group was 3.0±1.8 compared to 3.9±1.9 in the GDM group (p < 0.001) with a mean difference of 0.92 (CI 0.6–1.2). However, pregnancies one to four were associated most commonly without GDM: 14.8% and 33.0% of GDM cases were noted with five and six number of pregnancies, respectively.
Figure 6.
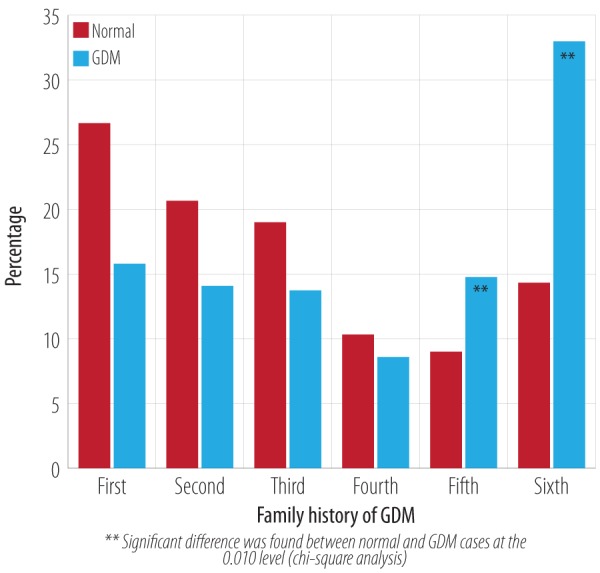
Percentage of normal and GDM cases associated with a number of pregnancies.
Spearman’s correlation analysis showed a significant (p < 0.010) relationship with a likelihood ratio of 52.6 and linear-by-linear association of 32.9 between the incidence of GDM and number of deliveries. The average number of pregnancies in the control group was 1.7±1.8 compared to GDM of 2.7±2.0 (p < 0.010) with a mean difference of 1.0 (CI 0.7–1.3). The risk of developing GDM was relatively higher in women with a history of three or more than three deliveries. The percentage of women with GDM with a history of three to six deliveries was 15.5, 12.7, 8.9, and 14.4 compared to control group 7.3, 8.6, 3.0, and 5.6 respectively [Figure 7].
Figure 7.
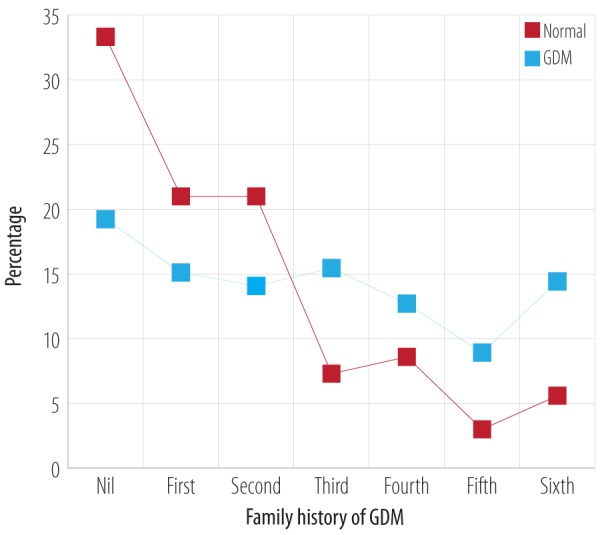
Comparison of number of past deliveries in normal and GDM cases.
A significantly (p < 0.010) higher number of GDM cases were associated with women having a history of pregnancy at ≤18 compared to the control group. However, there was no significant difference between GDM and the other age groups of first pregnancy [Table 2]. The average age of first pregnancy in the control group was 23.6±3.6 years and 23.2±4.3 in the GDM group (p > 0.050) with a mean difference of -0.3 (CI -1.0–0.3).
