Abstract
Objective We sought to assess the potential of a widely available source of electronic medication data to prevent medication history errors and resultant inpatient order errors.
Methods We used admission medication history (AMH) data from a recent clinical trial that identified 1017 AMH errors and 419 resultant inpatient order errors among 194 hospital admissions of predominantly older adult patients on complex medication regimens. Among the subset of patients for whom we could access current Surescripts electronic pharmacy claims data (SEPCD), two pharmacists independently assessed error severity and our main outcome, which was whether SEPCD (1) was unrelated to the medication error; (2) probably would not have prevented the error; (3) might have prevented the error; or (4) probably would have prevented the error.
Results Seventy patients had both AMH errors and current, accessible SEPCD. SEPCD probably would have prevented 110 (35%) of 315 AMH errors and 46 (31%) of 147 resultant inpatient order errors. When we excluded the least severe medication errors, SEPCD probably would have prevented 99 (47%) of 209 AMH errors and 37 (61%) of 61 resultant inpatient order errors. SEPCD probably would have prevented at least one AMH error in 42 (60%) of 70 patients.
Conclusion When current SEPCD was available for older adult patients on complex medication regimens, it had substantial potential to prevent AMH errors and resultant inpatient order errors, with greater potential to prevent more severe errors. Further study is needed to measure the benefit of SEPCD in actual use at hospital admission.
Keywords: medication reconciliation, adverse drug events, health information exchange
BACKGROUND AND SIGNIFICANCE
Each year, approximately 100 000 hospitalized patients in the United States die from over 2 million serious adverse drug events (ADEs).1 Nearly half of these ADEs are preventable,2 and avoidance of errors in medication histories obtained at hospital admission is one of the most important preventive tactics.3 This tactic not only aims to reduce inpatient ADEs, but also helps discharging providers to prescribe optimal discharge medication regimens, thus aiding the avoidance of subsequent outpatient ADEs.4
Despite multiple important research studies, quality improvement, and policy efforts directed at addressing this problem, several recent trends appear to be eroding prior progress. First, electronic health records (EHRs) frequently allow providers to click on one or two buttons that “order all home medications” at admission, even if the home medication regimen has not been adequately reviewed and updated. Similar functionality is available at discharge. Thus, with the increased use of EHRs, there is greater risk for medication history errors to affect not only inpatient ordering, but also discharge prescriptions. Even EHRs in integrated healthcare systems may not contain all of a patient’s medications,5 and most US patients receive fragmented care from multiple providers,6 such that a new medication history is always necessary at hospital admission. Second, the increasing prevalence of hospitalists means that admitting providers are less likely to be familiar with patients’ home medication regimens than the primary care physicians who previously admitted patients.7 Finally, the advancing age of the US population is increasing the prevalence of dementia and delirium that impairs patients’ recall of home medication regimens,8,9 increasing the complexity of medication regimens,10 and increasing patients’ physiologic susceptibility to ADEs when medication errors occur.
To help reduce medication history errors, several countries outside the United States allow providers to access outpatient electronic pharmaceutical claims data (EPCD). Indeed, several studies from such systems suggest potential for EPCD to contribute to improved medication histories.11–14 One area of benefit specifically noted was the identification of otherwise omitted medications,14 an error occurring in 10–61% of admission medication histories.15
In the United States, less is known about the potential of EPCD to improve medication histories. Given the historically fragmented nature of the US healthcare system, providers face major barriers in obtaining medication histories, especially at care transitions, as they often seek to aggregate multiple types of medication information from multiple providers across multiple health records and organizations.16 We thus suspected that EPCD might benefit US patients even more than patients served by the large, centrally controlled national health systems most likely to offer EPCD access.
US health information exchange organizations and commercially available databases have begun to integrate EPCD from several sources. Initial reports of such EPCD use in the United States focused on feasibility.17–20 Subsequently, availability of one EPCD data source (Surescripts, Arlington, VA, USA) has substantially increased.
Surescripts facilitates nationwide electronic exchange of prescription fill data between prescribers, pharmacies, and US payers. Many outpatient providers have access to Surescripts EPCD (SEPCD) as part of Surescripts’ package of electronic prescribing services. However, access to SEPCD in the inpatient setting for medication reconciliation at hospital admission is offered separately. Surescripts touts adoption of this latter product in 44% of US hospitals, with claims data available from 230 million patients. Nonetheless, we are aware of only one analysis of SEPCD, which compared SEPCD with clinical admission medication histories and found that SEPCD was generally accurate, albeit somewhat incomplete.21
OBJECTIVE
To go beyond assessing data accuracy and completeness, we developed a framework to focus on the error-prevention potential of SEPCD. We used this framework to assess the potential of SEPCD to reduce medication history errors and resultant medication order errors at hospital admission.
METHODS
Setting
The present study is set within a pragmatic randomized controlled trial (data collection ongoing) conducted at an 886 bed university-affiliated medical center. The studied medical center offers tertiary and multidisciplinary care to a large and socioeconomically diverse metropolitan population.
The Cedars-Sinai Health System (CSHS) Institutional Review Board approved the present study.
