Abstract
In order to protect against blood pressure, a mature artery is supported by mural cells which include vascular smooth muscle cells and pericytes. To regenerate a functional vascular system, arteries should be properly reconstructed with mural cells although the mechanisms underlying artery reconstruction remain unclear. In this study, we examined the process of artery reconstruction during regeneration of the zebrafish caudal fin as a model to study arterial formation in an adult setting. During fin regeneration, the arteries and veins form a net-like vasculature called the vascular plexus, and this plexus undergoes remodeling to form a new artery and 2 flanking veins. We found that the new vascular plexus originates mainly from venous cells in the stump but very rarely from the arterial cells. Interestingly, these vein-derived cells contributed to the reconstructed arteries. This arterialization was dependent on Notch signaling, and further analysis revealed that Notch signaling was required for the initiation of arterial gene expression. In contrast, venous remodeling did not require Notch signaling. These results provide new insights towards understanding mechanisms of vascular regeneration and illustrate the utility of the adult zebrafish fin to study this process.
Keywords: vascular regeneration, angiogenesis, arterialization, fin regeneration
INTRODUCTION
An adequate supply of oxygen-rich blood to the entire body is essential for life and impairment of arterial blood flow can cause severe diseases, including myocardial infarction and limb ischemia. To circulate blood throughout the entire body, the heart needs to pump blood forcefully enough, and arteries suffer high pressure from this blood flow. Therefore, the arterial conduits need to be constructed in a way to make them particularly suited for the quick, efficient, and sustainable delivery of blood. To ensure high blood pressure, arteries, but not veins, are intimately associated with mural cells. The mural cells, which include vascular smooth muscle cells and pericytes, support vessel function. Moreover, they are indispensable for the development of a mature and durable vasculature (Cheung and Sinha, 2011). For example, the loss of pericytes leads to the formation of numerous microaneurysms, hyperplasia of endothelial cells, and abnormal vascular formation (Hellström et al., 2001; Lindahl et al., 1997). Thus, the recruitment of mural cells is considered to be essential for the formation of durable arteries. Especially, in terms of developing vascular regeneration therapies, it is important to understand the mechanisms underlying the formation and regeneration of mature and durable arteries.
Compared to humans, zebrafish have a high capability of regeneration after an injury and can regenerate their fins, nephrons, heart, and other organs (Diep et al., 2011; Johnson and Weston, 1995; Poss et al., 2002). Given that mature and durable arteries should also be reconstructed during the regeneration of these organs, the zebrafish appears to be a suitable model organism to study artery regeneration. Furthermore, we believe that understanding the mechanisms underlying arterial reconstruction in zebrafish will contribute to the development of vascular regeneration therapies.
To reveal mechanisms of arterial reconstruction, fin regeneration provides us a remarkable system. As the caudal fin is thin and transparent, the vascular reconstruction process can be visualized in living fish. Moreover, the pattern of the arteries and veins in the caudal fins is quite simple. Each zebrafish caudal fin ray has a central artery and 2 flanking veins. Arteries are surrounded by smooth muscle actin-positive mural cells, and these vessels and mural cells are reconstructed during the process of fin regeneration.
Previous studies have revealed the cellular process of caudal regeneration in zebrafish. In this process, the regeneration of blood vessels starts with vascular healing. Then, vascular sprouting occurs from newly re-connected vessels, and around 2 days post amputation (dpa) the sprouts connect together to form a vascular plexus, whose structure resembles that of the primary capillary plexus formed during developmental vasculogenesis. Subsequently, this vascular plexus separates into veins and artery at 4 dpa . Furthermore, transposon-based clonal analysis revealed that reconstructed vessels in the regenerating zebrafish caudal fins are derived from endothelial progenitors in the stump (Tu and Johnson, 2011). In that study, the constituent lineages of cells in the adult fin were examined in zebrafish injected with a Tol2 transposon-based lineage tracer that drives GFP expression ubiquitously. Since Tol2 based transposons integrate at approximately the 4000 cell stage (dome stage; i.e., before gastrulation), it allowed these researchers to follow the descendants of individual cells after this stage. Although their study clearly showed that regenerated vessel cells are formed by the endothelial cells and their precursors in the stump, it also raised a further question as to the regeneration of the artery. Indeed, given that the endothelial lineage had not yet divided into arterial and venous sublineages at the time of Tol2 integration (Jin et al., 2005), it is unclear whether the zebrafish artery has the ability to construct a new durable artery, or whether other endothelial cells can contribute to the new artery.
To unveil the vascular reconstruction process precisely, in this study we first carefully characterized the arteries in the zebrafish caudal fins. Furthermore, by using 2 distinct transgenic lines, we were able to label arteries separately from veins. Unexpectedly, we found that the arterial endothelial cells in the regenerate come from venous endothelial cells in the stump, and that the venous endothelial cell-derived vascular plexus becomes rearranged into a new artery and veins. In contrast, the arterial endothelial cells did not contribute to artery formation in the regenerate. This study illustrates the utility of the adult zebrafish as a new model system to study not only the mechanisms of adult arterial formation, but also venous arterialization.