Table 2. Comparison of age at first pregnancy and age at marriage in GDM and normal control population (n = 290).
| Age at first pregnancy | Normal | GDM | ||
|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | |
| ≤ 18 | 19 | 6.3 | 39** | 13.4** |
| 19–24 | 174 | 58.0 | 140 | 48.1 |
| 25–29 | 90 | 30.0 | 90 | 30.9 |
| 30–34 | 15 | 5.0 | 20 | 6.9 |
| > 35 | 2 | 0.7 | 2 | 0.7 |
| Age at marriage | ||||
| ≤ 18 | 22 | 7.3 | 48* | 16.5* |
| 19–24 | 184 | 61.3 | 158 | 54.3 |
| 25–29 | 73 | 24.3 | 74 | 25.4 |
| 30–34 | 9 | 3.1 | 10 | 3.4 |
| > 35 | 2 | 1.7 | 1 | 1.3 |
*p < 0.050.
**p < 0.010 chi-square analysis of data.
As shown in Table 2, 16.5% of women with GDM were married at ≤ 18 years compared to 7.3% of normal cases. A significantly higher level of plasma glucose was found in women with GDM cases compared to women in the control group [Table 3]. The mean FPG level of patients with GDM was 5.7±1.1 mmol/L (p > 0.050) and 5.3±4.4 mmol/L in the control group. Similarly, random plasma glucose, one-hour OGTT (p < 0.050), two-hour OGTT (p < 0.001), and two-hour postprandial glucose (p < 0.001) were significantly higher compared to the control group.
Table 3. Plasma glucose profile of normal and GDM cases.
| Plasma glucose levels | Normal* | GDM* | Mean difference | CI (95%) | p-value |
|---|---|---|---|---|---|
| Fasting | 5.3±4.4 (4.8) | 5.7±1.1 (5.6) | 0.4 | -0.3–1.1 | > 0.050 |
| Random | 5.3±0.8 (5.2) | 6.8±4.1 (6.1) | 1.5 | 0.9–2.2 | < 0.001 |
| One-hour OGTT | 5.8±1.0 (5.5) | 6.9±0.9 (6.8) | 1.1 | 0.0–2.2 | < 0.050 |
| Two-hour OGTT | 5.5± 1 (5.3) | 6.7±1.8 (6.3) | 1.3 | 0.9–1.6 | < 0.001 |
| Two-hour PPG | 5.5±1.1 (5.6) | 8.6±1.1 (8.2) | 3.1 | 2.7–3.6 | < 0.001 |
*Data presented as mean±SD (median).
OGTT: oral glucose tolerance test; PPG: postprandial glucose.
Data was analyzed using the t-test and significant differences between control and GDM cases were confirmed by ANOVA. The mean difference in random plasma glucose, one-hour OGTT, two-hour OGTT, and two-hour postprandial glucose significantly higher in GDM cases compared to control indicating that the glucose intolerance is significantly associated with the incidence of GDM.
Discussion
The aim of the study was achieved by including a sufficient number of patients from different regions of Oman. The study design was validated as having significantly variable factors by calculating the computed Nagelkerke R-square value (0.58).
It is known from the data that to some extent risk factors in Omani women differ to the globally identified risk factors of GDM. However, there was no significant variation in risk factors between hospitals and regions in Oman. More than half of the population included in this study were from Al Batinah governorate, which may be because two of the three hospitals (Sohar and Rustaq) were located in this region.