Study design and participants
The present study is a retrospective observational study that estimates the potential that SEPCD would have had to prevent admission medication history (AMH) errors and resultant inpatient order errors that actually occurred and were subsequently identified as part of the aforementioned trial.22
Randomized Controlled Trial Inclusion and Exclusion Criteria
All adult patients admitted to Cedars-Sinai Medical Center through the emergency department during the trial were considered for inclusion. Because the trial tested intensive interventions, it focused on patients deemed high risk for AMH errors. Patients included in the trial met at least one inclusion criterion: ≥10 chronic prescription medications, acute myocardial infarction or congestive heart failure in the problem list, admission from a skilled nursing facility, history of transplant, or active anticoagulant, insulin, or narrow therapeutic index medications. Exclusion criteria were prior enrollment in the study, admission to pediatric or trauma services, or admission to a transplant service that already used pharmacists to obtain AMHs.
Inclusion and Exclusion Criteria for this Study
We began with the 283 patients who were enrolled in the trial from January 7, 2014 to February 13, 2014. We then excluded all patients whose AMHs had no identified errors. Next, we excluded patients for whom there had been no successful download of SEPCD within the prior 2 years. Lack of successful prior downloads may have been due to no outpatient EHR request for SEPCD (e.g., the patient had no interaction with clinicians using the CSHS outpatient EHR), no SEPCD for a patient (e.g., unsuccessful patient match between the EHR and Surescripts), or technical errors.
Finally, we excluded patients for whom we did not have at least one SEPCD download after their admission date. This final exclusion was done to ensure that SEPCD would not erroneously be deemed incomplete due to a relevant claim occurring after the most recent EHR download of SEPCD, but before the AMH was obtained (e.g., patient admitted on 1/10, but the last EHR download of SEPCD was on 1/1, such that pharmacy fills on 1/2–1/10 could not possibly have been contained in the downloaded SEPCD).
Data collection and extraction
AMH Data: Initial Clinical AMHs and Gold Standard AMHs
For the trial, patients were randomized in the emergency department to one of three arms: to have their initial clinical AMHs obtained by pharmacists, by trained pharmacy technicians working under the supervision of pharmacists, or by the usual care methods (nurses and physicians). Subsequently, usually within 1 day of admission, the trial required a gold standard AMH for patients in all arms to identify any medication history errors in these clinical AMHs. Gold standard AMHs were conducted by expert pharmacists using an accepted research protocol.23 They were more accurate due to more experienced personnel who were trained to use a systematic approach that utilized multiple sources for obtaining the AMH, and by virtue of using the initial AMHs as a starting point. Although even gold standard AMHs may not eliminate errors entirely, they are a widely accepted research method for identifying errors in clinical AMHs.
As part of the process of obtaining gold standard AMHs, errors in initial AMHs were recorded in a pharmacy database. These errors were the starting point for our data analysis described below. For study patients, inpatient pharmacists were instructed to defer any medication reconciliation to the expert pharmacist obtaining the gold standard AMH.
Surescripts Electronic Pharmacy Claims Data (SEPCD)
SEPCD is derived from insurer claims and includes the following medication-specific data elements: medication name, medication strength (e.g., 5 mg), medication route, medication form (e.g., tab, cream, capsule), dispense date, days of medication supplied, and quantity dispensed. Dose per administration and frequency of administration are not included, but can sometimes be deduced by dividing the quantity dispensed by the days of medication supplied.
Exclusively for this analysis, we accessed SEPCD with the Surescripts “Medication History Ambulatory” product. CSHS currently uses electronic prescribing in the outpatient setting, and thus receives Surescripts’ accompanying “Medication History Ambulatory” product. However, as is still the case for many other provider organizations, CSHS was not yet using the separate “Medication History for Hospitals” product that makes SEPCD available in the emergency department and inpatient settings at additional cost. Both products use the same SEPCD, which is provided by pharmaceutical benefit management companies and payers. Newer versions of both products also make use of dispensed medication data provided directly by pharmacies, but we did not have access to such data.
We queried the CSHS data warehouse to access SEPCD for medication claims occurring prior to admission. SEPCD was present only if it had been previously downloaded by the CSHS outpatient EHR (Epic Systems, Verona, WI) as a part of routine clinical care.
To assess the error prevention potential of this intervention, we compared retrospectively accessed SEPCD for each patient with the AMH errors and resultant inpatient order errors that had been identified among trial patients. Errors had already been identified in preliminary trial analysis by comparing the actual AMH obtained at hospital admission vs a gold standard AMH subsequently obtained from each patient.
Main Outcome and Definitions
The main outcome was classification of each AMH error into one of the following four categories: (1) SEPCD was unrelated to the AMH error; (2) SEPCD probably would not have prevented the error; (3) SEPCD might have prevented the error; or (4) SEPCD probably would have prevented the error. The classification was independently performed by two expert pharmacists. Classification was based on examination of the patient’s SEPCD, knowledge of the medication in question and the usual regimens, the type of AMH error, and the consistency and recentness of medication fills. Reviewers were presented with abstracted summaries, but they also reviewed charts when necessary. Reviewers erred on the side of trusting the gold standard AMH, but also used their experience to consider whether the gold standard might have been in error.
In cases where initial disagreements could not be resolved with discussion between the two initial raters, a third investigator was again used as a tiebreaker.
