Abstract
Clinical and pathological investigations were conducted on outbreaks of infectious bursal disease (IBD) in pullets under brooding using the battery cage system in a commercial poultry farm in Kaduna, Nigeria. Two consecutive outbreaks of IBD on the same farm were studied. The onset of the disease and morbidity and mortality rates were recorded. Postmortem examinations were conducted and gross lesions recorded. Tissues were collected and fixed in 10% buffered formalin and processed for histopathological examinations. In the first outbreak, 80 to 100% of the chicks were affected at the age of 4 to 5 weeks and mortality rate was 95.8% and lasted for 9 days. In the second outbreak, the mortality rate was 43.3% and it also lasted for 9 days. At the onset of the disease, the birds were also 4-week-old like in case 1. The disease was diagnosed based on clinical signs, pathology, and agar gel immunodiffusion test (AGID). Clinical signs, gross lesions, and histopathological findings were characteristic of virulent infectious bursal disease. After the first outbreak (case 1) the house was disinfected using polidine® (iodophor compound), V-ox® (inorganic peroxygen compounds), CID20® (quaternary ammonium chloride, aldehydes, and alcohol), terminator III® (phenols), and glutasan® (aldehyde and quaternary ammonium chloride). But they failed to eliminate the IBD virus from the poultry pen.
1. Introduction
Infectious bursal disease (IBD) is an acute, highly contagious viral disease of young chickens that primarily affects lymphoid tissues [1–3]. It is caused by a member of the genus Avibirnavirus in the family Birnaviridae [4, 5]. The disease was first described by Cosgrove in [6] around Gumboro, Delaware, USA.
Ojo et al. [7] first described the disease in South Western Nigeria and was confirmed by Onunkwo in [8]. Since then several studies have shown that the disease is of major concern to the poultry industry in the country [9–12]. Infectious bursal disease virus (IBDV) has tropism to actively dividing precursor B lymphocytes, primarily in the bursa of Fabricius, but other immune organs are also involved [13].
The disease is mainly controlled by rigorous sanitary measures and vaccination through the use of either live or killed vaccines. Infectious bursal disease intermedite or intermediate plus vaccines are commonly used to protect broilers and commercial pullets replacements from field IBDV exposure [14–18]. Despite vaccinations outbreaks of IBD are occasionally reported in vaccinated flocks of broiler and pullets with varying degree of mortality rates. Reversion of these attenuated vaccinal strains to more virulent phenotypes under field and experimental conditions has been frequently reported [19, 20] possibly due to a lack of IBDV polymerase fidelity during vaccine viral genome replication in the host cells.
Infectious bursal disease virus is very stable and has been reported to resist many disinfectants at certain concentrations and or conditions [3, 21]. The virus remained viable by exposure for 1 hour at 30°C to 0.5% phenol and 0.125% thimerosal [3]. Landgraf et al. [22] found that the virus survived 60°C but not 70°C for 30 minutes, and exposure to 0.5% chloramine after 10 minutes and 0.5% formalin for 6 hours destroyed the virus. In addition, iodine complex had deleterious effects on the virus at 23°C for 2 minutes [3].
Certainly, the hardy nature of this virus is one reason for its persistent survival in poultry houses even when thorough cleaning and disinfection procedures are followed [3, 23]. There are several claims by some practicing veterinarians and animal scientists that disinfectants like hypo, V-ox, and iodine are effective for the control of virulent IBD. Currently, there is no report available on the efficacy of these agents as treatment regimen for IBD in field or clinical trials in Nigeria. This paper describes the clinicopathological correlation of virulent IBD outbreaks in two successive flocks of commercial chickens raised under battery cage system in attempted treatments.
2. Materials and Methods
2.1. Study Area and Case History
Outbreaks of suspected IBD case were reported to the Avian Clinic of Veterinary Teaching Hospital, Ahmadu Bello University, Zaria, from a commercial poultry farm in Kaduna metropolis located at Km 3.7, Kaduna International Airport Road, Kaduna. Two subsequent outbreaks of acute IBD in pullets raised under battery cage system in the same farm were investigated.
2.2. Clinical Assessment and Postmortem Examination
Repeated farm visits were made and analysis was made on the clinical presentations of the disease, farm records including source of the chicks, breed, vaccination, age, major signs observed, intervention, and the mortality rate. Moribund and dead birds were collected and thorough postmortem examination was conducted. The gross lesions were recorded.