RESULTS
Characterization of the artery and veins in the zebrafish fin ray
We first characterized the arteries and veins in the zebrafish caudal fin by using Tg(fli1a:EGFP) fish, which express EGFP in both their arteries and veins (Fig. 1A, B, E). Zebrafish caudal fins are supported by bony fin rays, and each fin ray is associated with a single artery in the center of the ray and 2 veins adjacent to the bony ray (Huang et al., 2003) (Fig. 1A). Similar to mammalian arteries, we found that zebrafish arteries are highly associated with vascular smooth muscle cells, but that their veins are not (Bayliss et al., 2006) (Fig. 1A). Furthermore, we found that Tg(flt4:YFP) fish (Bussmann and Schulte-Merker, 2011) which express fluorescent protein YFP (citrine) under the control of the zebrafish flt4 promoter showed venous expression (Fig. 1D, G) while Tg(kdrl:EGFP)fish which express enhanced green fluorescent protein (EGFP) under the control of the zebrafish kdrl promoter showed strong arterial expression (Fig. 1C, F). In both transgenic lines, fluorescent protein-positive side branches were located between the artery and veins. Tg(kdrl:EGFP) was established by using the 6-kb promoter fragment of the kdrl gene (Jin et al., 2005). As the chromosomal location of the transgene integration site sometimes affects the expression pattern, especially when such a short promoter fragment is used, we also checked transgenic zebrafish that expressed mCherry (Tg(kdrl:ras-cherry); (Chi et al., 2008) and Kaede (Tg(kdrl:Kaede)) under the control of the zebrafish kdrl promoter. The expression of these 2 transgenes was also restricted to the artery (data not shown, Fig. 3). These results imply that kdrl expression in the adult caudal fin was restricted to the artery and not to the veins. The expression of these transgenes was retained after fin regeneration. The characteristics of the artery was gradually restored during fin regeneration, even though they had been once lost at the initial phase of regeneration (shown below, see Fig. 2A, C). In order to investigate the mechanism of vascular restoration, we first observed vascular reconstruction in detail by using these transgenic zebrafish. Due to the difficulty of whole-mount in situ hybridization using zebrafish caudal fins (Smith et al., 2008), we could not investigate the mRNA expression of those genes. Even though the actual gene expression pattern might be different, transgene expression was used as a differentiation marker.
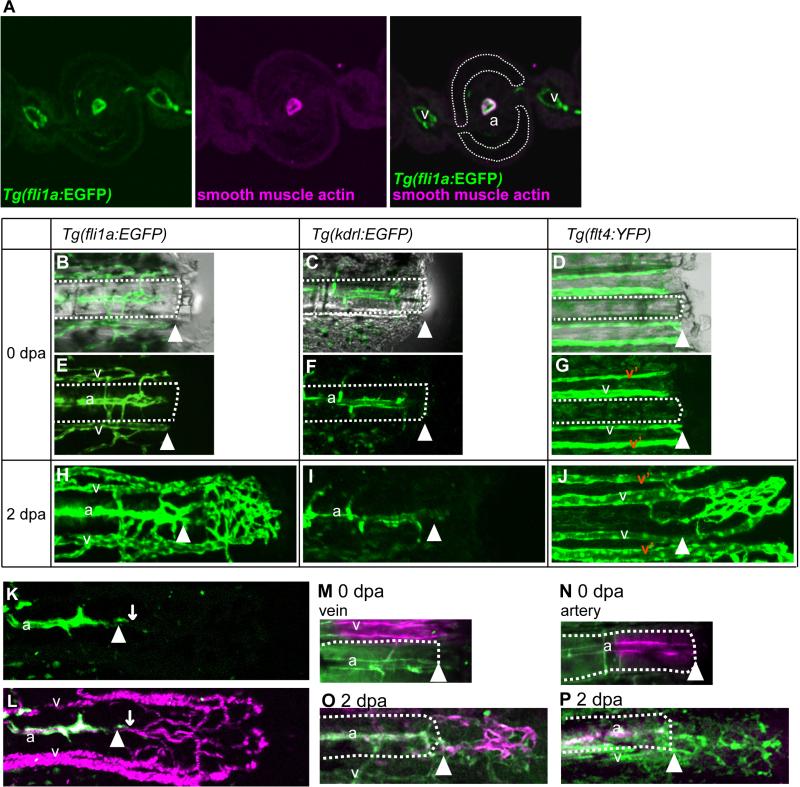
Fig. 1.
Veins, but not arteries, contributed to the formation of the vascular plexus during fin regeneration. (A) Immunostained cross section through the middle level of a Tg(fli1a:EGFP) fin ray showing the vasculature (green) and smooth muscle actin (red). Each fin ray (outlined with white dots) is associated with 1 artery (a) in the center of the ray and 2 veins (v) adjacent to the bony ray. The artery is intimately associated with smooth muscle cells, but the veins are not. (B-J) Time-lapse images from representative regenerating fin rays of Tg(fli1a:EGFP) (B, E, H), Tg(kdrl:EGFP) (C, F, I), and Tg(flt4:YFP) (D, G, J) at 0 dpa (day after amputation) (B-G), and 2 dpa (H, I, J). (K and L) Simultaneous imaging of Tg(kdrl:EGFP) –positive cells (green) and the trajectory of the blood cells (red) in living Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish. White arrowheads point to the amputation site. (M and O) Venous endothelial cells of a Tg(fli1a:kikGR) were labeled by photo-conversion (red) at 0 dpa (M). At 2 dpa, labeled cells were found in the vascular plexus (O). (N and P) Arterial endothelial cells of Tg(fli1a:kikGR) were labeled (red) at 0 dpa (N). At 2.5 dpa, the labeled cells did not contribute much to formation of the vascular plexus (P). White arrowheads point to the amputation site, and the right side of the amputation site is the regenerating fin (distal). The white-dotted lines outline the fin ray in the stump. a, artery; v, vein.