To better understand our main outcome definitions, consider a hypothetical patient with atrial fibrillation requiring anticoagulant medication (Table 1). Although the initial (clinical) AMH included no anticoagulant medications, the subsequent gold standard (research) AMH revealed that the patient was actually taking an anticoagulant medication, dabigatran etexilate, 150 mg by mouth twice daily. If SEPCD only showed that the patient had been taking amlodipine, an antihypertensive medication, the SEPCD would be classified as “unrelated” to this AMH error. If SEPCD showed one 6-month-old claim for warfarin, another anticoagulant medication in a different subclass, the determination would be that SEPCD “probably would not have” prevented the AMH error. However, if SEPCD showed that the patient had several claims over the last 6 months for warfarin, a provider might be prompted to ask the patient about anticoagulant medications, and might then have discovered that the patient had recently switched from warfarin to dabigatran for anticoagulation. In this case, the SEPCD “might have prevented” the AMH omission error. As this example demonstrates, SEPCD need not contain the exact medication, or even a medication in the same subclass, to prevent an AMH error. Finally, if the SEPCD showed several recent fills for dabigatran, it “probably would have” prevented the AMH error. To be classified as such, SEPCD would need to directly contradict the previously available AMH. In cases where SEPCD “probably would have” identified a commission error in the AMH, the SEPCD would need to match the AMH quite well, with an otherwise unexplainable gap for the medication erroneously included in the AMH. Less perfect AMH to SEPCD matches for other medications, and/or other potential explanations for SEPCD data containing a medication no longer part of the patient’s regimen (e.g., an anticoagulant discontinued prior to hospitalization for bleeding), would result in a “might have prevented” categorization. For simplicity, these four categories are also referred to as having error prevention potential of none, low, medium, and high, respectively.
Table 1:
Examples of Error Prevention Potential Categories.
| Examples of SEPCD | Definition | Error prevention potential |
|---|---|---|
| Several fills for amlodipine (an antihypertensive medication) only | Unrelated to error | None |
| One fill for warfarin 6 months ago | Probably would not have prevented error | Low |
| Several fills for warfarin | Might have prevented error (because knowing about these fills might have resulted in discussion about anticoagulation) | Medium |
| Several recent fills for dabigatran | Probably would have prevented error | High |
Background:
A patient with atrial fibrillation was admitted to the hospital. The initial clinical Admission Medication History (AMH) included no anticoagulant medications.
Subsequent gold standard AMH revealed that the patient had recently switched anticoagulant medications from warfarin sodium 3 mg by mouth daily to dabigatran etexilate 150 mg by mouth twice daily.
Various possible SEPCD results are shown with their error prevention potential.
To understand whether SEPCD error prevention potential differs for errors of varying severity, we also assessed the severity of AMH errors and any resultant inpatient order errors. Consistent with prior literature, all errors identified were independently classified by two pharmacists as significant, serious, or life-threatening.23 In a similar fashion, two physicians analyzed both AMH errors and associated medical records to independently determine whether each AMH error led to a medication order error, and to independently classify order error severity. In cases where initial disagreements could not be resolved with discussion between the two initial raters, a third investigator was brought in as a tiebreaker. For both the pharmacists and physicians, the earliest cases were independently reviewed in greater detail, with greater discussion and greater use of the third investigator, until reviewers achieved strong consensus regarding definitions.
Statistical Analysis and Pre-Specified Comparisons
To measure inter-rater reliability of these initial classifications, we calculated a quadratic weighted kappa value.24 Prevention potential was then analyzed in two categories: “none to low” vs “medium to high.” We compared the proportion of errors in each of the two categories across three predictor variables: AMH error severity (significant, serious, or life-threatening), AMH error type (omission, commission, incorrect dose/instructions, or other), and the provider type who obtained the AMH (pharmacy technicians, pharmacists, or the usual care providers). In cases where AMH errors led to inpatient order errors, we also examined how order error prevention potential varied by order error severity (significant, serious, or life-threatening). For AMH error types, we calculated the odds ratios of medium to high vs none to low prevention potential using a separate repeated measures logistic model for each of the four error types vs all other error types combined (e.g., the first of the four models was for odds of medium to high prevention potential for omission errors versus odds of medium to high prevention potential for all other error types combined).
Pre-specified hypotheses drove each comparison. Because SEPCD rarely contains over-the-counter medications, we expected it to have higher prevention potential for more severe errors. Based on prior literature, we hypothesized that SEPCD would be most useful in preventing omission errors.14,15 Lastly, we expected SEPCD to be equally useful in improving AMHs initially obtained using each of the three processes featured in a trial arm.
The units of analysis for the aforementioned hypotheses were AMH errors and resultant inpatient order errors. Because the data came from a large hospital with over 2000 attending physicians on staff, we did not adjust for clustering within clinicians. We adjusted P values for within-patient clustering using a Generalized estimating equations (GEE) repeated measures logistic model with the outcome as prevention potential (medium to high vs none to low). We modeled the covariate of interest as a categorical main effect with an exchangeable correlation structure for repeated errors within patients. We also accounted for within-patient clustering by calculating confidence intervals for proportions of medication error-level variables using a bootstrap procedure with 1000 samples.25
To understand differences between patients with and without current SEPCD, we used t-tests, chi-square, and Fisher’s exact tests to compare several patient-level characteristics. To understand whether lack of SEPCD was caused by certain insurers using pharmacy benefit managers that did not share claims with Surescripts, we compared patients’ insurers across the two groups. Confidence intervals for proportions of patient-level variables used the asymptotic binomial formula for a Wald interval. SAS version 9.3 was used for all analyses except for the bootstrap analyses, which used the “boot” package in R version 3.0.1.