The first outbreak was encountered in a flock of 8,413, 4.3-week-old brown pullets (ISA brown). The flock had received Newcastle disease vaccines at days 10 and 20 of age and IBD vaccines at days 8, 18, and 31 of age using Bioveta® Ornibur (intermediate strain, Bioveta, s.r.o. Ltd., Czech Republic) and B2K Indovax® (invasive intermediate strain, B.P. Vet., India) vaccines at 10 mL reconstitution per bird per os, respectively. Clinical signs such as dullness and ruffled feathers were observed at about 5 hours after Bursa B2K Indovax IBD vaccine administration. Twenty birds were lost on the first day and immediately Floricol® (Florfenicol, VIC Animal Health, Russia) at 1 mL/L of water was given for a day and V-ox (mixture of inorganic peroxygen compounds; Polchem Hygiene Laboratories Pvt. Limited, India) at 1 g/L of water, Vitavet® (multivitamins, vitamins A, Bs, E, D, and K, Pharma-Swede, Egypt) at 1 g/2 L of water, and glucose at 1 g/L of water were administered for 3 days. On days 2 and 3 of the outbreak, Floricol was changed to doxy-gen® (doxycycline and gentamycin, Kepro, Holland) at 1 g/2 L of water and on day 4 doxy-gen was switched to oxyfuravit® (Oxytetracycline HCl, Furaltadone HCl, vitamins (A, B2, B12, C, D3, E, and K3), Nicotinic Acid, DL-Methionine, and Lysine, Maridav, Ghana) at 1 g/L of water and substituted V-ox for polidine (iodine, alkylphenoxypolyglycol ether and phosphoric acid; Animal Care Services Konsult, Nigeria) at 1 mL per 2 liters of water for 5 days. Despite medications, the morbidity and mortality worsened and lasted for 9 days. The birds that survived (347) were disposed off and the house was thoroughly cleaned, washed, and disinfected.
The second outbreak occurred in a flock of 9,000, 4-week-old brown pullets. The chicks were vaccinated against ND at days 10 and 20 of age and IBD at days 8 and 18 of age using ABIC® IBD vaccines through oral and intranasal routes, respectively. During the outbreak, oxyfuravit was administered in feed and water at the rate of 10 g/5 kg and 1 g/L, respectively, together with polidine at 1 mL/L. V-ox, Neoceryl plus® (neomycin, erythromycin, oxytetracycline, colistin sulphate, streptomycin, and multivitamins, Alfasan International BV, Holland) at 1 g/L, and Tylodox (tylosin tartrate and doxycycline; Kepro, Holland) at 1 g/L together with multivitamins were administered for 3 days each.
However, despite the interventions, the morbidity and mortality also lasted for 9 days. Postmortem examination and sample collection were carried out in both cases.
2.3. House Cleaning and Disinfection
After the first outbreak, the house was rigorously cleaned, washed, and disinfected using CID20 (alkyldimethoylammonium chloride, glutaraldehyde, formaldehyde, glyoxal, and isopropanol; CID Lines, leper-Belgium), at dilution of 1 : 200, glutasan (glutaraldehyde and alkyldimethoylammonium chloride; Pine Oil, USA), at 1 : 400 of water, terminator III (ortho-phenylphenol, ortho-benzyl-para-chlorophenol, and para-tertiary-amylphenol; Neospark, India) at 2 mL/L of water, and hypo® (sodium hypochlorite; Multipro Enterprises Ltd., Nigeria) at 2 mL/L of water alternately. The house was left fallow for 40 days before restocking.
2.4. Sample Collection and Processing
Tissues were collected for virological, bacteriological, and histopathological examination. Bursae of Fabricius (BFs) were aseptically harvested into universal bottles and stored at −20°C for viral detection. Liver was sent for bacterial culture and identification. Tissues including skeletal muscles, spleen, BF, and kidneys were fixed in 10% neutral buffered formalin for histological examination. The fixed tissues were processed routinely for histopathology stained with haematoxylin and eosin and examined with the light microscope.
Frozen samples of the BFs were homogenised into 50% w/v suspension in phosphate buffered saline (PBS). The homogenate was centrifuged at 2000 rpm for 30 minutes and the supernatant was harvested and tested for IBD virus using AGID test as described by OIE [14].
3. Results
3.1. Clinical Evaluation
Clinical signs observed in chicks in the two outbreaks were ruffled feathers, depression, hurdling together, anorexia, prostration, and whitish diarrhoea. Mortality recorded spiked within 5 days of onset and then declined but lasted for 9 days in both cases. Morbidity and mortality recorded were 100% and 95.8%, respectively, for case 1 and 80% and 43.3%, respectively, for case 2. The mortality patterns are shown in Figure 1.