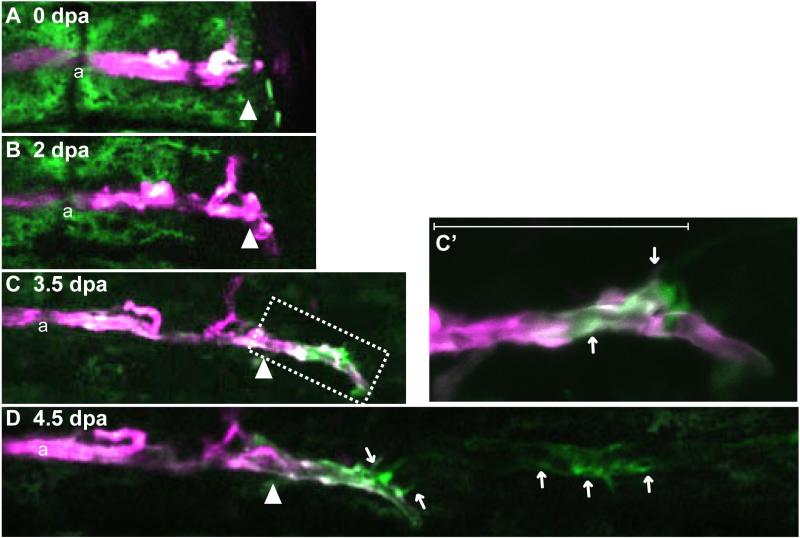
Fig. 3.
Arterial endothelial cells did not contribute much to arterial formation in the regenerate. (A-D) Time-lapse images from a representative Tg(kdrl:Kaede) regenerating fin ray from 0 dpa to 4.5 dpa. Kaede-expressing arterial cells were labeled (red) at 0 dpa by photo-conversion (A). At 2.5 dpa, when the vascular plexus is usually formed, labeled artery cells had penetrated a little bit into the regenerated region. Most of the vascular plexus seemed to comprise Tg(kdrl:Kaede) -negative cells (B). At 3.5 pda, non-labeled arterial cells (green) appeared at the tip of the labeled artery (C). C’ is an enlarged image of the boxed region in “C.” By 4.5 dpa, the number of non-labeled cells had increased, and sometimes these cells were not continuous with the artery in the stump (D). The amputation site is indicated by the white arrowhead. a, artery; v, vein.
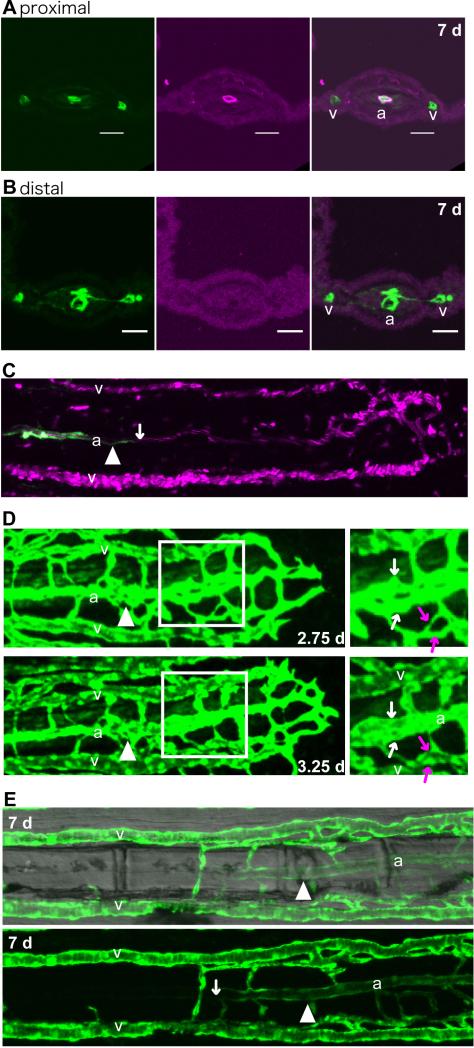
Fig. 2.
Artery and vein separation occurred by rearrangement of the vascular plexus.
(A and B) Frozen cross-sections of different regions of the same fin ray in a Tg(fli1a:EGFP) caudal fin at 7 dpa. The arterial vessel in the regenerate close to the stump (proximal) was associated with smooth muscle cells (red, A). In the distal region of the fin ray (B), the vasculature (green) had separated into 3 vessels, but smooth muscle cells (red) had not yet been recruited. As the fin rays in the regenerate were usually thicker than those in the mature fin rays, they could be easily distinguished. Scale bars, 20 μm. (C) Simultaneous imaging of Tg(kdrl:EGFP) (green) and the trajectory of the blood cells (red) in living Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish. The white arrow points to the distal extent of Tg(kdrl:EGFP) -positive cells. In the more distal side of the regenerate, Tg(kdrl:EGFP) -positive cells were not observed, although the blood flow showed 3 distinct streams, presumed to be the future artery and veins. (D) Representative image of remodeling of the vascular plexus. Time-lapse images of a Tg(fli1a:EGFP) regenerating fin ray at 2.75 and 3.25 dpa. Right panels are enlarged figures of the boxed regions in the left ones. Vascular fusion occurred close to the future artery (white arrows) and future veins (red arrows). (E) A representative regenerating fin ray of a Tg(flt4:YFP) at 7 dpa. A fluorescence image (bottom) and a same image merged with a bright-field image (top) are shown. In the stump, the artery did not have Tg(flt4: YFP) -positive cells except in a small region close to the artery in the regenerate. On the other hand, the artery in the regenerated region was Tg(flt4:YFP) positive. The amputation site is indicated by the white arrowhead. a, artery; v, vein.