RESULTS
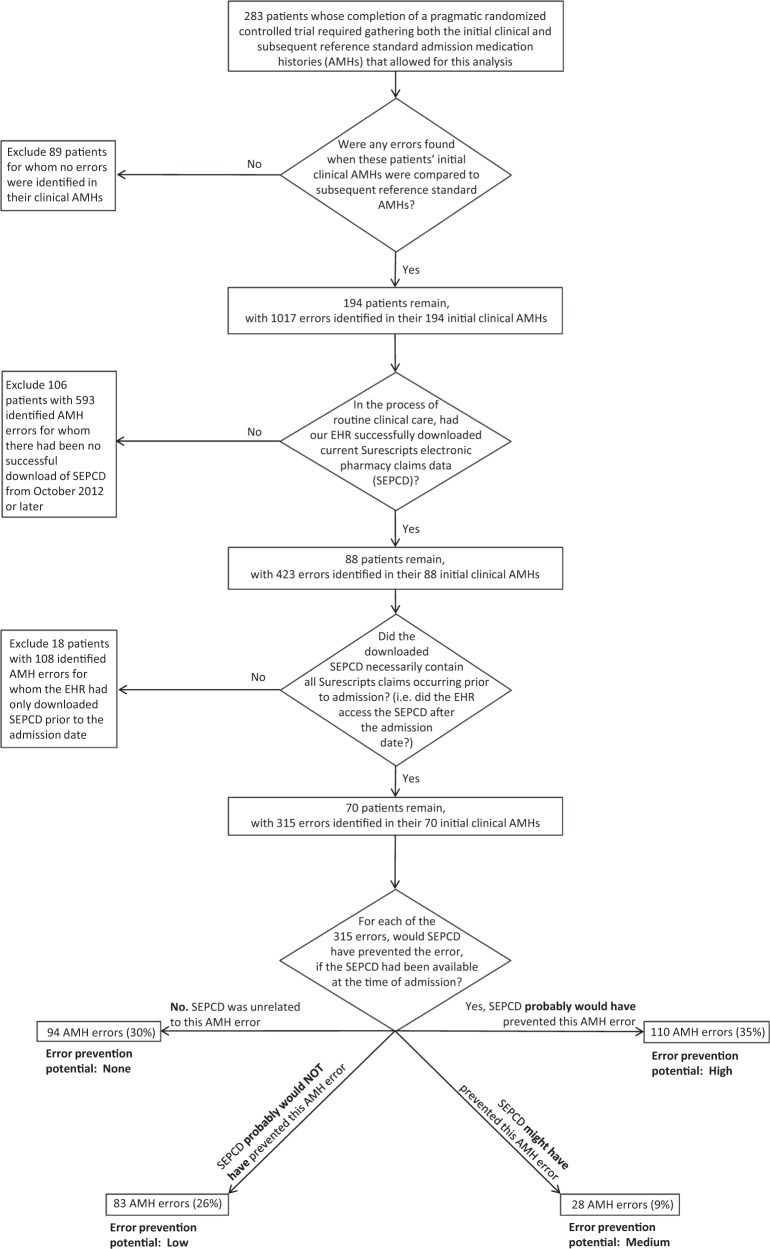
Of the 283 patients who had completed the trial, we excluded 89 patients from our analysis because no errors were detected in their AMHs. An additional 124 patients were excluded because we could not access current SEPCD (Figure 1).
Figure 1.
Patient selection process and initial results for surescripts electronic pharmaceutical claims data (SEPCD) error prevention potential analysis.
The 70 remaining patients had a mean age of 69 (SD 14), were 40% male, took a mean of 16 medications (SD 5.4), and predominantly used Medicare insurance (Table 2). They had a mean of 4.5 AMH errors (SD 3.3) and 2.1 resultant inpatient order errors (SD 2.0) per patient. The excluded 124 patients without current SEPCD had a higher mean age (74), percentage male, number of medications, AMH errors, and resultant inpatient order errors, but only age was significantly higher. Insurance status also differed significantly, and the largest difference was that no patients with Medicaid as their sole insurer had current SEPCD.
Table 2:
Characteristics of Analyzed and Excluded Patients
| Characteristic | Analyzed patients: with current SEPCD | Excluded patients: without current SEPCD | P valuea |
|---|---|---|---|
| Patients, n | 70 | 124 | |
| Mean age (SD) | 69 (14) | 74 (16) | 0.03 |
| Female, n (%) | 42 (60) | 62 (50) | 0.18 |
| Mean number of medications (SD) | 16 (5.4) | 15 (7.2) | 0.31 |
| Race, n (%) | |||
| White | 47 (67) | 85 (69) | 0.42 |
| Black | 19 (27) | 25 (20) | |
| Asian | 3 (4) | 7 (6) | |
| Other | 1 (1) | 7 (6) | |
| Insurance, n (%) | |||
| Commercial | 11 (16) | 11 (9) | 0.04 |
| Medicaid only | 0 | 11 (9) | |
| Medicare only | 1 (1) | 5 (4) | |
| Medicare + Medicaid | 31 (44) | 51 (41) | |
| Medicare + other secondary | 27 (39) | 44 (35) | |
| Other | 0 | 2 (2) | |
| Provider type that obtained initial AMH, n (%) | |||
| Usual care (physicians and nurses) | 33 (47) | 61 (49) | 0.41 |
| Usual care plus pharmacist | 15 (21) | 34 (27) | |
| Usual care plus pharmacy technician | 22 (31) | 29 (23) | |
| Admission medication history (AMH) errors | 315 | 702 | |
| AMH errors per patient (SD) | 4.5 (3.3) | 5.7 (5.7) | 0.12b |
| Resultant inpatient order errors | 147 | 272 | |
| Order errors per patient (SD) | 2.1 (2.0) | 2.2 (2.6) | 0.78† |
aBold results are statistically significant.