Figure 1.
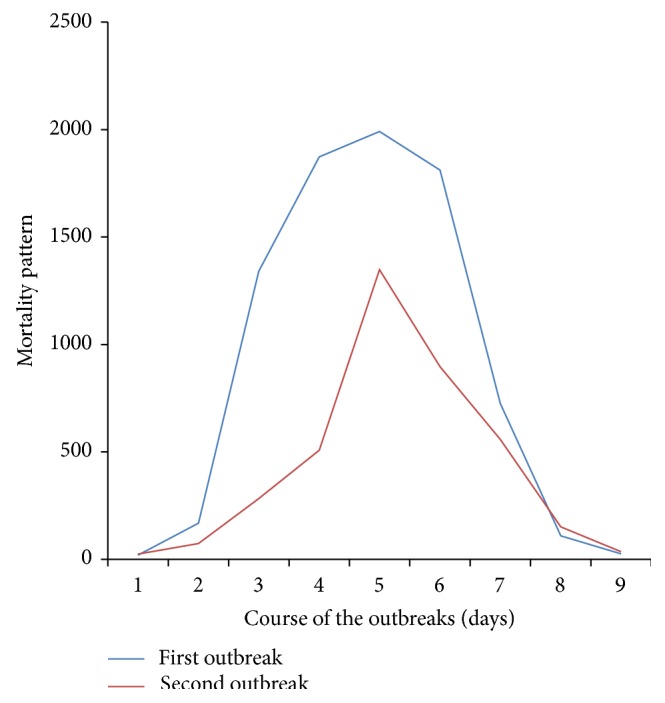
Mortality pattern in the two outbreaks of virulent infectious bursal disease in pullets on battery cage system.
3.2. Postmortem Findings
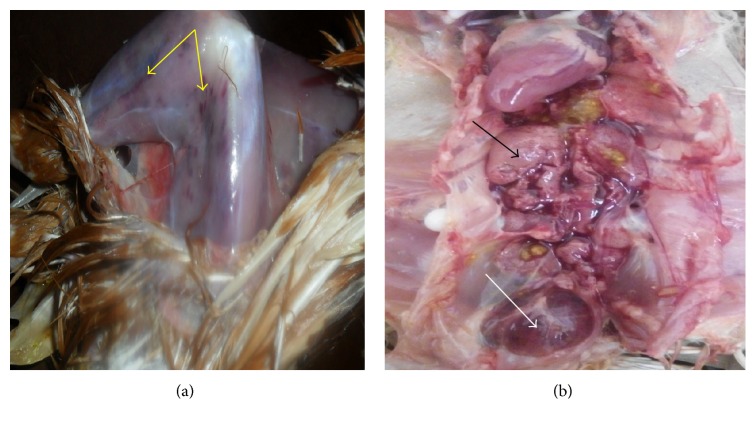
The carcasses were in good condition but moderately dehydrated. There were petechial and ecchymotic haemorrhages on the pectoral, thigh and leg muscles and caecal tonsils and at the junction between proventriculus and ventriculus. The liver was severely congested and the spleen was enlarged and mottled but in some cases atrophied. In most cases the bursa of Fabricius was edematous and haemorrhagic with yellowish gelatinous exudate on the mucosal surface. The kidneys were swollen and pale (Figure 2).
Figure 2.
(a) Ecchymotic haemorrhages on the thigh and leg muscles (yellow arrows), (b) enlarged pale kidneys (black arrow), and edematous and haemorrhagic bursa of Fabricius (white arrow) of 4-week-old brown pullets from case 2.