The maximum number of GDM cases were between 32–36 years and median age of incidence of GDM was 33 years old. This was significantly higher than the age of the control group (p < 0.050). These results are similar to a cross-sectional study implemented in two phases (retrospective and prospective)26 and supports the results of a study conducted to identify risk factors that used chart review methodology.27
GDM was a significantly (p < 0.001) associated with having a family history of diabetes (mother and father) with a likelihood ratio of 157.4. However, the maximum risk (20.3%) was found with a mother having a history of diabetes. These results are in line with a prospective cohort study conducted at an antenatal clinic in India from 2011 to 2012.28
Almost 84% of patients with GDM patients had a family history of GDM compared to 16% of normal cases. Spearman correlation analysis showed a significant correlation (0.010) and likelihood ratio of 224.6 with a family history of GDM. It also supports previous findings29,30 and confirms familial aggregation of diabetes among the Omani population and a maternal history influence.31
We observed no significant variation between normal and GDM cases with the mean height of patients. However, patients with a shorter height stature had a higher number of GDM cases. The number of GDM cases were significantly higher in women with height ≤ 155 cm supporting previously published results.32 These results also support those obtained in study conducted in Nordic women that looked at the prevalence of and risk factors for GDM using the World Health Organization and a simplified version of the new International Association of Diabetes in Pregnancy Study Groups criteria.9
Women married at ≤ 18 years old had a significantly (p < 0.050) higher risk for GDM compared to the control population. However, most women with GDM married between the ages of 19 and 24. Women who had their first pregnancy at ≤ 18 years old had a significantly (p < 0.010) higher risk of developing GDM. Early marriage may be associated with early and greater number of pregnancies and deliveries. Spearman's correlation analysis revealed a significant relationship (p < 0.010) between the incidence of GDM and women having five and six pregnancies compared to the control group with a likelihood ratio of 43.9.33 A higher number of pregnancies was associated with a higher number of successful deliveries. Spearman's correlation analysis showed a significant (p < 0.010) relationship with a likelihood ratio of 52.6 and linear-by-linear association of 32.9. The risk of developing GDM was relatively higher in women with a history of ≥ 3 live births. These results support studies carried out in Thailand.34
The mean FPG level of patients with GDM was 5.7±1.1 mmol/L (p > 0.050) and 5.3±4.4 mmol/L in the control group. These results are similar to a cross-sectional study carried out in Malaysia,35 and a cohort study from Brazil.28 Similarly, random plasma glucose, one-hour OGTT, two-hour OGTT, and two-hour postprandial glucose was also significantly higher than control groups indicating that the glucose intolerance is associated with the incidence of GDM. These results are in line with the results obtained in community-based screening study.36
Conclusion
The risk factors for GDM in Omani women includes pregnancy over 32 years of age, ≥ 5 pregnancies, ≥ 3 deliveries, abnormal blood glucose profile, height ≤ 155 cm, a family history of diabetes and gestational diabetes, marriage at ≤ 18 years, first pregnancy at ≤ 18 years, and prior history of pregnancy and delivery complications. Awareness of these risk factors can help to minimize glucose intolerance earlier than late pregnancy. We recommend healthcare professionals consider these findings to develop a comprehensive primary and secondary prevention and care program for gestational diabetes in Oman.
Disclosure
The authors declared no conflicts of interest. This study was funding by the Research Council of Oman (TRC).
Acknowledgements
We are thankful to the Research Council of Oman (TRC) for financial assistance under the FURAP grant to carry out the study. We appreciate and acknowledge the assistance of clinical and non-clinical staff of Sohar Hospital, Rustaq Hospital, and Nizwa Hospitals for their cooperation and assistance in collecting the data. We also thank Dr. Syed Gauhar Alam Rizvi Sultan Qaboos University, Muscat, for his kind assistance in statistical analysis of data.
References
- 1.Chen P, Wang S, Ji J, Ge A, Chen C, Zhu Y, et al. Risk factors and management of gestational diabetes. Cell Biochem Biophys 2015. Mar;71(2):689-694. 10.1007/s12013-014-0248-2 [DOI] [PubMed] [Google Scholar]
- 2.Kolu P, Raitanen J, Rissanen P, Luoto R. Health care costs associated with gestational diabetes mellitus among high-risk women–results from a randomised trial. BMC Pregnancy Childbirth 2012;12:71. 10.1186/1471-2393-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med 2014. Mar;31(3):292-301. 10.1111/dme.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 saudi women. Oman Med J 2012. Mar;27(2):140-144. 10.5001/omj.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Busaidi I, Al-Farsi Y, Ganguly S, Gowri V. Obstetric and non-obstetric risk factors for cesarean section in oman. Oman Med J 2012. Nov;27(6):478-481. 10.5001/omj.2012.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Riyami N, Al-Ruheili I, Al-Shezaw F, Al-Khabori M. Extreme preterm premature rupture of membranes: risk factors and feto maternal outcomes. Oman Med J 2013. Mar;28(2):108-111. 10.5001/omj.2013.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Khaduri MM, Abudraz RM, Rizvi SG, Al-Farsi YM. Risk factors profile of shoulder dystocia in oman: a case control study. Oman Med J 2014. Sep;29(5):325-329. 10.5001/omj.2014.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes in pregnancy: management from preconception to the postnatal period (NG3), NICE guideline Published: 25 February 2015 nice.org.uk/guidance/ng3, 17-66. [Cited 2016 July 8]. Available from: https://www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-of-diabetes-and-its-complications-from-preconception-to-the-postnatal-period-51038446021.