bUsing Wilcoxon rank sum tests, these p-values are 0.65 for AMH errors and 0.54 for order errors
The sample of errors analyzed included approximately twice as many serious and life-threatening AMH errors as significant (lowest-severity) errors. Examples of our severity ratings and error prevention potential ratings are shown in Supplementary Table 1a. For the two pharmacists’ initial independent assessments of SEPCD utility, quadratic weighted kappa was 0.64 (95% CI, 0.54-0.74).
In the 70 patients with 315 identified AMH errors, we found that SEPCD probably would have prevented 110 AMH errors (35%) representing high error prevention potential, might have prevented 28 AMH errors (9%) representing medium error prevention potential, probably would not have prevented 83 AMH errors (26%) representing low error prevention potential, and that SEPCD was unrelated to 94 AMH errors (30%) representing no error prevention potential (Figure 1). Of the 70 patients, SEPCD probably would have prevented AMH errors in 42 (60%, bootstrapped 95% CI, 49-71%) of patients. Figures 2 and 3 compare a combined “medium – high” category with a combined “none – low” category, whereas Tables 3 and 4 contain all categories.
Figure 2.
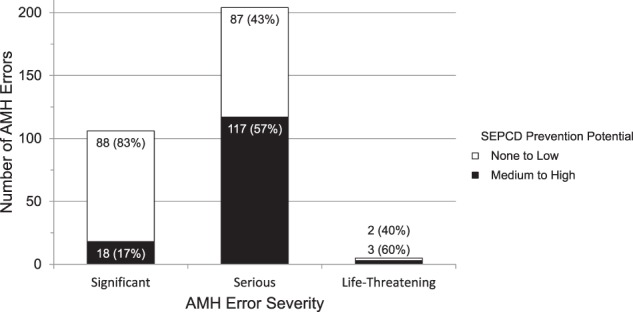
SEPCD potential to prevent admission medication history (AMH) errors by severity.
Figure 3.
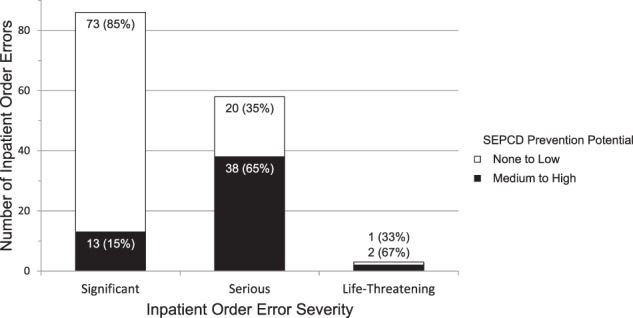
SEPCD potential to prevent inpatient order errors by severity.
Table 3:
SEPCD Potential to Prevent AMH Errors and Resultant Inpatient Order Errors of Varying Severity
| Error Prevention Potential of SEPCD | Significant | Serious | Life-Threatening | Total |
|---|---|---|---|---|
| Admission Medication History (AMH) Errors, n (%) | ||||
| None: SEPCD was unrelated to this AMH error | 73 (69) | 21 (10) | 0 (0) | 94 (30) |
| Low: SEPCD probably would not have prevented error | 15 (14) | 66 (32) | 2 (40) | 83 (26) |
| Medium: SEPCD might have prevented error | 7 (7) | 21 (10) | 0 (0) | 28 (9) |
| High: SEPCD probably would have prevented error | 11 (10) | 96 (47) | 3 (60) | 110 (35) |
| Total | 106 (100) | 204 (100) | 5 (100) | 315 (100) |
| Inpatient Order Errors due to AMH Errors, n (%) | ||||
| None: SEPCD was unrelated to this order error | 54 (63) | 4 (7) | 0 (0) | 58 (39) |
| Low: SEPCD probably would not have prevented error | 19 (22) | 16 (28) | 1 (33) | 36 (24) |
| Medium: SEPCD might have prevented error | 4 (5) | 2 (3) | 1 (33) | 7 (5) |
| High: SEPCD probably would have prevented error | 9 (10) | 36 (62) | 1 (33) | 46 (31) |
| Total | 86 (100) | 58 (100) | 3 (100) | 147 (100) |
Table 4:
SEPCD Potential to Prevent Different Types of AMH Errors
| AMH Error Prevention Potential of SEPCD | Omission, n (%) | Commission, n (%) | Incorrect dose/instructions, n (%) | Other, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| None: SEPCD was unrelated to this AMH error | 46 (42) | 14 (23) | 29 (24) | 5 (19) | 94 (30) |
| Low: SEPCD probably would not have prevented error | 8 (7) | 7 (11) | 65 (54) | 3 (12) | 83 (26) |
| Medium: SEPCD might have prevented error | 6 (6) | 16 (26) | 5 (4) | 1 (4) | 28 (9) |
| High: SEPCD probably would have prevented error | 48 (44) | 24 (39) | 21 (17) | 17 (65) | 110 (35) |
| Total | 108 (100) | 61 (100) | 120 (100) | 26 (100) | 315 (100) |
SEPCD had medium to high prevention potential for 53 (36%) of the 147 inpatient order errors. Of the 55 patients with 147 inpatient order errors, SEPCD had medium to high prevention potential for at least one order error in 31 (56%, bootstrapped 95% CI, 43-69%) patients.