Veins, but not the artery, contribute to the formation of the vascular plexus during regeneration
After fin amputation, the artery and veins in each fin ray heal and connect together by 24 hours post amputation (hpa) (Huang et al., 2003). Next, they form a net-like vascular network called the vascular plexus around 2 days post amputation (dpa, Fig. 1H). In the Tg(kdrl:EGFP) fish, which express EGFP in the fin-ray artery, EGFP-positive cells were scarce in the regenerate at 2 dpa (Fig. 1I). Double transgenic Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish were used for simultaneous imaging of Tg(kdrl:EGFP) -positive arterial cells and the tracts of blood flow, and this analysis revealed that vascular plexus formation was not disturbed in these double transgenics (Fig. 1K, L). On the other hand, almost all of the cells in the vascular plexus were Tg(flt4: YFP) -positive ones during the period of vascular plexus formation (Fig. 1J). These observations suggest that the vascular plexus had characteristics similar to those of the veins.
A previous report described lineage restriction in regenerating fins (Tu and Johnson, 2011), and the authors showed that the endothelial cells in the regenerate were derived from the endothelial cells in the stump. In light of their data and ours, we considered the possibility that the vascular plexus was derived from the venous cells in the stump. To examine this possibility, we first visualized the vascular healing process, as the vascular plexus is known to form from the site of vascular healing (Huang et al., 2003). For this visualization, we performed time-lapse imaging of Tg(flt4:YFP) -expressing cells before formation of the vascular plexus (supplementary material Fig. S1). At 18 hpa, Tg(flt4:YFP) -expressing venous cells migrated to the center of the fin ray. Around 24 hpa, the 2 veins associated with the same fin ray became connected together (supplementary material Fig. S1).
This observation supports the idea that the vascular plexus originates from the veins in the stump.
Next, we generated transgenic fish that express photo-convertible protein kikume-green and red (KIKGR (Tsutsui et al., 2005)) in both arteries and veins under the control of the fli1a promoter. When the venous cells were labeled by photo-conversion at 0 dpa (Fig. 1M), labeled cells were observed in the vascular plexus at 2 dpa (Fig. 1O). In contrast, when arterial endothelial cells were labeled at 0 dpa (Fig. 1N), a few (<10) labeled arterial cells were observed in the vascular plexus (Fig. 1P). These results suggest that the vascular plexus is derived from the venous cells in the stump and that the contribution of the arterial endothelial cells in the stump is minimal. As the transgenic fish expressed only a very low level of KIKGR, it was difficult to trace further the endothelial cells in the stump. After vascular plexus formation, the plexus becomes remodeled into 3 vessels having a spatial pattern similar to that of the artery and veins in the stump; but the vascular plexus is still present at the distal region (Huang et al., 2003; supplementary material Fig. S2A). Next, we examined whether the venous-derived cells in the vascular plexus actually contributed to the formation of the new artery in the regenerate. To approach this problem, we carefully visualized the arterio-venous reconstruction process.
Separation of the vascular plexus into artery and veins occurs by vascular remodeling
The 3 distinguishing features of the artery in the fin ray are its position, the transgene expression driven by the kdrl promoter, and smooth muscle recruitment. In order to examine the process of artery formation, we first examined the sequence of this process. As previously reported (Bayliss et al., 2006), smooth muscle recruitment starts around 7 dpa. Consistent with that report, our immunohistochemistry experiments using frozen sections showed that the artery in the regenerate was not covered by smooth muscle cells even though the vascular plexus had already separated into 3 vessels (Fig. 2B). On the other hand, the proximal side of the artery in the regenerate was covered by smooth muscle cells (Fig. 2A). This observation suggests that vascular separation occurred prior to smooth muscle recruitment. We next examined whether vessel separation occurred prior to arterial marker expression, by using Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish. At 4 dpa, the proximal side of the artery in the regenerate started to express EGFP in Tg(kdrl:EGFP); Tg(gata1a:dsRed) animals. However, the distal side of the vessels did not have Tg(kdrl:EGFP) -positive cells, although the blood flow had already begun in the 3 vessels (Fig. 2C). These results suggest that the vascular plexus has separated into the 3 vessels prior to kdrl promoter-driven transgene expression and smooth muscle recruitment.
Using time-lapse imaging of Tg(fli1a:EGFP) fish, we next examined how the net-like vasculature of the vascular plexus became rearranged into the 3 vessels (Fig. 2D). Most of the vessels in the vascular plexus gradually adjusted their angle, became aligned in the same direction as the vessels in the stump, and were then remodeled to form the vessels with large diameter (Fig. 2D). This vascular remodeling occurred close to the location of the future artery and future veins (Fig. 2D). These observations suggest that the vascular separation process occurs by the adjustment of vessel angle and vascular remodeling of the vascular plexus. Collectively, our results suggest that the artery in the regenerate originate from the vascular plexus, which derives from the veins in the stump. Consistent with our hypothesis, the regenerated artery was still Tg(flt4:YFP) positive (Fig. 2E) at 7 dpa, when the vasculature has already started to express the kdrl promoter-driven transgene (data not shown). The artery in the regenerate at this time still had characteristics similar to those of the veins, thus supporting the idea that the artery in the regenerate was derived from the veins in the stump. Therefore, we next examined whether the artery in the stump definitively did not contribute to arterial formation in the regenerate.