3.3. Histopathological Findings
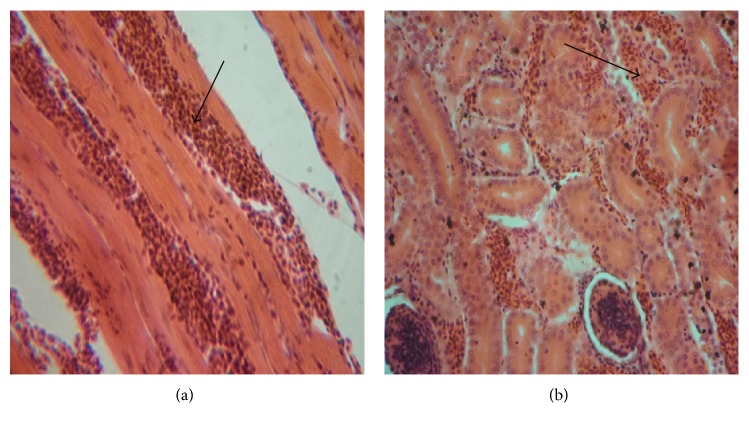
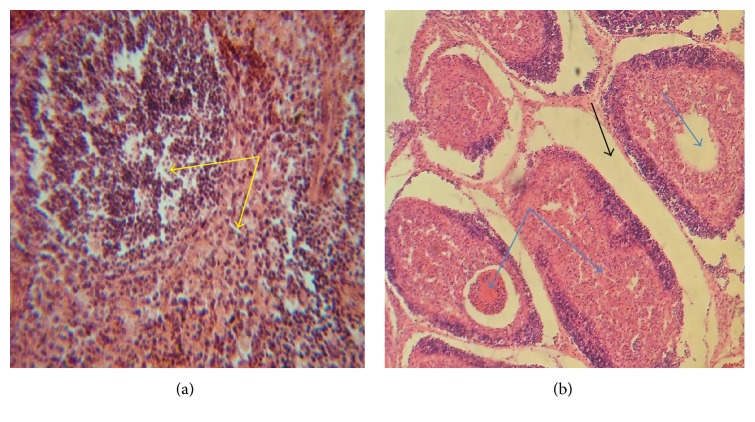
Microscopic examination of tissues showed moderate haemorrhages in the muscles and kidneys (Figures 3(a) and 3(b)) and the spleen showed moderate lymphoid depletion in the lymphoid nodules (Figure 4(a)). There was marked interfollicular oedema and depletion of lymphocytes from the lymphoid nodules in the BFs (Figure 4(b)). Other lymphoid nodules of the BF showed degeneration and necrosis of lymphocytes and cystic cavitations with heterophil infiltrates (Figure 4(b)).
Figure 3.
Photomicrographs of haemorrhage (arrows) in muscle (a) and kidney (b) of 4-week-old chicks affected with infectious bursal disease. H & E ×200.
Figure 4.
Photomicrographs of 4.3-week-old chicks affected with infectious bursal disease showing moderate lymphocytes depletion in the lymphoid nodules (yellow arrows) of the spleen (a), marked interfollicular oedema (black arrow), and cystic cavitation and necrosis (blue arrows) in the medullar of bursal follicles (b). H & E ×200.
3.4. Bacteriological and Virological Examinations
Escherichia coli were isolated from the liver and the bursal homogenate gave positive reactions to the IBDV known antiserum.
4. Discussion
The purpose of this investigation was to determine whether polidine or V-ox at 1 mL/L could significantly reduce typical IBD lesions in chicks infected with IBDV. However, the clinical manifestations and gross lesions observed in this study are similar to those reported previously [12, 24–32] that chickens infected with IBDV exhibit anorexia, prostration, and white diarrhoea while grossly the BFs appear yellowish, hemorrhagic, and turgid with prominent striations, oedema, and caseous material found and varying degrees of hemorrhages in the thigh and breast muscles and at the junction between gizzard and proventriculus. The microscopic lesions seen in this study are similar to those reported [3, 12, 25, 27, 28, 32–38] that found that bursae from IBDV exposed birds showed lymphoid depletion in the bursal follicles, interfollicular oedema, cellular debris in the medullary areas with necrosis, and/or eosinophilic cystic cavitations. These typical lesions confirmed that the attempted treatment claims cannot stand for control of IBD.
The observed morbidity and mortality are suggestive of vIBD and agree with the report by Asif et al. [39], Mbuko et al. [12, 28], El-Mahdy et al. [27], and Ezeibe et al. [40] that chickens infected with virulent IBDV could experience high morbidity rate of 80–100% and mortality rate of 40–90% depending on the presence of secondary bacterial complication. The sudden onset, high morbidity, spiking mortality pattern, and sharp recovery from clinical signs are suggestive of the disease. However, the course of the disease lasted longer than what was reported by Cosgrove [6], Cho and Edgar [41], Okoye and Uzoukwu [10], Mbuko et al. [12, 28], and Ezeibe et al. [40] that IBD runs its full course in about 7 days. During these outbreaks, mortality lasted for 9 days and peaked at day 5 in both cases. This could be attributed to the management system (battery cages) of the birds. The pullets in these cases were brooded under battery cages which provide minimum contacts of the chicks with one another and their droppings. Although aerosol route of the disease transmission exists, faeco-oral route is the major route by which susceptible chick can be infected [30]. The disease might spread very fast in deep litter management system due to free contact of the infected and noninfected birds. Also in the deep litter, the birds have direct access to their droppings, and as such the feed and water can be contaminated by the droppings of infected birds as suggested by Saif [42] and Eterradossi and Saif [3].