SEPCD had greater potential to prevent more severe errors (Figures 2 and 3, chi-square P < .0001 for AMH errors and resultant inpatient order errors). Furthermore, this statistical significance was robust to an approach accounting for clustering of errors within patients (logistic model P < .0001 for AMH errors, P < .01 for order errors). To measure the benefit of SEPCD in preventing clinically important errors, we excluded the least severe medication errors and found that SEPCD probably would have prevented 99 (47%, 95% CI, 40-54%) of 209 AMH errors and 37 (61%, 95% CI, 47-72%) of 61 resultant inpatient order errors (Table 3).
Error prevention potential varied by error type (Figure 4, logistic model P = .0005). However, our results did not support our hypothesis that SEPCD would have better prevention potential for AMH errors of omission than for other AMH error types (P = .08). We did find that SEPCD had 2.4 times higher odds of having medium to high prevention potential for AMH errors of commission, as compared to the three other error types combined (logistic model P = .01). Furthermore, SEPCD had worse prevention potential for AMH errors due to incorrect dose or instructions (OR 0.2 for medium to high prevention potential, logistic model P < .0001).
Figure 4.
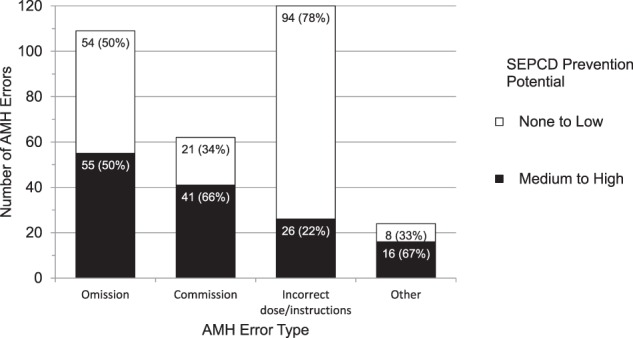
SEPCD prevention potential of admission medication history (AMH) errors by type.
Finally, SEPCD had a lower proportion of errors with medium to high prevention potential for AMHs obtained by pharmacy technicians (28%), as compared with pharmacists (50%) and the usual care methods (48%). An unadjusted comparison was statistically significant (P = .02), but accounting for multiple errors within patients did not result in a statistically significant difference between arms (P = .20).
DISCUSSION
In a sample of 70 patients on complex medication regimens, we measured the potential of medication data from a well-known, nationally available data service to prevent 315 identified AMH errors. We found that SEPCD probably would have prevented 35% and might have prevented an additional 9% of AMH errors. Furthermore, when we focused our analysis on the more downstream and patient-centric outcome of inpatient order errors resulting from AMH errors, prevention potential remained high for approximately one-third of order errors. Finally, there was greater prevention potential for more severe errors. Lower error prevention potential for less severe errors is likely related to known gaps in SEPCD for over-the-counter medications, which seldom generate insurance claims.
Several prior studies have analyzed similar but proprietary data from integrated health organizations or regional health information organizations.11,14,26 However, even though SEPCD is likely the most widely available and frequently used health information exchange data source in the United States, only one peer-reviewed study had assessed its benefit.21 By measuring SEPCD accuracy and completeness, Pfoh et al. took the critical first step of determining that SEPCD was generally accurate, albeit somewhat incomplete. Our study adds to these data-centric measures by providing information regarding the potential of this data to prevent AMH errors and resultant inpatient order errors.
We also examined the prevention potential of SEPCD across different AMH error types. SEPCD had the greatest potential benefit it preventing omission errors, but this was driven by their frequency. Although we had also expected greater relative benefit in preventing errors of omission, the rate of medium to high error prevention potential was not statistically different from that of other error types (P = .08), possibly due to lack of power. We did find that compared to other error types, SEPCD had 2.4 times higher odds of providing medium to high error prevention potential for errors of commission (P = .01). We had not expected SEPCD to prevent commission errors, because there are sundry reasons why SEPCD may be missing (e.g., patients’ insurers or pharmacy benefit managers not providing claims to Surescripts, patients using samples or medications not purchased with their insurance). Nonetheless, our findings demonstrate that SEPCD is frequently complete enough that providers may use it to identify medications inappropriately included in the AMH.
Compared to other error types, we found that SEPCD was less likely to have medium to high error prevention potential for AMH errors related to incorrect dosing or instructions (OR 0.2, P < .0001). We attribute some of this difference to the lack of dosing frequency information in SEPCD. Dosing frequency can sometimes be deduced from SEPCD by dividing the number of pills by the “days supplied,” but such inferences may be erroneous when a patient is instructed to take two pills at once or to split pills. Furthermore, this approach usually fails for medication dispensed in non-discrete units (e.g., creams, solutions, powders). The inclusion of patient instruction data with SEPCD would enhance its utility.
Finally, although we found potential for SEPCD to help all provider types, it is worth noting that there may have been less benefit for AMHs initially obtained by pharmacy technicians, depending on which statistical model was used. If future research supports such a difference, we would hypothesize that it may be due to higher pharmacy technician reliance on medication fill data obtained by contacting pharmacies.