Arterial cells in the stump do not contribute substantially to arterial formation in the regenerate
To investigate if the stump arterial cells contributed to the new artery in the regenerate, we used Tg(kdrl:Kaede) fish, which express the photo-convertible protein Kaede only in their arteries (Fig. 3A). The arterial endothelial cells in the stump were labeled at 0 dpa by photo-conversion (Fig. 3A). As had been observed in the Tg(kdrl:EGFP) fish, only a small number of arterial cells was found in the vascular plexus at 2.5 dpa (Fig. 3B). As almost all of the Tg(kdrl:Kaede)-expressing cells were labeled (red in Fig. 3B), these cells at 2.5 dpa were derived from the artery in the stump. At 3.5 dpa, a small number of arterial cells in the regenerate (green cells in Fig. 3C) newly appeared at the tip of the artery in the stump (Fig. 3C). As the number of these non-labeled cells (green cells in Fig.3C) in the regenerate was very small at 3.5 dpa and the labeled (red cells in Fig.3C) and non-labeled cells (green cells in Fig.3C) were complementary, the emergence of the non-labeled cells seemed not to have been due to the substitution of the photo-converted Kaede proteins with newly synthesized green ones in an artery-derived cell in the stump. These results strongly support our prediction that the artery in the stump did not contribute to regenerated arteries.
At 4.5 dpa, many cells started to express the Kaede transgene; however, labeled cells were not observed in vessels of the regenerate (Fig. 3D). These data suggest that the arterial endothelial cells in the stump did not make much of a contribution to the arterial formation in the regenerate and that venous arterialization seemed to have occurred. Interestingly, a contiguous part of the stump artery and the artery in the regenerate had both labeled and non-labeled cells. Tg(flt4:YFP) fish at 7 dpa also had Tg(flt4:YFP) -positive cells in the same region (Fig. 2E). These observations imply that the joint region between the artery in the regenerate and that in the stump was composed of both types of cells. Taken together, our data suggest that the origin of the vessels in the regenerate is the veins in the stump and that the artery in the stump does not contribute much to the formation of the new artery in the regenerate. In summary, the vascular plexus is derived from veins in the stump; and it forms the artery and the veins in the regenerate through vascular remodeling. Few arterial cells in the stump contribute to the regenerate.
Venous arterialization is regulated by Notch signaling
How did the vein-derived cells acquire their arterial identity? Notch signaling is known to be important for arterial differentiation (Krebs et al., 2004; Krebs et al., 2000; Lawson et al., 2001; Lawson et al., 2002). To reveal the molecular mechanisms of vascular plexus arterialization, we pharmacologically inhibited Notch signaling by using the γ-secretase inhibitor DAPT (Micchelli et al., 2003), which blocks Notch cleavage and signaling. DAPT treatment from 2 to 4 dpa revealed that Notch inhibition disturbed the vascular remodeling in the middle region of the fin ray, which was presumed to be the future artery (Fig. 4B, C, and supplementary material Fig. S3A). Interestingly, the diameter of the veins was comparable between the control and DAPT treated fish (Fig. 4B, C). These results suggest that Notch signaling is important for the arterial remodeling but not for venous remodeling. Furthermore, DAPT treatment inhibited the initiation of arterial marker expression (data not shown).
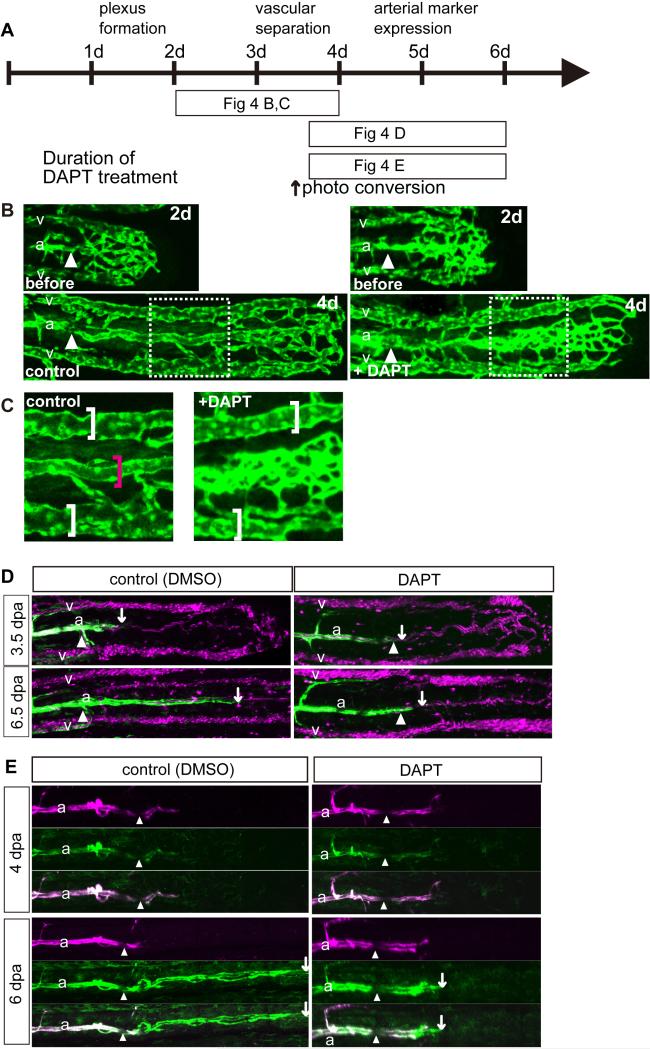
Fig. 4.