The high mortality recorded in the first outbreak may be due to the fact that it was the first outbreak of the disease in the farm and also the type of vaccines and vaccination programme employed may not have protected the birds against the field IBD virus. There is the likelihood that the intermediate vaccines administered at days 8 and 18 were interfered by maternally derived antibodies (MDAs) and therefore the chicks were unprotected and the intermediate plus vaccine given at day 31 may have exacerbated the condition. Several studies have indicated that high MDAs at the time of IBDV vaccination might interfere with the vaccine response, neutralise the vaccine virus, and delay or even prevent the induction of humoral immunity [15, 33, 43–45]. However, virulent strains of IBDV of same serotype have been reported to overcome high MDAs in commercial flocks vaccinated with vaccines developed from different variants, causing up to 60% to 70% mortality [46]. Although vaccination of chickens has remained the principal method to control this disease [27, 47], the effectiveness of vaccinations relys on variants of the virus circulating in the area [40]. Adamu et al. [48] studied the relationship between field and foreign vaccine strains in Nigeria and reported that when IBDV strains spread from their region of origin to a different region they mutate alongside indigenous field strains. The antigenic differences between field and vaccine viral strain could be responsible for vaccine failures. Therefore, vaccines being used in the country should be those made from strains of the virus circulating in Nigeria.
The source of the second outbreak for the subsequent batch of pullets may be due to the persistence of the virus in the environment between outbreaks, since IBD virus is very stable and resistant to many disinfectants [21, 42]. It is also pertinent to note that the brooding house was thoroughly cleaned and disinfected using the following disinfectants (polidine, V-ox, CID20, terminator, and glutasan) following the first outbreak before restocking. This further confirms previous report that once an outbreak of IBD occurs in a farm it will continue to occur with reduced mortality [3, 49] as seen in this investigation.
The Escherichia coli isolated from the bacteriological investigation was not surprising due to the immunosuppressive effects of the IBDV [50]. Consequences of immunosuppression due to IBD include poor response to vaccinations, gangrenous dermatitis, inclusion body hepatitis-anaemia syndrome, and Escherichia coli infection [51, 52]. Escherichia coli is known to be the abundant normal flora of gastrointestinal tracts of poultry. Part of the mechanism of immunosuppression in IBD is lymphocyte lysis and apoptosis [3].
There are several claims by field veterinarians and animal health workers that certain disinfectants (e.g., iodine, sodium hypoclorite, and V-ox) when given orally are effective for the treatment of IBD. In these cases, the chemicals administered seemed not to have been effective as the disease ran its normal course with a very high mortality rate in case 1 and mortality rate of about 40% in case 2.
5. Conclusion
In conclusion, the IBD vaccines currently being used to vaccinate birds against IBD in Nigeria may be antigenically different from the IBD virus circulating in our environment. There is therefore the need for effective sanitary measures and adequate decontamination with sufficient fallow period before restocking of birds after an IBD outbreak in a poultry farm. The findings of this study have shown that polidine and V-ox at 1 mL per litre of water given orally in chickens will not prevent typical IBD lesions in case of field exposure. Also, effort should be made to produce vaccine locally with the strains of the circulating IBD virus.
Competing Interests
The authors do not have any potential conflict of interests to declare.