- 9.Helseth R, Salvesen O, Stafne SN, Mørkved S, Salvesen KA, Carlsen SM. Gestational diabetes mellitus among Nordic Caucasian women: prevalence and risk factors according to WHO and simplified IADPSG criteria. Scand J Clin Lab Invest 2014. Oct;74(7):620-628. 10.3109/00365513.2014.928942 [DOI] [PubMed] [Google Scholar]
- 10.Barakat MN, Youssef RM, Al-Lawati JA. Pregnancy outcomes of diabetic women: charting Oman’s progress towards the goals of the Saint Vincent Declaration. Ann Saudi Med 2010. Jul-Aug;30(4):265-270. 10.4103/0256-4947.65253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health. Diabetes Mellitus: Management guideline for primary health care, Department of Surviellance and Non-communicable disease control, Directorate General of Health Affairs, 2003, p. 44. [Google Scholar]
- 12.D’Souza MS, Amirtharaj A, Venkatesaperumal R, Isac C, Maroof S. Risk-assessment score for screening diabetes mellitus among Omani adults. SAGE Open Med 2013;1:2050312113508390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutayeb A, Lamlili ME, Boutayeb W, Maamri A, Ziyyat A, Ramdani N. The rise of diabetes prevalence in the Arab region. Open J Epidemiol 2012;2:55-60 . 10.4236/ojepi.2012.22009 [DOI] [Google Scholar]
- 14.Kelly BB, Narula J, Fuster V. Recognizing global burden of cardiovascular disease and related chronic diseases. Mt Sinai J Med 2012. Nov-Dec;79(6):632-640. 10.1002/msj.21345 [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Warethi LO, Kumari AS, Haq A, Bakir A, Sainudeen A, Sedaghatian MR, et al. An evaluation of the latest ADA criteria for screening and diagnosing gestational diabetes at a tertiary care hospital in the United Arab Emirates. Int J Diabetes Metab 2006;14(1):55-60. [Google Scholar]
- 16.Mardi TG, Lutfi MF. Risk factors for gestational diabetes mellitus in Sudanese pregnant women. Int Med Biomed Res. 2012;1(1):79-84 . 10.14194/ijmbr.1113 [DOI] [Google Scholar]
- 17.Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of gestational diabetes mellitus in Bahrain from 2002 to 2010. Int J Gynaecol Obstet 2012. Apr;117(1):74-77. 10.1016/j.ijgo.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 18.Kirke AB, Evans SF, Walters BN. Gestational diabetes in a rural, regional centre in south Western Australia: predictors of risk. Rural Remote Health 2014;14(3):2667. [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012. Nov;8(11):639-649. 10.1038/nrendo.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. Br J Nutr 2010. Sep;104(6):775-787. 10.1017/S0007114510001741 [DOI] [PubMed] [Google Scholar]
- 21.Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015;350:h102. 10.1136/bmj.h102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn 2011. Feb;31(2):135-141. 10.1002/pd.2636 [DOI] [PubMed] [Google Scholar]
- 23.Report AH. 2012, Department of Health Information & Statistics, Directorate General of Planning, Ministry of Health, Sultanate of Oman, Chapter Seven: Utilization of Health Services, p.7-65. [Google Scholar]
- 24.Dode MA, Santos IS. Non-classical risk factors for gestational diabetes mellitus: a systematic review of the literature. Cad Saude Publica. Rio de Janeiro, 2009;25 Sup 3:S341-S356. [DOI] [PubMed]