Notch signaling was essential for vascular separation and arterial differentiation during fin regeneration. (B-D) Zebrafish caudal fins were clipped and then treated with the γ-secretase inhibitor DAPT or control DMSO as indicated in “A.” (B) Inhibition of Notch signaling caused vascular separation defects. DAPT treatment from 2 dpa (before ; 2d) to 4 dpa (+ DAPT; 4d) caused vascular separation defects in the artery but not in the veins. “C” is an enlarged image of the boxed region in “B.” Inhibition of Notch signaling from 3.5 to 6.5 dpa inhibited a transgene expression from kdrl promoter, which represents arterial gene expression (D). Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish were treated with DAPT from 3.5 to 6.5 dpa. White arrows point to the distal end of the area of Tg(kdrl:EGFP) positive cells. The blood flow had already separated into 3 vessels at 6 dpa in both the control and some fin rays of the DAPT-treated fish. However, Tg(kdrl:EGFP) -positive cells were not observed in the DAPT-treated fish. (E) Inhibition of Notch signaling in a Tg(kdrl:Kaede) fish from 4 to 6 dpa inhibited arterial gene expression. Kaede-expressing arterial cells were labeled (red) by photo-conversion at 4 dpa. Soon after conversion, the fish were treated with DAPT up to 6 dpa. The amputation site is indicated by the white arrowheads in all photos. White arrows point to the the distal end of the area of Tg(kdrl:Kaede) positive cells.
To assess whether the impaired initiation of arterial marker expression was caused by the failure of arterial remodeling, we performed a pharmacological Notch inhibition experiment after vascular separation at 3.5 dpa (Fig. 4D). After DAPT treatment, the blood flow at the middle of the fin was still observed in some vessels (Fig. 4D), but was disturbed in others. Even though blood flow was not disturbed in this middle region, a transgene expression from kdrl promoter, which represents arterial gene expression, was affected in the DAPT-treated fish, suggesting that the defective initiation of arterial marker expression is not due to the failure of arterial remodeling. In contrast, the arterial marker expression in the stump artery was not abnormal. These findings suggest that Notch signaling is important for the initiation of arterial marker expression but not for its maintenance. To further investigate our hypothesis, we performed a DAPT treatment experiment using Tg(kdrl;Kaede) fish. At 4 dpa, some of the arterial cells had already started kdrl promoter-driven transgene expression in the vessels in the regenerate. When the artery in the regenerate was labeled by photo -conversion, labeled arteries could be observed in the DAPT-treated fish although the initiation of the kdrl promoter-driven expression was inhibited (Fig. 4E and supplementary material Fig. S3B). Consistent with our results, it was reported that Notch signaling in the mouse retina is important for the initiation of arterial marker expression but dispensable for its maintenance (Ehling et al., 2013; Lawson et al., 2001). Our data suggest that arterialization of the vascular plexus occurs by a combination of vascular separation and arterial gene expression and that Notch signaling is essential for the initiation of arterial gene expression as well as arterial remodeling. In our experiments, the inhibition of Notch signaling affected arterial vessel fusion, but not venous fusion, implying that prior to vessel fusion, future arterial cells and venous cells had already acquired a different character.
DISCUSSION
To withstand and adapt to blood pressure, functional and durable arteries need to be supported by surrounding cells, i.e., the mural cells. However, it is unclear how arteries are formed in the adult. In this study, by careful analysis of regenerating zebrafish caudal fins, we found that newly formed arteries originated from the veins in the stump. In contrast, and to our surprise, the arterial endothelial cells in the stump contributed little to the newly formed arteries. Although arteries and veins are the 2 major vessels constituting the vascular system, they are quite different in many aspects. For instance, arteries are specifically associated with vascular smooth muscle cells in mammals and in fish as well; whereas veins, but not arteries, have ostial valves. Interestingly, we showed that vein-derived arteries were supported by smooth muscle cells, i.e., mural cells, comparable to the arteries in the stump, suggesting that veins had acquired the ability to induce mural cells. Thus, the results of this study raise several interesting questions as to how much developmental plasticity endothelial cells have and how they acquire such quite different characteristics during development and regeneration.
Previous evidence has suggested that venous cells retain plasticity. Experimental manipulations such as flow reversal (le Noble et al., 2004) and transplantation (Moyon et al., 2001; Othman-Hassan et al., 2001) have shown that embryonic venous cells can change into arterial cells. In the developing heart, some of the coronary arterial cells have been reported to be derived from the sinus venosus (Red-Horse et al., 2010). However, venous grafts do not acquire a full arterial phenotype (Kudo et al., 2007), which is probably due to their lower success rate compared to arterial grafts (Goldman et al., 2004; Sabik et al., 2005). Our results are consistent with these previous studies in the sense that venous cells have the required plasticity to transform into arterial ones and further indicate that functional arteries supported by mural cells can actually be generated from the veins, at least during fin regeneration. Thus, this study, as well as a series of previous studies, strongly suggests the possibility that understanding the mechanism of venous arterialization, especially that of the dedifferentiation and identification of reprogramming factors, is likely to contribute to the improvement of bypass grafts and vascular regeneration therapy.
Although this study indicates that venous cells could regenerate arterial cells, it still remains to be elucidated whether all of the venous endothelial cells have the ability to become arterial cells or not. On the other hand, it is also possible that veins contain endothelial stem cells and provide a niche for these cells. Supporting this possibility, a side population of vascular endothelial cells in mouse choroidal vessels has been suggested to be vessel-residing endothelial stem/progenitor cells that contribute mainly to angiogenesis (Wakabayashi et al., 2013). Precise tracing of individual venous cells would provide us a more clear answer to this question.