References
- 1.Lasher H. N., Shane S. M. Infectious bursal disease. World's Poultry Science Journal. 1994;50(02):133–166. doi: 10.1079/WPS19940013. [DOI] [Google Scholar]
- 2.Lukert P. D., Saif Y. M. Infectious bursal disease. In: Calnek B. W., editor. Diseases of Poultry. 11th. Ames, Iowa, USA: Iowa State University Press; 2003. pp. 161–179. [Google Scholar]
- 3.Eterradossi N., Saif Y. M. Newcastle disease. In: Saif Y. M., Fadly A. M., Glisson J. R., McDougald L. R., Nolan L. K., Swayne D. E., editors. Diseases of Poultry. 12th. Ames, Iowa, USA: Blackwell; 2008. pp. 185–208. [Google Scholar]
- 4.Kibenge F. S., Dhillon A. S., Russell R. G. Biochemistry and immunology of infectious bursal disease virus. Journal of General Virology. 1988;69:1757–1775. doi: 10.1099/0022-1317-69-8-1757. [DOI] [PubMed] [Google Scholar]
- 5.Müller H., Islam M. R., Raue R. Research on infectious bursal disease: the past, the present and the future. Veterinary Microbiology. 2003;97(1-2):153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove A. S. An apparently new disease of chickens: avian nephrosis. Avian Diseases. 1962;6(3):385–389. doi: 10.2307/1587909. [DOI] [Google Scholar]
- 7.Ojo M. O., Oduye O. O., Noibi L. M., Idowu A. L. Gumboro-like disease in Nigeria. Tropical Animal Health and Production. 1973;5(1):52–56. doi: 10.1007/BF02239686. [DOI] [Google Scholar]
- 8.Onunkwo O. An outbreak of infectious bursal disease (IBD) of chickens in Nigeria. Veterinary Record. 1975;97(22) doi: 10.1136/vr.97.22.433. [DOI] [PubMed] [Google Scholar]
- 9.Tong J. C., Umoh J. U., Abdu P. A., Sa'idu L. Retrospective studies of Gumboro disease seen in Ahmadu Bello University Veterinary Teaching Hospital, Zaria, Nigeria (1985–1990) Bulletin of Animal Health and Production in Africa. 1993;41:173–179. [Google Scholar]
- 10.Okoye J. O. A., Uzoukwu M. Histopathogenesis of local Nigerian isolates of infectious bursal disease virus in broilers. Proceedings of the International Symposium on IBD and CIA; June 2001; pp. 366–376. [Google Scholar]
- 11.Abdu P. A., Umoh J. U., Abdullhi S. U., Sa'idu L. Infectious bursal disease in chickens in Nigeria. Tropical Veterinarian. 2001;19(4):216–236. [Google Scholar]
- 12.Mbuko I. J., Musa W. I., Ibrahim S., et al. A retrospective analysis of infectious bursal disease diagnosed at poultry unit of Ahmadu Bello University, Nigeria. International Journal of Poultry Science. 2010;9(8):784–790. doi: 10.3923/ijps.2010.784.790. [DOI] [Google Scholar]
- 13.Wang A., Liu F., Wang Z., et al. Pathological study of SPF chickens experimentally infected with a chinese IBDV strain BC6/85. Asian Journal of Animal and Veterinary Advances. 2011;6(1):36–50. doi: 10.3923/ajava.2011.36.50. [DOI] [Google Scholar]
- 14.Office International des Epizooties (OIE) Terrestrial Manual. 2008. Infectious bursal disease; pp. 555–557. [Google Scholar]
- 15.Moraes H. L. S., Salle C. T. P., Nascimento V. P., et al. Infectious bursal disease: evaluation of maternal immunity and protection by vaccination of one-day old chicks against challenge with a very virulent virus isolate. Brazilian Journal of Poultry Science. 2005;7(1):51–57. doi: 10.1590/s1516-635x2005000100009. [DOI] [Google Scholar]
- 16.Miroljub D., Gordana Z., Jelena P. The effects of a Gumboro disease control program on the reduction of economic losses. Acta Veterinaria. 2008;58(1):53–62. doi: 10.2298/avb0801053d. [DOI] [Google Scholar]
- 17.Block H., Meyer-Block K., Rebeski D. E., et al. A field study on the significance of vaccination against infectious bursal disease virus (IBDV) at the optimal time point in broiler flocks with maternally derived IBDV antibodies. Avian Pathology. 2007;36(5):401–409. doi: 10.1080/03079450701589175. [DOI] [PubMed] [Google Scholar]
- 18.Müller H., Mundt E., Eterradossi N., Islam M. R. Current status of vaccines against infectious bursal disease. Avian Pathology. 2012;41(2):133–139. doi: 10.1080/03079457.2012.661403. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T., Setiyono A., Kobayashi M., Takigami S., Fukushi H., Hirai K. Infectious bursal disease live vaccines: changes in the virus population during serial passage in chickens and chicken embryo fibroblast cells. Avian Diseases. 2000;44(2):284–290. doi: 10.2307/1592541. [DOI] [PubMed] [Google Scholar]