Strengths and Limitations
The major strength of our analysis is that we took advantage of two conditions unique to our study setting. First, we had access to pragmatic trial data including hundreds of clinical AMHs with corresponding gold standard AMHs. These gold standard AMHs leveraged prior clinical AMHs and an accepted research protocol to identify 1017 errors across 194 clinical AMHs. This differs from most prior studies, which have assessed the accuracy and completeness of EPCD using AMHs obtained for clinical purposes.
Second, although SEPCD was retrospectively available for our analyses, it was not used, or even available, in obtaining our studied AMHs. This unique circumstance differs from the two situations observed at most institutions: some institutions lack access to SEPCD completely, and therefore cannot study its benefit. Other institutions have already adopted clinical use of SEPCD, and would thus have difficulty isolating its benefit. Neither situation would be conducive to this analysis. Even though we took advantage of unique conditions, our analysis framework could be replicated for other electronic data sources in cases where a sample of data could be obtained and retrospectively applied to a set of clinical and gold standard AMHs.
Our study setting also brought limitations. First, we could only measure the maximal potential benefit of SEPCD. To measure the benefit actually achieved, it would be necessary to study actual use of SEPCD. Nonetheless, each of these two analyses is independently important, because if SEPCD in everyday use is not found to achieve its hypothesized benefit, it will be crucial to explore the causes of this shortcoming. Indeed, prior work has suggested that despite its potential benefit, providers may over-rely on SEPCD, which could degrade AMH accuracy.27,28 If this concern surfaces with use of SEPCD, informaticians may need to design and reinforce provider workflows that encourage use of SEPCD as a supplementary, rather than exclusive, source of AMH data. Critical points will be ensuring that specific providers are designated to obtain AMH data from patients and/or caregivers, and ensuring that these providers have sufficient time and training to do so.
A second limitation is that current SEPCD was not available for a large proportion of patients. For the only other analysis of SEPCD of which we are aware, SEPCD for “essential” medications (defined as cardiovascular, anti-infective, hypoglycemic, and anticoagulant medications) was missing for 33% of patients studied at two community hospitals in upstate New York between September 2010 and April 2011.21 There is known geographic variation in SEPCD availability,29 which is likely due to geographic variation in the percentage of patients assigned to pharmacy benefit management companies that provide pharmaceutical claims data to Surescripts. Such variation likely accounts for the higher rate of SEPCD absence (106, or 55%, of the 194 patients with AMH errors) in our sample.
As noted in our methods, we could not confirm that the CSHS EHR had queried Surescripts for the SEPCD of each of those 106 excluded patients, so the actual number of patients without any SEPCD may have been lower. Nevertheless, patients included in the study were significantly different from excluded patients in only two ways: they were on average 5 years younger and never had Medicaid as their sole insurer. We suspect that the latter group did not have current SEPCD because this insurer does not provide claims data to Surescripts. Prospective study will be important to determine whether this lack of SEPCD results in more ADEs among these vulnerable patients. In the meantime, both our study and prior work suggest that other hospitals may be able to access SEPCD on one half to two thirds of their patients, unless they have large numbers of patients whose insurers do not provide claims to Surescripts. These proportions will likely be higher for hospitals accessing Surescripts dispensed medication data provided directly by pharmacies, but we are unaware of any study of this newer data source.
We chose not to focus on the causes of SEPCD absence, but rather to measure the benefit of SEPCD when present, in a population of patients at high risk for AMH errors. In the long run, we expect clinicians to electronically access several different sources of medication data, both at admission and during other care encounters, such that only a negligible percentage of these patients will lack any supplementary medication data. When any electronic medication data is present for a given patient, clinicians will rarely know if it is complete. Thus, such data will need to supplement, rather than replace, an interview and other data sources. We foresee this occurring both as part of a larger US trend of increased use of health information technology, and on the basis of the error prevention potential measured here.
Indeed, given the potential benefit demonstrated by SEPCD, we expect that as provider organizations accept increased accountability for patients’ health outcomes, it will become progressively more important to leverage electronic sources of medication data to avoid AMHs errors, so as to prevent ADEs both in the hospital and after discharge. Furthermore, as hospitals increasingly pay for the time necessary to obtain AMHs (via salary support for pharmacists and hospitalist physicians), they will have a financial incentive to minimize this time. This will likely also lead to further use of electronic medication data (EMD). Finally, we expect increased EMD adoption as the US population ages and becomes more similar to the population we studied here for several reasons. Patients will increasingly take more medications, be more at risk for altered mental status that limits recall of complex medication regimens, and become more physiologically susceptible to ADEs when medication errors occur.
Our analysis here focuses on one type of EMD, but future sources of EMD will likely also include data from health information exchange organizations and personal health records. To ascertain the benefit of various EMD sources across various healthcare settings, similar analyses may be required. Our study provides a methodological framework for estimating the benefit of other EMD sources, beyond just assessment of data accuracy and completeness. Indeed, we would assert that our more downstream and patient-centric assessment of an EMD’s potential to reduce medication errors is among the best possible tests that can be conducted before the data is actually used. For SEPCD, which has shown substantial potential benefit when subjected to this analysis, the next most important research task is to measure its benefit in practice.
Acknowledgments
The authors acknowledge Matthew Soneson and Ximin Li for their role in extracting the necessary data from the CSHS data warehouse. The authors acknowledge Anish Desai and Brian Doyle for their roles in adjudicating medication order errors.