Our study revealed that Notch signaling is important for arterialization of venous cells. Notch signaling has already been known to be important for arterial formation during development (Krebs et al., 2004; Krebs et al., 2000; Lawson et al., 2001; Lawson et al., 2002). However, because pattern formation of the zebrafish caudal fin vasculature is quite different between development and regeneration, it has not been elucidated whether the same signaling is involved in vascular regeneration. In this study, we showed that the same signaling was commonly involved in regulation of both vascular regeneration and development in spite of the difference in their morphologic procedures.
Furthermore, our experiments identified 2 different processes whereby Notch signaling played a role in vascular regeneration. The first one was arterial remodeling. Treatment with the Notch signaling inhibitor after vascular plexus formation disrupted arterial, but not venous, formation; besides, an avascular region was observed between the veins and putative arterial region. This observation implies that cells forming the vascular plexus were already fated into either the venous or non-venous cells and that Notch signaling was important for arterial remodeling of non-venous cells. An additional interesting point is that veins in the zebrafish fin regenerates were less sensitive to the Notch signaling inhibitor than were the arteries, thus suggesting the possibility that venous remodeling in the zebrafish caudal fin ray might differ from the arterial remodeling in terms of molecular mechanism. The second process in which Notch signaling was found to be essential was the initiation of expression of the arterial marker kdrl, as was recently reported to be the case in the developing mouse retina (Ehling et al., 2013). In addition, consistent with that previous study in mice, we found that Notch signaling was dispensable for the maintenance of arterial marker expression. These observations imply that, even in the adult vasculature, the signaling pathways used in the zebrafish vasculature are in common with those in mice; although there are some differences, such as regeneration capacity, between mouse and zebrafish.
In summary, we showed that venous arterialization depended on Notch signaling during fin regeneration. Our results also suggest that the adult zebrafish system can successfully model venous arterialization and adult arterial formation. Further extensive studies should reveal the precise mechanisms underlying venous arterialization and contribute to the development of vascular regeneration therapies.
MATERIALS AND METHODS
Zebrafish Strains and Husbandry
Zebrafish were maintained according to standard methods. The establishment and characterization of Tg(flt4:YFP)hu4881, Tg(gata1a:dsRed)sd2, Tg(fli1a:EGFP)y1, Tg(kdrl:EGFP)s843, and Tg(kdrl:ras-cherry)s896 fish were described elsewhere (Bussmann and Schulte-Merker, 2011; Jin et al., 2005; Lawson and Weinstein, 2002; Roman et al., 2002; Traver et al., 2003).
Regeneration and time-lapse experiments
Adult zebrafish were anesthetized with a standard solution of 0.02% tricaine (w/v; Tricaine Methane Sulfonate, MS-222, Sigma, A5040) or 500 ppm 2-Phenoxyethanol (SIGMA-Aldrich, 77699) for 5–15 min, until their gills stopped moving. Fish fins were amputated at approximately 50% of the proximal–distal level. The amputated fish were kept in 1-l fish tanks containing 500 ml of water or in individual 250-ml beakers (4-5 fish/beaker) containing 100 ml of water at 26-28C. At least 10 fish in each group were used in each experiment. Throughout the experiments, the fish were deprived of food. For vascular imaging, amputated fish were placed under a confocal microscope (Nikon A1R-A1 confocal laser Microscopes) for imaging and then immediately put back into a beaker. The same fin ray and vessels of each fish were photographed at different time intervals during the course of the time-lapse experiments.
Pharmacological Treatments
The amputated zebrafish were placed in a 250-ml beaker and treated with 50 μM DAPT (Sigma-Aldrich) or control DMSO. The γ-secretase inhibitor was dissolved in DMSO to make a stock concentration of 10 mM. For protection from light, the beaker containing the fish treated with inhibitor or control DMSO was wrapped in air-vented aluminum foil. The water in the beaker was replaced with fresh water every 2 days.
Establishment of the transgenic lines
A 6.8-kb fragment of the kdrl promoter (Beis et al., 2005) was subcloned upstream of the kaede gene to create the final Tg(kdrl:kaede) construct. We injected 200 pg of linearized DNA into one cell-stage embryos and selected individual transgenic carrier adults by screening for fluorescent progeny. Three Tg(kdrl:kaede) founders were recovered with identical expression patterns but with various levels of expression. Tg(kdrl:kaede)s960 exhibited the strongest expression and was subsequently used for experiments. Tg(fli1a:kikGR) was established by using the pTolfli1epDest vector obtained from the laboratory of Dr. Nathan Lawson. For the generation of the vectors, we followed the standard Gateway cloning technology (Invitrogen). As the transgene expression was very weak in the established transgenic fish, we used several lines to confirm the observations.
Immunostaining
For preparation of frozen sections, clipped zebrafish fins were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4 °C for 1 day, and then treated with 10% EDTA in 4% PFA at 4 °C for 1 day for decalcification. The fins were rinsed in PBS followed by immersion in a gradient of sucrose solution (15% for 2 hours, 20% for 2 more hours, 25% overnight), and then embedded in Tissue-Tek® O.C.T. Compound. Sectioning of the fins was performed by use of a cryostat. Antibodies used for immunostaining were anti-alpha smooth muscle actin antibody [B4] (abcam [ab40863]) and anti-GFP (Invitrogen).
For staining of whole mounts, clipped zebrafish fins were fixed in 4% paraformaldehyde overnight at 4 °C and subsequently stained with the anti-alpha smooth muscle actin for visualization of smooth muscle actin. The fins were rinsed in PBS, made permeable by immersion for 20 min in acetone at –20 °C, rinsed twice with PBS, and incubated in blocking serum for 1 h at 37 °C. They were then incubated with a 1:100 dilution of primary antibody in blocking serum overnight at 4 °C. The specimens were then washed extensively in PBS and subsequently incubated with a 1:200 dilution of Alexa-555-conjugated secondary antibody (Invitrogen).