- 20.Jackwood D. J., Sreedevi B., LeFever L. J., Sommer-Wagner S. E. Studies on naturally occurring infectious bursal disease viruses suggest that a single amino acid substitution at position 253 in VP2 increases pathogenicity. Virology. 2008;377(1):110–116. doi: 10.1016/j.virol.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Garriga D., Querol-Audí J., Abaitua F., et al. The 2.6-angstrom structure of infectious bursal disease virus-derived T = 1 particles reveals new stabilizing elements of the virus capsid. Journal of Virology. 2006;80(14):6895–6905. doi: 10.1128/jvi.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf H., Vielitz E., Kirsch R. Occurrence of an infectious disease affecting the bursa of Fabricius (Gumboro disease) Dtsch Tieraerztl Wochenschr. 1967;74:6–10. [PubMed] [Google Scholar]
- 23.Benton W. J., Cover M. S., Rosenberger J. K. Studies on the transmission of the infectious bursal agent (IBA) of chickens. Avian Diseases. 1967;11(3):430–438. doi: 10.2307/1588191. [DOI] [PubMed] [Google Scholar]
- 24.Mittal D., Jindal N., Gupta S. L., Kataria R. S., Tiwari A. K. Detection of infectious bursal disease virus in field outbreaks in broiler chickens by reverse transcription-polymerase chain reaction. International Journal of Poultry Science. 2005;4(4):239–243. doi: 10.3923/ijps.2005.239.243. [DOI] [Google Scholar]
- 25.Chansiripornchai N., Sasipreeyajan J. Comparison of the efficacy of the immune complex and conventionally live vaccine in broilers against infectious bursal disease infection. Thai Journal of Veterinary Medicine. 2009;39(2):115–120. [Google Scholar]
- 26.Musa I. W., Sai'du L., Abalaka E. S. Economic impact of recurrent outbreaks of gumboro disease in a commercial poultry farm in kano, Nigeria. Asian Journal of Poultry Science. 2012;6(4):152–159. doi: 10.3923/ajpsaj.2012.152.159. [DOI] [Google Scholar]
- 27.El-Mahdy S. S., Farouk H., El-Wanis N. A., Hamoud M. M. Comparative studies between different commercial types of live Infectious bursal disease [IBD] vaccine strains in Egypt. American Journal of Research Communication. 2013;1(10):113–129. [Google Scholar]
- 28.Mbuko I. J., Abdu P. A., Saidu L., Oladele S. B., Kazeem H. Investigation of outbreaks of infectious bursa disease in Zaria, Nigeria. International Journal of Tropical Disease and Health. 2014;4(4):411–426. doi: 10.9734/ijtdh/2014/6118. [DOI] [Google Scholar]
- 29.Murmu R., Islam M. N., Banu Juli M. S., et al. Pathogenicity and immunosuppresive properties of GM-97 strain of infectious bursal disease virus in commercial broiler chicken. Journal of Advanced Veterinary and Animal Research. 2014;1(1):1–7. doi: 10.5455/javar.v1i1p1-7. [DOI] [Google Scholar]
- 30.Tsegaye K., Mersha C. Review on the incidence and pathology of infectious bursal disease. British Journal of Poultry Sciences. 2014;3(3):68–77. [Google Scholar]
- 31.Teshome M., Fentahunand T., Admassu B. Infectious bursal disease (Gumboro disease) in Chickens. British Journal of Poultry Sciences. 2015;4(1):22–28. [Google Scholar]
- 32.Singh J., Banga H. S., Brar R. S., Singh N. D., Sodhi S., Leishangthem G. D. Histopathological and immunohistochemical diagnosis of infectious bursal disease in poultry birds. Veterinary World. 2015;8(11):1331–1339. doi: 10.14202/vetworld.2015.1331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Den Berg T. P. D. Acute infectious bursal disease in poultry: a review. Avian Pathology. 2000;29(3):175–194. doi: 10.1080/03079450050045431. [DOI] [PubMed] [Google Scholar]
- 34.Luengo A., Butcher G., Kozuka Y., Miles R. Histopathology and transmission electron microscopy of the bursa of fabricius following IBD vaccination and IBD virus challenge in chickens. Revista Cientifica de la Facultad de Ciencias Veterinarias de la Universidad del Zulia. 2001;11(6):533–544. [Google Scholar]
- 35.Oluwayelu D., Emikpe B., Ikheloa J., Fagbohun O., Adeniran G. The pathology of infectious bursal disease in crossbreeds of harco cocks and indigenous Nigerian hens. African Journal of Clinical and Experimental Microbiology. 2002;3(2):95–97. doi: 10.4314/ajcem.v3i2.7336. [DOI] [Google Scholar]