- 25.Shannon A, Wong CK. Risk factors associated with gestational diabetes mellitus. Int J Bioautomation 2010;14(1):15-26. [Google Scholar]
- 26.Fawole AO, Ezeasor C, Bello FA, Roberts A, Awoyinka BS, Tongo O, et al. Effectiveness of a structured checklist of risk factors in identifying pregnant women at risk of gestational diabetes mellitus: a cross-sectional study. Niger J Clin Pract 2014. Jul-Aug;17(4):495-501. 10.4103/1119-3077.134051 [DOI] [PubMed] [Google Scholar]
- 27.Crete JE, Anasti JN. Diagnosis of gestational diabetes mellitus: can we avoid the glucose challenge test? J Am Assoc Nurse Pract 2013. Jun;25(6):329-333. doi:. 10.1111/j.1745-7599.2012.00792.x [DOI] [PubMed] [Google Scholar]
- 28.Nayak PK, Mitra S, Sahoo JP, Daniel M, Mathew A, Padma A. Feto-maternal outcomes in women with and without gestational diabetes mellitus according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria. Diabetes Metab Syndr 2013. Oct-Dec;7(4):206-209. 10.1016/j.dsx.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 29.Cormier H, Vigneault J, Garneau V, Tchernof A, Vohl MC, Weisnagel SJ, et al. An explained variance-based genetic risk score associated with gestational diabetes antecedent and with progression to pre-diabetes and type 2 diabetes: a cohort study. BJOG 2015. Feb;122(3):411-419. 10.1111/1471-0528.12937 [DOI] [PubMed] [Google Scholar]
- 30.Kuti MA, Abbiyesuku FM, Akinlade KS, Akinosun OM, Adedapo KS, Adeleye JO, et al. Oral glucose tolerance testing outcomes among women at high risk for gestational diabetes mellitus. J Clin Pathol 2011. Aug;64(8):718-721. 10.1136/jcp.2010.087098 [DOI] [PubMed] [Google Scholar]
- 31.Al-Sinani S, Al-Shafaee M, Al-Mamari A, Woodhouse N, Al-Shafie O, Hassan M, et al. Familial Clustering of Type 2 Diabetes among Omanis. Oman Med J 2014. Jan;29(1):51-54. 10.5001/omj.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brite J, Shiroma EJ, Bowers K, Yeung E, Laughon SK, Grewal JG, et al. Height and the risk of gestational diabetes: variations by race/ethnicity. Diabet Med 2014. Mar;31(3):332-340. 10.1111/dme.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinert LS, Mastella LS, Oppermann ML, Silveiro SP, Guimarães LS, Reichelt AJ. Postpartum glucose tolerance status 6 to 12 weeks after gestational diabetes mellitus: a Brazilian cohort. Arq Bras Endocrinol Metabol 2014. Mar;58(2):197-204. 10.1590/0004-2730000003069 [DOI] [PubMed] [Google Scholar]
- 34.Youngwanichsetha S, Phumdoung S. Factors related to prediabetes among postpartum Thai women with a history of gestational diabetes mellitus. Nurs Health Sci 2013. Dec;15(4):449-453. 10.1111/nhs.12055 [DOI] [PubMed] [Google Scholar]
- 35.Chew WF, Rokiah P, Chan SP, Chee WS, Lee LF, Chan YM. Prevalence of glucose intolerance, and associated antenatal and historical risk factors among Malaysian women with a history of gestational diabetes mellitus. Singapore Med J 2012. Dec;53(12):814-820. [PubMed] [Google Scholar]
- 36.Balaji V, Madhuri BS, Paneerselvam A, Arthi T, Seshiah V. Comparison of venous plasma glucose and capillary whole blood glucose in the diagnosis of gestational diabetes mellitus: a community-based study. Diabetes Technol Ther 2012. Feb;14(2):131-134. 10.1089/dia.2011.0060 [DOI] [PubMed] [Google Scholar]