Supplementary Material
Figure S1 Time-lapse images from a representative Tg(flt4:YFP) regenerating fin ray (D, G, J) from 0 to 24 hpa. During vascular healing, Tg(flt4:YFP)-positive cells migrated to the center of the fin rays. White arrowheads point to the amputation site.
Figure S2 Representative image of a Tg(fli1a:EGFP) regenerating fin ray at 4 dpa. (B) Comparison of representative images among Tg(fli1a:EGFP), Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish, and Tg(flt4:YFP) at 4 dpa. White arrowheads point to the amputation site.
Figure S3 Notch signaling is essential for vascular separation and arterial differentiation during fin regeneration. (A) Inhibition of Notch signaling caused vascular separation defects. The Tg(fli1a:EGFP) fish were treated with DAPT (left) or control DMSO (right) from 2 dpa to 4 dpa caused vascular separation defects in the artery but not in the veins. (B) Inhibition of Notch signaling in Tg(kdrl:Kaede) from 4 dpa to 6 dpa disturbed a transgene expression from kdrl promoter, which represents arterial gene expression. Kaede-expressing arterial cells were labeled (red) by photo-conversion at 4 dpa. Soon after conversion, the fish were treated with DAPT (left) or control DMSO (right) up to 6 dpa.
Acknowledgements
We thank Ms. H.Utsumi, K.Takashiro, A.Ayala, K.Brand, and M.Alva for fish care; Functional Genomics Facility Spectrography and Bioimaging Facility, NIBB Core Research Facilities for technical support; Ms. S. Ukai and Ms. K. Yamamoto for assistant-secretary support, and all members of the laboratories of S.T. and D.Y.R.S lab for helpful discussions. This work was supported in part by grants from the National Institutes of Health to D.Y.R.S (HL54737) and N.C.C (HL104239, HD069305), Packard Foundation to D.Y.R.S, Human Frontier Science Program Organization to Y.K, and a grant for scientific research from the Okazaki Institute for Integrative Bioscience to S.T..
Footnotes
The authors declare no competing financial interests.
REFERENCES
- Bayliss PE, Bellavance KL, Whitehead GG, Abrams JM, Aegerter S, Robbins HS, Cowan DB, Keating MT, O'Reilly T, Wood JM, et al. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat Chem Biol. 2006;2:265–273. doi: 10.1038/nchembio778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin SW, Scott IC, D'Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Schulte-Merker S. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development. 2011;138:4327–4332. doi: 10.1242/dev.068080. [DOI] [PubMed] [Google Scholar]
- Cheung C, Sinha S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J Mol Cell Cardiol. 2011;51:651–664. doi: 10.1016/j.yjmcc.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lawson ND, Weinstein BM, Johnson SL. reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Dev Biol. 2003;264:263–274. doi: 10.1016/j.ydbio.2003.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Weston JA. Temperature-sensitive mutations that cause stage-specific defects in Zebrafish fin regeneration. Genetics. 1995;141:1583–1595. doi: 10.1093/genetics/141.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27:1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Bréant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Esler WP, Kimberly WT, Jack C, Berezovska O, Kornilova A, Hyman BT, Perrimon N, Wolfe MS. Gamma-secretase/presenilin inhibitors for Alzheimer's disease phenocopy Notch mutations in Drosophila. FASEB J. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Bréant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenführ M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ, et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Sabik JF, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–551. doi: 10.1016/j.athoracsur.2004.07.047. discussion 544-551. [DOI] [PubMed] [Google Scholar]
- Smith A, Zhang J, Guay D, Quint E, Johnson A, Akimenko MA. Gene expression analysis on sections of zebrafish regenerating fins reveals limitations in the whole-mount in situ hybridization method. Dev Dyn. 2008;237:417–425. doi: 10.1002/dvdy.21417. [DOI] [PubMed] [Google Scholar]
- Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 2005;6:233–238. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20:725–732. doi: 10.1016/j.devcel.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, Naito H, Takara K, Kidoya H, Sakimoto S, Oshima Y, Nishida K, Takakura N. Identification of vascular endothelial side population cells in the choroidal vessels and their potential role in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;1154(10):6686–93. doi: 10.1167/iovs.13-12342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Time-lapse images from a representative Tg(flt4:YFP) regenerating fin ray (D, G, J) from 0 to 24 hpa. During vascular healing, Tg(flt4:YFP)-positive cells migrated to the center of the fin rays. White arrowheads point to the amputation site.
Figure S2 Representative image of a Tg(fli1a:EGFP) regenerating fin ray at 4 dpa. (B) Comparison of representative images among Tg(fli1a:EGFP), Tg(kdrl:EGFP); Tg(gata1a:dsRed) fish, and Tg(flt4:YFP) at 4 dpa. White arrowheads point to the amputation site.
Figure S3 Notch signaling is essential for vascular separation and arterial differentiation during fin regeneration. (A) Inhibition of Notch signaling caused vascular separation defects. The Tg(fli1a:EGFP) fish were treated with DAPT (left) or control DMSO (right) from 2 dpa to 4 dpa caused vascular separation defects in the artery but not in the veins. (B) Inhibition of Notch signaling in Tg(kdrl:Kaede) from 4 dpa to 6 dpa disturbed a transgene expression from kdrl promoter, which represents arterial gene expression. Kaede-expressing arterial cells were labeled (red) by photo-conversion at 4 dpa. Soon after conversion, the fish were treated with DAPT (left) or control DMSO (right) up to 6 dpa.