COMPETING INTERESTS
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Numbers KL2TR000122 (Dr Pevnick) and UL1TR000124 (Dr Bell). The authors have no conflicts of interest to disclose.
CONTRIBUTORS
J.M.P. contributed to study design and drafting of the manuscript. J.M.P., K.P., and C.N.W. contributed to data acquisition, data analysis and interpretation, and statistical analysis. G.C.W. contributed to statistical analysis. J.M.P., K.P., C.N.W., R.S., D.B., G.C.W., and C.J. contributed to data interpretation and manuscript revisions. F.D. contributed to data acquisition and manuscript revisions.
REFERENCES
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. [DOI] [PubMed] [Google Scholar]
- 2.Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Safety. 2007;30(5):379–407. [DOI] [PubMed] [Google Scholar]
- 3.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Int Med. 2005;165(4):424–429. [DOI] [PubMed] [Google Scholar]
- 4.Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323. [DOI] [PubMed] [Google Scholar]
- 5.Kaboli PJ, McClimon BJ, Hoth AB, Barnett MJ. Assessing the accuracy of computerized medication histories. Am J Managed Care. 2004;10(11 Pt 2):872–877. [PubMed] [Google Scholar]
- 6.Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. The New Engl J Med. 2007;356(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 7.Pete Welch W, Stearns SC, Cuellar AE, Bindman AB. Use of hospitalists by medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2):mmrr2014.004.02.b01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dementia. 2013;9(1):63–75.e2. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, MD; 2014. [PubMed] [Google Scholar]
- 11.Glintborg B, Poulsen HE, Dalhoff KP. The use of nationwide on-line prescription records improves the drug history in hospitalized patients. Brit J Clin Pharmacol. 2008;65(2):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekedahl A, Brosius H, Jonsson J, Karlsson H, Yngvesson M. Discrepancies between the electronic medical record, the prescriptions in the Swedish national prescription repository and the current medication reported by patients. Pharmacoepidemiol Drug Safety. 2011;20(11):1177–1183. [DOI] [PubMed] [Google Scholar]
- 13.Grimes T, Fitzsimons M, Galvin M, Delaney T. Relative accuracy and availability of an Irish National Database of dispensed medication as a source of medication history information: observational study and retrospective record analysis. J Clin Pharmacy Therapeutics. 2013;38(3):219–224. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Poissant L, Huang A, et al. Estimating the information gap between emergency department records of community medication compared to on-line access to the community-based pharmacy records. J Am Med Inform Assoc. 2014;21(3):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hospital Med. 2010;5(8):477–485. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb LK, Stone EM, Stone D, Dunbrack LA, Calladine J. Regulatory and policy barriers to effective clinical data exchange: lessons learned from MedsInfo-ED. Health Affairs. 2005;24(5):1197–1204. [DOI] [PubMed] [Google Scholar]
- 18.Simonaitis L, Belsito A, Overhage JM. Enhancing an ePrescribing system by adding medication histories and formularies: the Regenstrief Medication Hub. AMIA Annual Symposium Proceedings/AMIA Symposium AMIA Symposium. 2008:677–681. [PMC free article] [PubMed] [Google Scholar]
- 19.Warholak TL, McCulloch M, Baumgart A, Smith M, Fink W, Fritz W. An exploratory comparison of medication lists at hospital admission with administrative database records. J Managed Care Pharmacy. 2009;15(9):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisse ME, Tang L, Belsito A, Overhage JM. Development and use of a medication history service associated with a health information exchange: architecture and preliminary findings. AMIA Annual Symposium Proceedings/AMIA Symposium AMIA Symposium. 2010;2010:242–245. [PMC free article] [PubMed] [Google Scholar]
- 21.Pfoh ER, Abramson E, Edwards A, et al. The Comparative Value of 3 Electronic Sources of Medication Data. Am J Pharm Benefits. 2014;6(5):217–224. [Google Scholar]
- 22.Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient-Centered Res Rev. 2015;2:93 http://dx.doi.org/10.17294/2330-0698.1089. [Google Scholar]
- 23.Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J General Int Med. 2008;23(9):1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster C. A note on the interpretation of weighted kappa and its relations to other rater agreement statistics for metric scales. Educ Psychol Meas. 2004;64(2):243–253. [Google Scholar]
- 25.Davison AC. Bootstrap Methods and their Application. Cambridge University Press; 1997. [Google Scholar]
- 26.Peterson JF, Shi Y, Denny JC, et al. Prevalence and clinical significance of discrepancies within three computerized pre-admission medication lists. AMIA Annual Symposium Proceedings/AMIA Symposium AMIA Symposium. 2010:642–646. [PMC free article] [PubMed] [Google Scholar]
- 27.Clay BJ, Halasyamani L, Stucky ER, Greenwald JL, Williams MV. Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hospital Med. 2008;3(6):465–472. [DOI] [PubMed] [Google Scholar]
- 28.Boockvar KS, Santos SL, Kushniruk A, Johnson C, Nebeker JR. Medication reconciliation: barriers and facilitators from the perspectives of resident physicians and pharmacists. J Hospital Med. 2011;6(6):329–337. [DOI] [PubMed] [Google Scholar]
- 29.Surescripts 2013 National Progress Report and Safe-Rx Rankings. Surescripts LLC; 2014 Arlington VA.