- 36.Guvenc T., Haziroglu R., Yarim M., Tunca R. Diagnosis of infectious bursal disease by immunoperoxidase technique. Ankara Üniversity of Veterinary Fak Derg. 2004;51:237–238. [Google Scholar]
- 37.AL-Zubeedy A. Z., Shamaun A. A., Al-Aalim A. M. Histopathological and immune response against infectious bursal disease in chickens vaccinated against newcastle disease. AL-Qadisiya Journal of Veterinary Medical Sciences. 2013;12(1):66–70. [Google Scholar]
- 38.Shekaro A. Infectious bursal disease outbreak in fifteen weeks old pullets in Kaduna, Nigeria. Journal of Animal Production Advances. 2015;5(3):636–644. doi: 10.5455/japa.20150315015216. [DOI] [Google Scholar]
- 39.Asif M., Lowenthal J. W., Ford M. E., Schat K. A., Kimpton W. G., Bean A. G. D. Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral Immunology. 2007;20(3):479–486. doi: 10.1089/vim.2006.0109. [DOI] [PubMed] [Google Scholar]
- 40.Ezeibe M. C., Okoye J. O., Ogunniran T. M., et al. Mortality rates from a Nigerian isolate of the Infectious Bursa Disease Virus and passive haemagglutination antibody titer that protects chicks against challenge with the virus isolate. Health. 2013;5(9):1355–1359. doi: 10.4236/health.2013.59184. [DOI] [Google Scholar]
- 41.Cho Y., Edgar S. A. Characterization of the infectious bursal agent. Poultry Science. 1969;48(6):2102–2109. doi: 10.3382/ps.0482102. [DOI] [PubMed] [Google Scholar]
- 42.Saif Y. M. Infectious Bursal disease and hemorrhagic enteritis. Poultry Science. 1998;77(8):1186–1189. doi: 10.1093/ps/77.8.1186. [DOI] [PubMed] [Google Scholar]
- 43.Alam J., Rahman M. M., Sil B. K., Khan M. S. R., Giasuddin, Sarker M. S. K. Effect of maternally derived antibody on vaccination against infectious bursal disease (Gumboro) with live vaccine in broiler. International Journal of Poultry Science. 2002;1(4):98–101. doi: 10.3923/ijps.2002.98.101. [DOI] [Google Scholar]
- 44.Hair-Bejo M., Ng M. K., Ng H. Y. Day old vaccination against infectious bursal disease in broiler chickens. International Journal of Poultry Science. 2004;3(2):124–128. doi: 10.3923/ijps.2004.124.128. [DOI] [Google Scholar]
- 45.Jung A. Pathogenesestudie eines intermediärvirulenten Gumborovirus in spezifiziert-pathogen-freien (SPF) Hühnern und kommerziellen Broiler [Ph.D. thesis] Hannover, Germany: University of Veterinary Medicine; 2006. [Google Scholar]
- 46.Etterradossi N. Major advances in infectious bursal disease virus (IBV) research seen the first international IBDV/CIAV symposium (Rauischholzhausen, Germany, 1994). Proceeding of 2nd International Symposium on Infectious Bursal Disease and Chicken Infectious Anaemia, Rauischholzhausen; July 2001; Ebsdorfergrund, Germany. pp. 6–23. [Google Scholar]
- 47.Okwor E. C., Eze D. C., Anyanwu M. U., Okpe C. B., Eze P. C. Effects of mixed vaccinations against newcastle disease and infectious bursal disease on immune response, feed consumption and weight gain in broilers. Journal of Agriculture and Veterinary Science. 2013;6(3):63–68. doi: 10.9790/2380-0636368. [DOI] [Google Scholar]
- 48.Adamu J., Owoade A. A., Abdu P. A., Kazeem H. M., Fatihu M. Y. Characterization of field and vaccine infectious bursal disease viruses from nigeria revealing possible virulence and regional markers in the VP2 minor hydrophilic peaks. Avian Pathology. 2013;42(5):420–433. doi: 10.1080/03079457.2013.822055. [DOI] [PubMed] [Google Scholar]
- 49.Lukert P. D., Saif Y. M. Infectious bursal disease. In: Calnek B. W., Barnes H. J., Beard C. W., McDougald L. R., Saif Y. M., editors. Diseases of Poultry. 10th. Ames, Iowa, USA: Iowa State University Press; 1997. pp. 721–738. [Google Scholar]
- 50.Wyeth P. J. Effect of infectious bursal disease on the response of chickens to S. typhimurium and E. coli infections. Veterinary Record. 1975;96(11):238–243. doi: 10.1136/vr.96.11.238. [DOI] [PubMed] [Google Scholar]
- 51.Chou K. C., Calnek B. W. Diseases of Poultry. 10th. Ames, Iowa, USA: Iowa State University Press; 1997. [Google Scholar]
- 52.Sharma J. M., Kim I.-J., Rautenschlein S., Yeh H.-Y. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Developmental and Comparative Immunology. 2000;24(2-3):223–235. doi: 10.1016/s0145-305x(99)00074-9. [DOI] [PubMed] [Google Scholar]